User login
CDC: Flu vaccine recommendations broaden for pregnant women and children
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM MMWR
AAP recommends hepatitis B vaccine within 24 hours of birth for all infants
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
FROM PEDIATRICS
Key clinical point: All infants should receive hepatitis B vaccine within 24 hours of birth.
Major finding: Hepatitis B vaccine prevents 75%-95% of perinatal hepatitis B infections.
Data source: A literature review of data on hepatitis B epidemiology in the United States.
Disclosures: The statement did not receive external funding, and the authors stated that they have no conflicts of interest.
Short Sleep Duration Increases the Risk of All-Cause Mortality
BOSTON—Insomnia with objective short sleep duration (ie, less than six hours) is not associated with an elevated risk of all-cause mortality, according to research presented at the 31st Annual Meeting of the Associated Professional Sleep Societies. Short sleep duration alone is associated with a higher risk of death, however.
To quantify the association between insomnia with objective short sleep duration and all-cause mortality, Suzanne Bertisch, MD, MPH, Assistant Professor of Medicine at Harvard Medical School and Clinical Investigator at Beth Israel Deaconess Medical Center in Boston, and colleagues conducted a time-to-event analysis of the Sleep Heart Health Study data. This prospective, community-based cohort study enrolled 6,441 participants age 40 and older between 1995 and 1998. At baseline, researchers administered questionnaires that queried participants about sleep symptoms, sleep patterns, medication, and medical history. In addition, participants underwent anthropometry and blood pressure measurements and had one night of unattended polysomnography at their home by certified and trained technicians. Researchers followed participants for a median of 11.6 years.
The primary exposure for Dr. Bertisch’s analysis was insomnia with short sleep duration. The investigators defined insomnia as self-report of difficulty falling asleep, difficulty getting back to sleep, early morning awakenings, or use of a sleeping pill for 16 to 30 nights/month. Short sleep duration was defined as total sleep time of less than six hours on single-night polysomnography. Covariates of interest included sex, race, smoking status, history of cardiovascular disease, apnea–hypopnea index, BMI, antidepressants used in the previous two weeks, hypertension, and diabetes.
Investigators used the propensity-score-adjusted Cox proportional hazards model to estimate the association between insomnia with short sleep duration and time to death. Researchers also performed a prespecified secondary analysis scoring hypertension and diabetes as potential mediators of an association between sleep variables and mortality. In addition, investigators stratified models by sex and examined insomnia with self-reported sleep duration. In secondary models, researchers used insomnia with self-reported duration as a secondary exposure.
Among 4,994 participants included in the study, researchers observed 1,163 deaths, of which 355 resulted from cardiovascular disease. The mean age of participants was 64. Participants with insomnia were more likely to be female, to smoke, and to use antidepressants, compared with those without insomnia.
Dr. Bertisch and colleagues also found that participants with short sleep duration had a 14% higher risk of mortality, compared with people without insomnia and with normal sleep duration. Insomnia with short sleep duration, however, was not associated with a higher risk of all-cause mortality. In the additional analysis, the researchers found no evidence that hypertension or diabetes mediated the association between sleep duration and mortality. Results also did not differ by sex, said Dr. Bertisch.
Limitations of this study include the fact that objective sleep duration was determined by a single night of polysomnography, and that researchers did not capture the duration of insomnia symptoms.
“Further work is needed to identify the mechanistic pathways by which insomnia with objective short sleep duration may confer increased risk for cardiovascular disease,” Dr. Bertisch concluded.
—Erica Tricarico
BOSTON—Insomnia with objective short sleep duration (ie, less than six hours) is not associated with an elevated risk of all-cause mortality, according to research presented at the 31st Annual Meeting of the Associated Professional Sleep Societies. Short sleep duration alone is associated with a higher risk of death, however.
To quantify the association between insomnia with objective short sleep duration and all-cause mortality, Suzanne Bertisch, MD, MPH, Assistant Professor of Medicine at Harvard Medical School and Clinical Investigator at Beth Israel Deaconess Medical Center in Boston, and colleagues conducted a time-to-event analysis of the Sleep Heart Health Study data. This prospective, community-based cohort study enrolled 6,441 participants age 40 and older between 1995 and 1998. At baseline, researchers administered questionnaires that queried participants about sleep symptoms, sleep patterns, medication, and medical history. In addition, participants underwent anthropometry and blood pressure measurements and had one night of unattended polysomnography at their home by certified and trained technicians. Researchers followed participants for a median of 11.6 years.
The primary exposure for Dr. Bertisch’s analysis was insomnia with short sleep duration. The investigators defined insomnia as self-report of difficulty falling asleep, difficulty getting back to sleep, early morning awakenings, or use of a sleeping pill for 16 to 30 nights/month. Short sleep duration was defined as total sleep time of less than six hours on single-night polysomnography. Covariates of interest included sex, race, smoking status, history of cardiovascular disease, apnea–hypopnea index, BMI, antidepressants used in the previous two weeks, hypertension, and diabetes.
Investigators used the propensity-score-adjusted Cox proportional hazards model to estimate the association between insomnia with short sleep duration and time to death. Researchers also performed a prespecified secondary analysis scoring hypertension and diabetes as potential mediators of an association between sleep variables and mortality. In addition, investigators stratified models by sex and examined insomnia with self-reported sleep duration. In secondary models, researchers used insomnia with self-reported duration as a secondary exposure.
Among 4,994 participants included in the study, researchers observed 1,163 deaths, of which 355 resulted from cardiovascular disease. The mean age of participants was 64. Participants with insomnia were more likely to be female, to smoke, and to use antidepressants, compared with those without insomnia.
Dr. Bertisch and colleagues also found that participants with short sleep duration had a 14% higher risk of mortality, compared with people without insomnia and with normal sleep duration. Insomnia with short sleep duration, however, was not associated with a higher risk of all-cause mortality. In the additional analysis, the researchers found no evidence that hypertension or diabetes mediated the association between sleep duration and mortality. Results also did not differ by sex, said Dr. Bertisch.
Limitations of this study include the fact that objective sleep duration was determined by a single night of polysomnography, and that researchers did not capture the duration of insomnia symptoms.
“Further work is needed to identify the mechanistic pathways by which insomnia with objective short sleep duration may confer increased risk for cardiovascular disease,” Dr. Bertisch concluded.
—Erica Tricarico
BOSTON—Insomnia with objective short sleep duration (ie, less than six hours) is not associated with an elevated risk of all-cause mortality, according to research presented at the 31st Annual Meeting of the Associated Professional Sleep Societies. Short sleep duration alone is associated with a higher risk of death, however.
To quantify the association between insomnia with objective short sleep duration and all-cause mortality, Suzanne Bertisch, MD, MPH, Assistant Professor of Medicine at Harvard Medical School and Clinical Investigator at Beth Israel Deaconess Medical Center in Boston, and colleagues conducted a time-to-event analysis of the Sleep Heart Health Study data. This prospective, community-based cohort study enrolled 6,441 participants age 40 and older between 1995 and 1998. At baseline, researchers administered questionnaires that queried participants about sleep symptoms, sleep patterns, medication, and medical history. In addition, participants underwent anthropometry and blood pressure measurements and had one night of unattended polysomnography at their home by certified and trained technicians. Researchers followed participants for a median of 11.6 years.
The primary exposure for Dr. Bertisch’s analysis was insomnia with short sleep duration. The investigators defined insomnia as self-report of difficulty falling asleep, difficulty getting back to sleep, early morning awakenings, or use of a sleeping pill for 16 to 30 nights/month. Short sleep duration was defined as total sleep time of less than six hours on single-night polysomnography. Covariates of interest included sex, race, smoking status, history of cardiovascular disease, apnea–hypopnea index, BMI, antidepressants used in the previous two weeks, hypertension, and diabetes.
Investigators used the propensity-score-adjusted Cox proportional hazards model to estimate the association between insomnia with short sleep duration and time to death. Researchers also performed a prespecified secondary analysis scoring hypertension and diabetes as potential mediators of an association between sleep variables and mortality. In addition, investigators stratified models by sex and examined insomnia with self-reported sleep duration. In secondary models, researchers used insomnia with self-reported duration as a secondary exposure.
Among 4,994 participants included in the study, researchers observed 1,163 deaths, of which 355 resulted from cardiovascular disease. The mean age of participants was 64. Participants with insomnia were more likely to be female, to smoke, and to use antidepressants, compared with those without insomnia.
Dr. Bertisch and colleagues also found that participants with short sleep duration had a 14% higher risk of mortality, compared with people without insomnia and with normal sleep duration. Insomnia with short sleep duration, however, was not associated with a higher risk of all-cause mortality. In the additional analysis, the researchers found no evidence that hypertension or diabetes mediated the association between sleep duration and mortality. Results also did not differ by sex, said Dr. Bertisch.
Limitations of this study include the fact that objective sleep duration was determined by a single night of polysomnography, and that researchers did not capture the duration of insomnia symptoms.
“Further work is needed to identify the mechanistic pathways by which insomnia with objective short sleep duration may confer increased risk for cardiovascular disease,” Dr. Bertisch concluded.
—Erica Tricarico
Immobility implicated in increased complications after bariatric surgery
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.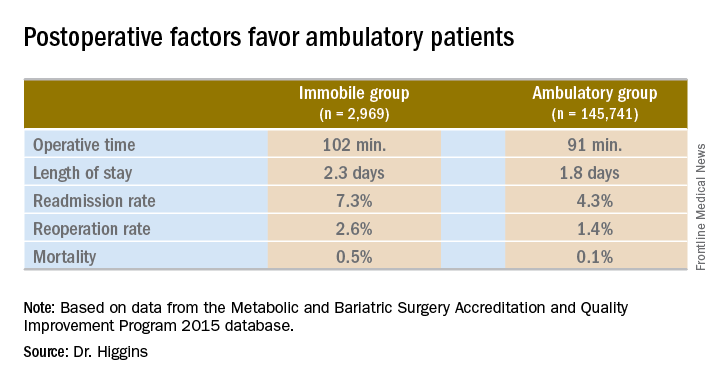
Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.
Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
NEW YORK –
“The importance of this study is to help us as an institution, but then also nationally, to try to focus on quality initiatives to improve the complication rate and safety profile of these patients, who are incredibly high risk for bariatric surgery,” said Rana Higgins, MD, a general surgeon at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee.
Dr. Higgins and her colleagues compared 2,969 immobile patients with 145,741 who were ambulatory before surgery. The most common bariatric procedure was sleeve gastrectomy at 56%. Another 30% had gastric bypass, 3% had the gastric band, and the remaining 1% underwent other procedures, such as biliopancreatic diversion with duodenal switch. The MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) defines immobility as a patient with limited ambulation who requires assistive devices, such as a scooter or wheelchair, to ambulate most or all of the time. In addition, with regard to negotiating stairs, immobile patients need a home lift or an elevator.
Only three complications evaluated by the researchers were not statistically different between groups: intraoperative or postoperative coma, stroke, and myocardial infarction.
Operative time was longer in the immobile group, about 102 minutes vs. 91 minutes (P less than .001). A meeting attendee asked what accounted for the difference. Dr. Higgins replied, “We’ll have to go back and look at our data. My hypothesis is that the immobile patients had a higher BMI [body mass index]. They may also have had other comorbidities that contributed to increased operative time.”
Hospital length of stay was also significantly longer among immobile patients at 2.3 days vs. 1.8 days in the ambulatory group (P less than .001).
The readmission rate was higher among immobile patients – 7.3% vs. 4.3% for the ambulatory group. The reoperation rate was higher at 2.6% vs. 1.4%. Both these findings were statistically significant as well (P less than .001).
Immobile patients had a statistically higher risk of mortality at 0.5%, compared with 0.1% among ambulatory patients (OR, 4.6).
A meeting attendee asked Dr. Higgins if her institution addresses mobility issues. She replied that there is preoperative education about the importance of ambulation, but the interventions are focused on ambulation in the postoperative period. “We order physical therapy, immediately postoperatively; typically the patients will receive it that day or the next day. We make sure patients are up and moving as much as possible, but there are limitations if they have limited mobility.”
The same attendee suggested preoperative physical therapy could help, even if only 2-4 weeks prior to surgery. Dr. Higgins agreed that would be a good quality initiative to explore in the future.
She had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Key clinical point: Patients immobile before bariatric surgery could require closer monitoring for postoperative complications.
Major finding: Thirty-day mortality after bariatric surgery in immobile patients was 0.5%, vs. 0.1% for an ambulatory group (P less than .0001).
Data source: A comparison of 2015 MBSAQIP data for 145,741 ambulatory patients and 2,969 immobile patients before bariatric surgery.
Disclosures: Dr. Higgins had no relevant financial disclosures.
Don’t omit extragenital gonorrhea, chlamydia testing
ESTES PARK, COLO. – Close to 80% of men who have sex with men who had gonorrhea or chlamydia in a recent study were infected only at extragenital sites – and therein lies a tale for primary care physicians.
“Five or six years ago my infectious diseases colleagues were pushing extragenital testing in MSM, and I thought then it was a little over the top and excessive. But I now think this is something we should be doing. Two studies from last year highlight this. I think we’re probably missing a lot of infections if we’re only doing genitourinary testing,” John Koeppe, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“It takes labs quite a while to get certified for extragenital testing. Many of my colleagues were sending samples to noncertified labs as urethral samples even though they were actually from the rectum or pharynx. The results were probably reliable. I’ll let you decide if that’s ok,” said Dr. Koeppe, an internist and infectious diseases specialist at the university.
He highlighted one recent potentially practice-changing study in which University of Pittsburgh investigators tested 224 MSM and 175 women with a history of receptive anal intercourse for genitourinary, rectal, and oral gonorrhea and chlamydia. A total of 22.8% of men and 3.4% of women had gonorrhea, while 21.9% of men and 12.6% of women had chlamydia. The major finding: 79.6% of the chlamydia infections and 76.5% of the gonorrhea infections in men were detected by NAAT only in the pharynx or rectum. So were 18.2% of chlamydia and 16.7% of gonorrhea infections in women (Sex Transm Dis. 2016 Feb;43[2]:105-9).
“So in gay men we’d be potentially missing more than three-quarters of infections by only doing genitourinary testing. And in women, it would be more than 16%,” Dr. Koeppe observed.
Moreover, in a national cross-sectional study of 1,071 MSM and bisexual men known as the One Thousand Strong Panel, the prevalence of gonorrhea and chlamydia in urine testing was 0.5% and 1.4%, respectively, whereas in rectal samples the rates were more than threefold higher at 1.8% for gonorrhea and 4.4% for chlamydia.
“Our finding that insertive CAS [condomless anal sex acts] was associated with rectal GC/CT highlights that providers should screen patients for GC/CT [gonococcus/Chlamydia trachomatis] via a full range of transmission routes, lest GC/CT go undiagnosed” the investigators concluded (Sex Transm Dis. 2016 Mar;43[3]:165-71).
Dr. Koeppe noted that major guidelines are in discord regarding chlamydia and gonorrhea screening in men. The U.S. Preventive Services Task Force and American Academy of Family Physicians don’t recommend the practice, while the Centers for Disease Control and Prevention and the Canadian STD guidelines do. The Canadian guidelines even include a series of specific questions to ask men to determine if they are at increased risk. If any of the answers raise a concern, then the guidelines urge testing, since chlamydia and gonorrhea are often asymptomatic.
Dr. Koeppe believes the CDC and the Canadians got it right.
“I think it makes sense to screen men. The CDC’s STD surveillance data indicate the incidence of chlamydia infection in U.S. women is twice as high as in men. That probably has a lot to do with the fact that all of the guidelines recommend screening sexually active women under age 25. I don’t think women are getting most of their chlamydia from other women, they’re probably getting it from men who we’re not screening,” said Dr. Koeppe.
He reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – Close to 80% of men who have sex with men who had gonorrhea or chlamydia in a recent study were infected only at extragenital sites – and therein lies a tale for primary care physicians.
“Five or six years ago my infectious diseases colleagues were pushing extragenital testing in MSM, and I thought then it was a little over the top and excessive. But I now think this is something we should be doing. Two studies from last year highlight this. I think we’re probably missing a lot of infections if we’re only doing genitourinary testing,” John Koeppe, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“It takes labs quite a while to get certified for extragenital testing. Many of my colleagues were sending samples to noncertified labs as urethral samples even though they were actually from the rectum or pharynx. The results were probably reliable. I’ll let you decide if that’s ok,” said Dr. Koeppe, an internist and infectious diseases specialist at the university.
He highlighted one recent potentially practice-changing study in which University of Pittsburgh investigators tested 224 MSM and 175 women with a history of receptive anal intercourse for genitourinary, rectal, and oral gonorrhea and chlamydia. A total of 22.8% of men and 3.4% of women had gonorrhea, while 21.9% of men and 12.6% of women had chlamydia. The major finding: 79.6% of the chlamydia infections and 76.5% of the gonorrhea infections in men were detected by NAAT only in the pharynx or rectum. So were 18.2% of chlamydia and 16.7% of gonorrhea infections in women (Sex Transm Dis. 2016 Feb;43[2]:105-9).
“So in gay men we’d be potentially missing more than three-quarters of infections by only doing genitourinary testing. And in women, it would be more than 16%,” Dr. Koeppe observed.
Moreover, in a national cross-sectional study of 1,071 MSM and bisexual men known as the One Thousand Strong Panel, the prevalence of gonorrhea and chlamydia in urine testing was 0.5% and 1.4%, respectively, whereas in rectal samples the rates were more than threefold higher at 1.8% for gonorrhea and 4.4% for chlamydia.
“Our finding that insertive CAS [condomless anal sex acts] was associated with rectal GC/CT highlights that providers should screen patients for GC/CT [gonococcus/Chlamydia trachomatis] via a full range of transmission routes, lest GC/CT go undiagnosed” the investigators concluded (Sex Transm Dis. 2016 Mar;43[3]:165-71).
Dr. Koeppe noted that major guidelines are in discord regarding chlamydia and gonorrhea screening in men. The U.S. Preventive Services Task Force and American Academy of Family Physicians don’t recommend the practice, while the Centers for Disease Control and Prevention and the Canadian STD guidelines do. The Canadian guidelines even include a series of specific questions to ask men to determine if they are at increased risk. If any of the answers raise a concern, then the guidelines urge testing, since chlamydia and gonorrhea are often asymptomatic.
Dr. Koeppe believes the CDC and the Canadians got it right.
“I think it makes sense to screen men. The CDC’s STD surveillance data indicate the incidence of chlamydia infection in U.S. women is twice as high as in men. That probably has a lot to do with the fact that all of the guidelines recommend screening sexually active women under age 25. I don’t think women are getting most of their chlamydia from other women, they’re probably getting it from men who we’re not screening,” said Dr. Koeppe.
He reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – Close to 80% of men who have sex with men who had gonorrhea or chlamydia in a recent study were infected only at extragenital sites – and therein lies a tale for primary care physicians.
“Five or six years ago my infectious diseases colleagues were pushing extragenital testing in MSM, and I thought then it was a little over the top and excessive. But I now think this is something we should be doing. Two studies from last year highlight this. I think we’re probably missing a lot of infections if we’re only doing genitourinary testing,” John Koeppe, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“It takes labs quite a while to get certified for extragenital testing. Many of my colleagues were sending samples to noncertified labs as urethral samples even though they were actually from the rectum or pharynx. The results were probably reliable. I’ll let you decide if that’s ok,” said Dr. Koeppe, an internist and infectious diseases specialist at the university.
He highlighted one recent potentially practice-changing study in which University of Pittsburgh investigators tested 224 MSM and 175 women with a history of receptive anal intercourse for genitourinary, rectal, and oral gonorrhea and chlamydia. A total of 22.8% of men and 3.4% of women had gonorrhea, while 21.9% of men and 12.6% of women had chlamydia. The major finding: 79.6% of the chlamydia infections and 76.5% of the gonorrhea infections in men were detected by NAAT only in the pharynx or rectum. So were 18.2% of chlamydia and 16.7% of gonorrhea infections in women (Sex Transm Dis. 2016 Feb;43[2]:105-9).
“So in gay men we’d be potentially missing more than three-quarters of infections by only doing genitourinary testing. And in women, it would be more than 16%,” Dr. Koeppe observed.
Moreover, in a national cross-sectional study of 1,071 MSM and bisexual men known as the One Thousand Strong Panel, the prevalence of gonorrhea and chlamydia in urine testing was 0.5% and 1.4%, respectively, whereas in rectal samples the rates were more than threefold higher at 1.8% for gonorrhea and 4.4% for chlamydia.
“Our finding that insertive CAS [condomless anal sex acts] was associated with rectal GC/CT highlights that providers should screen patients for GC/CT [gonococcus/Chlamydia trachomatis] via a full range of transmission routes, lest GC/CT go undiagnosed” the investigators concluded (Sex Transm Dis. 2016 Mar;43[3]:165-71).
Dr. Koeppe noted that major guidelines are in discord regarding chlamydia and gonorrhea screening in men. The U.S. Preventive Services Task Force and American Academy of Family Physicians don’t recommend the practice, while the Centers for Disease Control and Prevention and the Canadian STD guidelines do. The Canadian guidelines even include a series of specific questions to ask men to determine if they are at increased risk. If any of the answers raise a concern, then the guidelines urge testing, since chlamydia and gonorrhea are often asymptomatic.
Dr. Koeppe believes the CDC and the Canadians got it right.
“I think it makes sense to screen men. The CDC’s STD surveillance data indicate the incidence of chlamydia infection in U.S. women is twice as high as in men. That probably has a lot to do with the fact that all of the guidelines recommend screening sexually active women under age 25. I don’t think women are getting most of their chlamydia from other women, they’re probably getting it from men who we’re not screening,” said Dr. Koeppe.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
The future needs more surgeons
By 2030, the U.S. health care system could be facing a shortage of 19,800-29,000 surgeons, which means that things are looking up.
Last year, the Association of American Medical Colleges projected a shortage of 25,200-33,200 surgeons by the year 2025, which is larger than the 15,000- to 24,000-surgeon shortfall now projected for 2025 – larger even than the shortage that the AAMC is currently projecting for 2030.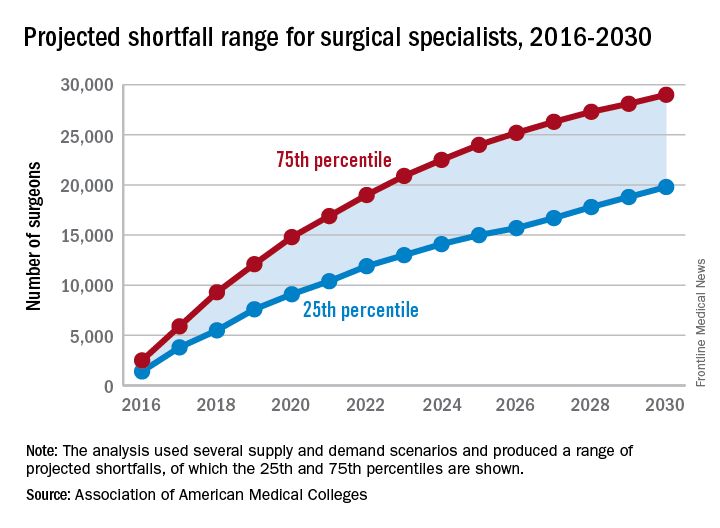
“On the basis of current trends, the number of newly trained surgeons is almost equal to projected future attrition, so there is little (if any) projected growth in supply,” the report’s authors wrote. Demand – mainly in the form of population growth and aging – is another story. From 2015 to 2030, “the U.S. population is projected to grow by close to 12%, from about 321 million to 359 million. The population under age 18 is projected to grow by only 5%, while the population aged 65 and over is projected to grow by 55%,” they said.
In other words, it may not be the best time to be a pediatrician.
By 2030, the U.S. health care system could be facing a shortage of 19,800-29,000 surgeons, which means that things are looking up.
Last year, the Association of American Medical Colleges projected a shortage of 25,200-33,200 surgeons by the year 2025, which is larger than the 15,000- to 24,000-surgeon shortfall now projected for 2025 – larger even than the shortage that the AAMC is currently projecting for 2030.
“On the basis of current trends, the number of newly trained surgeons is almost equal to projected future attrition, so there is little (if any) projected growth in supply,” the report’s authors wrote. Demand – mainly in the form of population growth and aging – is another story. From 2015 to 2030, “the U.S. population is projected to grow by close to 12%, from about 321 million to 359 million. The population under age 18 is projected to grow by only 5%, while the population aged 65 and over is projected to grow by 55%,” they said.
In other words, it may not be the best time to be a pediatrician.
By 2030, the U.S. health care system could be facing a shortage of 19,800-29,000 surgeons, which means that things are looking up.
Last year, the Association of American Medical Colleges projected a shortage of 25,200-33,200 surgeons by the year 2025, which is larger than the 15,000- to 24,000-surgeon shortfall now projected for 2025 – larger even than the shortage that the AAMC is currently projecting for 2030.
“On the basis of current trends, the number of newly trained surgeons is almost equal to projected future attrition, so there is little (if any) projected growth in supply,” the report’s authors wrote. Demand – mainly in the form of population growth and aging – is another story. From 2015 to 2030, “the U.S. population is projected to grow by close to 12%, from about 321 million to 359 million. The population under age 18 is projected to grow by only 5%, while the population aged 65 and over is projected to grow by 55%,” they said.
In other words, it may not be the best time to be a pediatrician.
VIDEO: Prescription-strength ibuprofen worsens blood pressure more than other NSAIDs
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Incident hypertension occurred within 4 months in 23.2% of arthritis patients on ibuprofen, compared with 10.3% taking celecoxib and 19% on naproxen.
Data source: This was a randomized, double-blind, multicenter, prospective trial including 444 arthritis patients at increased cardiovascular risk who underwent 4 months of ambulatory blood pressure monitoring after being assigned to prescription-strength ibuprofen, naproxen, or celecoxib.
Disclosures: The PRECISION-ABPM trial was sponsored by Pfizer. The presenter reported having no financial conflicts of interest.
FDA grants Priority Review to Gazyva for follicular lymphoma
Gazyva (obinutuzumab) has been granted a Priority Review by the Food and Drug Administration for the treatment of previously untreated follicular lymphoma, according to a press release from Genentech.
FDA approval was based on results from the GALLIUM study, a phase 3 trial comparing Gazyva to Rituxan (rituximab). Patients who received Gazyva plus chemotherapy followed by Gazyva therapy alone for 2 years had a 32% improvement in progression-free survival during the 41.1 month follow-up period, compared with the patient group who received Rituxan plus chemotherapy followed by 2 years of Rituxan therapy alone. Median progression-free survival has not been reached in either arm of the study.
The most common adverse events that occurred more often in the Gazyva arm of the study were low white blood cell count, infections, infusion-related reactions, low platelet count, new tumors, and cardiac events.
“Based on the GALLIUM study, Gazyva-based treatment significantly improved progression-free survival over the current standard of care, and we are committed to bringing this potential new option to patients as soon as possible,” Dr. Sandra Horning, chief medical officer and head of Genentech’s Global Product Development said in the press release.
The FDA is expected to make a decision on approval under Priority Review by Dec. 23, 2017.
Find the full press release on the Genentech website.
Gazyva (obinutuzumab) has been granted a Priority Review by the Food and Drug Administration for the treatment of previously untreated follicular lymphoma, according to a press release from Genentech.
FDA approval was based on results from the GALLIUM study, a phase 3 trial comparing Gazyva to Rituxan (rituximab). Patients who received Gazyva plus chemotherapy followed by Gazyva therapy alone for 2 years had a 32% improvement in progression-free survival during the 41.1 month follow-up period, compared with the patient group who received Rituxan plus chemotherapy followed by 2 years of Rituxan therapy alone. Median progression-free survival has not been reached in either arm of the study.
The most common adverse events that occurred more often in the Gazyva arm of the study were low white blood cell count, infections, infusion-related reactions, low platelet count, new tumors, and cardiac events.
“Based on the GALLIUM study, Gazyva-based treatment significantly improved progression-free survival over the current standard of care, and we are committed to bringing this potential new option to patients as soon as possible,” Dr. Sandra Horning, chief medical officer and head of Genentech’s Global Product Development said in the press release.
The FDA is expected to make a decision on approval under Priority Review by Dec. 23, 2017.
Find the full press release on the Genentech website.
Gazyva (obinutuzumab) has been granted a Priority Review by the Food and Drug Administration for the treatment of previously untreated follicular lymphoma, according to a press release from Genentech.
FDA approval was based on results from the GALLIUM study, a phase 3 trial comparing Gazyva to Rituxan (rituximab). Patients who received Gazyva plus chemotherapy followed by Gazyva therapy alone for 2 years had a 32% improvement in progression-free survival during the 41.1 month follow-up period, compared with the patient group who received Rituxan plus chemotherapy followed by 2 years of Rituxan therapy alone. Median progression-free survival has not been reached in either arm of the study.
The most common adverse events that occurred more often in the Gazyva arm of the study were low white blood cell count, infections, infusion-related reactions, low platelet count, new tumors, and cardiac events.
“Based on the GALLIUM study, Gazyva-based treatment significantly improved progression-free survival over the current standard of care, and we are committed to bringing this potential new option to patients as soon as possible,” Dr. Sandra Horning, chief medical officer and head of Genentech’s Global Product Development said in the press release.
The FDA is expected to make a decision on approval under Priority Review by Dec. 23, 2017.
Find the full press release on the Genentech website.
What’s in a Name? AIDS.gov Becomes HIV.gov
“HIV” has become a more common internet search term than “AIDS.” That change reflects the fact that more people now are living with HIV than with AIDS. What’s more, the number of annual HIV infections fell 18% between 2008 - 2014. In honor of the progress made in changing AIDS from an almost universally fatal disease to a manageable condition, AIDS.gov has changed its name to HIV.gov.
The name change comes 36 years after the CDC’s first report of the initial cases of what became known as acquired immune deficiency syndrome. “Much progress has been made in HIV/AIDS research since the disease was first recognized in 1981,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “Today, lifesaving antiretroviral therapies allow those living with HIV to enjoy longer, healthier lives—an outcome that once seemed unattainable.”
Organizations from the HIV/AIDS community are pleased with the name change. National Minority AIDS Council Executive Director Paul Kawata applauds it, saying it “honors the past while recognizing the power of words and acknowledging that their meanings change over time.”
“HIV” has become a more common internet search term than “AIDS.” That change reflects the fact that more people now are living with HIV than with AIDS. What’s more, the number of annual HIV infections fell 18% between 2008 - 2014. In honor of the progress made in changing AIDS from an almost universally fatal disease to a manageable condition, AIDS.gov has changed its name to HIV.gov.
The name change comes 36 years after the CDC’s first report of the initial cases of what became known as acquired immune deficiency syndrome. “Much progress has been made in HIV/AIDS research since the disease was first recognized in 1981,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “Today, lifesaving antiretroviral therapies allow those living with HIV to enjoy longer, healthier lives—an outcome that once seemed unattainable.”
Organizations from the HIV/AIDS community are pleased with the name change. National Minority AIDS Council Executive Director Paul Kawata applauds it, saying it “honors the past while recognizing the power of words and acknowledging that their meanings change over time.”
“HIV” has become a more common internet search term than “AIDS.” That change reflects the fact that more people now are living with HIV than with AIDS. What’s more, the number of annual HIV infections fell 18% between 2008 - 2014. In honor of the progress made in changing AIDS from an almost universally fatal disease to a manageable condition, AIDS.gov has changed its name to HIV.gov.
The name change comes 36 years after the CDC’s first report of the initial cases of what became known as acquired immune deficiency syndrome. “Much progress has been made in HIV/AIDS research since the disease was first recognized in 1981,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “Today, lifesaving antiretroviral therapies allow those living with HIV to enjoy longer, healthier lives—an outcome that once seemed unattainable.”
Organizations from the HIV/AIDS community are pleased with the name change. National Minority AIDS Council Executive Director Paul Kawata applauds it, saying it “honors the past while recognizing the power of words and acknowledging that their meanings change over time.”
Shorter DAPT appears safe, effective long-term
BARCELONA—Three-year follow-up of the NIPPON study showed no significant difference in net clinical benefit with short-term or long-term dual antiplatelet therapy (DAPT).
Overall, there was no significant difference in safety or efficacy outcomes between patients who received 6 months of DAPT or 18 months of DAPT after placement of a drug-eluting stent (DES).
However, the data did suggest certain patients may benefit from long-term DAPT.
Details of this study were presented at ESC Congress 2017 (abstract 3831).
“These findings support and strengthen the evidence for short-term DAPT after DES deployment and may help confirm that clinical benefits of extended DAPT are reduced in patients with newer-generation DES,” said study investigator Masato Nakamura MD, PhD, of Toho University Ohashi Medical Center in Tokyo, Japan.
“The findings are important because shorter duration of therapy is less expensive and could theoretically reduce the risk of side effects.”
NIPPON enrolled 3775 patients with coronary artery disease or acute myocardial infarction who had undergone percutaneous coronary intervention and DES placement at 130 Japanese institutions.
All patients had received the Nobori bioabsorbable abluminal-coated stent. They then received DAPT—consisting of aspirin (81–162 mg/day) and clopidogrel (75 mg/day) or ticlopidine (200 mg/day)—for 6 months or 18 months.
Initial results from this trial were presented last year at ESC Congress.
The 3-year follow-up included 3307 patients—1887 who received long-term DAPT and 1886 who received short-term DAPT.
Dr Nakamura first reported the net adverse clinical and cerebrovascular events (NACCEs)—which included all-cause mortality, myocardial infarction, stroke, and major bleeding—in each arm.
Three years from the start of the study, the cumulative incidence of NACCE was 7.03% in the short-term DAPT arm and 4.85% in the long-term DAPT arm (hazard ratio [HR]=1.20, P=0.27).
In the landmark analysis, which only included data from 6 months to 3 years, the incidence of NACCE was 5.60% in the short-term DAPT arm and 3.48% in the long-term DAPT arm (HR=1.49, P=0.06).
The investigators also looked at efficacy and safety endpoints separately.
The efficacy endpoint included cardiovascular death, myocardial infarction, stroke, and definite or probable stent thrombosis. The safety endpoint included BARC 2, 3, or 5 bleeding.
In the landmark analysis, the incidence of the efficacy endpoint was 2.58% in the short-term DAPT arm and 1.70% in the long-term DAPT arm (HR=1.53, P=0.17). The incidence of the safety endpoint was 2.22% and 2.01%, respectively (HR=1.02, P=0.94).
Although there were no significant differences between the arms in the overall cohort, the investigators did identify patients with a high risk of ischemic events who might benefit from long-term DAPT.
In patients older than 70 who had a SYNTAX score above 23.3, the rate of efficacy events was 0% in those on long-term DAPT and 18.8% in those on short-term DAPT (HR=13.24, P=0.01).
In patients age 70 to 76 who had diabetes, the rate of efficacy events was 2.0% in those on long-term DAPT and 6.0% in those on short-term DAPT (HR=2.89, P=0.04)
“In real-world practice, it is not easy to find the balance between risks and benefits of DAPT duration, and consensus criteria for individualization therapy have not been established,” Dr Nakamura said.
“The present findings may provide some assistance, although it is essential to obtain confirmation by further investigation.”
This study was sponsored by the Association for Establishment of Evidence in Interventions. Dr Nakamura disclosed research expert witness payment from Terumo Corporation, grant support from Daiichi Sankyo and Sanofi, and honoraria from Terumo Corporation, Daiichi Sankyo, and Sanofi. ![]()
BARCELONA—Three-year follow-up of the NIPPON study showed no significant difference in net clinical benefit with short-term or long-term dual antiplatelet therapy (DAPT).
Overall, there was no significant difference in safety or efficacy outcomes between patients who received 6 months of DAPT or 18 months of DAPT after placement of a drug-eluting stent (DES).
However, the data did suggest certain patients may benefit from long-term DAPT.
Details of this study were presented at ESC Congress 2017 (abstract 3831).
“These findings support and strengthen the evidence for short-term DAPT after DES deployment and may help confirm that clinical benefits of extended DAPT are reduced in patients with newer-generation DES,” said study investigator Masato Nakamura MD, PhD, of Toho University Ohashi Medical Center in Tokyo, Japan.
“The findings are important because shorter duration of therapy is less expensive and could theoretically reduce the risk of side effects.”
NIPPON enrolled 3775 patients with coronary artery disease or acute myocardial infarction who had undergone percutaneous coronary intervention and DES placement at 130 Japanese institutions.
All patients had received the Nobori bioabsorbable abluminal-coated stent. They then received DAPT—consisting of aspirin (81–162 mg/day) and clopidogrel (75 mg/day) or ticlopidine (200 mg/day)—for 6 months or 18 months.
Initial results from this trial were presented last year at ESC Congress.
The 3-year follow-up included 3307 patients—1887 who received long-term DAPT and 1886 who received short-term DAPT.
Dr Nakamura first reported the net adverse clinical and cerebrovascular events (NACCEs)—which included all-cause mortality, myocardial infarction, stroke, and major bleeding—in each arm.
Three years from the start of the study, the cumulative incidence of NACCE was 7.03% in the short-term DAPT arm and 4.85% in the long-term DAPT arm (hazard ratio [HR]=1.20, P=0.27).
In the landmark analysis, which only included data from 6 months to 3 years, the incidence of NACCE was 5.60% in the short-term DAPT arm and 3.48% in the long-term DAPT arm (HR=1.49, P=0.06).
The investigators also looked at efficacy and safety endpoints separately.
The efficacy endpoint included cardiovascular death, myocardial infarction, stroke, and definite or probable stent thrombosis. The safety endpoint included BARC 2, 3, or 5 bleeding.
In the landmark analysis, the incidence of the efficacy endpoint was 2.58% in the short-term DAPT arm and 1.70% in the long-term DAPT arm (HR=1.53, P=0.17). The incidence of the safety endpoint was 2.22% and 2.01%, respectively (HR=1.02, P=0.94).
Although there were no significant differences between the arms in the overall cohort, the investigators did identify patients with a high risk of ischemic events who might benefit from long-term DAPT.
In patients older than 70 who had a SYNTAX score above 23.3, the rate of efficacy events was 0% in those on long-term DAPT and 18.8% in those on short-term DAPT (HR=13.24, P=0.01).
In patients age 70 to 76 who had diabetes, the rate of efficacy events was 2.0% in those on long-term DAPT and 6.0% in those on short-term DAPT (HR=2.89, P=0.04)
“In real-world practice, it is not easy to find the balance between risks and benefits of DAPT duration, and consensus criteria for individualization therapy have not been established,” Dr Nakamura said.
“The present findings may provide some assistance, although it is essential to obtain confirmation by further investigation.”
This study was sponsored by the Association for Establishment of Evidence in Interventions. Dr Nakamura disclosed research expert witness payment from Terumo Corporation, grant support from Daiichi Sankyo and Sanofi, and honoraria from Terumo Corporation, Daiichi Sankyo, and Sanofi. ![]()
BARCELONA—Three-year follow-up of the NIPPON study showed no significant difference in net clinical benefit with short-term or long-term dual antiplatelet therapy (DAPT).
Overall, there was no significant difference in safety or efficacy outcomes between patients who received 6 months of DAPT or 18 months of DAPT after placement of a drug-eluting stent (DES).
However, the data did suggest certain patients may benefit from long-term DAPT.
Details of this study were presented at ESC Congress 2017 (abstract 3831).
“These findings support and strengthen the evidence for short-term DAPT after DES deployment and may help confirm that clinical benefits of extended DAPT are reduced in patients with newer-generation DES,” said study investigator Masato Nakamura MD, PhD, of Toho University Ohashi Medical Center in Tokyo, Japan.
“The findings are important because shorter duration of therapy is less expensive and could theoretically reduce the risk of side effects.”
NIPPON enrolled 3775 patients with coronary artery disease or acute myocardial infarction who had undergone percutaneous coronary intervention and DES placement at 130 Japanese institutions.
All patients had received the Nobori bioabsorbable abluminal-coated stent. They then received DAPT—consisting of aspirin (81–162 mg/day) and clopidogrel (75 mg/day) or ticlopidine (200 mg/day)—for 6 months or 18 months.
Initial results from this trial were presented last year at ESC Congress.
The 3-year follow-up included 3307 patients—1887 who received long-term DAPT and 1886 who received short-term DAPT.
Dr Nakamura first reported the net adverse clinical and cerebrovascular events (NACCEs)—which included all-cause mortality, myocardial infarction, stroke, and major bleeding—in each arm.
Three years from the start of the study, the cumulative incidence of NACCE was 7.03% in the short-term DAPT arm and 4.85% in the long-term DAPT arm (hazard ratio [HR]=1.20, P=0.27).
In the landmark analysis, which only included data from 6 months to 3 years, the incidence of NACCE was 5.60% in the short-term DAPT arm and 3.48% in the long-term DAPT arm (HR=1.49, P=0.06).
The investigators also looked at efficacy and safety endpoints separately.
The efficacy endpoint included cardiovascular death, myocardial infarction, stroke, and definite or probable stent thrombosis. The safety endpoint included BARC 2, 3, or 5 bleeding.
In the landmark analysis, the incidence of the efficacy endpoint was 2.58% in the short-term DAPT arm and 1.70% in the long-term DAPT arm (HR=1.53, P=0.17). The incidence of the safety endpoint was 2.22% and 2.01%, respectively (HR=1.02, P=0.94).
Although there were no significant differences between the arms in the overall cohort, the investigators did identify patients with a high risk of ischemic events who might benefit from long-term DAPT.
In patients older than 70 who had a SYNTAX score above 23.3, the rate of efficacy events was 0% in those on long-term DAPT and 18.8% in those on short-term DAPT (HR=13.24, P=0.01).
In patients age 70 to 76 who had diabetes, the rate of efficacy events was 2.0% in those on long-term DAPT and 6.0% in those on short-term DAPT (HR=2.89, P=0.04)
“In real-world practice, it is not easy to find the balance between risks and benefits of DAPT duration, and consensus criteria for individualization therapy have not been established,” Dr Nakamura said.
“The present findings may provide some assistance, although it is essential to obtain confirmation by further investigation.”
This study was sponsored by the Association for Establishment of Evidence in Interventions. Dr Nakamura disclosed research expert witness payment from Terumo Corporation, grant support from Daiichi Sankyo and Sanofi, and honoraria from Terumo Corporation, Daiichi Sankyo, and Sanofi. ![]()