User login
Pediatric Dermatology Consult - November 2017
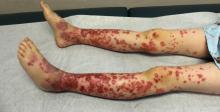
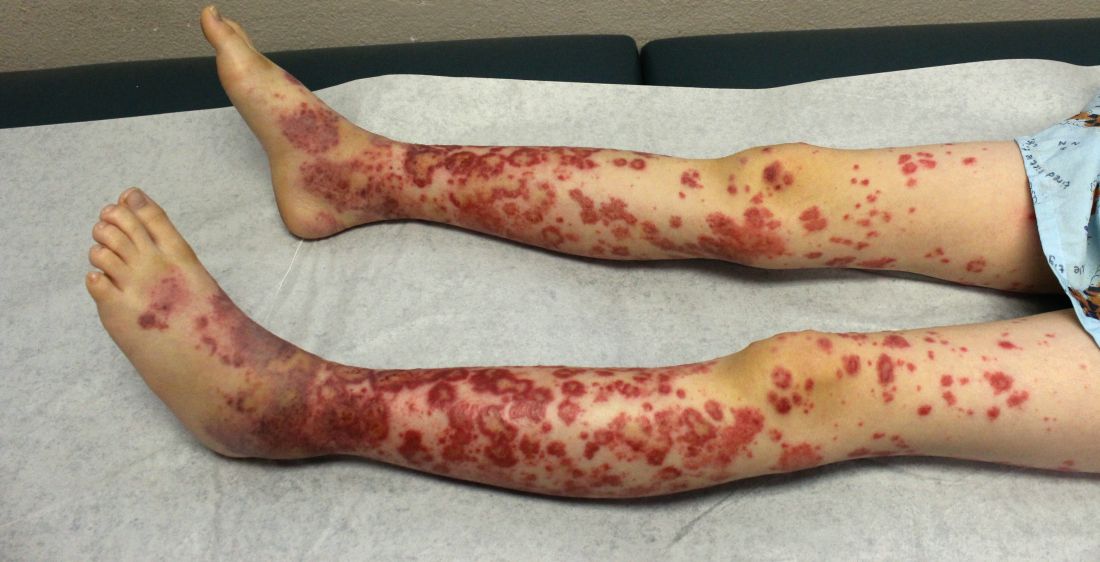
The patient was diagnosed with Henoch-Schönlein purpura (HSP) based on clinical presentation of the lesions and associated symptoms of arthralgia and abdominal pain. Urinalysis was obtained and found to be unremarkable, at presentation and follow-up, and treatment with naproxen 5 mg/kg divided into two doses per day was started for pain relief. A prednisone taper starting at 1 mg/kg per day for 3 weeks also was started due to the presence of severe abdominal pain and bullae on exam. The patient was followed with regular urine studies and blood pressure checks for 2 months, and these also were within normal limits.
HSP, also known as anaphylactoid purpura and immunoglobulin A (IgA) vasculitis, is a small vessel leukocytoclastic vasculitis characterized by the perivascular deposition of IgA1-based immune complexes in the walls of arterioles and postcapillary venules.1 In the vast majority of cases, the condition resolves spontaneously in 4-6 weeks and does not require any specific treatment,2 although NSAIDs and systemic corticosteroids can be used for mild-to-moderate and severe pain, respectively.3
HSP is the most common vasculitis in children, with a peak incidence in boys under the age of 5 years. It occurs worldwide, more commonly among whites and Asians, less commonly among blacks, and recent studies from the Czech Republic,4 Taiwan,5 Spain,6 France,7 South Korea,8 and the United Kingdom9 have shown similar incidence rates of 10-20 per 100,000 children. HSP does occur in adults, but is less common, and is known to carry a worse prognosis – in particular, a higher risk of progression to chronic kidney disease. The disease is more commonly seen in winter months,1 unsurprisingly as upper respiratory tract infections also are more common in these months.10
Pathogenesis
The exact pathogenesis of HSP is the subject of ongoing investigation and continued controversy. Mutations and polymorphisms in mannose-binding lectin, interleukins 1 and 8, vascular endothelial growth factor, and alpha-1-antitrypsin have been associated with HSP.3 Immunoglobulin A (IgA) normally exists in two heavily glycosylated forms – IgA1 and IgA2. Abnormal glycosylation, particularly undergalactosylation, of IgA1, the predominant form of IgA in serum and mucosal secretions, has been linked to HSP.11 HSP has been associated with group A streptococcal infections, Bartonella henselae (cat scratch fever) and numerous drugs,12 although no definitive causal or mechanistic explanation has been identified.
Diagnosis
Two major diagnostic criteria for HSP are widely in use, one developed by the American College of Rheumatology (ACR) in 199013 and the other by the European League Against Rheumatism (EULAR) in 2005.14 Both the ACR and EULAR criteria include acute abdominal pain, purpura, and microscopic evidence of vasculitis. Almost all patients with HSP have cutaneous purpura, and many of these patients have palpable purpura, which is pathognomonic of a leukocytoclastic vasculitis, but palpable purpura is not needed for diagnosis. The ACR criteria additionally include age of 20 years or younger, while the EULAR criteria include arthralgias and the presence of hematuria or proteinuria. Ancillary testing usually is not required to make the diagnosis, but when the diagnosis is not clear histopathologic analysis of a skin sample can identify leukocytoclastic vasculitis. Other laboratory studies that may be needed to rule out other conditions, as well as other organ involvement, include a complete blood count, which can be done to rule out thrombocytopenia as a cause of purpura, a metabolic panel, coagulation studies, occult blood test of stool, abdominal imaging, and urinalysis (UA), which can identify proteinuria or hematuria.
Abdominal pain in HSP is believed to be a result of vasculitis of the gastric, mesenteric, and/or colic vasculature. Bleeding from the inflamed vasculature rarely can lead to gross hematochezia, frank melena, or hematemesis. One serious, potential complication of HSP-related mesenteric vasculitis is intussusception, which is otherwise rare in children older than 2 years. Intussusception should be suspected if features of the classic triad of episodic abdominal pain, sausage-shaped abdominal mass, and currant jelly stool are present. Abdominal ultrasound can help to determine whether intussusception is present.
The purpura in HSP presents in waves or crops, and crops last 5-10 days each. Complete resolution takes 4-6 weeks. If biopsy is desired to confirm the diagnosis, it should be done on a lesion less than 24 hours old. This allows for identification of perivascular IgA on histopathology: beyond 24 hours, IgG and IgM also leak out, contributing to a less specific histopathologic picture.
Accurate diagnosis of HSP is important to guide therapy and anticipate potential complications. Wegener’s granulomatosis (A), also known as granulomatosis with polyangiitis, classically involves the upper and lower respiratory tract and the kidneys, leading to a presentation of epistaxis, cough, and hypertension. It occurs more commonly in adults than children. Finkelstein disease (B), also known as acute hemorrhagic edema of infancy (AHEI), is characterized by the development of petechial, urticarial, or targetoid plaques over 24-48 hours with tender edema and fever in children aged less than 2 years. Unlike HSP, AHEI typically does not involve the gastrointestinal tract, kidneys, or joints. Biopsy of skin lesions of AHEI reveals IgA deposition and leukocytoclastic vasculitis, leading some authors to consider it a closely related entity to HSP. Microscopic polyangiitis (D) is an uncommon pauci-immune vasculitis similar to Wegener’s granulomatosis, but lacking granulomas. It presents typically in the 5th decade of life with fever, fatigue, weight loss, and renal involvement. IgA nephropathy (E), also known as synpharyngitic nephritis and Berger disease, is less likely than HSP to cause a rash, joint pain, or abdominal pain. The nomenclature of HSP (whose alternate name is IgA vasculitis) reflects the multi-organ nature of HSP in comparison to IgA nephropathy, which is more likely to be limited to the kidneys.
Treatment
Aside from intussusception and renal disease, which may result from HSP, treatment is not typically required for HSP as it resolves spontaneously. Patients with significant arthralgias are likely to benefit from NSAIDs such as naproxen 5-20 mg/kg per day, although NSAIDs should be avoided if there is significant renal dysfunction or GI bleeding. Patients with severe abdominal pain or joint pain may be more likely to benefit from oral corticosteroids, particularly prednisone 1-2 mg/kg per day. A meta-analysis showed that corticosteroids significantly reduce the duration of symptoms if given early in the course of disease.15
The prognosis is usually excellent, except for a very small sample of the population (5%) that can develop end-stage renal disease. It is recommended that all children with HSP continue monitoring blood pressure and UA either weekly or biweekly for the first 2 months and then once a month for 6-12 months.16
First described in 1801 by a British physician, HSP is a common and usually self-limited disease for which our understanding has advanced greatly over the past 2 centuries, yet for which many important questions regarding pathophysiology remain unanswered. No diagnostic tests or treatments are needed for the majority of patients. Providers should include HSP in the differential diagnosis for the child with unexplained abdominal pain, renal dysfunction, or nonthrombocytopenic purpura.
Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz is a practicing dermatologist at Southern California Permanente Medical Group in La Mesa, California. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. “Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence”, 5th ed. (New York: Elsevier, 2016).
2. Lancet. 2007;369(9566):976-8.
3. “Dermatology”, 3rd ed. (Philadelphia: Elsevier Saunders, 2012).
4. J Rheumatol. 2004 Nov;31(11):2295-9.
5. Rheumatology (Oxford). 2005 May;44(5):618-22.
6. Medicine (Baltimore). 2014 Mar;93(2):106-13.
7. Rheumatology (Oxford). 2017;56(8):1358-66.
8. J Korean Med Sci. 2014 Feb;29(2):198-203.
9. Lancet. 2002 Oct 19;360(9341):1197-202.
10. Rhinology. 2015 Jun;53(2):99-106.
11. PLoS One. 2016 Nov 21;11(11):e0166700.
12. Pediatr Infect Dis J. 2002 Jan;21(1):28-31.
13. Arthritis Rheum. 1990 Aug;33(8):1114-21.
14. Ann Rheum Dis. 2006 Jul;65(7):936-41.
15. Pediatrics. 2007 Nov;120(5):1079-87.
16. Arch Dis Child. 2010 Nov;95(11):877-82.
The patient was diagnosed with Henoch-Schönlein purpura (HSP) based on clinical presentation of the lesions and associated symptoms of arthralgia and abdominal pain. Urinalysis was obtained and found to be unremarkable, at presentation and follow-up, and treatment with naproxen 5 mg/kg divided into two doses per day was started for pain relief. A prednisone taper starting at 1 mg/kg per day for 3 weeks also was started due to the presence of severe abdominal pain and bullae on exam. The patient was followed with regular urine studies and blood pressure checks for 2 months, and these also were within normal limits.
HSP, also known as anaphylactoid purpura and immunoglobulin A (IgA) vasculitis, is a small vessel leukocytoclastic vasculitis characterized by the perivascular deposition of IgA1-based immune complexes in the walls of arterioles and postcapillary venules.1 In the vast majority of cases, the condition resolves spontaneously in 4-6 weeks and does not require any specific treatment,2 although NSAIDs and systemic corticosteroids can be used for mild-to-moderate and severe pain, respectively.3
HSP is the most common vasculitis in children, with a peak incidence in boys under the age of 5 years. It occurs worldwide, more commonly among whites and Asians, less commonly among blacks, and recent studies from the Czech Republic,4 Taiwan,5 Spain,6 France,7 South Korea,8 and the United Kingdom9 have shown similar incidence rates of 10-20 per 100,000 children. HSP does occur in adults, but is less common, and is known to carry a worse prognosis – in particular, a higher risk of progression to chronic kidney disease. The disease is more commonly seen in winter months,1 unsurprisingly as upper respiratory tract infections also are more common in these months.10
Pathogenesis
The exact pathogenesis of HSP is the subject of ongoing investigation and continued controversy. Mutations and polymorphisms in mannose-binding lectin, interleukins 1 and 8, vascular endothelial growth factor, and alpha-1-antitrypsin have been associated with HSP.3 Immunoglobulin A (IgA) normally exists in two heavily glycosylated forms – IgA1 and IgA2. Abnormal glycosylation, particularly undergalactosylation, of IgA1, the predominant form of IgA in serum and mucosal secretions, has been linked to HSP.11 HSP has been associated with group A streptococcal infections, Bartonella henselae (cat scratch fever) and numerous drugs,12 although no definitive causal or mechanistic explanation has been identified.
Diagnosis
Two major diagnostic criteria for HSP are widely in use, one developed by the American College of Rheumatology (ACR) in 199013 and the other by the European League Against Rheumatism (EULAR) in 2005.14 Both the ACR and EULAR criteria include acute abdominal pain, purpura, and microscopic evidence of vasculitis. Almost all patients with HSP have cutaneous purpura, and many of these patients have palpable purpura, which is pathognomonic of a leukocytoclastic vasculitis, but palpable purpura is not needed for diagnosis. The ACR criteria additionally include age of 20 years or younger, while the EULAR criteria include arthralgias and the presence of hematuria or proteinuria. Ancillary testing usually is not required to make the diagnosis, but when the diagnosis is not clear histopathologic analysis of a skin sample can identify leukocytoclastic vasculitis. Other laboratory studies that may be needed to rule out other conditions, as well as other organ involvement, include a complete blood count, which can be done to rule out thrombocytopenia as a cause of purpura, a metabolic panel, coagulation studies, occult blood test of stool, abdominal imaging, and urinalysis (UA), which can identify proteinuria or hematuria.
Abdominal pain in HSP is believed to be a result of vasculitis of the gastric, mesenteric, and/or colic vasculature. Bleeding from the inflamed vasculature rarely can lead to gross hematochezia, frank melena, or hematemesis. One serious, potential complication of HSP-related mesenteric vasculitis is intussusception, which is otherwise rare in children older than 2 years. Intussusception should be suspected if features of the classic triad of episodic abdominal pain, sausage-shaped abdominal mass, and currant jelly stool are present. Abdominal ultrasound can help to determine whether intussusception is present.
The purpura in HSP presents in waves or crops, and crops last 5-10 days each. Complete resolution takes 4-6 weeks. If biopsy is desired to confirm the diagnosis, it should be done on a lesion less than 24 hours old. This allows for identification of perivascular IgA on histopathology: beyond 24 hours, IgG and IgM also leak out, contributing to a less specific histopathologic picture.
Accurate diagnosis of HSP is important to guide therapy and anticipate potential complications. Wegener’s granulomatosis (A), also known as granulomatosis with polyangiitis, classically involves the upper and lower respiratory tract and the kidneys, leading to a presentation of epistaxis, cough, and hypertension. It occurs more commonly in adults than children. Finkelstein disease (B), also known as acute hemorrhagic edema of infancy (AHEI), is characterized by the development of petechial, urticarial, or targetoid plaques over 24-48 hours with tender edema and fever in children aged less than 2 years. Unlike HSP, AHEI typically does not involve the gastrointestinal tract, kidneys, or joints. Biopsy of skin lesions of AHEI reveals IgA deposition and leukocytoclastic vasculitis, leading some authors to consider it a closely related entity to HSP. Microscopic polyangiitis (D) is an uncommon pauci-immune vasculitis similar to Wegener’s granulomatosis, but lacking granulomas. It presents typically in the 5th decade of life with fever, fatigue, weight loss, and renal involvement. IgA nephropathy (E), also known as synpharyngitic nephritis and Berger disease, is less likely than HSP to cause a rash, joint pain, or abdominal pain. The nomenclature of HSP (whose alternate name is IgA vasculitis) reflects the multi-organ nature of HSP in comparison to IgA nephropathy, which is more likely to be limited to the kidneys.
Treatment
Aside from intussusception and renal disease, which may result from HSP, treatment is not typically required for HSP as it resolves spontaneously. Patients with significant arthralgias are likely to benefit from NSAIDs such as naproxen 5-20 mg/kg per day, although NSAIDs should be avoided if there is significant renal dysfunction or GI bleeding. Patients with severe abdominal pain or joint pain may be more likely to benefit from oral corticosteroids, particularly prednisone 1-2 mg/kg per day. A meta-analysis showed that corticosteroids significantly reduce the duration of symptoms if given early in the course of disease.15
The prognosis is usually excellent, except for a very small sample of the population (5%) that can develop end-stage renal disease. It is recommended that all children with HSP continue monitoring blood pressure and UA either weekly or biweekly for the first 2 months and then once a month for 6-12 months.16
First described in 1801 by a British physician, HSP is a common and usually self-limited disease for which our understanding has advanced greatly over the past 2 centuries, yet for which many important questions regarding pathophysiology remain unanswered. No diagnostic tests or treatments are needed for the majority of patients. Providers should include HSP in the differential diagnosis for the child with unexplained abdominal pain, renal dysfunction, or nonthrombocytopenic purpura.
Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz is a practicing dermatologist at Southern California Permanente Medical Group in La Mesa, California. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. “Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence”, 5th ed. (New York: Elsevier, 2016).
2. Lancet. 2007;369(9566):976-8.
3. “Dermatology”, 3rd ed. (Philadelphia: Elsevier Saunders, 2012).
4. J Rheumatol. 2004 Nov;31(11):2295-9.
5. Rheumatology (Oxford). 2005 May;44(5):618-22.
6. Medicine (Baltimore). 2014 Mar;93(2):106-13.
7. Rheumatology (Oxford). 2017;56(8):1358-66.
8. J Korean Med Sci. 2014 Feb;29(2):198-203.
9. Lancet. 2002 Oct 19;360(9341):1197-202.
10. Rhinology. 2015 Jun;53(2):99-106.
11. PLoS One. 2016 Nov 21;11(11):e0166700.
12. Pediatr Infect Dis J. 2002 Jan;21(1):28-31.
13. Arthritis Rheum. 1990 Aug;33(8):1114-21.
14. Ann Rheum Dis. 2006 Jul;65(7):936-41.
15. Pediatrics. 2007 Nov;120(5):1079-87.
16. Arch Dis Child. 2010 Nov;95(11):877-82.
The patient was diagnosed with Henoch-Schönlein purpura (HSP) based on clinical presentation of the lesions and associated symptoms of arthralgia and abdominal pain. Urinalysis was obtained and found to be unremarkable, at presentation and follow-up, and treatment with naproxen 5 mg/kg divided into two doses per day was started for pain relief. A prednisone taper starting at 1 mg/kg per day for 3 weeks also was started due to the presence of severe abdominal pain and bullae on exam. The patient was followed with regular urine studies and blood pressure checks for 2 months, and these also were within normal limits.
HSP, also known as anaphylactoid purpura and immunoglobulin A (IgA) vasculitis, is a small vessel leukocytoclastic vasculitis characterized by the perivascular deposition of IgA1-based immune complexes in the walls of arterioles and postcapillary venules.1 In the vast majority of cases, the condition resolves spontaneously in 4-6 weeks and does not require any specific treatment,2 although NSAIDs and systemic corticosteroids can be used for mild-to-moderate and severe pain, respectively.3
HSP is the most common vasculitis in children, with a peak incidence in boys under the age of 5 years. It occurs worldwide, more commonly among whites and Asians, less commonly among blacks, and recent studies from the Czech Republic,4 Taiwan,5 Spain,6 France,7 South Korea,8 and the United Kingdom9 have shown similar incidence rates of 10-20 per 100,000 children. HSP does occur in adults, but is less common, and is known to carry a worse prognosis – in particular, a higher risk of progression to chronic kidney disease. The disease is more commonly seen in winter months,1 unsurprisingly as upper respiratory tract infections also are more common in these months.10
Pathogenesis
The exact pathogenesis of HSP is the subject of ongoing investigation and continued controversy. Mutations and polymorphisms in mannose-binding lectin, interleukins 1 and 8, vascular endothelial growth factor, and alpha-1-antitrypsin have been associated with HSP.3 Immunoglobulin A (IgA) normally exists in two heavily glycosylated forms – IgA1 and IgA2. Abnormal glycosylation, particularly undergalactosylation, of IgA1, the predominant form of IgA in serum and mucosal secretions, has been linked to HSP.11 HSP has been associated with group A streptococcal infections, Bartonella henselae (cat scratch fever) and numerous drugs,12 although no definitive causal or mechanistic explanation has been identified.
Diagnosis
Two major diagnostic criteria for HSP are widely in use, one developed by the American College of Rheumatology (ACR) in 199013 and the other by the European League Against Rheumatism (EULAR) in 2005.14 Both the ACR and EULAR criteria include acute abdominal pain, purpura, and microscopic evidence of vasculitis. Almost all patients with HSP have cutaneous purpura, and many of these patients have palpable purpura, which is pathognomonic of a leukocytoclastic vasculitis, but palpable purpura is not needed for diagnosis. The ACR criteria additionally include age of 20 years or younger, while the EULAR criteria include arthralgias and the presence of hematuria or proteinuria. Ancillary testing usually is not required to make the diagnosis, but when the diagnosis is not clear histopathologic analysis of a skin sample can identify leukocytoclastic vasculitis. Other laboratory studies that may be needed to rule out other conditions, as well as other organ involvement, include a complete blood count, which can be done to rule out thrombocytopenia as a cause of purpura, a metabolic panel, coagulation studies, occult blood test of stool, abdominal imaging, and urinalysis (UA), which can identify proteinuria or hematuria.
Abdominal pain in HSP is believed to be a result of vasculitis of the gastric, mesenteric, and/or colic vasculature. Bleeding from the inflamed vasculature rarely can lead to gross hematochezia, frank melena, or hematemesis. One serious, potential complication of HSP-related mesenteric vasculitis is intussusception, which is otherwise rare in children older than 2 years. Intussusception should be suspected if features of the classic triad of episodic abdominal pain, sausage-shaped abdominal mass, and currant jelly stool are present. Abdominal ultrasound can help to determine whether intussusception is present.
The purpura in HSP presents in waves or crops, and crops last 5-10 days each. Complete resolution takes 4-6 weeks. If biopsy is desired to confirm the diagnosis, it should be done on a lesion less than 24 hours old. This allows for identification of perivascular IgA on histopathology: beyond 24 hours, IgG and IgM also leak out, contributing to a less specific histopathologic picture.
Accurate diagnosis of HSP is important to guide therapy and anticipate potential complications. Wegener’s granulomatosis (A), also known as granulomatosis with polyangiitis, classically involves the upper and lower respiratory tract and the kidneys, leading to a presentation of epistaxis, cough, and hypertension. It occurs more commonly in adults than children. Finkelstein disease (B), also known as acute hemorrhagic edema of infancy (AHEI), is characterized by the development of petechial, urticarial, or targetoid plaques over 24-48 hours with tender edema and fever in children aged less than 2 years. Unlike HSP, AHEI typically does not involve the gastrointestinal tract, kidneys, or joints. Biopsy of skin lesions of AHEI reveals IgA deposition and leukocytoclastic vasculitis, leading some authors to consider it a closely related entity to HSP. Microscopic polyangiitis (D) is an uncommon pauci-immune vasculitis similar to Wegener’s granulomatosis, but lacking granulomas. It presents typically in the 5th decade of life with fever, fatigue, weight loss, and renal involvement. IgA nephropathy (E), also known as synpharyngitic nephritis and Berger disease, is less likely than HSP to cause a rash, joint pain, or abdominal pain. The nomenclature of HSP (whose alternate name is IgA vasculitis) reflects the multi-organ nature of HSP in comparison to IgA nephropathy, which is more likely to be limited to the kidneys.
Treatment
Aside from intussusception and renal disease, which may result from HSP, treatment is not typically required for HSP as it resolves spontaneously. Patients with significant arthralgias are likely to benefit from NSAIDs such as naproxen 5-20 mg/kg per day, although NSAIDs should be avoided if there is significant renal dysfunction or GI bleeding. Patients with severe abdominal pain or joint pain may be more likely to benefit from oral corticosteroids, particularly prednisone 1-2 mg/kg per day. A meta-analysis showed that corticosteroids significantly reduce the duration of symptoms if given early in the course of disease.15
The prognosis is usually excellent, except for a very small sample of the population (5%) that can develop end-stage renal disease. It is recommended that all children with HSP continue monitoring blood pressure and UA either weekly or biweekly for the first 2 months and then once a month for 6-12 months.16
First described in 1801 by a British physician, HSP is a common and usually self-limited disease for which our understanding has advanced greatly over the past 2 centuries, yet for which many important questions regarding pathophysiology remain unanswered. No diagnostic tests or treatments are needed for the majority of patients. Providers should include HSP in the differential diagnosis for the child with unexplained abdominal pain, renal dysfunction, or nonthrombocytopenic purpura.
Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz is a practicing dermatologist at Southern California Permanente Medical Group in La Mesa, California. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. “Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence”, 5th ed. (New York: Elsevier, 2016).
2. Lancet. 2007;369(9566):976-8.
3. “Dermatology”, 3rd ed. (Philadelphia: Elsevier Saunders, 2012).
4. J Rheumatol. 2004 Nov;31(11):2295-9.
5. Rheumatology (Oxford). 2005 May;44(5):618-22.
6. Medicine (Baltimore). 2014 Mar;93(2):106-13.
7. Rheumatology (Oxford). 2017;56(8):1358-66.
8. J Korean Med Sci. 2014 Feb;29(2):198-203.
9. Lancet. 2002 Oct 19;360(9341):1197-202.
10. Rhinology. 2015 Jun;53(2):99-106.
11. PLoS One. 2016 Nov 21;11(11):e0166700.
12. Pediatr Infect Dis J. 2002 Jan;21(1):28-31.
13. Arthritis Rheum. 1990 Aug;33(8):1114-21.
14. Ann Rheum Dis. 2006 Jul;65(7):936-41.
15. Pediatrics. 2007 Nov;120(5):1079-87.
16. Arch Dis Child. 2010 Nov;95(11):877-82.
Clinical presentation
A healthy 9-year-old boy presents with 1 week history of a rash that began as “bruises” on both ankles that subsequently ascended over a few days to the proximal lower extremities and upper extremities. The rash has been painful and pruritic at times. The patient’s mother reports regular application of hydrocortisone cream for itch and pain relief, and this has been somewhat successful.
The patient has a history of longstanding constipation and abdominal pain, but over the past week has reported abdominal pain that is different and more severe than his usual abdominal pain. This abdominal pain has limited oral intake over the past 2 days. The patient and family also report bilateral pain of the wrists and elbows, which has limited his daily activities. The patient and mother deny fevers, chills, cough, coryza, and any sick contacts.
His vital signs are stable. On physical examination there is mild conjunctival injection, no intraoral lesions, and no lymphadenopathy or hepatosplenomegaly. The abdomen is not distended but it is tender to deep palpation. Bowel sounds are present. On skin examination, there are multiple purpuric annular plaques with central clearing, some with bullae and petechiae, on the bilateral buttocks and legs. There is bilateral pedal edema. On the arms, there are a few polymorphic pink and red annular to targetoid plaques.
Can McArdle’s Sign Help Diagnose MS?
PARIS—McArdle’s sign, a rapidly reversible motor weakness induced by head flexion in patients with suspected multiple sclerosis (MS), may facilitate diagnosis in certain clinical situations, according to a study presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. “McArdle’s sign, when defined as a greater than 10% neck flexion-induced reduction using isoinertial finger extension on a measurement device, is highly specific and moderately sensitive for a diagnosis of MS,” said Brian G. Weinshenker, MD, and colleagues. Dr. Weinshenker is Professor of Neurology at the Mayo Clinic in Rochester, Minnesota.
Dr. Weinshenker and colleagues quantified McArdle’s sign in finger extensors using a torque measuring device and assessed its specificity for MS. They enrolled 25 healthy controls and 76 patients with detectable finger extensor weakness, 52 with MS, 24 with other myelopathies, and five with peripheral nerve lesions. Patients were not selected for having McArdle’s sign. Dr. Weinshenker and his team evaluated McArdle’s sign blinded to diagnosis by measuring change in finger extensor strength in successive trials of head extension and flexion, first clinically and then with a torque measuring device. McArdle’s sign was clinically rated from zero (absent) to three (marked). In the quantitative measurement, the patient applied maximum extension strength of four fingers on a bar using isometric (against fixed object) and isoinertial (against a constant resistance) maneuvers. The researchers then averaged the percentage decrease in strength over four trials.
Baseline strength was similar in the three patient groups. The median clinical McArdle’s sign was one (range, zero to three) in patients with MS, zero (range, zero to two) in other myelopathies, zero (range, zero to one) in healthy controls, and zero in all patients with peripheral nerve lesions. The isometric and isoinertial maneuvers provided similar quantitative results, but the isoinertial maneuver had superior diagnostic performance. Head flexion resulted in 17% (± 17%) isoinertial strength reduction in patients with MS versus 1% (± 6%) in other myelopathies, 1% (± 5%) in healthy controls and -3% (± 10%) in patients with peripheral nerve lesions.
A multivariate regression analysis eliminated confounding by baseline strength. Receiver operator curves were generated to assess the diagnostic properties of the test; the area under the curve was 0.82 in patients with MS versus healthy controls and 0.83 in patients with MS versus other myelopathies for isoinertial testing. A 10% drop in strength with flexion was 100% specific and 62% sensitive for MS compared with other myelopathies and a 6% drop, 92% specific and 73% sensitive, for MS compared with healthy controls. Quantitative McArdle’s sign correlated with clinical McArdle’s sign by referring physician and technician (r = 0.58). McArdle’s sign correlated with Expanded Disability Status Scale (r = 0.41) and pyramidal score (r = 0.49) in patients with MS, but was evident in some patients in very early phases of MS and minor disability.
PARIS—McArdle’s sign, a rapidly reversible motor weakness induced by head flexion in patients with suspected multiple sclerosis (MS), may facilitate diagnosis in certain clinical situations, according to a study presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. “McArdle’s sign, when defined as a greater than 10% neck flexion-induced reduction using isoinertial finger extension on a measurement device, is highly specific and moderately sensitive for a diagnosis of MS,” said Brian G. Weinshenker, MD, and colleagues. Dr. Weinshenker is Professor of Neurology at the Mayo Clinic in Rochester, Minnesota.
Dr. Weinshenker and colleagues quantified McArdle’s sign in finger extensors using a torque measuring device and assessed its specificity for MS. They enrolled 25 healthy controls and 76 patients with detectable finger extensor weakness, 52 with MS, 24 with other myelopathies, and five with peripheral nerve lesions. Patients were not selected for having McArdle’s sign. Dr. Weinshenker and his team evaluated McArdle’s sign blinded to diagnosis by measuring change in finger extensor strength in successive trials of head extension and flexion, first clinically and then with a torque measuring device. McArdle’s sign was clinically rated from zero (absent) to three (marked). In the quantitative measurement, the patient applied maximum extension strength of four fingers on a bar using isometric (against fixed object) and isoinertial (against a constant resistance) maneuvers. The researchers then averaged the percentage decrease in strength over four trials.
Baseline strength was similar in the three patient groups. The median clinical McArdle’s sign was one (range, zero to three) in patients with MS, zero (range, zero to two) in other myelopathies, zero (range, zero to one) in healthy controls, and zero in all patients with peripheral nerve lesions. The isometric and isoinertial maneuvers provided similar quantitative results, but the isoinertial maneuver had superior diagnostic performance. Head flexion resulted in 17% (± 17%) isoinertial strength reduction in patients with MS versus 1% (± 6%) in other myelopathies, 1% (± 5%) in healthy controls and -3% (± 10%) in patients with peripheral nerve lesions.
A multivariate regression analysis eliminated confounding by baseline strength. Receiver operator curves were generated to assess the diagnostic properties of the test; the area under the curve was 0.82 in patients with MS versus healthy controls and 0.83 in patients with MS versus other myelopathies for isoinertial testing. A 10% drop in strength with flexion was 100% specific and 62% sensitive for MS compared with other myelopathies and a 6% drop, 92% specific and 73% sensitive, for MS compared with healthy controls. Quantitative McArdle’s sign correlated with clinical McArdle’s sign by referring physician and technician (r = 0.58). McArdle’s sign correlated with Expanded Disability Status Scale (r = 0.41) and pyramidal score (r = 0.49) in patients with MS, but was evident in some patients in very early phases of MS and minor disability.
PARIS—McArdle’s sign, a rapidly reversible motor weakness induced by head flexion in patients with suspected multiple sclerosis (MS), may facilitate diagnosis in certain clinical situations, according to a study presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. “McArdle’s sign, when defined as a greater than 10% neck flexion-induced reduction using isoinertial finger extension on a measurement device, is highly specific and moderately sensitive for a diagnosis of MS,” said Brian G. Weinshenker, MD, and colleagues. Dr. Weinshenker is Professor of Neurology at the Mayo Clinic in Rochester, Minnesota.
Dr. Weinshenker and colleagues quantified McArdle’s sign in finger extensors using a torque measuring device and assessed its specificity for MS. They enrolled 25 healthy controls and 76 patients with detectable finger extensor weakness, 52 with MS, 24 with other myelopathies, and five with peripheral nerve lesions. Patients were not selected for having McArdle’s sign. Dr. Weinshenker and his team evaluated McArdle’s sign blinded to diagnosis by measuring change in finger extensor strength in successive trials of head extension and flexion, first clinically and then with a torque measuring device. McArdle’s sign was clinically rated from zero (absent) to three (marked). In the quantitative measurement, the patient applied maximum extension strength of four fingers on a bar using isometric (against fixed object) and isoinertial (against a constant resistance) maneuvers. The researchers then averaged the percentage decrease in strength over four trials.
Baseline strength was similar in the three patient groups. The median clinical McArdle’s sign was one (range, zero to three) in patients with MS, zero (range, zero to two) in other myelopathies, zero (range, zero to one) in healthy controls, and zero in all patients with peripheral nerve lesions. The isometric and isoinertial maneuvers provided similar quantitative results, but the isoinertial maneuver had superior diagnostic performance. Head flexion resulted in 17% (± 17%) isoinertial strength reduction in patients with MS versus 1% (± 6%) in other myelopathies, 1% (± 5%) in healthy controls and -3% (± 10%) in patients with peripheral nerve lesions.
A multivariate regression analysis eliminated confounding by baseline strength. Receiver operator curves were generated to assess the diagnostic properties of the test; the area under the curve was 0.82 in patients with MS versus healthy controls and 0.83 in patients with MS versus other myelopathies for isoinertial testing. A 10% drop in strength with flexion was 100% specific and 62% sensitive for MS compared with other myelopathies and a 6% drop, 92% specific and 73% sensitive, for MS compared with healthy controls. Quantitative McArdle’s sign correlated with clinical McArdle’s sign by referring physician and technician (r = 0.58). McArdle’s sign correlated with Expanded Disability Status Scale (r = 0.41) and pyramidal score (r = 0.49) in patients with MS, but was evident in some patients in very early phases of MS and minor disability.
Serum Neurofilament Light Levels May Reflect the Efficacy of MS Treatments
PARIS—It is possible to gauge the effects of disease-modifying therapies (DMTs) for multiple sclerosis (MS) by measuring serum levels of neurofilament light (NFL), according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. DMTs with greater efficacy appear to be associated with lower serum NFL concentrations.
NFL is a biomarker of axonal damage that has been measured primarily in CSF. In patients with MS, the CSF NFL concentration reflects disease activity and the efficacy of DMTs. Investigators recently developed an ultrasensitive immunoassay that can determine NFL levels in serum.
The investigators measured serum NFL concentrations using an in-house ultrasensitive single molecule array immunoassay. The intra-assay and inter-assay coefficient of variation was less than 10%.
Median serum NFL concentration decreased significantly in treatment-naïve patients who initiated second-line DMTs (ie, from 22.7 ng/L to 18.5 ng/L) or escalated from a first-line to a second-line DMT (ie, from 17.9 ng/L to 12.6 ng/L). The median serum NFL concentration was stable in patients who switched between second-line DMTs (14.9 ng/L before the switch and 13.7 ng/L after the switch). Similarly, the median serum NFL concentration did not change significantly in patients who stayed untreated (40.7 ng/L at first measurement and 37.1 ng/L at second measurement), initiated first-line treatment (20.6 ng/L vs 25.5 ng/L), or switched between first-line DMTs (17.3 ng/L vs 16.7 ng/L).
“The goal of DMTs in MS is to reduce axonal degeneration,” said Dr. Nováková. “Repeated analysis of serum NFL may represent a new possibility to monitor this process and may provide objective support in treatment decisions.”
PARIS—It is possible to gauge the effects of disease-modifying therapies (DMTs) for multiple sclerosis (MS) by measuring serum levels of neurofilament light (NFL), according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. DMTs with greater efficacy appear to be associated with lower serum NFL concentrations.
NFL is a biomarker of axonal damage that has been measured primarily in CSF. In patients with MS, the CSF NFL concentration reflects disease activity and the efficacy of DMTs. Investigators recently developed an ultrasensitive immunoassay that can determine NFL levels in serum.
The investigators measured serum NFL concentrations using an in-house ultrasensitive single molecule array immunoassay. The intra-assay and inter-assay coefficient of variation was less than 10%.
Median serum NFL concentration decreased significantly in treatment-naïve patients who initiated second-line DMTs (ie, from 22.7 ng/L to 18.5 ng/L) or escalated from a first-line to a second-line DMT (ie, from 17.9 ng/L to 12.6 ng/L). The median serum NFL concentration was stable in patients who switched between second-line DMTs (14.9 ng/L before the switch and 13.7 ng/L after the switch). Similarly, the median serum NFL concentration did not change significantly in patients who stayed untreated (40.7 ng/L at first measurement and 37.1 ng/L at second measurement), initiated first-line treatment (20.6 ng/L vs 25.5 ng/L), or switched between first-line DMTs (17.3 ng/L vs 16.7 ng/L).
“The goal of DMTs in MS is to reduce axonal degeneration,” said Dr. Nováková. “Repeated analysis of serum NFL may represent a new possibility to monitor this process and may provide objective support in treatment decisions.”
PARIS—It is possible to gauge the effects of disease-modifying therapies (DMTs) for multiple sclerosis (MS) by measuring serum levels of neurofilament light (NFL), according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. DMTs with greater efficacy appear to be associated with lower serum NFL concentrations.
NFL is a biomarker of axonal damage that has been measured primarily in CSF. In patients with MS, the CSF NFL concentration reflects disease activity and the efficacy of DMTs. Investigators recently developed an ultrasensitive immunoassay that can determine NFL levels in serum.
The investigators measured serum NFL concentrations using an in-house ultrasensitive single molecule array immunoassay. The intra-assay and inter-assay coefficient of variation was less than 10%.
Median serum NFL concentration decreased significantly in treatment-naïve patients who initiated second-line DMTs (ie, from 22.7 ng/L to 18.5 ng/L) or escalated from a first-line to a second-line DMT (ie, from 17.9 ng/L to 12.6 ng/L). The median serum NFL concentration was stable in patients who switched between second-line DMTs (14.9 ng/L before the switch and 13.7 ng/L after the switch). Similarly, the median serum NFL concentration did not change significantly in patients who stayed untreated (40.7 ng/L at first measurement and 37.1 ng/L at second measurement), initiated first-line treatment (20.6 ng/L vs 25.5 ng/L), or switched between first-line DMTs (17.3 ng/L vs 16.7 ng/L).
“The goal of DMTs in MS is to reduce axonal degeneration,” said Dr. Nováková. “Repeated analysis of serum NFL may represent a new possibility to monitor this process and may provide objective support in treatment decisions.”
Can a Saliva Test Provide a New Biomarker for MS?
PARIS—A noninvasive test that assesses immunoglobulin (Ig) free light chains in saliva may detect immunopathologic changes in the multiple sclerosis (MS) disease state and evaluate response to treatment, according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The technique has a specificity of 80% and sensitivity of 89% for diagnosing active MS, said lead author Esther Ganelin-Cohen, MD, PhD, Director of the Neuro-Immunology Clinic at Schneider Children’s Medical Center of Israel, Petach Tikva, Israel, on behalf of her research colleagues.
The complexity of MS requires different biomarkers to evaluate the various aspects of the disease. CSF analysis is commonly used, but the need for lumbar puncture makes CSF tests impractical for monitoring disease activity and response to treatment. In their search for noninvasive diagnostic methods, Dr. Ganelin-Cohen and colleagues hypothesized that Ig free light chain analysis in saliva may help detect immunopathologic changes in patients with MS. This assumption relied on prior reports indicating changes in mucosal immunity in patients with MS, and on a growing body of evidence for a potential diagnostic role of free light chains in MS.
A new technique based on Western blot analysis was developed to study kappa (k) and lambda (λ) free light chain monomers and dimers in saliva. Normal saliva showed high proportion of dimeric free light chains compared to that in the serum. “This finding might be explained by structural peculiarities of Ig in saliva,” Dr. Ganelin-Cohen said. “In contrast to most serum Ig, saliva IgA2 molecules incorporate the dimeric (not monomeric) light chains that may require production of larger amounts of dimeric light chains by the B cells synthesizing IgA2.”
Dr. Ganelin-Cohen and her colleagues compared free light chain monomer and dimer patterns in the saliva of patients with MS with those in healthy subjects. The intensity of the immunoreactive free light chain was measured, and the free light chain indices accounting for the total free light chain level and for monomer/dimer ratios (k monomer/dimer index and λ monomer/dimer index) were computed.
Most patients with active MS showed abnormally high free light chain levels, or a high proportion of monomeric free light chains. The reasons for such pathologic free light chain changes in patients with active MS are not clear, but they might be due to peripheral B lymphocytes penetrating oral mucosa and producing larger amounts of monomeric free light chains. Statistical analysis of these indices showed significant differences not only between patients with active MS (n = 27) and healthy subjects (n = 28), but also between patients with active MS (n = 27) and those in remission (n = 58).
Cut-off values were established to distinguish a healthy state from the pathologic conditions in MS: total free light chain level index = 17, k monomer/dimer index = 4.0, λ monomer/dimer index = 2.4. Most patients with active MS showed free light chain indices above these cut-off values.
The high specificity and sensitivity of the technique for diagnosing active MS enable this test to become a new noninvasive complementary tool to evaluate MS, Dr. Ganelin-Cohen and colleagues concluded.
PARIS—A noninvasive test that assesses immunoglobulin (Ig) free light chains in saliva may detect immunopathologic changes in the multiple sclerosis (MS) disease state and evaluate response to treatment, according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The technique has a specificity of 80% and sensitivity of 89% for diagnosing active MS, said lead author Esther Ganelin-Cohen, MD, PhD, Director of the Neuro-Immunology Clinic at Schneider Children’s Medical Center of Israel, Petach Tikva, Israel, on behalf of her research colleagues.
The complexity of MS requires different biomarkers to evaluate the various aspects of the disease. CSF analysis is commonly used, but the need for lumbar puncture makes CSF tests impractical for monitoring disease activity and response to treatment. In their search for noninvasive diagnostic methods, Dr. Ganelin-Cohen and colleagues hypothesized that Ig free light chain analysis in saliva may help detect immunopathologic changes in patients with MS. This assumption relied on prior reports indicating changes in mucosal immunity in patients with MS, and on a growing body of evidence for a potential diagnostic role of free light chains in MS.
A new technique based on Western blot analysis was developed to study kappa (k) and lambda (λ) free light chain monomers and dimers in saliva. Normal saliva showed high proportion of dimeric free light chains compared to that in the serum. “This finding might be explained by structural peculiarities of Ig in saliva,” Dr. Ganelin-Cohen said. “In contrast to most serum Ig, saliva IgA2 molecules incorporate the dimeric (not monomeric) light chains that may require production of larger amounts of dimeric light chains by the B cells synthesizing IgA2.”
Dr. Ganelin-Cohen and her colleagues compared free light chain monomer and dimer patterns in the saliva of patients with MS with those in healthy subjects. The intensity of the immunoreactive free light chain was measured, and the free light chain indices accounting for the total free light chain level and for monomer/dimer ratios (k monomer/dimer index and λ monomer/dimer index) were computed.
Most patients with active MS showed abnormally high free light chain levels, or a high proportion of monomeric free light chains. The reasons for such pathologic free light chain changes in patients with active MS are not clear, but they might be due to peripheral B lymphocytes penetrating oral mucosa and producing larger amounts of monomeric free light chains. Statistical analysis of these indices showed significant differences not only between patients with active MS (n = 27) and healthy subjects (n = 28), but also between patients with active MS (n = 27) and those in remission (n = 58).
Cut-off values were established to distinguish a healthy state from the pathologic conditions in MS: total free light chain level index = 17, k monomer/dimer index = 4.0, λ monomer/dimer index = 2.4. Most patients with active MS showed free light chain indices above these cut-off values.
The high specificity and sensitivity of the technique for diagnosing active MS enable this test to become a new noninvasive complementary tool to evaluate MS, Dr. Ganelin-Cohen and colleagues concluded.
PARIS—A noninvasive test that assesses immunoglobulin (Ig) free light chains in saliva may detect immunopathologic changes in the multiple sclerosis (MS) disease state and evaluate response to treatment, according to research presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The technique has a specificity of 80% and sensitivity of 89% for diagnosing active MS, said lead author Esther Ganelin-Cohen, MD, PhD, Director of the Neuro-Immunology Clinic at Schneider Children’s Medical Center of Israel, Petach Tikva, Israel, on behalf of her research colleagues.
The complexity of MS requires different biomarkers to evaluate the various aspects of the disease. CSF analysis is commonly used, but the need for lumbar puncture makes CSF tests impractical for monitoring disease activity and response to treatment. In their search for noninvasive diagnostic methods, Dr. Ganelin-Cohen and colleagues hypothesized that Ig free light chain analysis in saliva may help detect immunopathologic changes in patients with MS. This assumption relied on prior reports indicating changes in mucosal immunity in patients with MS, and on a growing body of evidence for a potential diagnostic role of free light chains in MS.
A new technique based on Western blot analysis was developed to study kappa (k) and lambda (λ) free light chain monomers and dimers in saliva. Normal saliva showed high proportion of dimeric free light chains compared to that in the serum. “This finding might be explained by structural peculiarities of Ig in saliva,” Dr. Ganelin-Cohen said. “In contrast to most serum Ig, saliva IgA2 molecules incorporate the dimeric (not monomeric) light chains that may require production of larger amounts of dimeric light chains by the B cells synthesizing IgA2.”
Dr. Ganelin-Cohen and her colleagues compared free light chain monomer and dimer patterns in the saliva of patients with MS with those in healthy subjects. The intensity of the immunoreactive free light chain was measured, and the free light chain indices accounting for the total free light chain level and for monomer/dimer ratios (k monomer/dimer index and λ monomer/dimer index) were computed.
Most patients with active MS showed abnormally high free light chain levels, or a high proportion of monomeric free light chains. The reasons for such pathologic free light chain changes in patients with active MS are not clear, but they might be due to peripheral B lymphocytes penetrating oral mucosa and producing larger amounts of monomeric free light chains. Statistical analysis of these indices showed significant differences not only between patients with active MS (n = 27) and healthy subjects (n = 28), but also between patients with active MS (n = 27) and those in remission (n = 58).
Cut-off values were established to distinguish a healthy state from the pathologic conditions in MS: total free light chain level index = 17, k monomer/dimer index = 4.0, λ monomer/dimer index = 2.4. Most patients with active MS showed free light chain indices above these cut-off values.
The high specificity and sensitivity of the technique for diagnosing active MS enable this test to become a new noninvasive complementary tool to evaluate MS, Dr. Ganelin-Cohen and colleagues concluded.
Cancer patients prefer no computer at physician visit
Patients with cancer perceived physicians who did not use a computer as more compassionate, more professional, and better at communication, according to results of a randomized, video-based study presented at the Palliative and Supportive Care in Oncology Symposium.
In addition, the majority of patients said they would prefer having a doctor who communicated face to face (in other words, without aid of a computer) to be their provider, said Ali Haider, MD, of the University of Texas MD Anderson Cancer Center, Houston.
This is one of the few, if not only, studies to evaluate how the presence of a computer affects exam room interactions between physicians and patients, Dr. Haider said in a press conference held during the meeting.
To test the impact of the computer in the exam room, Dr. Haider and his colleagues created four different 3-minute video vignettes featuring two different actors playing physicians in an encounter with a patient. Each actor created one video in which he used a computer and one in which he did not. To minimize potential bias, the videos had identical scripts, and actors were careful to use the same gestures, expressions, and nonverbal communication in each video.
A total of 120 cancer patients were randomized to view two of the videos and fill out validated questionnaires rating their perception of the physician’s compassion, communication skills, and professionalism.
The face-to-face clinical encounter videos were associated with a median compassion score of 9 on a scale of 0-50 where 0 is best and 50 is worst; by comparison, the encounters with computers scored worse, at a median of 20 out of 50 (P = .0003). Likewise, the patients rated the face-to-face encounter videos significantly higher on communication skills (P = .0001) and professionalism (P = .013).
After watching both videos, the patients were asked which encounter they would personally prefer, and 86 (72%) said they liked the face-to-face communication video better.
Actors and patients were all blinded to the purpose of the study, according to the researchers.
Further research is required to confirm these findings in other clinical settings and populations, according to Dr. Haider.
“We believe these results may be different if we choose a younger population, or patients with high computer literacy,” he explained.
While more research may be needed, “face-to-face communication seems quite possibly the preferred route, despite the pressures clinicians have to search and document in the medical record,” said medical oncologist Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, who was not involved with the study.
“In an age of ubiquitous technology, this study is an important reminder of the need to address the potential for technology to interfere with the patient-physician interface,” said Dr. Epstein, who moderated the press conference from the palliative care symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
Patients with cancer perceived physicians who did not use a computer as more compassionate, more professional, and better at communication, according to results of a randomized, video-based study presented at the Palliative and Supportive Care in Oncology Symposium.
In addition, the majority of patients said they would prefer having a doctor who communicated face to face (in other words, without aid of a computer) to be their provider, said Ali Haider, MD, of the University of Texas MD Anderson Cancer Center, Houston.
This is one of the few, if not only, studies to evaluate how the presence of a computer affects exam room interactions between physicians and patients, Dr. Haider said in a press conference held during the meeting.
To test the impact of the computer in the exam room, Dr. Haider and his colleagues created four different 3-minute video vignettes featuring two different actors playing physicians in an encounter with a patient. Each actor created one video in which he used a computer and one in which he did not. To minimize potential bias, the videos had identical scripts, and actors were careful to use the same gestures, expressions, and nonverbal communication in each video.
A total of 120 cancer patients were randomized to view two of the videos and fill out validated questionnaires rating their perception of the physician’s compassion, communication skills, and professionalism.
The face-to-face clinical encounter videos were associated with a median compassion score of 9 on a scale of 0-50 where 0 is best and 50 is worst; by comparison, the encounters with computers scored worse, at a median of 20 out of 50 (P = .0003). Likewise, the patients rated the face-to-face encounter videos significantly higher on communication skills (P = .0001) and professionalism (P = .013).
After watching both videos, the patients were asked which encounter they would personally prefer, and 86 (72%) said they liked the face-to-face communication video better.
Actors and patients were all blinded to the purpose of the study, according to the researchers.
Further research is required to confirm these findings in other clinical settings and populations, according to Dr. Haider.
“We believe these results may be different if we choose a younger population, or patients with high computer literacy,” he explained.
While more research may be needed, “face-to-face communication seems quite possibly the preferred route, despite the pressures clinicians have to search and document in the medical record,” said medical oncologist Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, who was not involved with the study.
“In an age of ubiquitous technology, this study is an important reminder of the need to address the potential for technology to interfere with the patient-physician interface,” said Dr. Epstein, who moderated the press conference from the palliative care symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
Patients with cancer perceived physicians who did not use a computer as more compassionate, more professional, and better at communication, according to results of a randomized, video-based study presented at the Palliative and Supportive Care in Oncology Symposium.
In addition, the majority of patients said they would prefer having a doctor who communicated face to face (in other words, without aid of a computer) to be their provider, said Ali Haider, MD, of the University of Texas MD Anderson Cancer Center, Houston.
This is one of the few, if not only, studies to evaluate how the presence of a computer affects exam room interactions between physicians and patients, Dr. Haider said in a press conference held during the meeting.
To test the impact of the computer in the exam room, Dr. Haider and his colleagues created four different 3-minute video vignettes featuring two different actors playing physicians in an encounter with a patient. Each actor created one video in which he used a computer and one in which he did not. To minimize potential bias, the videos had identical scripts, and actors were careful to use the same gestures, expressions, and nonverbal communication in each video.
A total of 120 cancer patients were randomized to view two of the videos and fill out validated questionnaires rating their perception of the physician’s compassion, communication skills, and professionalism.
The face-to-face clinical encounter videos were associated with a median compassion score of 9 on a scale of 0-50 where 0 is best and 50 is worst; by comparison, the encounters with computers scored worse, at a median of 20 out of 50 (P = .0003). Likewise, the patients rated the face-to-face encounter videos significantly higher on communication skills (P = .0001) and professionalism (P = .013).
After watching both videos, the patients were asked which encounter they would personally prefer, and 86 (72%) said they liked the face-to-face communication video better.
Actors and patients were all blinded to the purpose of the study, according to the researchers.
Further research is required to confirm these findings in other clinical settings and populations, according to Dr. Haider.
“We believe these results may be different if we choose a younger population, or patients with high computer literacy,” he explained.
While more research may be needed, “face-to-face communication seems quite possibly the preferred route, despite the pressures clinicians have to search and document in the medical record,” said medical oncologist Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, who was not involved with the study.
“In an age of ubiquitous technology, this study is an important reminder of the need to address the potential for technology to interfere with the patient-physician interface,” said Dr. Epstein, who moderated the press conference from the palliative care symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
AT PALLONC 2017
Key clinical point: Patients rate physicians who communicate face to face, without using a computer, as more compassionate, more professional, and better at communication.
Major finding: A total of 72% of patients preferred videos in which physicians did not use a computer during the conversation.
Data source: Randomized study including 120 adults who watched two short video vignettes depicting two different physician-patient encounters.
Disclosures: Dr. Haider reported no disclosures. The study was funded by the University of Texas MD Anderson Cancer Center.
Sunburn Purpura
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
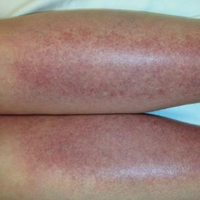
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.
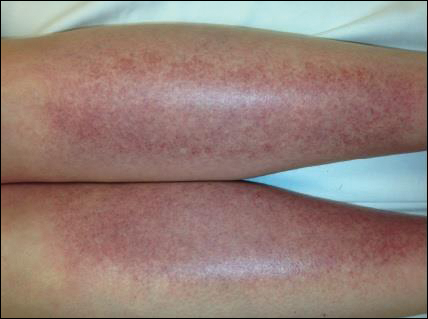
Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.
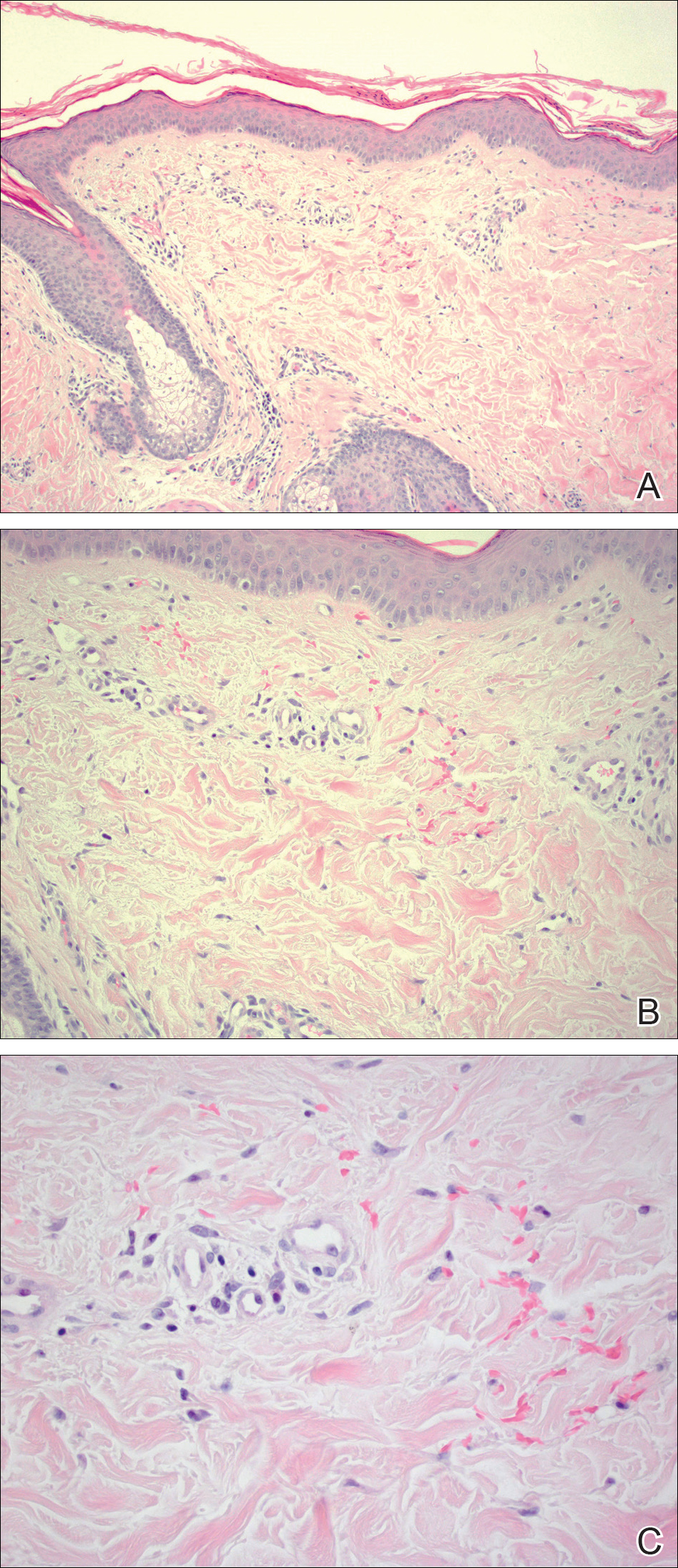
Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.

Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.

Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.

Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.

Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
Practice Points
- Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
- Idiopathic sunburn purpura should only be diagnosed after vasculitis and/or thrombocytopenia is ruled out, which is best achieved through skin biopsy and a platelet count, respectively.
- The rash typically resolves without treatment in 1 to 2 weeks; however, a variety of UV protection modalities and education should be offered to the patient.
Naloxone: Difficult conversations about a potential lifesaver
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
Evidence-backed questions can guide a GERD vs. NERD differential diagnosis
CHICAGO – when doing a differential diagnosis. Fortunately, five questions backed by increasing evidence can help you make the call.
“Everyone in the room knows babies puke, and babies can puke a lot,” Barry K. Wershil, MD, said at the annual meeting of the American Academy of Pediatrics. The approach to diagnosing GERD is age specific. “Kids who puke tend to outgrow it over time. With development, 95% or more are no longer refluxing at 18 months of age.”
“So generally there is no reason to initially refer older children and teenagers to a gastroenterologist,” Dr. Wershil said. “One of the essential things [you] do is consider all the causes of vomiting that are not GERD. If your first go-to is GERD, you’re going to miss other issues.”
Dr. Wershil reviewed the definitions: Gastroesophageal reflux is passage of gastric contents into the esophagus. GERD, on the other hand, is defined by the troublesome symptoms or complications associated with reflux of gastric material into the esophagus. In contrast, NERD is the presence of reflux symptoms with no evidence of mucosal erosion or mucosal breaks.
Considerations backed by evidence
Unfortunately, symptoms alone do not always differentiate erosive versus nonerosive esophagitis, Dr. Wershil said, although recurrent vomiting, poor weight gain, anemia, feeding problems, and respiratory problems can be signs of complicated GERD.
He recommended the following five considerations to distinguish GERD from NERD:
- Is the patient exhibiting normal weight gain? If not, ask questions about how the child is being fed. Have the parents started diluting the formula because they think that will take care of the vomiting? Have they begun limiting the amount of formula after observing that the child throws up at 4 ounces but not at 2 ounces?
- Is the patient bleeding or anemic? Hematemesis is rarely the presentation of infants with GERD, but anemia may be.
- Does the patient have respiratory problems (for example, a history of aspiration, recurring wheezing, or cough)?
- Is the patient neurologically normal? If so, that can present a special class of patients in which vomiting may not be just normal infant vomiting.
- Is the patient older than 2 years? We expect 95% of children to outgrow reflux by 18 months, and most children who have physiological reflux will outgrow it by 2 years.
“Those five questions in 1983 had little evidence, but in 2017 there is more evidence that these are the questions to focus on,” Dr. Wershil said.
The role of diagnostic testing
Diagnostic testing, such as pH monitoring, impedance testing, and endoscopy, can be useful in specific situations but carry limitations for widespread use, Dr. Wershil said. “Each test has reasons and limitations.”
An upper GI tract series looks only for anatomic anomalies, for example. pH monitoring is still used in many centers, but in general, impedance monitoring has become more common because it can detect both acid and nonacid reflux: “You can get a very detailed analysis of events happening in the esophagus over time.” One caveat Dr. Wershil added is that, “in some instances, we’re unsure how to define this in the pediatric age ranges we treat.”
Endoscopy has a limited role for the rare patients with mucosal changes or erosive esophagitis, he added. Endoscopy is ordered to detect mucosal changes that confirm esophageal erosion. “In all the kids we scope with positive pH, we rarely find erosive esophagitis,” Dr. Wershil said. “What we find more often is NERD. That really represents more of what we see in our patient population.”
“I hope this information is a good starting point to understand the algorithms that get generated,” Dr. Wershil said.
He recommended an algorithm for gastroesophageal reflux prepared by the American Academy of Pediatrics’ Section on Gastroenterology, Hepatology, and Nutrition (Pediatrics. 2013 May. doi: 10.1542/peds.2013-0421). “I think this information is really solidly grounded in evidence.”
Dr. Wershil is a consultant for Alexion Pharmaceuticals; is a member of the speakers bureau for Abbott Nutrition, Mead Johnson Nutrition, and Nutricia; and receives funding from the National Institutes of Health Consortium of Eosinophilic Gastrointestinal Disease Researchers.
CHICAGO – when doing a differential diagnosis. Fortunately, five questions backed by increasing evidence can help you make the call.
“Everyone in the room knows babies puke, and babies can puke a lot,” Barry K. Wershil, MD, said at the annual meeting of the American Academy of Pediatrics. The approach to diagnosing GERD is age specific. “Kids who puke tend to outgrow it over time. With development, 95% or more are no longer refluxing at 18 months of age.”
“So generally there is no reason to initially refer older children and teenagers to a gastroenterologist,” Dr. Wershil said. “One of the essential things [you] do is consider all the causes of vomiting that are not GERD. If your first go-to is GERD, you’re going to miss other issues.”
Dr. Wershil reviewed the definitions: Gastroesophageal reflux is passage of gastric contents into the esophagus. GERD, on the other hand, is defined by the troublesome symptoms or complications associated with reflux of gastric material into the esophagus. In contrast, NERD is the presence of reflux symptoms with no evidence of mucosal erosion or mucosal breaks.
Considerations backed by evidence
Unfortunately, symptoms alone do not always differentiate erosive versus nonerosive esophagitis, Dr. Wershil said, although recurrent vomiting, poor weight gain, anemia, feeding problems, and respiratory problems can be signs of complicated GERD.
He recommended the following five considerations to distinguish GERD from NERD:
- Is the patient exhibiting normal weight gain? If not, ask questions about how the child is being fed. Have the parents started diluting the formula because they think that will take care of the vomiting? Have they begun limiting the amount of formula after observing that the child throws up at 4 ounces but not at 2 ounces?
- Is the patient bleeding or anemic? Hematemesis is rarely the presentation of infants with GERD, but anemia may be.
- Does the patient have respiratory problems (for example, a history of aspiration, recurring wheezing, or cough)?
- Is the patient neurologically normal? If so, that can present a special class of patients in which vomiting may not be just normal infant vomiting.
- Is the patient older than 2 years? We expect 95% of children to outgrow reflux by 18 months, and most children who have physiological reflux will outgrow it by 2 years.
“Those five questions in 1983 had little evidence, but in 2017 there is more evidence that these are the questions to focus on,” Dr. Wershil said.
The role of diagnostic testing
Diagnostic testing, such as pH monitoring, impedance testing, and endoscopy, can be useful in specific situations but carry limitations for widespread use, Dr. Wershil said. “Each test has reasons and limitations.”
An upper GI tract series looks only for anatomic anomalies, for example. pH monitoring is still used in many centers, but in general, impedance monitoring has become more common because it can detect both acid and nonacid reflux: “You can get a very detailed analysis of events happening in the esophagus over time.” One caveat Dr. Wershil added is that, “in some instances, we’re unsure how to define this in the pediatric age ranges we treat.”
Endoscopy has a limited role for the rare patients with mucosal changes or erosive esophagitis, he added. Endoscopy is ordered to detect mucosal changes that confirm esophageal erosion. “In all the kids we scope with positive pH, we rarely find erosive esophagitis,” Dr. Wershil said. “What we find more often is NERD. That really represents more of what we see in our patient population.”
“I hope this information is a good starting point to understand the algorithms that get generated,” Dr. Wershil said.
He recommended an algorithm for gastroesophageal reflux prepared by the American Academy of Pediatrics’ Section on Gastroenterology, Hepatology, and Nutrition (Pediatrics. 2013 May. doi: 10.1542/peds.2013-0421). “I think this information is really solidly grounded in evidence.”
Dr. Wershil is a consultant for Alexion Pharmaceuticals; is a member of the speakers bureau for Abbott Nutrition, Mead Johnson Nutrition, and Nutricia; and receives funding from the National Institutes of Health Consortium of Eosinophilic Gastrointestinal Disease Researchers.
CHICAGO – when doing a differential diagnosis. Fortunately, five questions backed by increasing evidence can help you make the call.
“Everyone in the room knows babies puke, and babies can puke a lot,” Barry K. Wershil, MD, said at the annual meeting of the American Academy of Pediatrics. The approach to diagnosing GERD is age specific. “Kids who puke tend to outgrow it over time. With development, 95% or more are no longer refluxing at 18 months of age.”
“So generally there is no reason to initially refer older children and teenagers to a gastroenterologist,” Dr. Wershil said. “One of the essential things [you] do is consider all the causes of vomiting that are not GERD. If your first go-to is GERD, you’re going to miss other issues.”
Dr. Wershil reviewed the definitions: Gastroesophageal reflux is passage of gastric contents into the esophagus. GERD, on the other hand, is defined by the troublesome symptoms or complications associated with reflux of gastric material into the esophagus. In contrast, NERD is the presence of reflux symptoms with no evidence of mucosal erosion or mucosal breaks.
Considerations backed by evidence
Unfortunately, symptoms alone do not always differentiate erosive versus nonerosive esophagitis, Dr. Wershil said, although recurrent vomiting, poor weight gain, anemia, feeding problems, and respiratory problems can be signs of complicated GERD.
He recommended the following five considerations to distinguish GERD from NERD:
- Is the patient exhibiting normal weight gain? If not, ask questions about how the child is being fed. Have the parents started diluting the formula because they think that will take care of the vomiting? Have they begun limiting the amount of formula after observing that the child throws up at 4 ounces but not at 2 ounces?
- Is the patient bleeding or anemic? Hematemesis is rarely the presentation of infants with GERD, but anemia may be.
- Does the patient have respiratory problems (for example, a history of aspiration, recurring wheezing, or cough)?
- Is the patient neurologically normal? If so, that can present a special class of patients in which vomiting may not be just normal infant vomiting.
- Is the patient older than 2 years? We expect 95% of children to outgrow reflux by 18 months, and most children who have physiological reflux will outgrow it by 2 years.
“Those five questions in 1983 had little evidence, but in 2017 there is more evidence that these are the questions to focus on,” Dr. Wershil said.
The role of diagnostic testing
Diagnostic testing, such as pH monitoring, impedance testing, and endoscopy, can be useful in specific situations but carry limitations for widespread use, Dr. Wershil said. “Each test has reasons and limitations.”
An upper GI tract series looks only for anatomic anomalies, for example. pH monitoring is still used in many centers, but in general, impedance monitoring has become more common because it can detect both acid and nonacid reflux: “You can get a very detailed analysis of events happening in the esophagus over time.” One caveat Dr. Wershil added is that, “in some instances, we’re unsure how to define this in the pediatric age ranges we treat.”
Endoscopy has a limited role for the rare patients with mucosal changes or erosive esophagitis, he added. Endoscopy is ordered to detect mucosal changes that confirm esophageal erosion. “In all the kids we scope with positive pH, we rarely find erosive esophagitis,” Dr. Wershil said. “What we find more often is NERD. That really represents more of what we see in our patient population.”
“I hope this information is a good starting point to understand the algorithms that get generated,” Dr. Wershil said.
He recommended an algorithm for gastroesophageal reflux prepared by the American Academy of Pediatrics’ Section on Gastroenterology, Hepatology, and Nutrition (Pediatrics. 2013 May. doi: 10.1542/peds.2013-0421). “I think this information is really solidly grounded in evidence.”
Dr. Wershil is a consultant for Alexion Pharmaceuticals; is a member of the speakers bureau for Abbott Nutrition, Mead Johnson Nutrition, and Nutricia; and receives funding from the National Institutes of Health Consortium of Eosinophilic Gastrointestinal Disease Researchers.
EXPERT ANALYSIS FROM AAP 2017
Rheumatoid arthritis increases risk of COPD hospitalizations
Individuals with rheumatoid arthritis (RA) had an increased risk of hospitalizations from chronic obstructive pulmonary disease (COPD) when compared with the general population in a Canadian retrospective, population-based cohort study.
The risk of COPD hospitalizations was 47% higher in individuals with RA. “This finding emphasizes the need to control inflammation in rheumatoid arthritis, not only to prevent joint damage, but also to prevent complications of systemic inflammation, including the development of comorbidities such as cardiovascular diseases and COPD,” wrote Diane Lacaille, MD, of the University of British Columbia, Vancouver, and her coauthors (Arthritis Care Res. 2017 Oct 19. doi: 10.1002/acr.23410).
Several previous studies have suggested a link between COPD and inflammation, Dr. Lacaille and her colleagues said. Accordingly, they sought to evaluate the risk of COPD hospitalizations in a cohort of 24,625 individuals with RA as compared with 25,396 general population controls randomly selected and matched based on age, sex, and index year. Most subjects in the analysis were female, and the mean age at onset of RA was 57.2 years.
The investigators reported an increased incidence of COPD in individuals with RA, compared with controls, based on an incident rate ratio (IRR) of 1.58 (95% confidence interval, 1.34-1.87) that dropped to 1.47 (95% CI, 1.24-1.74) after adjustment for potential confounders, including comorbidities and health services usage at baseline. The overall incidence rate for COPD was 2.07 per 1,000 patient-years for RA patients and 1.31 per 1,000 patient-years for controls.
When the model was stratified based on sex, COPD hospitalization risk was significantly increased in women (adjusted hazard ratio [HR], 1.61; 95% CI, 1.30-1.98), but not in men (adjusted HR, 1.25; 95% CI, 0.95-1.66), they said.
Data were not available on smoking, the main COPD risk factor, for the patients in this study; however, the increased risk of COPD hospitalizations in the RA group remained significant after modeling for smoking, according to the investigators.
Combined, these results have “notable implications for the clinical care of RA and COPD,” Dr. Lacaille and her coinvestigators said.
Both clinicians and people living with RA “should be aware of the increased risk of developing COPD and be vigilant in watching for early symptoms of COPD, so that appropriate diagnostic tests can be administered at the onset of early symptoms,” they wrote. “Early detection of COPD is essential so that effective treatments can be initiated before irreversible damage to the lungs occurs, to improve long-term outcomes.”
These findings strengthen the conclusions of two previous cross-sectional studies showing an association between RA and COPD prevalence, according to the investigators. In one study, RA patients in Israel who were receiving disease-modifying antirheumatic drugs had double the prevalence of COPD, compared with general population controls, according to authors of that study (Immunol Res. 2013;56[2-3]:261-6). Similarly, U.K. investigators compared 421 RA patients against controls and reported a twofold increase in obstructive pattern on screening spirometry in the RA group (Ann Rheum Dis. 2013;72:1517-23).
The current study from Dr. Lacaille and her coinvestigators was supported by funding from the Canadian Institute for Health Research. The authors reported that they had no financial disclosures, conflicts of interest, or benefits from commercial sources.
Individuals with rheumatoid arthritis (RA) had an increased risk of hospitalizations from chronic obstructive pulmonary disease (COPD) when compared with the general population in a Canadian retrospective, population-based cohort study.
The risk of COPD hospitalizations was 47% higher in individuals with RA. “This finding emphasizes the need to control inflammation in rheumatoid arthritis, not only to prevent joint damage, but also to prevent complications of systemic inflammation, including the development of comorbidities such as cardiovascular diseases and COPD,” wrote Diane Lacaille, MD, of the University of British Columbia, Vancouver, and her coauthors (Arthritis Care Res. 2017 Oct 19. doi: 10.1002/acr.23410).
Several previous studies have suggested a link between COPD and inflammation, Dr. Lacaille and her colleagues said. Accordingly, they sought to evaluate the risk of COPD hospitalizations in a cohort of 24,625 individuals with RA as compared with 25,396 general population controls randomly selected and matched based on age, sex, and index year. Most subjects in the analysis were female, and the mean age at onset of RA was 57.2 years.
The investigators reported an increased incidence of COPD in individuals with RA, compared with controls, based on an incident rate ratio (IRR) of 1.58 (95% confidence interval, 1.34-1.87) that dropped to 1.47 (95% CI, 1.24-1.74) after adjustment for potential confounders, including comorbidities and health services usage at baseline. The overall incidence rate for COPD was 2.07 per 1,000 patient-years for RA patients and 1.31 per 1,000 patient-years for controls.
When the model was stratified based on sex, COPD hospitalization risk was significantly increased in women (adjusted hazard ratio [HR], 1.61; 95% CI, 1.30-1.98), but not in men (adjusted HR, 1.25; 95% CI, 0.95-1.66), they said.
Data were not available on smoking, the main COPD risk factor, for the patients in this study; however, the increased risk of COPD hospitalizations in the RA group remained significant after modeling for smoking, according to the investigators.
Combined, these results have “notable implications for the clinical care of RA and COPD,” Dr. Lacaille and her coinvestigators said.
Both clinicians and people living with RA “should be aware of the increased risk of developing COPD and be vigilant in watching for early symptoms of COPD, so that appropriate diagnostic tests can be administered at the onset of early symptoms,” they wrote. “Early detection of COPD is essential so that effective treatments can be initiated before irreversible damage to the lungs occurs, to improve long-term outcomes.”
These findings strengthen the conclusions of two previous cross-sectional studies showing an association between RA and COPD prevalence, according to the investigators. In one study, RA patients in Israel who were receiving disease-modifying antirheumatic drugs had double the prevalence of COPD, compared with general population controls, according to authors of that study (Immunol Res. 2013;56[2-3]:261-6). Similarly, U.K. investigators compared 421 RA patients against controls and reported a twofold increase in obstructive pattern on screening spirometry in the RA group (Ann Rheum Dis. 2013;72:1517-23).
The current study from Dr. Lacaille and her coinvestigators was supported by funding from the Canadian Institute for Health Research. The authors reported that they had no financial disclosures, conflicts of interest, or benefits from commercial sources.
Individuals with rheumatoid arthritis (RA) had an increased risk of hospitalizations from chronic obstructive pulmonary disease (COPD) when compared with the general population in a Canadian retrospective, population-based cohort study.
The risk of COPD hospitalizations was 47% higher in individuals with RA. “This finding emphasizes the need to control inflammation in rheumatoid arthritis, not only to prevent joint damage, but also to prevent complications of systemic inflammation, including the development of comorbidities such as cardiovascular diseases and COPD,” wrote Diane Lacaille, MD, of the University of British Columbia, Vancouver, and her coauthors (Arthritis Care Res. 2017 Oct 19. doi: 10.1002/acr.23410).
Several previous studies have suggested a link between COPD and inflammation, Dr. Lacaille and her colleagues said. Accordingly, they sought to evaluate the risk of COPD hospitalizations in a cohort of 24,625 individuals with RA as compared with 25,396 general population controls randomly selected and matched based on age, sex, and index year. Most subjects in the analysis were female, and the mean age at onset of RA was 57.2 years.
The investigators reported an increased incidence of COPD in individuals with RA, compared with controls, based on an incident rate ratio (IRR) of 1.58 (95% confidence interval, 1.34-1.87) that dropped to 1.47 (95% CI, 1.24-1.74) after adjustment for potential confounders, including comorbidities and health services usage at baseline. The overall incidence rate for COPD was 2.07 per 1,000 patient-years for RA patients and 1.31 per 1,000 patient-years for controls.
When the model was stratified based on sex, COPD hospitalization risk was significantly increased in women (adjusted hazard ratio [HR], 1.61; 95% CI, 1.30-1.98), but not in men (adjusted HR, 1.25; 95% CI, 0.95-1.66), they said.
Data were not available on smoking, the main COPD risk factor, for the patients in this study; however, the increased risk of COPD hospitalizations in the RA group remained significant after modeling for smoking, according to the investigators.
Combined, these results have “notable implications for the clinical care of RA and COPD,” Dr. Lacaille and her coinvestigators said.
Both clinicians and people living with RA “should be aware of the increased risk of developing COPD and be vigilant in watching for early symptoms of COPD, so that appropriate diagnostic tests can be administered at the onset of early symptoms,” they wrote. “Early detection of COPD is essential so that effective treatments can be initiated before irreversible damage to the lungs occurs, to improve long-term outcomes.”
These findings strengthen the conclusions of two previous cross-sectional studies showing an association between RA and COPD prevalence, according to the investigators. In one study, RA patients in Israel who were receiving disease-modifying antirheumatic drugs had double the prevalence of COPD, compared with general population controls, according to authors of that study (Immunol Res. 2013;56[2-3]:261-6). Similarly, U.K. investigators compared 421 RA patients against controls and reported a twofold increase in obstructive pattern on screening spirometry in the RA group (Ann Rheum Dis. 2013;72:1517-23).
The current study from Dr. Lacaille and her coinvestigators was supported by funding from the Canadian Institute for Health Research. The authors reported that they had no financial disclosures, conflicts of interest, or benefits from commercial sources.
FROM Arthritis Care AND Research
Key clinical point:
Major finding: The risk of COPD hospitalizations was 47% higher in individuals with rheumatoid arthritis (adjusted hazard ratio, 1.47; 95% confidence interval, 1.34-1.87).
Data source: A retrospective cohort study including approximately 25,000 RA patients seen in British Columbia and a roughly equal number of controls.
Disclosures: The Canadian Institute for Health Research provided funding for the study. The authors reported that they had no financial disclosures, conflicts of interest, or benefits from commercial sources.
A ‘game changer’ for pediatric HIV
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at pdnews@frontlinemedcom.com.
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at pdnews@frontlinemedcom.com.
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at pdnews@frontlinemedcom.com.