User login
Biomarkers come up empty for ribociclib targeting
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
EXPERT ANALYSIS FROM IMPAKT 2017 BREAST CANCER CONFERENCE
Key clinical point:
Major finding: Hazard ratio benefits from ribociclib plus letrozole, compared with letrozole alone, were similar regardless of the levels of seven biomarkers.
Data source: Exploratory analysis of tumor specimens collected from 668 patients with breast cancer enrolled in the MONALEESA-2 study.
Disclosures: MONALEESA-2 was sponsored by Novartis, the company that markets ribociclib (Kisqali). Dr. Bedard has received honoraria from Novartis, Pfizer, Roche, and Sanofi, and he has received research funding from Novartis and several other companies. Dr. André has received research funding from Novartis, AstraZeneca, Lilly, and Pfizer.
Breast density is no reason to perform MRI
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
AT ASBS 2017
Key clinical point: High breast density is probably not cause enough to order preoperative MRI.
Major finding: Freedom from recurrence rates were 90% in high density and 91% in low.
Data source: Retrospective analysis of 683 women at a single institution.
Disclosures: The study was funded internally. Dr. McLaughlin and Dr. Boughey reported having no relevant financial relationships.
Radiation bests mastectomy for occult breast cancer
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
AT ASBS 2017
Key clinical point:
Major finding: Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation.
Data source: Review of 934 cases in the National Cancer Database.
Disclosures: The investigators said they had no financial disclosures.
Novel agent varlilumab shows activity in RCC
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A novel, first-in-class, agonist anti-CD27 monoclonal antibody showed activity in solid tumors when used as monotherapy.
Major finding: Two patients with advanced renal cell carcinoma experienced tumor shrinkage and durable responses while eight achieved stable disease.
Data source: Phase I first-in-human study comprising 56 patients with advanced colorectal cancer, renal cell carcinoma, and melanoma.
Disclosures: The study was supported by Celldex Therapeutics. Dr. Burris has no disclosures, and several coauthors have declared relationships with the industry.
Low-histamine diet reduces disease activity in chronic urticaria
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A low-histamine diet for 3-4 weeks may be a simple therapeutic option for patients suffering with chronic spontaneous urticaria (CsU).
Major finding: Three-quarters of patients with CsU showed an improvement in disease activity score after 3 weeks on a low-histamine diet.
Data source: A prospective 3-week study evaluating the impact of a low-histamine diet on 56 patients with CsU.
Disclosures: No conflicts of interest or study funding source were declared.
Recalcitrant Solitary Erythematous Scaly Patch on the Foot
The Diagnosis: Pagetoid Reticulosis
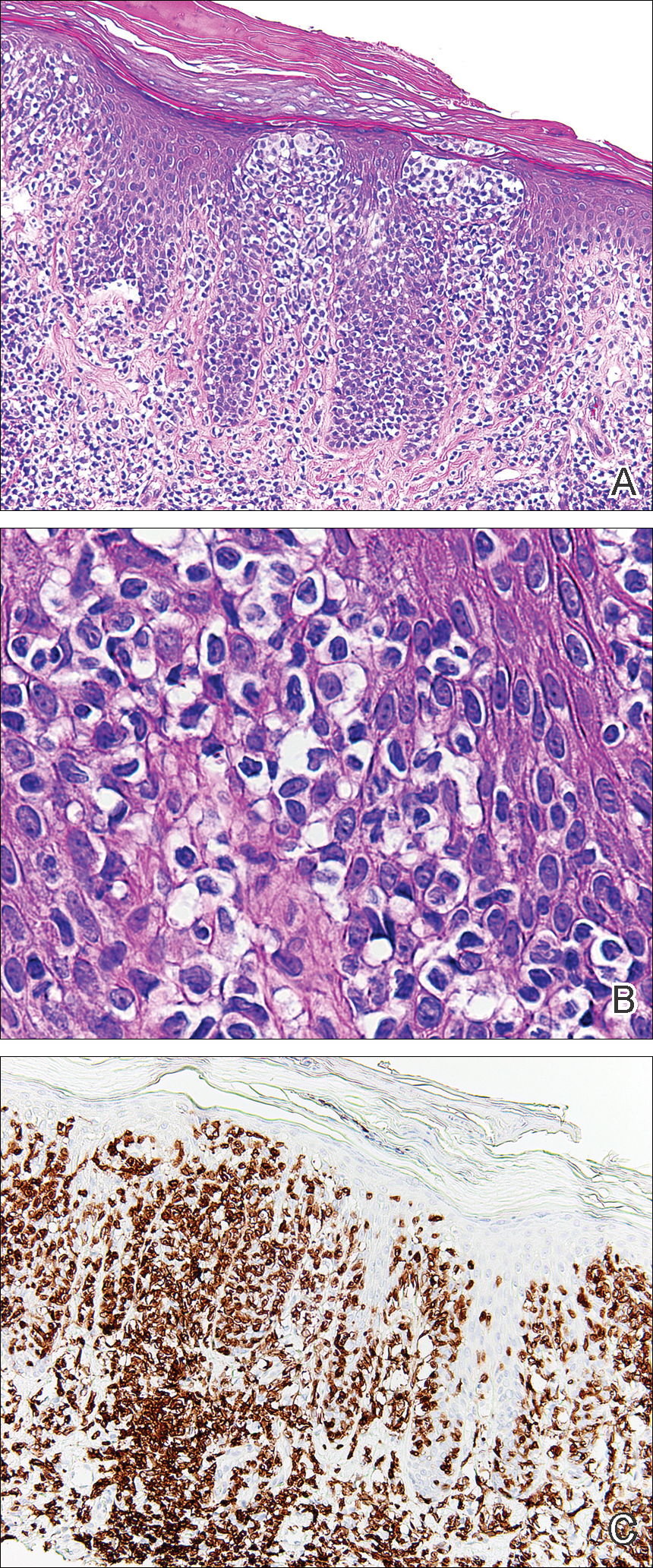
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.
Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
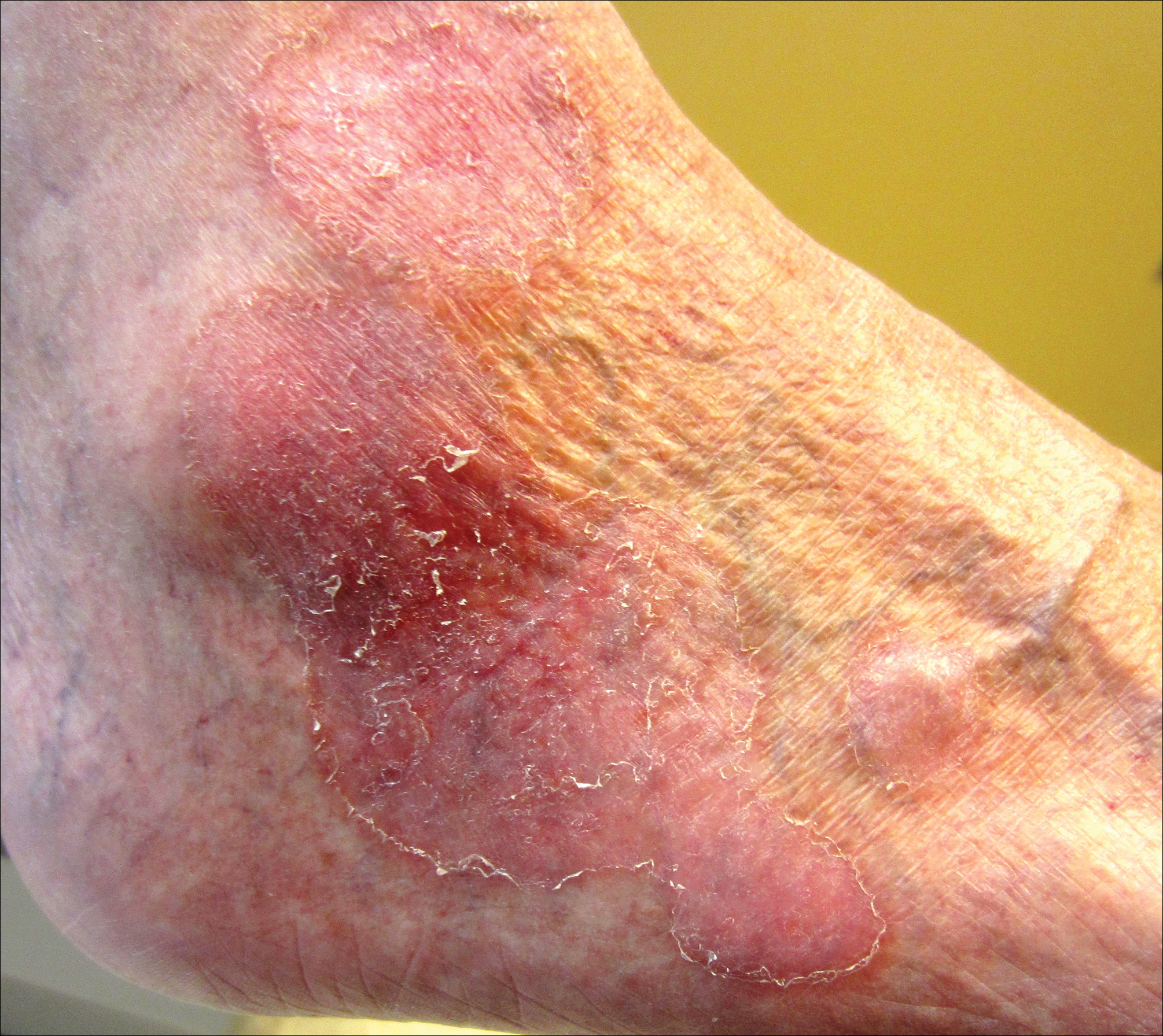
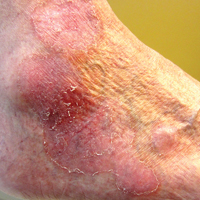
An 80-year-old man with a history of malignant melanoma and squamous cell carcinoma presented to the dermatology clinic with a chronic rash of 20 years' duration on the right ankle that extended to the instep of the right foot. His medical history was notable for hypertension and hyperlipidemia. Family history was unremarkable. The patient described the rash as red and scaly but denied associated pain or pruritus. Over the last 2 to 3 years he had tried treating the affected area with petroleum jelly, topical and oral antifungals, and mild topical steroids with minimal improvement. Complete review of systems was performed and was negative other than some mild constipation. Physical examination revealed an erythematous scaly patch on the dorsal aspect of the right ankle. Potassium hydroxide preparation and fungal culture swab yielded negative results, and a shave biopsy was performed.
Amie Hiller, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Total-Body Photography in Skin Cancer Screening: The Clinical Utility of Standardized Imaging
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Practice Points
- Advances in technology have the potential to provide affordable standardized total-body photography platforms.
- Total-body photography augments the clinical examination and plays a role in clinical decision-making.
- Total-body photography has the potential to become a part of the total-body skin examination and increase access to dermatologic care.
Anup Patel, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3
A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3
A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3
A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.