User login
Endoscopic weight loss surgery cuts costs, side effects
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
FROM DDW
Key clinical point: Endoscopic sleeve gastroplasty is a viable option for patients seeking weight loss but wishing to avoid major surgery.
Major finding: After 1 year, 1% of patients who underwent endoscopic sleeve gastroplasty experienced adverse events, compared with 8% of laparoscopic sleeve gastrectomy patients, and 9% of laparoscopic band placement patients.
Data source: A randomized trial of 278 obese adults who underwent one of three weight loss procedures.
Disclosures: Dr. Sharaiha had no relevant financial conflicts to disclose.
2017 Update on cervical disease
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
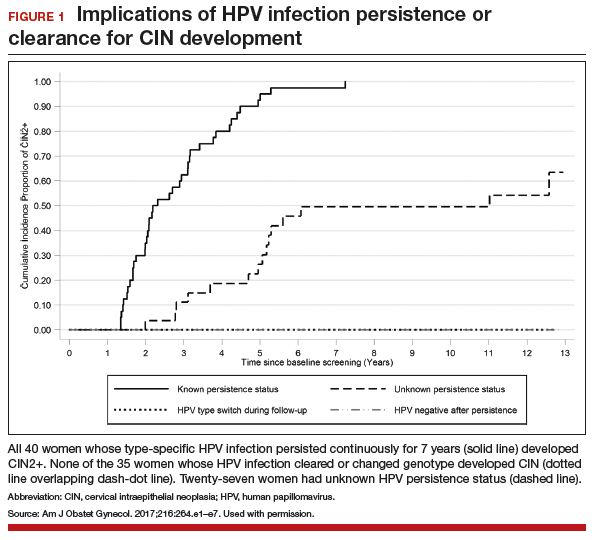
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
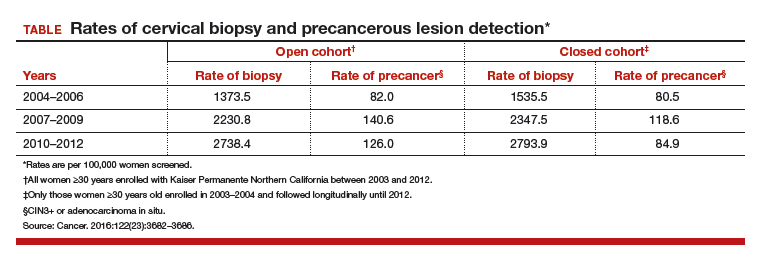
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
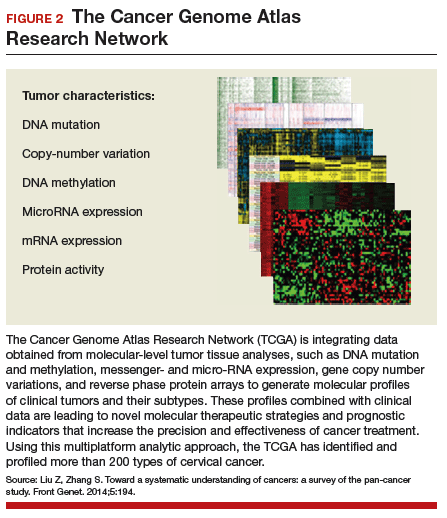
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
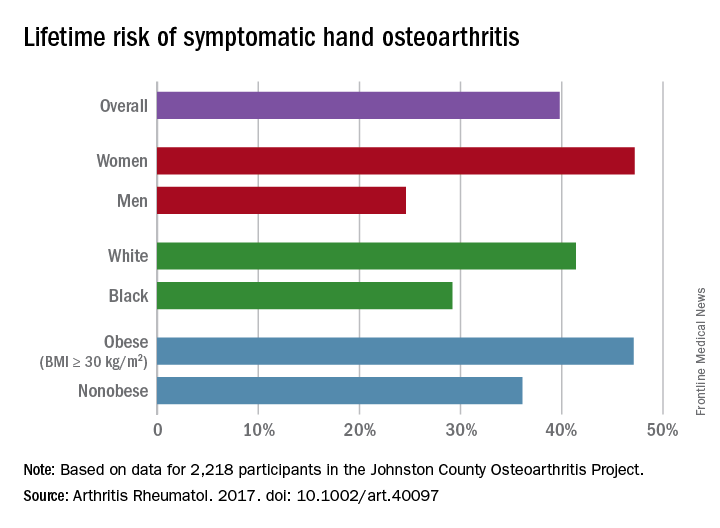
Lifetime risk of hand OA comes close to 40%
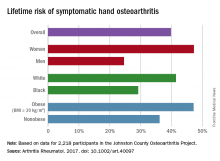
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
FROM ARTHRITIS & RHEUMATOLOGY
FDA approves first new drug for ALS in decades
The Food and Drug Administration approved the antioxidant drug edaravone on May 5 for the treatment of amyotrophic lateral sclerosis, making it only the second drug ever to be approved by the agency for the motor neuron disease.
The FDA granted approval for edaravone, to be marketed by Mitsubishi Tanabe Pharma America under the brand name Radicava, through its orphan drug pathway, which is meant for drugs used to treat rare diseases or conditions. The Centers for Disease Control and Prevention estimates that amyotrophic lateral sclerosis (ALS) affects 12,000-15,000 Americans.
Mitsubishi Tanabe Pharma America demonstrated the efficacy of edaravone in a 6-month trial of 137 Japanese ALS patients. At 24 weeks, individuals who received edaravone had less decline on a clinical assessment of daily functioning, the ALS Functional Rating Scale-Revised (ALSFRS-R), compared with those who received a placebo. The difference in decline between the two groups was 33%, or a total of 2.49 points, on the ALSFRS-R. Most of the patients in the study also received the only other drug approved for ALS, riluzole (Rilutek).
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity.
The adverse events most often reported by clinical trial participants who took edaravone included bruising and gait disturbance. The FDA also warned that edaravone is associated with hives, swelling, or shortness of breath, and allergic reactions to an ingredient in the drug, sodium bisulfite, which may cause anaphylactic symptoms that can be life-threatening in people with sulfite sensitivity.
The drug is administered via intravenous infusion with an initial treatment cycle of daily dosing for 14 days, followed by a 14-day drug-free period. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug-free.
Mitsubishi Tanabe Pharma America said in a statement that it has created a patient access program called Searchlight Support for people with ALS who are prescribed the drug. The program provides personal case management, reimbursement support, and 24/7 clinical support.
In 2015, edaravone was approved for use as a treatment for ALS in Japan and South Korea.
The Food and Drug Administration approved the antioxidant drug edaravone on May 5 for the treatment of amyotrophic lateral sclerosis, making it only the second drug ever to be approved by the agency for the motor neuron disease.
The FDA granted approval for edaravone, to be marketed by Mitsubishi Tanabe Pharma America under the brand name Radicava, through its orphan drug pathway, which is meant for drugs used to treat rare diseases or conditions. The Centers for Disease Control and Prevention estimates that amyotrophic lateral sclerosis (ALS) affects 12,000-15,000 Americans.
Mitsubishi Tanabe Pharma America demonstrated the efficacy of edaravone in a 6-month trial of 137 Japanese ALS patients. At 24 weeks, individuals who received edaravone had less decline on a clinical assessment of daily functioning, the ALS Functional Rating Scale-Revised (ALSFRS-R), compared with those who received a placebo. The difference in decline between the two groups was 33%, or a total of 2.49 points, on the ALSFRS-R. Most of the patients in the study also received the only other drug approved for ALS, riluzole (Rilutek).
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity.
The adverse events most often reported by clinical trial participants who took edaravone included bruising and gait disturbance. The FDA also warned that edaravone is associated with hives, swelling, or shortness of breath, and allergic reactions to an ingredient in the drug, sodium bisulfite, which may cause anaphylactic symptoms that can be life-threatening in people with sulfite sensitivity.
The drug is administered via intravenous infusion with an initial treatment cycle of daily dosing for 14 days, followed by a 14-day drug-free period. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug-free.
Mitsubishi Tanabe Pharma America said in a statement that it has created a patient access program called Searchlight Support for people with ALS who are prescribed the drug. The program provides personal case management, reimbursement support, and 24/7 clinical support.
In 2015, edaravone was approved for use as a treatment for ALS in Japan and South Korea.
The Food and Drug Administration approved the antioxidant drug edaravone on May 5 for the treatment of amyotrophic lateral sclerosis, making it only the second drug ever to be approved by the agency for the motor neuron disease.
The FDA granted approval for edaravone, to be marketed by Mitsubishi Tanabe Pharma America under the brand name Radicava, through its orphan drug pathway, which is meant for drugs used to treat rare diseases or conditions. The Centers for Disease Control and Prevention estimates that amyotrophic lateral sclerosis (ALS) affects 12,000-15,000 Americans.
Mitsubishi Tanabe Pharma America demonstrated the efficacy of edaravone in a 6-month trial of 137 Japanese ALS patients. At 24 weeks, individuals who received edaravone had less decline on a clinical assessment of daily functioning, the ALS Functional Rating Scale-Revised (ALSFRS-R), compared with those who received a placebo. The difference in decline between the two groups was 33%, or a total of 2.49 points, on the ALSFRS-R. Most of the patients in the study also received the only other drug approved for ALS, riluzole (Rilutek).
Edaravone is thought to confer neuroprotection in part through its free radical–scavenging activity.
The adverse events most often reported by clinical trial participants who took edaravone included bruising and gait disturbance. The FDA also warned that edaravone is associated with hives, swelling, or shortness of breath, and allergic reactions to an ingredient in the drug, sodium bisulfite, which may cause anaphylactic symptoms that can be life-threatening in people with sulfite sensitivity.
The drug is administered via intravenous infusion with an initial treatment cycle of daily dosing for 14 days, followed by a 14-day drug-free period. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug-free.
Mitsubishi Tanabe Pharma America said in a statement that it has created a patient access program called Searchlight Support for people with ALS who are prescribed the drug. The program provides personal case management, reimbursement support, and 24/7 clinical support.
In 2015, edaravone was approved for use as a treatment for ALS in Japan and South Korea.
BTK inhibitor staves off progression in CLL
Long-term follow-up of a phase 1 study suggests the BTK inhibitor ONO/GS-4059 can stave off progression in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Roughly 60% of the patients studied were progression-free and still taking ONO/GS-4059 at last follow-up, with the longest time on treatment exceeding 3 years.
In addition, researchers said the extended follow-up revealed no new safety concerns, and the maximum tolerated dose of ONO/GS-4059 has not been reached.
Martin Dyer, DPhil, of the University of Leicester in the UK, and his colleagues reported these results in Blood.
The research was funded by Gilead Sciences, Inc., and ONO Pharmaceuticals helped with data analysis.
The study enrolled 90 patients with relapsed or refractory B-cell malignancies, 28 of whom had CLL. Dr Dyer and his colleagues reported follow-up results in CLL patients only.
The patients’ median number of prior treatments was 4 (range, 2-9), and 11 patients were refractory to their last line of therapy. None had received prior treatment with a BTK inhibitor.
The patients received ONO/GS-4059 at varying doses, from 20 mg once daily (QD) to 600 mg QD and a twice-daily (BID) regimen of 300 mg. Six patients were also taking anticoagulant therapy while on study.
Patients were allowed to continue treatment with ONO/GS-4059 if they responded to the drug or maintained stable disease.
Initially, 25 patients were evaluable for response, and 24 of them responded to ONO/GS-4059, for an overall response rate of 96%.
At last follow-up on June 8, 2016, 17 patients were still receiving ONO/GS-4059, and all had a very good partial response.
Dr Dyer said the responses have been similar to those seen with other irreversible BTK inhibitors. Most have involved rapid and almost complete resolution of lymph node masses and rapid improvement in hematological indexes.
“It is clear . . . that the major responses occur rapidly, within the first 3 months of drug, and that, thereafter, improvement occurs at a much slower rate,” Dr Dyer said. “It will be of interest, I think, to look at the remaining patients on study to assess whether responses deepen with time on drug.”
The duration of treatment for these patients ranged from 302 days to 1160 days at last follow-up. They were receiving ONO/GS-4059 at doses ranging from 40 mg QD to 600 mg QD or 300 mg BID, and no maximum tolerated dose had been identified.
Eleven patients (39.3%) discontinued ONO/GS-4059 due to death (n=3), disease progression (n=4), adverse events (AEs, n=3), and sponsor decision due to extended drug interruption (n=1). One of the patients included in the AE group also had concurrent disease progression.
The median progression-free survival was 38.5 months, and the median overall survival was 44.9 months. The median time on study was 32.5 months.
The most common treatment-emergent AEs were bruising (35.7%), neutropenia (35.7%), anemia (32.1%), nasopharyngitis (32.1%), fall (32.1%), cough (28.6%), arthralgia (28.6%), and basal cell carcinoma (28.6%).
The most common grade 3/4 AEs included neutropenia (25%), thrombocytopenia (14.3%), lower respiratory tract infection (14.3%), and anemia (10.7%).
“Our long-term follow-up shows maintained efficacy without toxicity,” Dr Dyer said. “This study is the first report of long-term follow-up of a selective BTK inhibitor, and it is excellent news for patients. We are now doing studies of ONO/GS-4059 in combination with other precision medicines to assess whether these results can be enhanced in patients with CLL and other B-cell malignancies.” ![]()
Long-term follow-up of a phase 1 study suggests the BTK inhibitor ONO/GS-4059 can stave off progression in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Roughly 60% of the patients studied were progression-free and still taking ONO/GS-4059 at last follow-up, with the longest time on treatment exceeding 3 years.
In addition, researchers said the extended follow-up revealed no new safety concerns, and the maximum tolerated dose of ONO/GS-4059 has not been reached.
Martin Dyer, DPhil, of the University of Leicester in the UK, and his colleagues reported these results in Blood.
The research was funded by Gilead Sciences, Inc., and ONO Pharmaceuticals helped with data analysis.
The study enrolled 90 patients with relapsed or refractory B-cell malignancies, 28 of whom had CLL. Dr Dyer and his colleagues reported follow-up results in CLL patients only.
The patients’ median number of prior treatments was 4 (range, 2-9), and 11 patients were refractory to their last line of therapy. None had received prior treatment with a BTK inhibitor.
The patients received ONO/GS-4059 at varying doses, from 20 mg once daily (QD) to 600 mg QD and a twice-daily (BID) regimen of 300 mg. Six patients were also taking anticoagulant therapy while on study.
Patients were allowed to continue treatment with ONO/GS-4059 if they responded to the drug or maintained stable disease.
Initially, 25 patients were evaluable for response, and 24 of them responded to ONO/GS-4059, for an overall response rate of 96%.
At last follow-up on June 8, 2016, 17 patients were still receiving ONO/GS-4059, and all had a very good partial response.
Dr Dyer said the responses have been similar to those seen with other irreversible BTK inhibitors. Most have involved rapid and almost complete resolution of lymph node masses and rapid improvement in hematological indexes.
“It is clear . . . that the major responses occur rapidly, within the first 3 months of drug, and that, thereafter, improvement occurs at a much slower rate,” Dr Dyer said. “It will be of interest, I think, to look at the remaining patients on study to assess whether responses deepen with time on drug.”
The duration of treatment for these patients ranged from 302 days to 1160 days at last follow-up. They were receiving ONO/GS-4059 at doses ranging from 40 mg QD to 600 mg QD or 300 mg BID, and no maximum tolerated dose had been identified.
Eleven patients (39.3%) discontinued ONO/GS-4059 due to death (n=3), disease progression (n=4), adverse events (AEs, n=3), and sponsor decision due to extended drug interruption (n=1). One of the patients included in the AE group also had concurrent disease progression.
The median progression-free survival was 38.5 months, and the median overall survival was 44.9 months. The median time on study was 32.5 months.
The most common treatment-emergent AEs were bruising (35.7%), neutropenia (35.7%), anemia (32.1%), nasopharyngitis (32.1%), fall (32.1%), cough (28.6%), arthralgia (28.6%), and basal cell carcinoma (28.6%).
The most common grade 3/4 AEs included neutropenia (25%), thrombocytopenia (14.3%), lower respiratory tract infection (14.3%), and anemia (10.7%).
“Our long-term follow-up shows maintained efficacy without toxicity,” Dr Dyer said. “This study is the first report of long-term follow-up of a selective BTK inhibitor, and it is excellent news for patients. We are now doing studies of ONO/GS-4059 in combination with other precision medicines to assess whether these results can be enhanced in patients with CLL and other B-cell malignancies.” ![]()
Long-term follow-up of a phase 1 study suggests the BTK inhibitor ONO/GS-4059 can stave off progression in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Roughly 60% of the patients studied were progression-free and still taking ONO/GS-4059 at last follow-up, with the longest time on treatment exceeding 3 years.
In addition, researchers said the extended follow-up revealed no new safety concerns, and the maximum tolerated dose of ONO/GS-4059 has not been reached.
Martin Dyer, DPhil, of the University of Leicester in the UK, and his colleagues reported these results in Blood.
The research was funded by Gilead Sciences, Inc., and ONO Pharmaceuticals helped with data analysis.
The study enrolled 90 patients with relapsed or refractory B-cell malignancies, 28 of whom had CLL. Dr Dyer and his colleagues reported follow-up results in CLL patients only.
The patients’ median number of prior treatments was 4 (range, 2-9), and 11 patients were refractory to their last line of therapy. None had received prior treatment with a BTK inhibitor.
The patients received ONO/GS-4059 at varying doses, from 20 mg once daily (QD) to 600 mg QD and a twice-daily (BID) regimen of 300 mg. Six patients were also taking anticoagulant therapy while on study.
Patients were allowed to continue treatment with ONO/GS-4059 if they responded to the drug or maintained stable disease.
Initially, 25 patients were evaluable for response, and 24 of them responded to ONO/GS-4059, for an overall response rate of 96%.
At last follow-up on June 8, 2016, 17 patients were still receiving ONO/GS-4059, and all had a very good partial response.
Dr Dyer said the responses have been similar to those seen with other irreversible BTK inhibitors. Most have involved rapid and almost complete resolution of lymph node masses and rapid improvement in hematological indexes.
“It is clear . . . that the major responses occur rapidly, within the first 3 months of drug, and that, thereafter, improvement occurs at a much slower rate,” Dr Dyer said. “It will be of interest, I think, to look at the remaining patients on study to assess whether responses deepen with time on drug.”
The duration of treatment for these patients ranged from 302 days to 1160 days at last follow-up. They were receiving ONO/GS-4059 at doses ranging from 40 mg QD to 600 mg QD or 300 mg BID, and no maximum tolerated dose had been identified.
Eleven patients (39.3%) discontinued ONO/GS-4059 due to death (n=3), disease progression (n=4), adverse events (AEs, n=3), and sponsor decision due to extended drug interruption (n=1). One of the patients included in the AE group also had concurrent disease progression.
The median progression-free survival was 38.5 months, and the median overall survival was 44.9 months. The median time on study was 32.5 months.
The most common treatment-emergent AEs were bruising (35.7%), neutropenia (35.7%), anemia (32.1%), nasopharyngitis (32.1%), fall (32.1%), cough (28.6%), arthralgia (28.6%), and basal cell carcinoma (28.6%).
The most common grade 3/4 AEs included neutropenia (25%), thrombocytopenia (14.3%), lower respiratory tract infection (14.3%), and anemia (10.7%).
“Our long-term follow-up shows maintained efficacy without toxicity,” Dr Dyer said. “This study is the first report of long-term follow-up of a selective BTK inhibitor, and it is excellent news for patients. We are now doing studies of ONO/GS-4059 in combination with other precision medicines to assess whether these results can be enhanced in patients with CLL and other B-cell malignancies.” ![]()
Psychological account of Robert Lowell’s life is magnificent
Robert Lowell knew civic valor. Sixteen times and more he had been down on his knees in madness, he said. Sixteen times and more he had gotten up. He had gone back to his work, entered back into life. He had faced down uncertainty and madness, had created new forms when pushed to stay with the old, had brought back imaginative order from chaos. It was a different kind of courage, this civic courage, and the rules of engagement were unclear. Lowell’s life, as his daughter observed, was a messy one, difficult for him and for those who knew him. But it was lived with iron, and often with grace. He kept always in the front of his mind what he thought he ought to be, even when he couldn’t be it; he believed in what his country could be, even if it wasn’t. He worked hard at his art.
–Kay Redfield Jamison, PhD, in “Robert Lowell, Setting the River on Fire: A Study of Genius, Mania, and Character” (New York: Alfred A. Knopf, 2017).
Lowell, who lived from 1917 to 1977, was a two-time Pulitzer Prize winner, deemed to be the greatest American poet of his time. He studied the classics and was obsessed with Napoleon as a child, and he drew on the work of other great poets and classicists as influences for his own work. I must confess, I came to this psychological study having never read the work of Robert Lowell. My only familiarity with the poet came directly from the author. I heard Dr. Jamison, a professor of psychiatry at Johns Hopkins University, Baltimore, speak several years ago at the Johns Hopkins Annual Mood Disorders Symposium about her then work-in-progress as she was researching this book. What I heard was intriguing enough that I was eager to read and review a long and solid book about a great poet whose work I had never read.
As I began “Setting The River On Fire,” my first thought was that the writing itself was astounding. Dr. Jamison’s words flow, her metaphors never fall flat or feel artificial, the ride itself is lovely. I looked for a few lines to quote as an example, and I was left at a standstill. One line was more gracious than the next. I finally settled on the quote I used at the beginning of this piece, benignly chosen from page 403 because it encapsulated not just the beautiful writing but a synopsis of who Lowell was and what he had achieved, set in the context of attempted differentiation between the man, the madness, and the interplay of the two.
Dr. Jamison’s research on Lowell’s life is nothing short of astounding and was clearly a labor that took both sustained passion and years of her time. Dr. Jamison quoted the poet at length. She is an expert on his many volumes of poetry and prose, as well as his life and loves – three marriages and many intimate friendships – documented through letters and conversations. In addition, she quoted many other poets as examples of how their work influenced Lowell. Beyond the literature and correspondence, Dr. Jamison interviewed those who knew Lowell well. She unearthed his medical and psychiatric records, and she plotted out the course of his life in an uncanny way, linking so much of his work to the ebbs and flows of his illness. My only “criticism” of the book would be in how extensive it is. She sometimes makes a point by quoting several sources, each of whom drive at the same idea. It makes for very strong rhetoric.
His second wife, Elizabeth Hardwick, had a striking understanding of his illness as a biological disorder beyond his control. Her sympathy for his behavior as a product of illness allowed her to tolerate actions that many people would not, even with our current day emphasis on disease states, including sexual indiscretions. His friends, too, saw the uncharacteristic chaos of his manias as the result of a state of illness, and, as such, as forgivable. These were often not subtle indiscretions: Jamison describes intense delusional states, combative behavior, police with straightjackets, often at very public and professional events worldwide. If psychoanalytic thinking weighed in on an understanding of Lowell’s motivations, Dr. Jamison did not include it in her study of Lowell, and she makes a point at the end of saying that she focused on his illness and did not include the content of psychotherapy notes. Still, I was struck by the understanding of his depressions and manias as a state of illness by lay people in his life and thought that, given the time period, it was noteworthy.
On a similar vein, I wondered if Lowell could live his life now as he lived his life then. A crucial arena for his career was Harvard College, where he returned over and over to teach. Dr. Jamison says that Lowell lectured in an acutely psychotic and disorganized state. She says that, while students clamored to take his classes, so, too, they were afraid of him. I cannot quite imagine that, in our world of “trigger warnings,” microaggressions, and college safe spaces, we might ever allow an openly ill genius to reign in a classroom of students. I am never certain if we are aimed forward or backward in our struggle against stigma, and “Setting The River On Fire” may be one more example in which we have lost ground in a quest for tolerance.
Once again, Dr. Jamison pulled me into her world. “Setting The River On Fire” is no one’s version of a light or happy read, it is a serious study of an intensely brilliant and often desperately ill poet – and it does not disappoint.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Robert Lowell knew civic valor. Sixteen times and more he had been down on his knees in madness, he said. Sixteen times and more he had gotten up. He had gone back to his work, entered back into life. He had faced down uncertainty and madness, had created new forms when pushed to stay with the old, had brought back imaginative order from chaos. It was a different kind of courage, this civic courage, and the rules of engagement were unclear. Lowell’s life, as his daughter observed, was a messy one, difficult for him and for those who knew him. But it was lived with iron, and often with grace. He kept always in the front of his mind what he thought he ought to be, even when he couldn’t be it; he believed in what his country could be, even if it wasn’t. He worked hard at his art.
–Kay Redfield Jamison, PhD, in “Robert Lowell, Setting the River on Fire: A Study of Genius, Mania, and Character” (New York: Alfred A. Knopf, 2017).
Lowell, who lived from 1917 to 1977, was a two-time Pulitzer Prize winner, deemed to be the greatest American poet of his time. He studied the classics and was obsessed with Napoleon as a child, and he drew on the work of other great poets and classicists as influences for his own work. I must confess, I came to this psychological study having never read the work of Robert Lowell. My only familiarity with the poet came directly from the author. I heard Dr. Jamison, a professor of psychiatry at Johns Hopkins University, Baltimore, speak several years ago at the Johns Hopkins Annual Mood Disorders Symposium about her then work-in-progress as she was researching this book. What I heard was intriguing enough that I was eager to read and review a long and solid book about a great poet whose work I had never read.
As I began “Setting The River On Fire,” my first thought was that the writing itself was astounding. Dr. Jamison’s words flow, her metaphors never fall flat or feel artificial, the ride itself is lovely. I looked for a few lines to quote as an example, and I was left at a standstill. One line was more gracious than the next. I finally settled on the quote I used at the beginning of this piece, benignly chosen from page 403 because it encapsulated not just the beautiful writing but a synopsis of who Lowell was and what he had achieved, set in the context of attempted differentiation between the man, the madness, and the interplay of the two.
Dr. Jamison’s research on Lowell’s life is nothing short of astounding and was clearly a labor that took both sustained passion and years of her time. Dr. Jamison quoted the poet at length. She is an expert on his many volumes of poetry and prose, as well as his life and loves – three marriages and many intimate friendships – documented through letters and conversations. In addition, she quoted many other poets as examples of how their work influenced Lowell. Beyond the literature and correspondence, Dr. Jamison interviewed those who knew Lowell well. She unearthed his medical and psychiatric records, and she plotted out the course of his life in an uncanny way, linking so much of his work to the ebbs and flows of his illness. My only “criticism” of the book would be in how extensive it is. She sometimes makes a point by quoting several sources, each of whom drive at the same idea. It makes for very strong rhetoric.
His second wife, Elizabeth Hardwick, had a striking understanding of his illness as a biological disorder beyond his control. Her sympathy for his behavior as a product of illness allowed her to tolerate actions that many people would not, even with our current day emphasis on disease states, including sexual indiscretions. His friends, too, saw the uncharacteristic chaos of his manias as the result of a state of illness, and, as such, as forgivable. These were often not subtle indiscretions: Jamison describes intense delusional states, combative behavior, police with straightjackets, often at very public and professional events worldwide. If psychoanalytic thinking weighed in on an understanding of Lowell’s motivations, Dr. Jamison did not include it in her study of Lowell, and she makes a point at the end of saying that she focused on his illness and did not include the content of psychotherapy notes. Still, I was struck by the understanding of his depressions and manias as a state of illness by lay people in his life and thought that, given the time period, it was noteworthy.
On a similar vein, I wondered if Lowell could live his life now as he lived his life then. A crucial arena for his career was Harvard College, where he returned over and over to teach. Dr. Jamison says that Lowell lectured in an acutely psychotic and disorganized state. She says that, while students clamored to take his classes, so, too, they were afraid of him. I cannot quite imagine that, in our world of “trigger warnings,” microaggressions, and college safe spaces, we might ever allow an openly ill genius to reign in a classroom of students. I am never certain if we are aimed forward or backward in our struggle against stigma, and “Setting The River On Fire” may be one more example in which we have lost ground in a quest for tolerance.
Once again, Dr. Jamison pulled me into her world. “Setting The River On Fire” is no one’s version of a light or happy read, it is a serious study of an intensely brilliant and often desperately ill poet – and it does not disappoint.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Robert Lowell knew civic valor. Sixteen times and more he had been down on his knees in madness, he said. Sixteen times and more he had gotten up. He had gone back to his work, entered back into life. He had faced down uncertainty and madness, had created new forms when pushed to stay with the old, had brought back imaginative order from chaos. It was a different kind of courage, this civic courage, and the rules of engagement were unclear. Lowell’s life, as his daughter observed, was a messy one, difficult for him and for those who knew him. But it was lived with iron, and often with grace. He kept always in the front of his mind what he thought he ought to be, even when he couldn’t be it; he believed in what his country could be, even if it wasn’t. He worked hard at his art.
–Kay Redfield Jamison, PhD, in “Robert Lowell, Setting the River on Fire: A Study of Genius, Mania, and Character” (New York: Alfred A. Knopf, 2017).
Lowell, who lived from 1917 to 1977, was a two-time Pulitzer Prize winner, deemed to be the greatest American poet of his time. He studied the classics and was obsessed with Napoleon as a child, and he drew on the work of other great poets and classicists as influences for his own work. I must confess, I came to this psychological study having never read the work of Robert Lowell. My only familiarity with the poet came directly from the author. I heard Dr. Jamison, a professor of psychiatry at Johns Hopkins University, Baltimore, speak several years ago at the Johns Hopkins Annual Mood Disorders Symposium about her then work-in-progress as she was researching this book. What I heard was intriguing enough that I was eager to read and review a long and solid book about a great poet whose work I had never read.
As I began “Setting The River On Fire,” my first thought was that the writing itself was astounding. Dr. Jamison’s words flow, her metaphors never fall flat or feel artificial, the ride itself is lovely. I looked for a few lines to quote as an example, and I was left at a standstill. One line was more gracious than the next. I finally settled on the quote I used at the beginning of this piece, benignly chosen from page 403 because it encapsulated not just the beautiful writing but a synopsis of who Lowell was and what he had achieved, set in the context of attempted differentiation between the man, the madness, and the interplay of the two.
Dr. Jamison’s research on Lowell’s life is nothing short of astounding and was clearly a labor that took both sustained passion and years of her time. Dr. Jamison quoted the poet at length. She is an expert on his many volumes of poetry and prose, as well as his life and loves – three marriages and many intimate friendships – documented through letters and conversations. In addition, she quoted many other poets as examples of how their work influenced Lowell. Beyond the literature and correspondence, Dr. Jamison interviewed those who knew Lowell well. She unearthed his medical and psychiatric records, and she plotted out the course of his life in an uncanny way, linking so much of his work to the ebbs and flows of his illness. My only “criticism” of the book would be in how extensive it is. She sometimes makes a point by quoting several sources, each of whom drive at the same idea. It makes for very strong rhetoric.
His second wife, Elizabeth Hardwick, had a striking understanding of his illness as a biological disorder beyond his control. Her sympathy for his behavior as a product of illness allowed her to tolerate actions that many people would not, even with our current day emphasis on disease states, including sexual indiscretions. His friends, too, saw the uncharacteristic chaos of his manias as the result of a state of illness, and, as such, as forgivable. These were often not subtle indiscretions: Jamison describes intense delusional states, combative behavior, police with straightjackets, often at very public and professional events worldwide. If psychoanalytic thinking weighed in on an understanding of Lowell’s motivations, Dr. Jamison did not include it in her study of Lowell, and she makes a point at the end of saying that she focused on his illness and did not include the content of psychotherapy notes. Still, I was struck by the understanding of his depressions and manias as a state of illness by lay people in his life and thought that, given the time period, it was noteworthy.
On a similar vein, I wondered if Lowell could live his life now as he lived his life then. A crucial arena for his career was Harvard College, where he returned over and over to teach. Dr. Jamison says that Lowell lectured in an acutely psychotic and disorganized state. She says that, while students clamored to take his classes, so, too, they were afraid of him. I cannot quite imagine that, in our world of “trigger warnings,” microaggressions, and college safe spaces, we might ever allow an openly ill genius to reign in a classroom of students. I am never certain if we are aimed forward or backward in our struggle against stigma, and “Setting The River On Fire” may be one more example in which we have lost ground in a quest for tolerance.
Once again, Dr. Jamison pulled me into her world. “Setting The River On Fire” is no one’s version of a light or happy read, it is a serious study of an intensely brilliant and often desperately ill poet – and it does not disappoint.
Dr. Miller, who practices in Baltimore, is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care,” (Baltimore: Johns Hopkins University Press, 2016).
Finally, a reproducible quality measure for Mohs
As chair of the American Academy of Dermatology’s Patient Access and Payer Relations Committee, I traveled around the United States for three years with other well-versed dermatologists and explained the value of dermatology to insurance company medical directors (thanks to James Zalla, Scott Collins, Howard Rogers, Alexa Kimball, Clifford Lober, Sabra Sullivan, Mark Lebwohl, Beth Lertzman, Bruce Brod, Carrie Kovarik, Brent Moody, George Hruza, and Carl Johnson).
We showed them the statistics, clinical guidelines, and clinical photos and explained how cost effective dermatologists are in treating skin disease. We argued against using blunt tools, like average provider expense, as a proxy for quality. I thought it was a pretty compelling story, but the medical directors always asked for a reproducible quality metric. Almost no one in specialty medicine has reproducible quality metrics, and these are very difficult to develop.
The average number of layers taken for Mohs surgery of head, neck, hands, feet, and genitalia was calculated for all physicians reporting the codes to Medicare from 2012 to 2014. The Accreditation Council for Graduate Medical Education training programs were separately analyzed, since theoretically, they should get referrals of the more complex and difficult cases.
The average proved to be 1.74 stages per case, with a median of 1.69. Of 2,305 physicians billing for Mohs surgery, there were 137 extremely high outliers in at least 1 of 3 years, and 49 persistent high outliers (greater than two standard deviations in all three years), who averaged more than 2.41 layers per case. There were also 92 extremely low outliers (1.28 stages per case, in at least 1 of the 3 years), 20 of whom were persistent in all 3 years. High outliers were more likely to work in a solo practice setting.
The Improving Wisely program is based on the concept that reducing unnecessary variations in care can improve patient safety and quality of care while also reducing costs. It works on the premise that many outliers are unaware they are an outlier, no one wants to be an outlier, and confidential, collegial education and peer mentoring within a medical specialty society can reduce unnecessary variations in care.
Last month, all ACMS members received confidential data reports with their own personal ratio in relation to the entire cohort. Educational and mentoring resources are available for members, and outliers are encouraged to engage with the ACMS to identify opportunities for modifying and improving their practice patterns.
Now, these numbers must not be taken as an indictment of anyone. They are for educational purposes, and the goal is to help identify physicians who are unaware of their deviation and bring these outliers back into the norm. Supporting this premise, solo practitioners were at greatest risk of being outliers, which may be explained by lack of collegial interaction, peer review, and feedback.
In addition, there may be good reasons for being an outlier depending on one’s patient population, and the ACMS is interested in examples. The Mohs College is devoting considerable resources to help outliers. Much of this variation may also result from incorrect coding or processing of specimens. Nonetheless, no patient, payer, or physician wants unnecessary surgery or avoidable charges.
Low outliers are particularly puzzling, since someone would need godlike abilities to almost never have a positive margin in Mohs. I have heard of some practices whose patients all present in the morning, one layer is performed, and the rest of the day is spent processing and interpreting their slides. (Mohs is time consuming.)
I am also aware of some rural providers who travel to distant sites, take a layer, return to the city to process the tissue, and return a few days later to complete the case. This may not indicate bad care, just an unusual practice pattern or adaptation to difficult circumstances. However, it must also be noted that not completing cases on the same day could result in increased payments because of the coding system that reimburses more for first stages than for additional ones.
You must be aware that all these numbers, with a two-year lag, are available to physicians, payers, and patients. If you don’t know your ratio of first to additional Mohs layers, I encourage you to look your numbers up and calculate your ratio (CPT code 17311 plus 17312/code 17311). The easiest website to use is provided by the Wall Street Journal. If you are an outlier, you should ask yourself why, and consider some peer review and other appropriate changes. If your patterns change, they will be noticed quickly, since Johns Hopkins and the Improving Wisely program has leveraged their relationship with the Robert Wood Johnson Foundation to gain more immediate access to current Medicare data.
Everyone is hoping we see normalization of the patterns in layer usage, since this will give great credibility to Mohs surgeons and be better for our patients and the health care system in general. Kudos to the ACMS and the Jama Dermatology paper’s senior author, John Albertini, MD, of Winston-Salem, NC, in particular, for making this benchmark become a reality.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
As chair of the American Academy of Dermatology’s Patient Access and Payer Relations Committee, I traveled around the United States for three years with other well-versed dermatologists and explained the value of dermatology to insurance company medical directors (thanks to James Zalla, Scott Collins, Howard Rogers, Alexa Kimball, Clifford Lober, Sabra Sullivan, Mark Lebwohl, Beth Lertzman, Bruce Brod, Carrie Kovarik, Brent Moody, George Hruza, and Carl Johnson).
We showed them the statistics, clinical guidelines, and clinical photos and explained how cost effective dermatologists are in treating skin disease. We argued against using blunt tools, like average provider expense, as a proxy for quality. I thought it was a pretty compelling story, but the medical directors always asked for a reproducible quality metric. Almost no one in specialty medicine has reproducible quality metrics, and these are very difficult to develop.
The average number of layers taken for Mohs surgery of head, neck, hands, feet, and genitalia was calculated for all physicians reporting the codes to Medicare from 2012 to 2014. The Accreditation Council for Graduate Medical Education training programs were separately analyzed, since theoretically, they should get referrals of the more complex and difficult cases.
The average proved to be 1.74 stages per case, with a median of 1.69. Of 2,305 physicians billing for Mohs surgery, there were 137 extremely high outliers in at least 1 of 3 years, and 49 persistent high outliers (greater than two standard deviations in all three years), who averaged more than 2.41 layers per case. There were also 92 extremely low outliers (1.28 stages per case, in at least 1 of the 3 years), 20 of whom were persistent in all 3 years. High outliers were more likely to work in a solo practice setting.
The Improving Wisely program is based on the concept that reducing unnecessary variations in care can improve patient safety and quality of care while also reducing costs. It works on the premise that many outliers are unaware they are an outlier, no one wants to be an outlier, and confidential, collegial education and peer mentoring within a medical specialty society can reduce unnecessary variations in care.
Last month, all ACMS members received confidential data reports with their own personal ratio in relation to the entire cohort. Educational and mentoring resources are available for members, and outliers are encouraged to engage with the ACMS to identify opportunities for modifying and improving their practice patterns.
Now, these numbers must not be taken as an indictment of anyone. They are for educational purposes, and the goal is to help identify physicians who are unaware of their deviation and bring these outliers back into the norm. Supporting this premise, solo practitioners were at greatest risk of being outliers, which may be explained by lack of collegial interaction, peer review, and feedback.
In addition, there may be good reasons for being an outlier depending on one’s patient population, and the ACMS is interested in examples. The Mohs College is devoting considerable resources to help outliers. Much of this variation may also result from incorrect coding or processing of specimens. Nonetheless, no patient, payer, or physician wants unnecessary surgery or avoidable charges.
Low outliers are particularly puzzling, since someone would need godlike abilities to almost never have a positive margin in Mohs. I have heard of some practices whose patients all present in the morning, one layer is performed, and the rest of the day is spent processing and interpreting their slides. (Mohs is time consuming.)
I am also aware of some rural providers who travel to distant sites, take a layer, return to the city to process the tissue, and return a few days later to complete the case. This may not indicate bad care, just an unusual practice pattern or adaptation to difficult circumstances. However, it must also be noted that not completing cases on the same day could result in increased payments because of the coding system that reimburses more for first stages than for additional ones.
You must be aware that all these numbers, with a two-year lag, are available to physicians, payers, and patients. If you don’t know your ratio of first to additional Mohs layers, I encourage you to look your numbers up and calculate your ratio (CPT code 17311 plus 17312/code 17311). The easiest website to use is provided by the Wall Street Journal. If you are an outlier, you should ask yourself why, and consider some peer review and other appropriate changes. If your patterns change, they will be noticed quickly, since Johns Hopkins and the Improving Wisely program has leveraged their relationship with the Robert Wood Johnson Foundation to gain more immediate access to current Medicare data.
Everyone is hoping we see normalization of the patterns in layer usage, since this will give great credibility to Mohs surgeons and be better for our patients and the health care system in general. Kudos to the ACMS and the Jama Dermatology paper’s senior author, John Albertini, MD, of Winston-Salem, NC, in particular, for making this benchmark become a reality.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
As chair of the American Academy of Dermatology’s Patient Access and Payer Relations Committee, I traveled around the United States for three years with other well-versed dermatologists and explained the value of dermatology to insurance company medical directors (thanks to James Zalla, Scott Collins, Howard Rogers, Alexa Kimball, Clifford Lober, Sabra Sullivan, Mark Lebwohl, Beth Lertzman, Bruce Brod, Carrie Kovarik, Brent Moody, George Hruza, and Carl Johnson).
We showed them the statistics, clinical guidelines, and clinical photos and explained how cost effective dermatologists are in treating skin disease. We argued against using blunt tools, like average provider expense, as a proxy for quality. I thought it was a pretty compelling story, but the medical directors always asked for a reproducible quality metric. Almost no one in specialty medicine has reproducible quality metrics, and these are very difficult to develop.
The average number of layers taken for Mohs surgery of head, neck, hands, feet, and genitalia was calculated for all physicians reporting the codes to Medicare from 2012 to 2014. The Accreditation Council for Graduate Medical Education training programs were separately analyzed, since theoretically, they should get referrals of the more complex and difficult cases.
The average proved to be 1.74 stages per case, with a median of 1.69. Of 2,305 physicians billing for Mohs surgery, there were 137 extremely high outliers in at least 1 of 3 years, and 49 persistent high outliers (greater than two standard deviations in all three years), who averaged more than 2.41 layers per case. There were also 92 extremely low outliers (1.28 stages per case, in at least 1 of the 3 years), 20 of whom were persistent in all 3 years. High outliers were more likely to work in a solo practice setting.
The Improving Wisely program is based on the concept that reducing unnecessary variations in care can improve patient safety and quality of care while also reducing costs. It works on the premise that many outliers are unaware they are an outlier, no one wants to be an outlier, and confidential, collegial education and peer mentoring within a medical specialty society can reduce unnecessary variations in care.
Last month, all ACMS members received confidential data reports with their own personal ratio in relation to the entire cohort. Educational and mentoring resources are available for members, and outliers are encouraged to engage with the ACMS to identify opportunities for modifying and improving their practice patterns.
Now, these numbers must not be taken as an indictment of anyone. They are for educational purposes, and the goal is to help identify physicians who are unaware of their deviation and bring these outliers back into the norm. Supporting this premise, solo practitioners were at greatest risk of being outliers, which may be explained by lack of collegial interaction, peer review, and feedback.
In addition, there may be good reasons for being an outlier depending on one’s patient population, and the ACMS is interested in examples. The Mohs College is devoting considerable resources to help outliers. Much of this variation may also result from incorrect coding or processing of specimens. Nonetheless, no patient, payer, or physician wants unnecessary surgery or avoidable charges.
Low outliers are particularly puzzling, since someone would need godlike abilities to almost never have a positive margin in Mohs. I have heard of some practices whose patients all present in the morning, one layer is performed, and the rest of the day is spent processing and interpreting their slides. (Mohs is time consuming.)
I am also aware of some rural providers who travel to distant sites, take a layer, return to the city to process the tissue, and return a few days later to complete the case. This may not indicate bad care, just an unusual practice pattern or adaptation to difficult circumstances. However, it must also be noted that not completing cases on the same day could result in increased payments because of the coding system that reimburses more for first stages than for additional ones.
You must be aware that all these numbers, with a two-year lag, are available to physicians, payers, and patients. If you don’t know your ratio of first to additional Mohs layers, I encourage you to look your numbers up and calculate your ratio (CPT code 17311 plus 17312/code 17311). The easiest website to use is provided by the Wall Street Journal. If you are an outlier, you should ask yourself why, and consider some peer review and other appropriate changes. If your patterns change, they will be noticed quickly, since Johns Hopkins and the Improving Wisely program has leveraged their relationship with the Robert Wood Johnson Foundation to gain more immediate access to current Medicare data.
Everyone is hoping we see normalization of the patterns in layer usage, since this will give great credibility to Mohs surgeons and be better for our patients and the health care system in general. Kudos to the ACMS and the Jama Dermatology paper’s senior author, John Albertini, MD, of Winston-Salem, NC, in particular, for making this benchmark become a reality.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
Diversity in Dermatology: A Society Devoted to Skin of Color
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
Practice Points
- The mission of the Skin of Color Society (SOCS) is to improve education of young dermatologists relevant to skin of color patients.
- Educational resources on many different diseases important to patients with skin of color are available to patients and providers on the SOCS website.
Restoring the promise of (really) meaningful use
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Early-stage HL patients fare well 10 years after lower-intensity regimens
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
koakes@frontlinemedcom.com
On Twitter @karioakes
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
koakes@frontlinemedcom.com
On Twitter @karioakes
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
koakes@frontlinemedcom.com
On Twitter @karioakes
FROM JCO
Key clinical point:
Major finding: Early-stage favorable HL patients had identical progression-free survival, whether they received a more or less intense chemotherapy and radiation regimen (10-year PFS, 87% in each arm).
Data source: Long-term follow-up data from 4,276 patients in four arms of the German Hodgkin Study Group trials.
Disclosures: Several study authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.