User login
Order entry tool halves prescription drug costs
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
EXPERT ANALYSIS AT ACP INTERNAL MEDICINE
VIDEO: GI innovators dive into the Shark Tank
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
FROM THE AGA 2017 TECH SUMMIT
Three strategies target high quality care with new technologies
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
FROM THE 2017 AGA TECH SUMMIT
VIDEO: Awardee's work targets mucosal stem cells in Crohn’s, ulcerative colitis
BOSTON – A series of important technological advances in cloning stem cells of the gastrointestinal tract is rewriting the basic concepts surrounding these cells and has the potential to advance understanding of GI conditions such as Barrett’s esophagus and Crohn’s disease, according to the winner of the 2016 AGA-Medtronic Research and Development Pilot Award in Technology.
The award is sponsored by Medtronic and administered through the AGA Research Foundation. “Medtronic is pleased to partner with the AGA Research Foundation in supporting research to improve patient outcomes,” said Vafa Jamali, president of Early Technologies’ business with Medtronic. “We are dedicated to support patient-friendly innovations enabling the early detection and treatment of chronic GI diseases and cancers.”
“The enabling technology we developed is the means of cloning and propagating single mucosal stem cells from discrete regions of the gastrointestinal tract in their most immature form. What we have shown is that these stem cells from normal individuals are essentially immortal, are genetically stable, and, critically, are rigorously regiospecific,” Dr. Xian of the Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center, Houston, said in an interview.
“This regiospecificity – duodenum stem cells always yield duodenum epithelia, ascending colon stem cells produce ascending colon epithelia – has important implications for both regenerative medicine and drug discovery,” she added.
With regard to the dominant stem cell technologies in use today, including induced pluripotent stem cells and organoid cultures of the gastrointestinal tract, Dr. Xian’s technology represents a paradigm shift.
Dr. Xian’s system generates pure “ground state” stem cells that differentiate in 3-D culture to an epithelium indistinguishable from textbook images of in situ epithelia, at rates 250 times faster than intestinal organoid systems.
This system is being applied in major investigations into Barrett’s esophagus and inflammatory bowel disease in collaboration with investigators at multiple centers in the United States and Singapore.
For Barrett’s, Dr. Xian has developed patient-matched stem cells from endoscopic biopsies of esophageal, Barrett’s, and gastric cardia to show that each arises from distinct lineages, and that the Barrett’s stem cells accumulate genetic alterations not found in those of the matched normal epithelia. These stem cells have been adapted to a drug-screening platform to identify leads that specifically eliminate Barrett’s with an eye on preemptive therapies.
Her work on mucosal stem cells in patients with Crohn’s and ulcerative colitis is revealing a coexistence of normal and pathogenic stem cells in these patients that may alter concepts of both the etiology and treatment of these conditions.
Dr. Xian suggests that treatment of Crohn’s may become analogous to treatment of cancer – emphasizing preservation of healthy cells while eliminating diseased cells – and move therapy from the current effort to induce a quiescent inflammatory state to one in which the goal is cure.
“These findings appear to indicate that some of the chronic inflammatory conditions so prevalent in gastroenterology, as well as the cancers arising from them, may have as their basis profound alterations in mucosal stem cell biology,” she added.
Though it’s still early days with this technology, its single-cell resolution naturally melds with other emerging genomic and genome editing technologies into a powerful means of dissecting the molecular basis of disease and realizing the potential of autologous regenerative medicine, according to Dr. Xian.
Following Dr. Xian’s presentation, Michael L. Kochman, MD, who is executive committee chair of the AGA Center for GI Innovation and Technology, announced the winner of the 2017 AGA-Medtronic Research and Development Pilot Award in Technology. The award was presented to Bani Chander Roland, MD, of Lenox Hill Hospital & Northwell Health System, New York. Her research will involve assessment of the ileocecal valve.
“She has several aims in this proposal to really understand better the causes of ileosphincter dysfunction using a novel method of measuring ileocecal junction pressures,” reported Dr. Kochman. He explained that other parts of the proposal included ileocecal evaluation with wireless capsule endoscopy as well as an evaluation of flora across the GI tract.
A new award to foster innovation in GI medtech is also planned. In partnership with Boston Scientific, the AGA has established the AGA-Boston Scientific Technology and Innovation Pilot Award. This $30,000 research grant will support investigators at any career stage who are developing and testing new medical devices and technologies with applications in gastroenterology and hepatology. The details of the award will be available on the AGA’s website in May. Applications for the award will be accepted beginning in the fall.
BOSTON – A series of important technological advances in cloning stem cells of the gastrointestinal tract is rewriting the basic concepts surrounding these cells and has the potential to advance understanding of GI conditions such as Barrett’s esophagus and Crohn’s disease, according to the winner of the 2016 AGA-Medtronic Research and Development Pilot Award in Technology.
The award is sponsored by Medtronic and administered through the AGA Research Foundation. “Medtronic is pleased to partner with the AGA Research Foundation in supporting research to improve patient outcomes,” said Vafa Jamali, president of Early Technologies’ business with Medtronic. “We are dedicated to support patient-friendly innovations enabling the early detection and treatment of chronic GI diseases and cancers.”
“The enabling technology we developed is the means of cloning and propagating single mucosal stem cells from discrete regions of the gastrointestinal tract in their most immature form. What we have shown is that these stem cells from normal individuals are essentially immortal, are genetically stable, and, critically, are rigorously regiospecific,” Dr. Xian of the Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center, Houston, said in an interview.
“This regiospecificity – duodenum stem cells always yield duodenum epithelia, ascending colon stem cells produce ascending colon epithelia – has important implications for both regenerative medicine and drug discovery,” she added.
With regard to the dominant stem cell technologies in use today, including induced pluripotent stem cells and organoid cultures of the gastrointestinal tract, Dr. Xian’s technology represents a paradigm shift.
Dr. Xian’s system generates pure “ground state” stem cells that differentiate in 3-D culture to an epithelium indistinguishable from textbook images of in situ epithelia, at rates 250 times faster than intestinal organoid systems.
This system is being applied in major investigations into Barrett’s esophagus and inflammatory bowel disease in collaboration with investigators at multiple centers in the United States and Singapore.
For Barrett’s, Dr. Xian has developed patient-matched stem cells from endoscopic biopsies of esophageal, Barrett’s, and gastric cardia to show that each arises from distinct lineages, and that the Barrett’s stem cells accumulate genetic alterations not found in those of the matched normal epithelia. These stem cells have been adapted to a drug-screening platform to identify leads that specifically eliminate Barrett’s with an eye on preemptive therapies.
Her work on mucosal stem cells in patients with Crohn’s and ulcerative colitis is revealing a coexistence of normal and pathogenic stem cells in these patients that may alter concepts of both the etiology and treatment of these conditions.
Dr. Xian suggests that treatment of Crohn’s may become analogous to treatment of cancer – emphasizing preservation of healthy cells while eliminating diseased cells – and move therapy from the current effort to induce a quiescent inflammatory state to one in which the goal is cure.
“These findings appear to indicate that some of the chronic inflammatory conditions so prevalent in gastroenterology, as well as the cancers arising from them, may have as their basis profound alterations in mucosal stem cell biology,” she added.
Though it’s still early days with this technology, its single-cell resolution naturally melds with other emerging genomic and genome editing technologies into a powerful means of dissecting the molecular basis of disease and realizing the potential of autologous regenerative medicine, according to Dr. Xian.
Following Dr. Xian’s presentation, Michael L. Kochman, MD, who is executive committee chair of the AGA Center for GI Innovation and Technology, announced the winner of the 2017 AGA-Medtronic Research and Development Pilot Award in Technology. The award was presented to Bani Chander Roland, MD, of Lenox Hill Hospital & Northwell Health System, New York. Her research will involve assessment of the ileocecal valve.
“She has several aims in this proposal to really understand better the causes of ileosphincter dysfunction using a novel method of measuring ileocecal junction pressures,” reported Dr. Kochman. He explained that other parts of the proposal included ileocecal evaluation with wireless capsule endoscopy as well as an evaluation of flora across the GI tract.
A new award to foster innovation in GI medtech is also planned. In partnership with Boston Scientific, the AGA has established the AGA-Boston Scientific Technology and Innovation Pilot Award. This $30,000 research grant will support investigators at any career stage who are developing and testing new medical devices and technologies with applications in gastroenterology and hepatology. The details of the award will be available on the AGA’s website in May. Applications for the award will be accepted beginning in the fall.
BOSTON – A series of important technological advances in cloning stem cells of the gastrointestinal tract is rewriting the basic concepts surrounding these cells and has the potential to advance understanding of GI conditions such as Barrett’s esophagus and Crohn’s disease, according to the winner of the 2016 AGA-Medtronic Research and Development Pilot Award in Technology.
The award is sponsored by Medtronic and administered through the AGA Research Foundation. “Medtronic is pleased to partner with the AGA Research Foundation in supporting research to improve patient outcomes,” said Vafa Jamali, president of Early Technologies’ business with Medtronic. “We are dedicated to support patient-friendly innovations enabling the early detection and treatment of chronic GI diseases and cancers.”
“The enabling technology we developed is the means of cloning and propagating single mucosal stem cells from discrete regions of the gastrointestinal tract in their most immature form. What we have shown is that these stem cells from normal individuals are essentially immortal, are genetically stable, and, critically, are rigorously regiospecific,” Dr. Xian of the Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center, Houston, said in an interview.
“This regiospecificity – duodenum stem cells always yield duodenum epithelia, ascending colon stem cells produce ascending colon epithelia – has important implications for both regenerative medicine and drug discovery,” she added.
With regard to the dominant stem cell technologies in use today, including induced pluripotent stem cells and organoid cultures of the gastrointestinal tract, Dr. Xian’s technology represents a paradigm shift.
Dr. Xian’s system generates pure “ground state” stem cells that differentiate in 3-D culture to an epithelium indistinguishable from textbook images of in situ epithelia, at rates 250 times faster than intestinal organoid systems.
This system is being applied in major investigations into Barrett’s esophagus and inflammatory bowel disease in collaboration with investigators at multiple centers in the United States and Singapore.
For Barrett’s, Dr. Xian has developed patient-matched stem cells from endoscopic biopsies of esophageal, Barrett’s, and gastric cardia to show that each arises from distinct lineages, and that the Barrett’s stem cells accumulate genetic alterations not found in those of the matched normal epithelia. These stem cells have been adapted to a drug-screening platform to identify leads that specifically eliminate Barrett’s with an eye on preemptive therapies.
Her work on mucosal stem cells in patients with Crohn’s and ulcerative colitis is revealing a coexistence of normal and pathogenic stem cells in these patients that may alter concepts of both the etiology and treatment of these conditions.
Dr. Xian suggests that treatment of Crohn’s may become analogous to treatment of cancer – emphasizing preservation of healthy cells while eliminating diseased cells – and move therapy from the current effort to induce a quiescent inflammatory state to one in which the goal is cure.
“These findings appear to indicate that some of the chronic inflammatory conditions so prevalent in gastroenterology, as well as the cancers arising from them, may have as their basis profound alterations in mucosal stem cell biology,” she added.
Though it’s still early days with this technology, its single-cell resolution naturally melds with other emerging genomic and genome editing technologies into a powerful means of dissecting the molecular basis of disease and realizing the potential of autologous regenerative medicine, according to Dr. Xian.
Following Dr. Xian’s presentation, Michael L. Kochman, MD, who is executive committee chair of the AGA Center for GI Innovation and Technology, announced the winner of the 2017 AGA-Medtronic Research and Development Pilot Award in Technology. The award was presented to Bani Chander Roland, MD, of Lenox Hill Hospital & Northwell Health System, New York. Her research will involve assessment of the ileocecal valve.
“She has several aims in this proposal to really understand better the causes of ileosphincter dysfunction using a novel method of measuring ileocecal junction pressures,” reported Dr. Kochman. He explained that other parts of the proposal included ileocecal evaluation with wireless capsule endoscopy as well as an evaluation of flora across the GI tract.
A new award to foster innovation in GI medtech is also planned. In partnership with Boston Scientific, the AGA has established the AGA-Boston Scientific Technology and Innovation Pilot Award. This $30,000 research grant will support investigators at any career stage who are developing and testing new medical devices and technologies with applications in gastroenterology and hepatology. The details of the award will be available on the AGA’s website in May. Applications for the award will be accepted beginning in the fall.
FROM THE AGA 2017 TECH SUMMIT
Debulking called reasonable for unresectable liver cancer
MIAMI BEACH – Cytoreductive debulking surgery for neuroendocrine liver metastases provides a lower but “reasonable” long-term survival, compared with curative intent surgery, according to results of a study presented at the annual meeting of the Americas Hepatico-Pancreato-Biliary Association.
Liver debulking for patients with grossly unresectable disease is therefore an option that may extend survival, the study researchers said.
“Surgical resection offers the best chance for long-term survival but may not be technically feasible for all our patients,” said Fabio Bagante, MD, a resident at the University of Verona (Italy). “Debulking neuroendocrine liver metastases has been proposed as an alternative.”
Dr. Bagante and coinvestigators compared outcomes between 180 patients who had debulking surgery (defined as an R2 outcome) and 449 who underwent curative intent resection (R0 or R1). Patients had surgery between 1990 and 2015 at eight institutions, and the primary outcome was overall survival. The mean follow-up was 51 months; during that time, 174 patients died.
The 5-year overall survival was 72% in the debulking group and 89% in the curative intent group. The 10-year overall survival was 41% in the debulking group and 77% among the curative intent surgery patients.
“Debulking operations for neuroendocrine liver metastases provide lower but reasonable long-term survival, compared with patients who underwent curative intent,” Dr. Bagante said.
“Nearly 3 in 10 patients underwent debulking surgery,” Dr. Bagante said. “It was more common among the elderly, males with symptomatic disease, and patients with greater liver involvement.”
Patients in the liver-debulking groups were also significantly more likely to have high–tumor grade disease, 35%, versus 13% of the curative intent resection group (P less than .001). They were also more likely to have metastatic disease, with an N1 rate of 73% versus 52% (P less than .001).
“Great work. Thanks for presenting this topic. It’s quite a debated topic,” said study discussant Aaron Lee, DO, of the department of general surgery at Cleveland Clinic Florida in Weston. “I looked at your data on asymptomatic patients who had a 5-year survival of 60%; U.K. data show [the] same survival without any intervention.” He asked Dr. Bagante to comment on operating on patients without any symptoms.
“Regarding the role of symptoms as a predictor of survival, there are many papers in the literature debating what are the true predictors of survival,” Dr. Bagante noted. “We don’t think [symptoms have an] impact; our data do not demonstrate that. We think the biologic behavior of the disease, the impact of the progression of the disease, and the grade of the tumor, [indicated by] Ki-67, could have more of a role.”
MIAMI BEACH – Cytoreductive debulking surgery for neuroendocrine liver metastases provides a lower but “reasonable” long-term survival, compared with curative intent surgery, according to results of a study presented at the annual meeting of the Americas Hepatico-Pancreato-Biliary Association.
Liver debulking for patients with grossly unresectable disease is therefore an option that may extend survival, the study researchers said.
“Surgical resection offers the best chance for long-term survival but may not be technically feasible for all our patients,” said Fabio Bagante, MD, a resident at the University of Verona (Italy). “Debulking neuroendocrine liver metastases has been proposed as an alternative.”
Dr. Bagante and coinvestigators compared outcomes between 180 patients who had debulking surgery (defined as an R2 outcome) and 449 who underwent curative intent resection (R0 or R1). Patients had surgery between 1990 and 2015 at eight institutions, and the primary outcome was overall survival. The mean follow-up was 51 months; during that time, 174 patients died.
The 5-year overall survival was 72% in the debulking group and 89% in the curative intent group. The 10-year overall survival was 41% in the debulking group and 77% among the curative intent surgery patients.
“Debulking operations for neuroendocrine liver metastases provide lower but reasonable long-term survival, compared with patients who underwent curative intent,” Dr. Bagante said.
“Nearly 3 in 10 patients underwent debulking surgery,” Dr. Bagante said. “It was more common among the elderly, males with symptomatic disease, and patients with greater liver involvement.”
Patients in the liver-debulking groups were also significantly more likely to have high–tumor grade disease, 35%, versus 13% of the curative intent resection group (P less than .001). They were also more likely to have metastatic disease, with an N1 rate of 73% versus 52% (P less than .001).
“Great work. Thanks for presenting this topic. It’s quite a debated topic,” said study discussant Aaron Lee, DO, of the department of general surgery at Cleveland Clinic Florida in Weston. “I looked at your data on asymptomatic patients who had a 5-year survival of 60%; U.K. data show [the] same survival without any intervention.” He asked Dr. Bagante to comment on operating on patients without any symptoms.
“Regarding the role of symptoms as a predictor of survival, there are many papers in the literature debating what are the true predictors of survival,” Dr. Bagante noted. “We don’t think [symptoms have an] impact; our data do not demonstrate that. We think the biologic behavior of the disease, the impact of the progression of the disease, and the grade of the tumor, [indicated by] Ki-67, could have more of a role.”
MIAMI BEACH – Cytoreductive debulking surgery for neuroendocrine liver metastases provides a lower but “reasonable” long-term survival, compared with curative intent surgery, according to results of a study presented at the annual meeting of the Americas Hepatico-Pancreato-Biliary Association.
Liver debulking for patients with grossly unresectable disease is therefore an option that may extend survival, the study researchers said.
“Surgical resection offers the best chance for long-term survival but may not be technically feasible for all our patients,” said Fabio Bagante, MD, a resident at the University of Verona (Italy). “Debulking neuroendocrine liver metastases has been proposed as an alternative.”
Dr. Bagante and coinvestigators compared outcomes between 180 patients who had debulking surgery (defined as an R2 outcome) and 449 who underwent curative intent resection (R0 or R1). Patients had surgery between 1990 and 2015 at eight institutions, and the primary outcome was overall survival. The mean follow-up was 51 months; during that time, 174 patients died.
The 5-year overall survival was 72% in the debulking group and 89% in the curative intent group. The 10-year overall survival was 41% in the debulking group and 77% among the curative intent surgery patients.
“Debulking operations for neuroendocrine liver metastases provide lower but reasonable long-term survival, compared with patients who underwent curative intent,” Dr. Bagante said.
“Nearly 3 in 10 patients underwent debulking surgery,” Dr. Bagante said. “It was more common among the elderly, males with symptomatic disease, and patients with greater liver involvement.”
Patients in the liver-debulking groups were also significantly more likely to have high–tumor grade disease, 35%, versus 13% of the curative intent resection group (P less than .001). They were also more likely to have metastatic disease, with an N1 rate of 73% versus 52% (P less than .001).
“Great work. Thanks for presenting this topic. It’s quite a debated topic,” said study discussant Aaron Lee, DO, of the department of general surgery at Cleveland Clinic Florida in Weston. “I looked at your data on asymptomatic patients who had a 5-year survival of 60%; U.K. data show [the] same survival without any intervention.” He asked Dr. Bagante to comment on operating on patients without any symptoms.
“Regarding the role of symptoms as a predictor of survival, there are many papers in the literature debating what are the true predictors of survival,” Dr. Bagante noted. “We don’t think [symptoms have an] impact; our data do not demonstrate that. We think the biologic behavior of the disease, the impact of the progression of the disease, and the grade of the tumor, [indicated by] Ki-67, could have more of a role.”
AT AHPBA 2017
Key clinical point:
Major finding: The 10-year overall survival was 77% in the curative intent surgery group, compared with 41% among the debulking patients.
Data source: A retrospective database study of 629 people with neuroendocrine liver metastases.
Disclosures: Dr. Bagante and Dr. Lee had no relevant financial disclosures.
Combining teamwork and technology when tragedy strikes
I’m the new chief of service for the department of dermatology at Kaiser Permanente San Diego. We are hiring, so I’ve been working on my answers to astute questions about how we at Kaiser Permanente differ from other health systems and why I love our medical group. Many points of differentiation involve how we work as an integrated system and how we are compensated for effective care instead of simply volume of care.
There are more than 70 dermatologists and staff in our department, and we all play a role in meeting the access needs of our patients. When one of our docs emailed me at 4 a.m. to tell me of a terrible catastrophe that struck her family, it set off a somber day for our team. In addition to offering our sympathy to her, we got right to work to help her. She needed time off to be with her family, and like all of us, she had full schedules booked for weeks ahead.
By 6 a.m., our administrative team was aware and working to recover. We canceled her clinic, and, using scheduling software, identified dermatologists in our department who might be able to help. With a few clicks, we reassigned patients from her to me and others who immediately volunteered. This was seamless as far as the patients would be concerned. Patients coming in within hours that morning were picked up by other doctors; one by one, they added them to their schedules.
Every doctor in San Diego has a Kaiser Permanente–issued smartphone. These allowed us to quickly email, text, and message to coordinate our efforts. Each of us dermatologists connected to her in-basket in our electronic medical record and set to work sending out her biopsy results, answering her secure email messages, and calling her patients. Others volunteered to cover her call, and we reassigned her teledermatology shifts with just a click. By noon, all her responsibilities as a dermatologist had been accounted for, allowing her to focus on her family. Teamwork was enabled by our digital system of care.
This story isn’t a sales pitch. We wish it had never happened. But it might be the best answer to the question of why we love working here. When we combine team plus technology to care for our patients and to care for each other, there’s no medical group we’d rather be.
I hope this is the last tragedy to befall us as a department. And if it is not, I hope to have just this same team around me to cope.
I’m sure others have similar stories of how technology helped them work as a team. Please send them to me at dermnews@frontlinemedcom.com; I’d like to write a follow-up piece.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. He is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com.
I’m the new chief of service for the department of dermatology at Kaiser Permanente San Diego. We are hiring, so I’ve been working on my answers to astute questions about how we at Kaiser Permanente differ from other health systems and why I love our medical group. Many points of differentiation involve how we work as an integrated system and how we are compensated for effective care instead of simply volume of care.
There are more than 70 dermatologists and staff in our department, and we all play a role in meeting the access needs of our patients. When one of our docs emailed me at 4 a.m. to tell me of a terrible catastrophe that struck her family, it set off a somber day for our team. In addition to offering our sympathy to her, we got right to work to help her. She needed time off to be with her family, and like all of us, she had full schedules booked for weeks ahead.
By 6 a.m., our administrative team was aware and working to recover. We canceled her clinic, and, using scheduling software, identified dermatologists in our department who might be able to help. With a few clicks, we reassigned patients from her to me and others who immediately volunteered. This was seamless as far as the patients would be concerned. Patients coming in within hours that morning were picked up by other doctors; one by one, they added them to their schedules.
Every doctor in San Diego has a Kaiser Permanente–issued smartphone. These allowed us to quickly email, text, and message to coordinate our efforts. Each of us dermatologists connected to her in-basket in our electronic medical record and set to work sending out her biopsy results, answering her secure email messages, and calling her patients. Others volunteered to cover her call, and we reassigned her teledermatology shifts with just a click. By noon, all her responsibilities as a dermatologist had been accounted for, allowing her to focus on her family. Teamwork was enabled by our digital system of care.
This story isn’t a sales pitch. We wish it had never happened. But it might be the best answer to the question of why we love working here. When we combine team plus technology to care for our patients and to care for each other, there’s no medical group we’d rather be.
I hope this is the last tragedy to befall us as a department. And if it is not, I hope to have just this same team around me to cope.
I’m sure others have similar stories of how technology helped them work as a team. Please send them to me at dermnews@frontlinemedcom.com; I’d like to write a follow-up piece.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. He is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com.
I’m the new chief of service for the department of dermatology at Kaiser Permanente San Diego. We are hiring, so I’ve been working on my answers to astute questions about how we at Kaiser Permanente differ from other health systems and why I love our medical group. Many points of differentiation involve how we work as an integrated system and how we are compensated for effective care instead of simply volume of care.
There are more than 70 dermatologists and staff in our department, and we all play a role in meeting the access needs of our patients. When one of our docs emailed me at 4 a.m. to tell me of a terrible catastrophe that struck her family, it set off a somber day for our team. In addition to offering our sympathy to her, we got right to work to help her. She needed time off to be with her family, and like all of us, she had full schedules booked for weeks ahead.
By 6 a.m., our administrative team was aware and working to recover. We canceled her clinic, and, using scheduling software, identified dermatologists in our department who might be able to help. With a few clicks, we reassigned patients from her to me and others who immediately volunteered. This was seamless as far as the patients would be concerned. Patients coming in within hours that morning were picked up by other doctors; one by one, they added them to their schedules.
Every doctor in San Diego has a Kaiser Permanente–issued smartphone. These allowed us to quickly email, text, and message to coordinate our efforts. Each of us dermatologists connected to her in-basket in our electronic medical record and set to work sending out her biopsy results, answering her secure email messages, and calling her patients. Others volunteered to cover her call, and we reassigned her teledermatology shifts with just a click. By noon, all her responsibilities as a dermatologist had been accounted for, allowing her to focus on her family. Teamwork was enabled by our digital system of care.
This story isn’t a sales pitch. We wish it had never happened. But it might be the best answer to the question of why we love working here. When we combine team plus technology to care for our patients and to care for each other, there’s no medical group we’d rather be.
I hope this is the last tragedy to befall us as a department. And if it is not, I hope to have just this same team around me to cope.
I’m sure others have similar stories of how technology helped them work as a team. Please send them to me at dermnews@frontlinemedcom.com; I’d like to write a follow-up piece.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. He is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com.
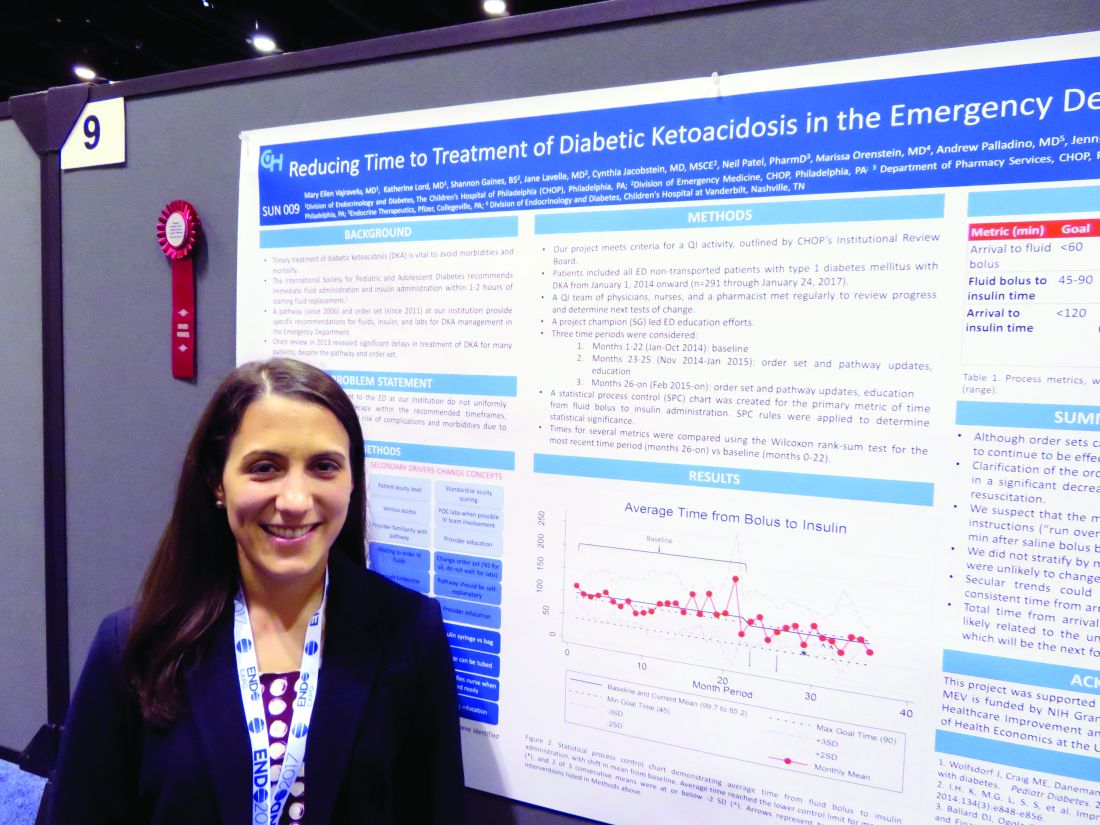
Simple changes shorten door-to-insulin time for diabetic ketoacidosis
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Overall door-to-insulin time decreased from 168 minutes to 146 minutes.
Data source: A 2-year quality improvement project involving 291 patients.
Disclosures: Dr. Vajravelu had no relevant financial disclosures.
Using genetics to predict CRC outcomes
BOSTON – Gastroenterologists and GI surgeons had the opportunity to learn more about “Predicting Clinical Outcomes in CRC with Genetics” at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The topic is extremely significant for two reasons,” said presenter C. Richard Boland, MD, of the University of California in San Diego. “First, we are now able to test colorectal cancer (CRC) for genetic alternations – allowing us to gauge prognosis and, eventually, target specific therapies to the actual tumor,” he said in an interview in advance of the meeting. “Second, the issue of detecting germline mutations in familial lines of CRC is an important factor in diagnosis.”
“All cancers are genetic diseases, developing as a consequence of cumulative mutations. Each cancer is different, both in terms of total mutational burden, as well as the specific mutations that ‘drive’ the tumor,” explained Dr. Boland. “We will be able to stop tumor growth by inhibiting the driver mutation, once we know what that driver is. This will allow us to make more accurate prognoses and predict tumor responses to specific therapies. The hard part will be having effective therapies for each driver mutation.”
The presentation began with a discussion of optimizing CRC care using genetics, predictive tests (for defective DNA mismatch repair – dMMR), specific tumor mutations and blood tests (for miRNAs or other noncoding RNAs). It also covered the sensitivity of immunohistochemistry (IHC) for dMMR and potential problems with IHC.
Then it detailed an innovative technical proposal whose elements include developing a next-generation sequencing platform for CRC and performing microsatellite instability and other mutational testing for specific genes as test-guided therapies emerge. The session also provided an overview of germline mutation testing, including a description of genes that cause hereditary CRC, are on Myriad’s myRisk panel, and are included on Ambry’s 8 Test Panels.
“We think about 3% or so of CRCs have a strong genetic basis, but probably a lot more have weaker genetic bases,” said Dr. Boland. “When we find strongly penetrant germline mutations, it permits case finding and preventive intervention in family members. It also tells us a lot about how to care for the index individual, since all of these familial syndromes have a wide range of effects on patients.
“The technology driving this type of patient assessment is rapidly developing, and making it easier to find these mutations” he continued. “But it is also confusing when we find unexpected mutations in people who don’t seem to fit the classic disease descriptions.”
Attendees were taught why genetic test of CRCs can help direct patient care, how the tests can be efficiently done using a new diagnostic platform, and the state of germline testing and its interpretation.
Dr. Boland has given a lecture for Ambry Genetics (a genetics testing company) and may give others in the future.
BOSTON – Gastroenterologists and GI surgeons had the opportunity to learn more about “Predicting Clinical Outcomes in CRC with Genetics” at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The topic is extremely significant for two reasons,” said presenter C. Richard Boland, MD, of the University of California in San Diego. “First, we are now able to test colorectal cancer (CRC) for genetic alternations – allowing us to gauge prognosis and, eventually, target specific therapies to the actual tumor,” he said in an interview in advance of the meeting. “Second, the issue of detecting germline mutations in familial lines of CRC is an important factor in diagnosis.”
“All cancers are genetic diseases, developing as a consequence of cumulative mutations. Each cancer is different, both in terms of total mutational burden, as well as the specific mutations that ‘drive’ the tumor,” explained Dr. Boland. “We will be able to stop tumor growth by inhibiting the driver mutation, once we know what that driver is. This will allow us to make more accurate prognoses and predict tumor responses to specific therapies. The hard part will be having effective therapies for each driver mutation.”
The presentation began with a discussion of optimizing CRC care using genetics, predictive tests (for defective DNA mismatch repair – dMMR), specific tumor mutations and blood tests (for miRNAs or other noncoding RNAs). It also covered the sensitivity of immunohistochemistry (IHC) for dMMR and potential problems with IHC.
Then it detailed an innovative technical proposal whose elements include developing a next-generation sequencing platform for CRC and performing microsatellite instability and other mutational testing for specific genes as test-guided therapies emerge. The session also provided an overview of germline mutation testing, including a description of genes that cause hereditary CRC, are on Myriad’s myRisk panel, and are included on Ambry’s 8 Test Panels.
“We think about 3% or so of CRCs have a strong genetic basis, but probably a lot more have weaker genetic bases,” said Dr. Boland. “When we find strongly penetrant germline mutations, it permits case finding and preventive intervention in family members. It also tells us a lot about how to care for the index individual, since all of these familial syndromes have a wide range of effects on patients.
“The technology driving this type of patient assessment is rapidly developing, and making it easier to find these mutations” he continued. “But it is also confusing when we find unexpected mutations in people who don’t seem to fit the classic disease descriptions.”
Attendees were taught why genetic test of CRCs can help direct patient care, how the tests can be efficiently done using a new diagnostic platform, and the state of germline testing and its interpretation.
Dr. Boland has given a lecture for Ambry Genetics (a genetics testing company) and may give others in the future.
BOSTON – Gastroenterologists and GI surgeons had the opportunity to learn more about “Predicting Clinical Outcomes in CRC with Genetics” at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The topic is extremely significant for two reasons,” said presenter C. Richard Boland, MD, of the University of California in San Diego. “First, we are now able to test colorectal cancer (CRC) for genetic alternations – allowing us to gauge prognosis and, eventually, target specific therapies to the actual tumor,” he said in an interview in advance of the meeting. “Second, the issue of detecting germline mutations in familial lines of CRC is an important factor in diagnosis.”
“All cancers are genetic diseases, developing as a consequence of cumulative mutations. Each cancer is different, both in terms of total mutational burden, as well as the specific mutations that ‘drive’ the tumor,” explained Dr. Boland. “We will be able to stop tumor growth by inhibiting the driver mutation, once we know what that driver is. This will allow us to make more accurate prognoses and predict tumor responses to specific therapies. The hard part will be having effective therapies for each driver mutation.”
The presentation began with a discussion of optimizing CRC care using genetics, predictive tests (for defective DNA mismatch repair – dMMR), specific tumor mutations and blood tests (for miRNAs or other noncoding RNAs). It also covered the sensitivity of immunohistochemistry (IHC) for dMMR and potential problems with IHC.
Then it detailed an innovative technical proposal whose elements include developing a next-generation sequencing platform for CRC and performing microsatellite instability and other mutational testing for specific genes as test-guided therapies emerge. The session also provided an overview of germline mutation testing, including a description of genes that cause hereditary CRC, are on Myriad’s myRisk panel, and are included on Ambry’s 8 Test Panels.
“We think about 3% or so of CRCs have a strong genetic basis, but probably a lot more have weaker genetic bases,” said Dr. Boland. “When we find strongly penetrant germline mutations, it permits case finding and preventive intervention in family members. It also tells us a lot about how to care for the index individual, since all of these familial syndromes have a wide range of effects on patients.
“The technology driving this type of patient assessment is rapidly developing, and making it easier to find these mutations” he continued. “But it is also confusing when we find unexpected mutations in people who don’t seem to fit the classic disease descriptions.”
Attendees were taught why genetic test of CRCs can help direct patient care, how the tests can be efficiently done using a new diagnostic platform, and the state of germline testing and its interpretation.
Dr. Boland has given a lecture for Ambry Genetics (a genetics testing company) and may give others in the future.
FROM THE 2017 AGA TECH SUMMIT
Crohn’s hospitalizations up significantly … or not
Hospitalizations for Crohn’s disease were up by a statistically significant 35% from 2003 to 2013 … or they were up just 5%, according to the Centers for Disease Control and Prevention.
It depends on how you look at it. In 2013, age-adjusted hospitalization was 59.7 stays per 100,000 population for Crohn’s as any-listed diagnosis – indicating that patients had Crohn’s disease but that it was not necessarily the main reason they were being hospitalized – compared with 44.2 per 100,000 in 2003. That’s an increase of 35%, the CDC said (MMWR. 2017 Apr 14;66[14]:377-81).
Hospitalizations with Crohn’s as the first-listed diagnosis – making it the main reason for the admission – were up 5% over the same period: 18.2 stays per 100,000 pop. in 2003 and 19.1 per 100,000 in 2013. That increase is not statistically significant, the CDC noted.
The increase in any-listed diagnosis “might represent greater physician awareness and diagnosis of Crohn’s disease or more complete coding of secondary diagnoses by physicians,” CDC investigators suggested.
Increases in hospitalizations for any-listed Crohn’s were significant for both men (40.5%) and women (31.6%). For first-listed Crohn’s, women saw a slight but not significant decrease of 1% while men had a significant 14.5% increase, according to the report, which was based on data from the National Inpatient Sample.
Hospitalizations for Crohn’s disease were up by a statistically significant 35% from 2003 to 2013 … or they were up just 5%, according to the Centers for Disease Control and Prevention.
It depends on how you look at it. In 2013, age-adjusted hospitalization was 59.7 stays per 100,000 population for Crohn’s as any-listed diagnosis – indicating that patients had Crohn’s disease but that it was not necessarily the main reason they were being hospitalized – compared with 44.2 per 100,000 in 2003. That’s an increase of 35%, the CDC said (MMWR. 2017 Apr 14;66[14]:377-81).
Hospitalizations with Crohn’s as the first-listed diagnosis – making it the main reason for the admission – were up 5% over the same period: 18.2 stays per 100,000 pop. in 2003 and 19.1 per 100,000 in 2013. That increase is not statistically significant, the CDC noted.
The increase in any-listed diagnosis “might represent greater physician awareness and diagnosis of Crohn’s disease or more complete coding of secondary diagnoses by physicians,” CDC investigators suggested.
Increases in hospitalizations for any-listed Crohn’s were significant for both men (40.5%) and women (31.6%). For first-listed Crohn’s, women saw a slight but not significant decrease of 1% while men had a significant 14.5% increase, according to the report, which was based on data from the National Inpatient Sample.
Hospitalizations for Crohn’s disease were up by a statistically significant 35% from 2003 to 2013 … or they were up just 5%, according to the Centers for Disease Control and Prevention.
It depends on how you look at it. In 2013, age-adjusted hospitalization was 59.7 stays per 100,000 population for Crohn’s as any-listed diagnosis – indicating that patients had Crohn’s disease but that it was not necessarily the main reason they were being hospitalized – compared with 44.2 per 100,000 in 2003. That’s an increase of 35%, the CDC said (MMWR. 2017 Apr 14;66[14]:377-81).
Hospitalizations with Crohn’s as the first-listed diagnosis – making it the main reason for the admission – were up 5% over the same period: 18.2 stays per 100,000 pop. in 2003 and 19.1 per 100,000 in 2013. That increase is not statistically significant, the CDC noted.
The increase in any-listed diagnosis “might represent greater physician awareness and diagnosis of Crohn’s disease or more complete coding of secondary diagnoses by physicians,” CDC investigators suggested.
Increases in hospitalizations for any-listed Crohn’s were significant for both men (40.5%) and women (31.6%). For first-listed Crohn’s, women saw a slight but not significant decrease of 1% while men had a significant 14.5% increase, according to the report, which was based on data from the National Inpatient Sample.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Medicaid reform: Work-based waivers may not fly
The Trump administration may not be able to successfully implement the work requirements and other Medicaid eligibility caveats proffered by Health and Human Services Secretary Tom Price, MD, according to Jane Perkins, legal director for the National Health Law Program.
In March, Secretary Price and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, wrote to state governors, letting them know that the HHS would support states’ efforts to increase employment, community engagement, and work requirements for Medicaid recipients. The letter also was supportive of aligning Medicaid benefits with private insurance via alternative benefits, cost-sharing, and premium payments.
High mandatory premiums, cost sharing, lifetime limits, and drug testing “are of concern to us,” Ms. Perkins said at an April 13 press briefing. “They really change the complexion of Medicaid and Medicaid coverage for low-income people.”
These requirements “are not typically seen in Medicaid programs,” she said.
While Section 1115 of the Social Security Act “allows states to test novel approaches to providing medical assistance” via Medicaid waivers, it does not allow the HHS or the states to “ignore congressional mandates; to cut eligibility, services, or provider payments; or to use section 1115 to save money,” according to an issue brief by Ms. Perkins.
When asked how work requirements harm people, Ms. Perkins responded that adding a work requirement to Medicaid eligibility gets things “backwards,” because a sick person needs health care before being able to return to work.
The work requirement would not save states much money, as nearly 8 in 10 adults on Medicaid are in a household that includes a worker and 59% of recipients work themselves, according to a Kaiser Family Foundation study. The adults affected by the work requirement would make up only a drop in the ocean of Medicaid spending. About two-thirds of that spending goes toward senior citizens, people with disabilities, children, and people in long term care, according to projections from the Congressional Budget Office.
The National Health Law program, which advocates for low-income Medicaid recipients, is following the waivers state-by-state with a network of lawyers who work with people with disabilities in each state.
“With this new openness to flexibility, we are certainly watching what is going on in the states,” Ms. Perkins said.
The Trump administration may not be able to successfully implement the work requirements and other Medicaid eligibility caveats proffered by Health and Human Services Secretary Tom Price, MD, according to Jane Perkins, legal director for the National Health Law Program.
In March, Secretary Price and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, wrote to state governors, letting them know that the HHS would support states’ efforts to increase employment, community engagement, and work requirements for Medicaid recipients. The letter also was supportive of aligning Medicaid benefits with private insurance via alternative benefits, cost-sharing, and premium payments.
High mandatory premiums, cost sharing, lifetime limits, and drug testing “are of concern to us,” Ms. Perkins said at an April 13 press briefing. “They really change the complexion of Medicaid and Medicaid coverage for low-income people.”
These requirements “are not typically seen in Medicaid programs,” she said.
While Section 1115 of the Social Security Act “allows states to test novel approaches to providing medical assistance” via Medicaid waivers, it does not allow the HHS or the states to “ignore congressional mandates; to cut eligibility, services, or provider payments; or to use section 1115 to save money,” according to an issue brief by Ms. Perkins.
When asked how work requirements harm people, Ms. Perkins responded that adding a work requirement to Medicaid eligibility gets things “backwards,” because a sick person needs health care before being able to return to work.
The work requirement would not save states much money, as nearly 8 in 10 adults on Medicaid are in a household that includes a worker and 59% of recipients work themselves, according to a Kaiser Family Foundation study. The adults affected by the work requirement would make up only a drop in the ocean of Medicaid spending. About two-thirds of that spending goes toward senior citizens, people with disabilities, children, and people in long term care, according to projections from the Congressional Budget Office.
The National Health Law program, which advocates for low-income Medicaid recipients, is following the waivers state-by-state with a network of lawyers who work with people with disabilities in each state.
“With this new openness to flexibility, we are certainly watching what is going on in the states,” Ms. Perkins said.
The Trump administration may not be able to successfully implement the work requirements and other Medicaid eligibility caveats proffered by Health and Human Services Secretary Tom Price, MD, according to Jane Perkins, legal director for the National Health Law Program.
In March, Secretary Price and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, wrote to state governors, letting them know that the HHS would support states’ efforts to increase employment, community engagement, and work requirements for Medicaid recipients. The letter also was supportive of aligning Medicaid benefits with private insurance via alternative benefits, cost-sharing, and premium payments.
High mandatory premiums, cost sharing, lifetime limits, and drug testing “are of concern to us,” Ms. Perkins said at an April 13 press briefing. “They really change the complexion of Medicaid and Medicaid coverage for low-income people.”
These requirements “are not typically seen in Medicaid programs,” she said.
While Section 1115 of the Social Security Act “allows states to test novel approaches to providing medical assistance” via Medicaid waivers, it does not allow the HHS or the states to “ignore congressional mandates; to cut eligibility, services, or provider payments; or to use section 1115 to save money,” according to an issue brief by Ms. Perkins.
When asked how work requirements harm people, Ms. Perkins responded that adding a work requirement to Medicaid eligibility gets things “backwards,” because a sick person needs health care before being able to return to work.
The work requirement would not save states much money, as nearly 8 in 10 adults on Medicaid are in a household that includes a worker and 59% of recipients work themselves, according to a Kaiser Family Foundation study. The adults affected by the work requirement would make up only a drop in the ocean of Medicaid spending. About two-thirds of that spending goes toward senior citizens, people with disabilities, children, and people in long term care, according to projections from the Congressional Budget Office.
The National Health Law program, which advocates for low-income Medicaid recipients, is following the waivers state-by-state with a network of lawyers who work with people with disabilities in each state.
“With this new openness to flexibility, we are certainly watching what is going on in the states,” Ms. Perkins said.