User login
Primary analysis confirms interim findings of CTL019 in DLBCL
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene. ![]()
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene. ![]()
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene. ![]()
CRP drives bone destruction in myeloma, team says
New research suggests that C-reactive protein (CRP) drives multiple myeloma (MM) to destroy bone.
Researchers found that CRP accelerated the onset of bone destruction and made bone damage more severe in mouse models of MM.
The team also observed an association between elevated serum CRP levels and greater degree of bone damage in newly diagnosed MM patients.
The researchers therefore believe that CRP might be targeted to prevent or treat MM-associated bone disease.
Qing Yi, MD, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his colleagues conducted this research and reported the results in Science Translational Medicine.
The researchers noted that high levels of circulating CRP have been associated with poor prognosis in many cancers, including MM.
In a previous study, the team found that CRP enhanced MM cell proliferation under stressed conditions and protected MM cells from chemotherapy-induced apoptosis.
Now, the researchers have found that CRP activates MM cells to promote osteoclastogenesis and bone destruction.
In experiments with mouse models, the team found that CRP promoted MM-cell-mediated lytic bone disease. The researchers said CRP enhanced osteoclast differentiation and bone resorption activity.
In vitro experiments showed that CRP stimulates MM cells to produce osteoclast activators such as RANKL, MCP-1, and MIP-1a.
Further investigation revealed that CRP binds to CD32 on MM cells. This activates a pathway mediated by the kinase p38 MAPK and the transcription factor Twist, which increases MM cells’ production of osteolytic cytokines.
Finally, the researchers analyzed samples from newly diagnosed MM patients.
The team found that serum CRP levels “significantly and positively” correlated with the number of bone lesions patients had. And CRP was abundant in lesion biopsies from individuals with severe skeletal disease. ![]()
New research suggests that C-reactive protein (CRP) drives multiple myeloma (MM) to destroy bone.
Researchers found that CRP accelerated the onset of bone destruction and made bone damage more severe in mouse models of MM.
The team also observed an association between elevated serum CRP levels and greater degree of bone damage in newly diagnosed MM patients.
The researchers therefore believe that CRP might be targeted to prevent or treat MM-associated bone disease.
Qing Yi, MD, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his colleagues conducted this research and reported the results in Science Translational Medicine.
The researchers noted that high levels of circulating CRP have been associated with poor prognosis in many cancers, including MM.
In a previous study, the team found that CRP enhanced MM cell proliferation under stressed conditions and protected MM cells from chemotherapy-induced apoptosis.
Now, the researchers have found that CRP activates MM cells to promote osteoclastogenesis and bone destruction.
In experiments with mouse models, the team found that CRP promoted MM-cell-mediated lytic bone disease. The researchers said CRP enhanced osteoclast differentiation and bone resorption activity.
In vitro experiments showed that CRP stimulates MM cells to produce osteoclast activators such as RANKL, MCP-1, and MIP-1a.
Further investigation revealed that CRP binds to CD32 on MM cells. This activates a pathway mediated by the kinase p38 MAPK and the transcription factor Twist, which increases MM cells’ production of osteolytic cytokines.
Finally, the researchers analyzed samples from newly diagnosed MM patients.
The team found that serum CRP levels “significantly and positively” correlated with the number of bone lesions patients had. And CRP was abundant in lesion biopsies from individuals with severe skeletal disease. ![]()
New research suggests that C-reactive protein (CRP) drives multiple myeloma (MM) to destroy bone.
Researchers found that CRP accelerated the onset of bone destruction and made bone damage more severe in mouse models of MM.
The team also observed an association between elevated serum CRP levels and greater degree of bone damage in newly diagnosed MM patients.
The researchers therefore believe that CRP might be targeted to prevent or treat MM-associated bone disease.
Qing Yi, MD, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his colleagues conducted this research and reported the results in Science Translational Medicine.
The researchers noted that high levels of circulating CRP have been associated with poor prognosis in many cancers, including MM.
In a previous study, the team found that CRP enhanced MM cell proliferation under stressed conditions and protected MM cells from chemotherapy-induced apoptosis.
Now, the researchers have found that CRP activates MM cells to promote osteoclastogenesis and bone destruction.
In experiments with mouse models, the team found that CRP promoted MM-cell-mediated lytic bone disease. The researchers said CRP enhanced osteoclast differentiation and bone resorption activity.
In vitro experiments showed that CRP stimulates MM cells to produce osteoclast activators such as RANKL, MCP-1, and MIP-1a.
Further investigation revealed that CRP binds to CD32 on MM cells. This activates a pathway mediated by the kinase p38 MAPK and the transcription factor Twist, which increases MM cells’ production of osteolytic cytokines.
Finally, the researchers analyzed samples from newly diagnosed MM patients.
The team found that serum CRP levels “significantly and positively” correlated with the number of bone lesions patients had. And CRP was abundant in lesion biopsies from individuals with severe skeletal disease. ![]()
Providers endorse medical marijuana for kids with cancer
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
A survey of nearly 300 US medical providers revealed that many were open to helping children with cancer access medical marijuana (MM).
However, most of the providers surveyed did not know state-specific regulations pertaining to MM.
Providers who were legally eligible to certify (ETC) for MM were less open to endorsing its use.
The lack of standards on formulations, dosing, and potency of MM was identified as the greatest barrier to recommending MM for children with cancer.
Kelly Michelson, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago in Illinois, and her colleagues reported these findings in Pediatrics.
The researchers used a 32-item survey to assess MM practices, knowledge, attitudes, and barriers for pediatric oncology providers in Illinois, Massachusetts, and Washington.
The survey was sent to providers at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Seattle Children’s Cancer and Blood Disorders Center, and Lurie Children’s Center for Cancer and Blood Disorders.
There were 288 respondents, and 33% were legally ETC for MM. Eighty-six percent of ETC providers were physicians, and 14% were nurse practitioners or physician assistants.
Of the non-ETC providers, 89% were nurses, 8% were nurse practitioners or physician assistants, 2% were psychosocial providers, and 2% were “other” providers.
Thirty percent of all providers said they had received at least 1 request for MM in the previous month. And 14% of these providers facilitated patient access to MM.
Ninety-two percent of providers said they were willing to help pediatric cancer patients access MM. Fifty-seven percent of providers approved of patients smoking MM, 89% approved of oral formulations, 67% approved of using MM as cancer-directed therapy, and 92% approved of using MM to manage symptoms.
Fifty-nine percent of providers knew that MM is against federal laws, and 86% knew that their state had legalized MM, but only 5% knew state-specific regulations.
ETC providers were less likely to report willingness to help patients access MM. These providers were also less likely to approve of MM use by smoking, oral formulations, as cancer-directed therapy, or to manage symptoms.
“It is not surprising that providers who are eligible to certify for medical marijuana were more cautious about recommending it, given that their licensure could be jeopardized due to federal prohibition,” Dr Michelson said.
“Institutional policies also may have influenced their attitudes. Lurie Children’s, for example, prohibits pediatric providers from facilitating medical marijuana access in accordance with the federal law, even though it is legal in Illinois.”
Most providers considered MM more permissible for use in children with advanced cancer or near the end of life than in earlier stages of cancer treatment. This is consistent with the current American Academy of Pediatrics position that sanctions MM use for “children with life-limiting or seriously debilitating conditions.”
Only 2% of providers reported that MM was never appropriate for a child with cancer.
Most providers (63%) were not concerned about substance abuse in children who receive MM or about being prosecuted for helping patients access MM (80%).
The greatest concern (listed by 46% of providers) was the absence of standards around prescribing MM to children with cancer.
“In addition to unclear dosage guidelines, the lack of high quality scientific data that medical marijuana benefits outweigh possible harm is a huge concern for providers accustomed to evidence-based practice,” Dr Michelson said. “We need rigorously designed clinical trials on the use of medical marijuana in children with cancer.” ![]()
Bladder Complications in MS
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
Q) My patient has multiple sclerosis and complains of feeling weaker, but denies urinary symptoms. Why have I been told to check for urinary tract infection and not just administer steroids?
Bladder complications are extremely common in patients living with multiple sclerosis (MS), occurring in around 80% of this population.1 These complications—which include urinary urgency, failure to fully empty the bladder, incontinence, and difficulty getting to a toilet in time—can increase risk for urinary tract infection (UTI). And because many patients with MS also have sensory problems (eg, neurogenic bladder), they do not always present with the hallmark UTI symptoms of burning or pain with urination.
Often, presenting symptoms include generalized weakness, increased spasticity, or intensified neurologic issues. These can lead patients to believe they are having a relapse, when in fact, a UTI is causing a pseudoexacerbation of their baseline neurologic issues. In addition, frequent nocturia can disrupt sleep and further contribute to MS-related fatigue. Patients may self-induce dehydration by limiting their daytime fluid intake in an effort to avoid bathroom visits.1
In partnership with urology colleagues, you can help mitigate bladder complications in patients with MS; this can entail use of medication or interventions such as in-and-out or straight catheterization, timed voids, Botox, or pelvic floor physical therapy. Behavior modifications—ie, minimizing caffeine intake, limiting alcohol consumption, and stopping fluids early in the evening—can also be beneficial.1,2
Before initiating bladder medication, it is important to review potential adverse effects with the patient. It’s also crucial to ensure that patients are fully emptying their bladders before starting anticholinergic medications, as these can worsen retention.
Which treatment should you choose? Insurance companies tend to prefer generic, older-generation anticholinergics, but bear in mind that these can cause or contribute to cognitive issues (which many patients with MS already have).3 Another medication, such as mirabegron, may be preferable; it’s less likely than anticholinergics to cause dry mouth, which may help with compliance. Also, be aware that anticholinergics can cause blurred vision, which might lead patients to believe they are having optic neuritis or another MS-related visual change.4
That said, it is possible for patients to have a relapse and a UTI simultaneously. Due to potential adverse effects, it is essential to balance the risks and benefits of steroid therapy. Steroids could worsen an untreated infection and may not be appropriate for the patient’s symptoms or chief complaint.
Addressing bladder symptoms can not only help prevent UTIs but can also improve skin integrity, sleep quality, independence, and overall quality of life. A thorough exam and history-taking can alleviate secondary and tertiary urinary complications, as well as avoid unnecessary use of corticosteroids. -DRB
Denise R. Bruen, MSN, APRN-BC, MSCN
University of Virgina, Charlottesville
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
1. Sheehan J. Coping with MS bladder dysfunction. www.everydayhealth.com/multiple-sclerosis/symptoms/coping-with-bladder-dysfunction/. Accessed November 18, 2017.
2. Mayo Clinic. Bladder control: medications for urinary problems. www.mayoclinic.org/diseases-conditions/urinary-incontinence/in-depth/bladder-control-problems/art-20044220. Accessed November 18, 2017.
3. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: are we confused? Rev Urol. 2007;9(4):191-196.
4. Geller EJ, Crane AK, Wells EC, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32(10):697-705.
You aren’t (necessarily) a walking petri dish!
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
Clinical question: Does exposure to a patient with a multidrug-resistant organism result in colonization of a health care provider?
Background: Multidrug-resistant organisms (MDROs) are growing threats in our hospitals, particularly vancomycin-resistant enterococci (VRE) and resistant gram-negative bacteria. The role of the health care team in preventing infection transmission is paramount. If a team member who is caring for a patient with an MDRO or handling lab specimens becomes colonized with these bacteria, he or she could potentially transmit them to the next patient.
Setting: Large academic research hospital.
Synopsis: Staff submitted self-collected rectal swabs, which were then cultured for MDROs. 379 health care personnel (which they defined as having had self-reported exposure to MDROs) were compared with 376 staff members in the control group, who reported no exposure to MDROs. There was a nonsignificant difference between growth of multidrug-resistant organisms between the groups (4.0% vs 3.2%).
Bottom line: This study suggests that occupational exposure to an MDRO does not result in subsequent colonization of the health care provider and may not be a major risk factor for nosocomial transmission.
Citation: Decker BK et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect. 2017 May 12. pii:S1198-743X(17)30270-7.
Dr. Sata is a medical instructor, Duke University Hospital.
After 6 weeks, HealthCare.gov activity still ahead of last year
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.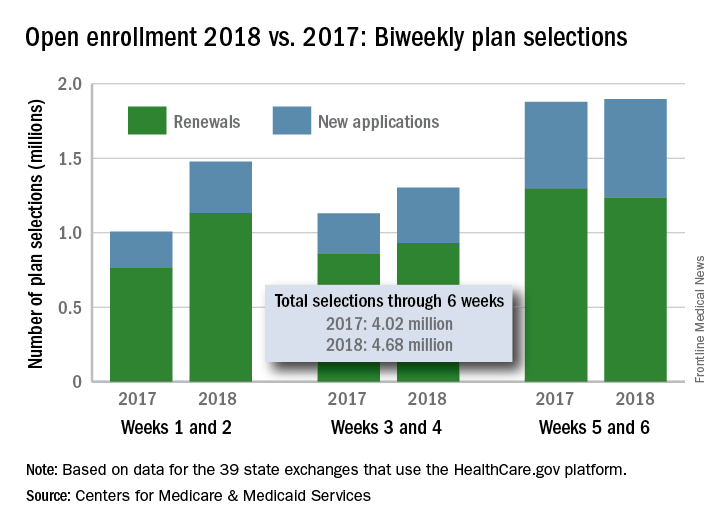
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.
compared with the total at the end of week 4, according to the Centers for Medicare & Medicaid Services.
The 1.89 million plans that consumers selected over the 2-week period ending Dec. 9 brought this year’s total to 4.68 million after 6 weeks. That’s 16.5% higher than last year’s 6-week total of 4.02 million, but the difference has been getting smaller: After week 2 (enrollment figures were released only biweekly last year), the 2018 open season’s tally was higher than the 2017 open season’s week 2 tally by almost 47%, but after 4 weeks, the difference was only 30%, the CMS data show.
Carotid-axillary bypass for revascularization of the left subclavian artery in zone-2 TEVAR
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
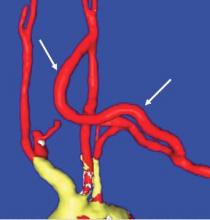
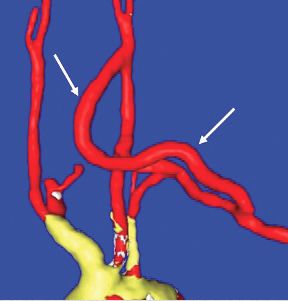
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
Stent-graft coverage of the left subclavian artery (LSA) is often performed during TEVAR treatment of thoracic aortic pathologies and, consequently, debranching of the LSA is frequently performed in such settings. The carotid-subclavian bypass (CSB) is undoubtedly the cervical bypass option preferred by most surgeons for this purpose.1,2 The technique was first described by Lyons and Galbraith in 1957,3 and popularized by Diethrich et al. who reported their large experience in a well-known article published 10 years later.4 In the ensuing decades, CSB became the overwhelming favorite of surgeons everywhere performing LSA revascularization for management of arterial occlusive disease and, more recently, in the context of zone-2 TEVAR. Well- documented good results would seem to justify such preference,5 but some level of concern has been voiced consistently over the years about some technical complexities and potential complications such as phrenic nerve and thoracic duct injuries.6 My own personal experience substantiated these reservations early on, prompting adoption of an alternative operative solution with use of the carotid-axillary bypass (CAB),7 an operation first reported by Shumacker in 1973.8 In my hands, it has produced equivalent results to the carotid-subclavian technique in terms of efficacy and durability, and with the additional appeal of distinct practical advantages – mainly because the axillary artery tends to be an easier vessel to expose and handle, and through the avoidance of complications resulting from damage to anatomical structures that are often in harm’s way when exposing the LSA.
Technical aspects
End-to-side anastomoses proximally and distally are constructed in routine manner (Fig. 2). We do not use carotid shunting for this procedure.
Occasionally one may want to combine a carotid endarterectomy with the cervical bypass, in which case the proximal vascular graft anastomosis is constructed at the endarterectomy site (Fig. 3). Close attention must be paid to careful length-tailoring the conduit to achieve the desirable gently curving course without undue tension or redundancy.
Proximal ligation of the LSA, often performed during the CSB, cannot be a component of the carotid-axillary operation because of inaccessibility. Some experts look upon this as a disadvantage, but I tend to view such limitation as advantageous because it eliminates the potential for a misplaced ligation distal to the left vertebral artery origin which present-day CTA studies show it to be the case more frequently than previously suspected. If interruption of the LSA is deemed necessary, it is arguably best to use an endovascular (retrograde trans-brachial) approach with precise deployment of a vascular plug device under angiographic guidance (Fig. 4). ■
Dr. Criado is at MedStar Union Memorial Hospital, Baltimore.
References
1. J Endovasc Ther 2002;9(suppl 2):1132-1138.
2. Ann Cardiothorac Surg 2013;2:247-260.
3. Ann Surg 1957;146:487-494.
4. Am J Surg 1967;114:800-808.
5. J Vasc Surg 2008;48:555-560.
6. Ann Vasc Surg 2008;22:70-78.
7. J Vasc Surg 1995;22:717-723.
8. Surg Gynecol Obstet 1973;136:447-8.
9. J Vasc Surg 1999;30:1106-1112.
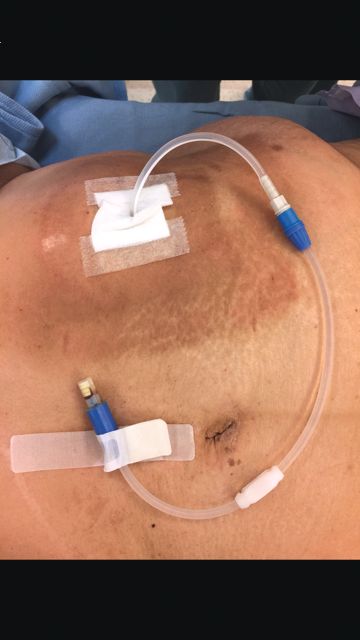
Tips and Tricks: Dealing with a troublesome peritoneal dialysis catheter
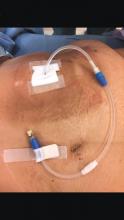
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
When faced with a poorly performing or nonfunctional peritoneal dialysis catheter, there is a very simple trick to make laparoscopic exploration easier. Prep the catheter into the field and take extra care to prep the cover of the catheter. It is usually easier to remove the extended portion of the catheter (A) and just leave the shorter piece (B).
As soon as the port is in, simply switch the CO2 over to it from the catheter. This technique comes in handy quite often, as many patients with difficult PD catheters have undergone multiple explorations or laparoscopies. Not rocket science, but it can definitely save you and the patient some difficulty!
Dr. Rigberg is Clinical Professor of Surgery and Program Director, University of California, Los Angeles, Division of Vascular Surgery, and an associate medical editor for Vascular Specialist.
The men and women of vascular surgery
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
From the Editor
Recent news events have detailed the many humiliations and abuses, both verbal and physical, that women, and some men, have to endure in the workforce. It would not surprise me if some vascular surgeons admit that they have heard of similar instances of egregious behavior occurring in our workplaces. The people that have been impacted have predominantly been women and have come from all walks of life. They have been patients, colleagues, our employees or those of the many institutions in which we work. Unfortunately, the demands of our profession and the pace of our lives may diminish our relationships with these persons. This facelessness and disconnection may allow some surgeons to justify their poor behavior whereas others may not realize that they are negatively impacting these individuals’ lives. The fact that these injustices persist is made more upsetting because we are so indebted for all that these nurses, technologists, office personnel, and even patients, do for us.
Just think how much we owe the nurses on the hospital floors. It is to nurses that we entrust the postoperative care of our patients. They make sure to call us when they detect that a pulse is weakening or suddenly absent, or that a neck is expanding as a hematoma threatens breathing. They timely diagnose a retroperitoneal bleed that may endanger the patient’s life. Dialysis nurses notify us that a puncture looks like it may suddenly bleed out. Our patients’ lives are often entirely dependent on the astute observation of an accomplished nurse.
And what about the operating nurses and scrub technicians? They lay out our surgical tray perfectly with all the tools that we are wont to use. They are there to assist when a sudden event requires the steady hand of an observant nurse who knows just what instrument we need without us having to ask. When you are in a difficult area, an encouraging word will often inspire the confidence required to accomplish a successful outcome. When a procedure is going poorly and tension mounts, their silence accepts our sometimes curt requests. There is a bond that develops between two professionals who recognize each other’s expertise.
Vascular technologists work tirelessly, often in darkened rooms, frequently under challenging positions straining eyes and limbs to detect pathology that may be life or limb saving. Their diagnostic acumen can be the difference between a subsequent procedure’s success or failure. Indeed, the vascular surgeon has to make the final interpretation, but if the technologist fails to show the pathology, even the most erudite physician may miss the diagnosis.
Front-desk personnel who sit at check-in and check-out in an office are the face of our practice. Their friendly attitude welcomes our patients and reassures them that they have come to a well-run, professional workplace. A smiling, personal greeting will calm even the most worried patient. Of course, their attention to detail assures that collections will not be misplaced.
Our office nurses exude compassion for the many patients who face immense hurdles in living with vascular disease. They assist in teaching wound care, explain medications, and help in arranging social services. They cry with those that have recently lost a spouse or child and get excited to hear of the birth of a patient’s grandchild. Without their organizational skills, office hours would be interminable, and patients who are kept waiting would complain, or worse, leave the practice. They have learned to laugh at the same joke that they have heard us tell innumerable times, and to ignore the sometimes lousy mood we may bring into the office after a brutal night on call.
The spouses or significant others of our patients also play an important role since it is often from them that we get the most accurate history. They will ask to speak to us privately to make sure we do not cause despair when we discuss treatment options or to ensure that we firmly admonish their loved one to stop smoking, exercise or watch their weight. Unfortunately, they will sometimes have to accept a disparaging remark or gesture from their “spouse” to make sure that we are supplied all the necessary information to come to an appropriate diagnosis.
I can go on about other medical personnel that contribute to our success, but I believe I have made the point. The men and women with whom we interact as vascular surgeons deserve the same respect we grant ourselves. Any insult to them demeans not only the recipient but more so the abuser and those of us who stand by silently.
Finally, there are many female colleagues whose interest and drive has allowed them to not only break into but achieve leadership positions in a specialty that was almost uniformly male and unwelcoming. Their aptitudes and attitudes have broadened the specialty’s ability to help our patients. However, recently the news has been replete with evidence that women have been abused as they tried to enter other male-dominated professions and so it is likely that this has happened in ours.
Other recent news items suggest that these physical and emotional abuses are inflicted not only on women but also men. We may never know the scope of this mistreatment, but we must assure that it stops immediately.
Ethical behavior must be gender neutral. Further, condescending attitudes, cruel language, and a lack of appreciation sometimes can be as damaging as physical or sexual abuse and must be abolished from our workplace. ■
Should carotid body tumors be embolized preoperatively?
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.
Preop embolization is safe and effective
To embolize or not to embolize ... that is the question when it comes to the management of carotid body tumors. Carotid body tumors (CBTs) are rare benign neoplasms that are almost always nonfunctional and account for up to 80% of all head and neck paragangliomas. Radiographic characteristics include tumor location at the carotid bifurcation, splaying apart the internal and external carotid arteries. Surgical resection is the mainstay of definitive treatment and is preferably performed at diagnosis. CBTs are categorized based upon their involvement with surrounding structures: Shamblin I tumors are small, without encasement of the carotid arteries; Shamblin II tumors partially encase the vessels; and Shamblin III tumors completely encase the vessels. When it comes to operative complications, advanced Shamblin stage is associated with a higher rate of cranial nerve injury when the tumor is resected.1
Pertinent to the technical aspects of surgical resection, CBTs are characteristically encased with an elaborate network of friable vessels which may contribute to significant intraoperative bleeding, especially with resection of larger tumors. The blood supply to CBTs typically originates from the branches of the external carotid artery. These branches may be sacrificed with minimal consequence using preoperative embolization. This common practice is felt by many to facilitate safe resection while minimizing intraoperative blood loss. Although the reduction of blood loss has not been observed universally, there is enough evidence of this in the literature to support the use of selective preoperative embolization. Several embolic agents have been described in this setting, including polyvinyl alcohol particles (150-1000 microns), polymerized glue (n-butyl cyanoacrylate), and more recently, ethylene-vinyl alcohol copolymer (Onyx, ev3, Irvine, Calif.). Risks of complications related to CBT embolization in experienced hands are quite low with no adverse events reported in a number of reports describing the technique.2,3
Although embolization is argued to decrease blood loss, its impact on rates of neurologic complications is admittedly insignificant. One might question the added cost of the embolization procedure in the current atmosphere of focus on cost-containment, but this may be defrayed by lower rates of postoperative hematoma and operative times. Although blood loss has been shown to be decreased in a number of reports, translating this to decreased need for transfusion has unfortunately yet to be demonstrated. Until large-volume prospective randomized studies of the outcomes associated with preoperative embolization of CBTs are available, its use should be considered mainly when risks of blood loss are significant in advanced stage tumors and only if the associated complication risks associated with its use can be kept to acceptably low rates. As with other adjunctive procedures whose merit has not yet been convincingly sorted out in the available data, personal experience and preference often play a role in the decision making process we all face. For those who have experienced the added challenge of the meticulous dissection of the CBT in the face of a bloodied field, having such tools as embolization at our fingertips is a reassuring adjunct to be considered.
References
1. J Vasc Surg. 2013;57:64-8S.
2. J Vasc Interv Neurol. 2008;1(2):37-41.
3. Am J Neuroradiol. 2009;30:1594-97.
4. J Vasc Surg. 2012;56:979-89.
5. Vasc Endovasc Surg. 2009;43:457-61.
Too many downsides
As vascular surgeons, we are frequently confronting situations where the “best” approach to a problem is far from established. The evidence to make a clinical decision may simply not exist, and we need to choose a course of therapy based more on personal preference than science. Such is the case with preoperative embolization for the resection of carotid body tumors (CBT).
It has been over 35 years since the technique for preoperative embolization of CBT was described, the aim being reduction in blood loss, decreased operative time, and improved visualization for safer tumor resection. This is based on the fact that most of the arterial supply to these tumors arises from external carotid branches (particularly the ascending pharyngeal), and these that can be safely sacrificed. Although this technique is certainly now a standard part of treatment for many vascular surgeons, the evidence of benefit is certainly not overwhelming, and like all interventions, nothing comes without risks.
So what are the downsides of preoperative embolization? The cost of the procedure itself is not insignificant, in some cases doubling the expense of the intervention. And there can also be organizational issues with coordinating the time in the endovascular suite versus the operating room. In my experience, we have taken patients directly from embolization for the CBT resection, and there are frequently delays and transport issues. Although advocates may claim decreased operative time as a benefit of embolization, this is attained at an overall increase in anesthetic time for the combined procedures. If a day or two separates the procedures, some patients do report the pain or fevers that can accompany any tumor embolization.
While the above may be nuisances, there are serious adverse effects from preoperative embolization as well. A review of 11 studies of preoperative embolization for CBT found a 2.5% rate of complications, including temporary aphasia, vocal cord paralysis, and arterial dissection. Stroke with permanent deficit has also been reported secondary to CBT embolization. Access site hematoma has also been reported as with any catheter-based intervention.
With regards to “selective” preoperative embolization, the literature again offers a mixed bag. Many advocates of embolization rely upon the Shamblin classification for selecting candidates, reserving embolization for Shamblin II and III tumors. However, the extent of carotid involvement is sometimes a surgical diagnosis. Although size of tumor generally correlates with Shamblin class, even smaller tumors can demonstrate arterial wall involvement. Abu-Ghanem provides a succinct review of the uncertainty regarding size, Shamblin class, and the impact these have on cranial nerve injury.2 The bottom line is that there is no data-based algorithm for deciding whom to select for preoperative embolization.
In the absence of compelling data for the use of preoperative embolization, this technique is difficult to recommend. There are undoubtedly cases with giant CBTs where a surgeon will want to take advantage of any preoperative adjuvant therapy that is available. However, for the vast majority of CBTs, the important principles are to stay in the correct plane, identify and protect the nerves at risk, and control the tumor blood supply in a systematic fashion. In the absence of a forthcoming randomized prospective study to evaluate preoperative embolization for CBT resection, I will rely on the wisdom of my mentor, Dr. Wesley Moore. He never utilized preoperative embolization for these cases. At least not that he will admit to me.
References
1. Surgery. 1980;87:459-464.
2. Head Neck. 2016;38:E2386-E2394.
3. J Vasc Surg. 2015;61:1081-91.
4. Otolaryngol Head Neck Surg. 2015;153:942-950.