User login
Genetic profiling can guide HSCT in MDS, team says

Genetic profiling can be used to determine which patients with myelodysplastic syndrome (MDS) are likely to benefit from allogeneic hematopoietic stem cell transplant (HSCT), according to research published in NEJM.
Targeted sequencing of 129 genes revealed mutations that, after adjustment for clinical variables, were associated with shorter survival and/or relapse after HSCT.
Patients with mutations in TP53, JAK2, and the RAS pathway tended to have worse outcomes after HSCT than patients without such mutations.
“Although donor stem cell transplantation is the only curative therapy for MDS, many patients die after transplantation, largely due to relapse of the disease or complications relating to the transplant itself,” said study author R. Coleman Lindsley, MD, PhD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
“As physicians, one of our major challenges is to be able to predict which patients are most likely to benefit from a transplant. Improving our ability to identify patients who are most likely to have a relapse or to experience life-threatening complications from a transplant could lead to better pre-transplant therapies and strategies for preventing relapse.”
Researchers have long known that specific genetic mutations are closely related to the course MDS takes. With this study, Dr Lindsley and his colleagues sought to discover whether mutations can be used to predict how patients will fare following allogeneic HSCT.
The team analyzed blood samples from 1514 MDS patients, performing targeted sequencing of 129 genes. The genes were selected based on their known or suspected involvement in the pathogenesis of myeloid cancers or bone marrow failure syndromes.
Dr Lindsley and his colleagues then evaluated the association between mutations and HSCT outcomes, including overall survival, relapse, and death without relapse.
After adjusting for significant clinical variables, the researchers found that having mutated TP53 was significantly associated with shorter survival and shorter time to relapse after HSCT (P<0.001 for both comparisons). This was true whether patients received standard conditioning or reduced-intensity conditioning.
In patients age 40 and older who did not have TP53 mutations, mutations in RAS pathway genes (P=0.004) or JAK2 (P=0.001) were significantly associated with shorter survival.
The shorter survival in patients with mutated RAS pathway genes was due to a higher risk of relapse, while the shorter survival in patients with JAK2 mutations was due to a higher risk of death without relapse.
In contrast to TP53 mutations, the adverse effect of RAS mutations on survival and risk of relapse was evident only in patients who received reduced-intensity conditioning (P<0.001). This suggests these patients may benefit from higher intensity conditioning regimens, the researchers said.
This study also yielded insights about the biology of MDS in specific groups of patients.
For example, the researchers found that 4% of MDS patients between the ages of 18 and 40 had mutations associated with Shwachman-Diamond syndrome (in the SBDS gene), but most of them had not previously been diagnosed with the syndrome.
In each case, the patients had acquired a TP53 mutation, suggesting not only how MDS develops in patients with Schwachman-Diamond syndrome but also what underlies their poor prognosis after HSCT.
The researchers also analyzed patients with therapy-related MDS. The team found that TP53 mutations and mutations in PPM1D, a gene that regulates TP53 function, were far more common in these patients than in those with primary MDS (15% and 3%, respectively, P<0.001).
“In deciding whether a stem cell transplant is appropriate for a patient with MDS, it’s always necessary to balance the potential benefit with the risk of complications,” Dr Lindsley noted.
“Our findings offer physicians a guide—based on the genetic profile of the disease and certain clinical factors—to identifying patients for whom a transplant is appropriate, and the intensity of treatment most likely to be effective.” ![]()

Genetic profiling can be used to determine which patients with myelodysplastic syndrome (MDS) are likely to benefit from allogeneic hematopoietic stem cell transplant (HSCT), according to research published in NEJM.
Targeted sequencing of 129 genes revealed mutations that, after adjustment for clinical variables, were associated with shorter survival and/or relapse after HSCT.
Patients with mutations in TP53, JAK2, and the RAS pathway tended to have worse outcomes after HSCT than patients without such mutations.
“Although donor stem cell transplantation is the only curative therapy for MDS, many patients die after transplantation, largely due to relapse of the disease or complications relating to the transplant itself,” said study author R. Coleman Lindsley, MD, PhD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
“As physicians, one of our major challenges is to be able to predict which patients are most likely to benefit from a transplant. Improving our ability to identify patients who are most likely to have a relapse or to experience life-threatening complications from a transplant could lead to better pre-transplant therapies and strategies for preventing relapse.”
Researchers have long known that specific genetic mutations are closely related to the course MDS takes. With this study, Dr Lindsley and his colleagues sought to discover whether mutations can be used to predict how patients will fare following allogeneic HSCT.
The team analyzed blood samples from 1514 MDS patients, performing targeted sequencing of 129 genes. The genes were selected based on their known or suspected involvement in the pathogenesis of myeloid cancers or bone marrow failure syndromes.
Dr Lindsley and his colleagues then evaluated the association between mutations and HSCT outcomes, including overall survival, relapse, and death without relapse.
After adjusting for significant clinical variables, the researchers found that having mutated TP53 was significantly associated with shorter survival and shorter time to relapse after HSCT (P<0.001 for both comparisons). This was true whether patients received standard conditioning or reduced-intensity conditioning.
In patients age 40 and older who did not have TP53 mutations, mutations in RAS pathway genes (P=0.004) or JAK2 (P=0.001) were significantly associated with shorter survival.
The shorter survival in patients with mutated RAS pathway genes was due to a higher risk of relapse, while the shorter survival in patients with JAK2 mutations was due to a higher risk of death without relapse.
In contrast to TP53 mutations, the adverse effect of RAS mutations on survival and risk of relapse was evident only in patients who received reduced-intensity conditioning (P<0.001). This suggests these patients may benefit from higher intensity conditioning regimens, the researchers said.
This study also yielded insights about the biology of MDS in specific groups of patients.
For example, the researchers found that 4% of MDS patients between the ages of 18 and 40 had mutations associated with Shwachman-Diamond syndrome (in the SBDS gene), but most of them had not previously been diagnosed with the syndrome.
In each case, the patients had acquired a TP53 mutation, suggesting not only how MDS develops in patients with Schwachman-Diamond syndrome but also what underlies their poor prognosis after HSCT.
The researchers also analyzed patients with therapy-related MDS. The team found that TP53 mutations and mutations in PPM1D, a gene that regulates TP53 function, were far more common in these patients than in those with primary MDS (15% and 3%, respectively, P<0.001).
“In deciding whether a stem cell transplant is appropriate for a patient with MDS, it’s always necessary to balance the potential benefit with the risk of complications,” Dr Lindsley noted.
“Our findings offer physicians a guide—based on the genetic profile of the disease and certain clinical factors—to identifying patients for whom a transplant is appropriate, and the intensity of treatment most likely to be effective.” ![]()

Genetic profiling can be used to determine which patients with myelodysplastic syndrome (MDS) are likely to benefit from allogeneic hematopoietic stem cell transplant (HSCT), according to research published in NEJM.
Targeted sequencing of 129 genes revealed mutations that, after adjustment for clinical variables, were associated with shorter survival and/or relapse after HSCT.
Patients with mutations in TP53, JAK2, and the RAS pathway tended to have worse outcomes after HSCT than patients without such mutations.
“Although donor stem cell transplantation is the only curative therapy for MDS, many patients die after transplantation, largely due to relapse of the disease or complications relating to the transplant itself,” said study author R. Coleman Lindsley, MD, PhD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
“As physicians, one of our major challenges is to be able to predict which patients are most likely to benefit from a transplant. Improving our ability to identify patients who are most likely to have a relapse or to experience life-threatening complications from a transplant could lead to better pre-transplant therapies and strategies for preventing relapse.”
Researchers have long known that specific genetic mutations are closely related to the course MDS takes. With this study, Dr Lindsley and his colleagues sought to discover whether mutations can be used to predict how patients will fare following allogeneic HSCT.
The team analyzed blood samples from 1514 MDS patients, performing targeted sequencing of 129 genes. The genes were selected based on their known or suspected involvement in the pathogenesis of myeloid cancers or bone marrow failure syndromes.
Dr Lindsley and his colleagues then evaluated the association between mutations and HSCT outcomes, including overall survival, relapse, and death without relapse.
After adjusting for significant clinical variables, the researchers found that having mutated TP53 was significantly associated with shorter survival and shorter time to relapse after HSCT (P<0.001 for both comparisons). This was true whether patients received standard conditioning or reduced-intensity conditioning.
In patients age 40 and older who did not have TP53 mutations, mutations in RAS pathway genes (P=0.004) or JAK2 (P=0.001) were significantly associated with shorter survival.
The shorter survival in patients with mutated RAS pathway genes was due to a higher risk of relapse, while the shorter survival in patients with JAK2 mutations was due to a higher risk of death without relapse.
In contrast to TP53 mutations, the adverse effect of RAS mutations on survival and risk of relapse was evident only in patients who received reduced-intensity conditioning (P<0.001). This suggests these patients may benefit from higher intensity conditioning regimens, the researchers said.
This study also yielded insights about the biology of MDS in specific groups of patients.
For example, the researchers found that 4% of MDS patients between the ages of 18 and 40 had mutations associated with Shwachman-Diamond syndrome (in the SBDS gene), but most of them had not previously been diagnosed with the syndrome.
In each case, the patients had acquired a TP53 mutation, suggesting not only how MDS develops in patients with Schwachman-Diamond syndrome but also what underlies their poor prognosis after HSCT.
The researchers also analyzed patients with therapy-related MDS. The team found that TP53 mutations and mutations in PPM1D, a gene that regulates TP53 function, were far more common in these patients than in those with primary MDS (15% and 3%, respectively, P<0.001).
“In deciding whether a stem cell transplant is appropriate for a patient with MDS, it’s always necessary to balance the potential benefit with the risk of complications,” Dr Lindsley noted.
“Our findings offer physicians a guide—based on the genetic profile of the disease and certain clinical factors—to identifying patients for whom a transplant is appropriate, and the intensity of treatment most likely to be effective.” ![]()
Algorithm predicts NRM, GVHD after HSCT
A biomarker algorithm can identify patients with a high risk of graft-vs-host disease (GVHD) and non-relapse mortality (NRM) after hematopoietic stem cell transplant (HSCT), according to researchers.
The team found evidence to suggest that 2 proteins—ST2 and REG3α—present in blood drawn a week after HSCT can predict the likelihood of GVHD, including lethal GVHD, and NRM in patients with hematologic disorders.
James L.M. Ferrara, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and his colleagues reported these findings in JCI Insight.
The researchers analyzed blood samples collected on day 7 after HSCT from 1287 patients. Of these, 620 samples were designated the training set.
The team measured the concentrations of 4 GVHD biomarkers—ST2, REG3α, TNFR1, and IL-2Rα—in the training set and used them to model 6-month NRM in an attempt to identify the best algorithm that defined 2 distinct risk groups.
The researchers applied the resulting algorithm to the test set of samples (n=309) and the validation set of samples (n=358).
The final algorithm used ST2 and REG3α concentrations to identify patients with a high and low risk of NRM at 6 months. Sixteen percent of patients in the training set belonged to the high-risk group, as did 17% of the test set and 20% of the validation set.
In the training set, the cumulative incidence of NRM at 6 months was 28% in the high-risk group and 7% in the low-risk group (P<0.001). The incidence was 33% and 7%, respectively (P<0.001), in the test set and 26% and 10%, respectively (P<0.001), in the validation set.
The high-risk patients were 3 times more likely to die from GVHD than low-risk patients in the overall cohort. The incidence of lethal GVHD was 19% and 6%, respectively (P<0.001).
GVHD-related mortality in the high-risk and low-risk groups, respectively, was 18% and 5% (P<0.001) in the training set, 24% and 4% (P<0.001) in the test set, and 14% and 5% (P<0.001) in the validation set.
The researchers said their algorithm can also be adapted to define 3 distinct risk groups at GVHD onset—Ann Arbor scores 1, 2, and 3.
The team dubbed their algorithm the “MAGIC algorithm,” after the Mount Sinai Acute GVHD International Consortium (MAGIC).
“The MAGIC algorithm gives doctors a roadmap to save many lives in the future,” Dr Ferrara said. “This simple blood test can determine which bone marrow transplant patients are at high risk for a lethal complication before it occurs. It will allow early intervention and potentially save many lives.”
Doctors at Mount Sinai are now designing clinical trials to determine whether immunotherapy drugs would benefit patients if the MAGIC algorithm determines they are at high risk for severe GVHD.
The researchers believe that if patients receive the drugs once the blood test is administered, which is well before symptoms develop, they would be spared the full force of GVHD, and fewer of them would die.
“This test will make bone marrow transplant safer and more effective for patients because it will guide adjustment of medications to protect against graft-vs-host disease,” said study author John Levine, MD, of the Icahn School of Medicine at Mount Sinai.
“If successful, the early use of the drugs would become a standard of care for bone marrow transplant patients.” ![]()
A biomarker algorithm can identify patients with a high risk of graft-vs-host disease (GVHD) and non-relapse mortality (NRM) after hematopoietic stem cell transplant (HSCT), according to researchers.
The team found evidence to suggest that 2 proteins—ST2 and REG3α—present in blood drawn a week after HSCT can predict the likelihood of GVHD, including lethal GVHD, and NRM in patients with hematologic disorders.
James L.M. Ferrara, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and his colleagues reported these findings in JCI Insight.
The researchers analyzed blood samples collected on day 7 after HSCT from 1287 patients. Of these, 620 samples were designated the training set.
The team measured the concentrations of 4 GVHD biomarkers—ST2, REG3α, TNFR1, and IL-2Rα—in the training set and used them to model 6-month NRM in an attempt to identify the best algorithm that defined 2 distinct risk groups.
The researchers applied the resulting algorithm to the test set of samples (n=309) and the validation set of samples (n=358).
The final algorithm used ST2 and REG3α concentrations to identify patients with a high and low risk of NRM at 6 months. Sixteen percent of patients in the training set belonged to the high-risk group, as did 17% of the test set and 20% of the validation set.
In the training set, the cumulative incidence of NRM at 6 months was 28% in the high-risk group and 7% in the low-risk group (P<0.001). The incidence was 33% and 7%, respectively (P<0.001), in the test set and 26% and 10%, respectively (P<0.001), in the validation set.
The high-risk patients were 3 times more likely to die from GVHD than low-risk patients in the overall cohort. The incidence of lethal GVHD was 19% and 6%, respectively (P<0.001).
GVHD-related mortality in the high-risk and low-risk groups, respectively, was 18% and 5% (P<0.001) in the training set, 24% and 4% (P<0.001) in the test set, and 14% and 5% (P<0.001) in the validation set.
The researchers said their algorithm can also be adapted to define 3 distinct risk groups at GVHD onset—Ann Arbor scores 1, 2, and 3.
The team dubbed their algorithm the “MAGIC algorithm,” after the Mount Sinai Acute GVHD International Consortium (MAGIC).
“The MAGIC algorithm gives doctors a roadmap to save many lives in the future,” Dr Ferrara said. “This simple blood test can determine which bone marrow transplant patients are at high risk for a lethal complication before it occurs. It will allow early intervention and potentially save many lives.”
Doctors at Mount Sinai are now designing clinical trials to determine whether immunotherapy drugs would benefit patients if the MAGIC algorithm determines they are at high risk for severe GVHD.
The researchers believe that if patients receive the drugs once the blood test is administered, which is well before symptoms develop, they would be spared the full force of GVHD, and fewer of them would die.
“This test will make bone marrow transplant safer and more effective for patients because it will guide adjustment of medications to protect against graft-vs-host disease,” said study author John Levine, MD, of the Icahn School of Medicine at Mount Sinai.
“If successful, the early use of the drugs would become a standard of care for bone marrow transplant patients.” ![]()
A biomarker algorithm can identify patients with a high risk of graft-vs-host disease (GVHD) and non-relapse mortality (NRM) after hematopoietic stem cell transplant (HSCT), according to researchers.
The team found evidence to suggest that 2 proteins—ST2 and REG3α—present in blood drawn a week after HSCT can predict the likelihood of GVHD, including lethal GVHD, and NRM in patients with hematologic disorders.
James L.M. Ferrara, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York, and his colleagues reported these findings in JCI Insight.
The researchers analyzed blood samples collected on day 7 after HSCT from 1287 patients. Of these, 620 samples were designated the training set.
The team measured the concentrations of 4 GVHD biomarkers—ST2, REG3α, TNFR1, and IL-2Rα—in the training set and used them to model 6-month NRM in an attempt to identify the best algorithm that defined 2 distinct risk groups.
The researchers applied the resulting algorithm to the test set of samples (n=309) and the validation set of samples (n=358).
The final algorithm used ST2 and REG3α concentrations to identify patients with a high and low risk of NRM at 6 months. Sixteen percent of patients in the training set belonged to the high-risk group, as did 17% of the test set and 20% of the validation set.
In the training set, the cumulative incidence of NRM at 6 months was 28% in the high-risk group and 7% in the low-risk group (P<0.001). The incidence was 33% and 7%, respectively (P<0.001), in the test set and 26% and 10%, respectively (P<0.001), in the validation set.
The high-risk patients were 3 times more likely to die from GVHD than low-risk patients in the overall cohort. The incidence of lethal GVHD was 19% and 6%, respectively (P<0.001).
GVHD-related mortality in the high-risk and low-risk groups, respectively, was 18% and 5% (P<0.001) in the training set, 24% and 4% (P<0.001) in the test set, and 14% and 5% (P<0.001) in the validation set.
The researchers said their algorithm can also be adapted to define 3 distinct risk groups at GVHD onset—Ann Arbor scores 1, 2, and 3.
The team dubbed their algorithm the “MAGIC algorithm,” after the Mount Sinai Acute GVHD International Consortium (MAGIC).
“The MAGIC algorithm gives doctors a roadmap to save many lives in the future,” Dr Ferrara said. “This simple blood test can determine which bone marrow transplant patients are at high risk for a lethal complication before it occurs. It will allow early intervention and potentially save many lives.”
Doctors at Mount Sinai are now designing clinical trials to determine whether immunotherapy drugs would benefit patients if the MAGIC algorithm determines they are at high risk for severe GVHD.
The researchers believe that if patients receive the drugs once the blood test is administered, which is well before symptoms develop, they would be spared the full force of GVHD, and fewer of them would die.
“This test will make bone marrow transplant safer and more effective for patients because it will guide adjustment of medications to protect against graft-vs-host disease,” said study author John Levine, MD, of the Icahn School of Medicine at Mount Sinai.
“If successful, the early use of the drugs would become a standard of care for bone marrow transplant patients.” ![]()
Study shows no increased risk of mutations with iPSCs

Image from Salk Institute
The use of induced pluripotent stem cells (iPSCs) in biomedical research and medicine has been slowed by concerns that these cells are prone to increased numbers of genetic mutations.
However, a new study suggests iPSCs do not develop more mutations than cells that are duplicated by subcloning, a technique where single cells are cultured individually and then grown into a cell line.
Subcloning is similar to the technique used to create iPSCs, except the subcloned cells are not treated with the reprogramming factors that have been thought to cause mutations in iPSCs.
“These findings suggest that the question of safety shouldn’t impede research using iPSCs,” said study author Paul Liu, MD, PhD, of the National Human Genome Research Institute, part of the National Institutes of Health, in Bethesda, Maryland.
Dr Liu and his colleagues reported the findings in PNAS.
For this study, the researchers examined 2 sets of donated cells. One set came from a healthy individual, and the second came from a person with familial platelet disorder.
Using fibroblasts from each of the donors, the researchers created genetically identical copies of the cells using both the iPSC and subcloning techniques.
The team then sequenced the DNA of the fibroblasts as well as the iPSCs and the subcloned cells and determined that mutations occurred at the same rate in cells that were reprogrammed and cells that were subcloned.
More than 90% of the genetic variants detected in the iPSCs and subclones were rare variants inherited from the parent cells.
This suggests that most mutations in iPSCs are not generated during the reprogramming or iPSC production phase and provides evidence that iPSCs are stable and safe to use for both basic and clinical research, Dr Liu said.
“Based on this data, we plan to start using iPSCs to gain a deeper understanding of how diseases start and progress,” said study author Erika Mijin Kwon, PhD, also of the National Human Genome Research Institute.
“We eventually hope to develop new therapies to treat patients with leukemia using their own iPSCs. We encourage other researchers to embrace the use of iPSCs.” ![]()

Image from Salk Institute
The use of induced pluripotent stem cells (iPSCs) in biomedical research and medicine has been slowed by concerns that these cells are prone to increased numbers of genetic mutations.
However, a new study suggests iPSCs do not develop more mutations than cells that are duplicated by subcloning, a technique where single cells are cultured individually and then grown into a cell line.
Subcloning is similar to the technique used to create iPSCs, except the subcloned cells are not treated with the reprogramming factors that have been thought to cause mutations in iPSCs.
“These findings suggest that the question of safety shouldn’t impede research using iPSCs,” said study author Paul Liu, MD, PhD, of the National Human Genome Research Institute, part of the National Institutes of Health, in Bethesda, Maryland.
Dr Liu and his colleagues reported the findings in PNAS.
For this study, the researchers examined 2 sets of donated cells. One set came from a healthy individual, and the second came from a person with familial platelet disorder.
Using fibroblasts from each of the donors, the researchers created genetically identical copies of the cells using both the iPSC and subcloning techniques.
The team then sequenced the DNA of the fibroblasts as well as the iPSCs and the subcloned cells and determined that mutations occurred at the same rate in cells that were reprogrammed and cells that were subcloned.
More than 90% of the genetic variants detected in the iPSCs and subclones were rare variants inherited from the parent cells.
This suggests that most mutations in iPSCs are not generated during the reprogramming or iPSC production phase and provides evidence that iPSCs are stable and safe to use for both basic and clinical research, Dr Liu said.
“Based on this data, we plan to start using iPSCs to gain a deeper understanding of how diseases start and progress,” said study author Erika Mijin Kwon, PhD, also of the National Human Genome Research Institute.
“We eventually hope to develop new therapies to treat patients with leukemia using their own iPSCs. We encourage other researchers to embrace the use of iPSCs.” ![]()

Image from Salk Institute
The use of induced pluripotent stem cells (iPSCs) in biomedical research and medicine has been slowed by concerns that these cells are prone to increased numbers of genetic mutations.
However, a new study suggests iPSCs do not develop more mutations than cells that are duplicated by subcloning, a technique where single cells are cultured individually and then grown into a cell line.
Subcloning is similar to the technique used to create iPSCs, except the subcloned cells are not treated with the reprogramming factors that have been thought to cause mutations in iPSCs.
“These findings suggest that the question of safety shouldn’t impede research using iPSCs,” said study author Paul Liu, MD, PhD, of the National Human Genome Research Institute, part of the National Institutes of Health, in Bethesda, Maryland.
Dr Liu and his colleagues reported the findings in PNAS.
For this study, the researchers examined 2 sets of donated cells. One set came from a healthy individual, and the second came from a person with familial platelet disorder.
Using fibroblasts from each of the donors, the researchers created genetically identical copies of the cells using both the iPSC and subcloning techniques.
The team then sequenced the DNA of the fibroblasts as well as the iPSCs and the subcloned cells and determined that mutations occurred at the same rate in cells that were reprogrammed and cells that were subcloned.
More than 90% of the genetic variants detected in the iPSCs and subclones were rare variants inherited from the parent cells.
This suggests that most mutations in iPSCs are not generated during the reprogramming or iPSC production phase and provides evidence that iPSCs are stable and safe to use for both basic and clinical research, Dr Liu said.
“Based on this data, we plan to start using iPSCs to gain a deeper understanding of how diseases start and progress,” said study author Erika Mijin Kwon, PhD, also of the National Human Genome Research Institute.
“We eventually hope to develop new therapies to treat patients with leukemia using their own iPSCs. We encourage other researchers to embrace the use of iPSCs.” ![]()
Anticoagulants: more harm than good in isolated calf DVT
Clinical question: Is therapeutic anticoagulation superior to placebo in patients with symptomatic acute calf deep venous thrombosis (DVT)?
Background: Medical evidence supporting the usage of therapeutic anticoagulation in symptomatic acute isolated calf DVT is lacking. This type of DVT has a low embolic potential. The bleeding risk of anticoagulation might therefore be higher than its benefit.
Study design: Double-blind, placebo-controlled trial.
Setting: Twenty-three centers in Canada, France and Switzerland.
Synopsis: A total of 259 outpatients with a first acute symptomatic objectively confirmed isolated calf DVT were enrolled to receive either a therapeutic dose of the low-molecular weight heparin nadroparin (122 patients), or a placebo (130 patients).
The primary efficacy outcome (a composite endpoint of extension of calf DVT to proximal veins, contralateral proximal DVT and symptomatic pulmonary embolism) was not statistically significant between the two groups (3% in the nadroparin group and 5% in the placebo group, P = .54). The primary safety outcome (the number of patients with major or clinically relevant non-major bleeding) was significantly higher in the nadroparin group (4% in nadroparin group, 0 patients in the placebo group, P = .0255).
The study was limited by the relative low number of patients (goal was 286 patients). The results of the study do not apply to inpatients and to cancer patients as patients with high risk for extension or recurrence of their DVT were excluded.
Bottom line: Therapeutic anticoagulation in low-risk outpatients with isolated calf DVT will likely cause more harm from bleeding than benefit.
Citation: Righini M, Galanaud J, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): A randomised, double-blind, placebo-controlled trial. The Lancet Haematology. 2016;3(12):e556-e562. doi: 10.1016/S2352-3026(16)30131-4.
Dr. Badr is a hospitalist at Cooper University Hospital in Camden, N.J., and an assistant professor of clinical medicine at the Cooper Medical School of Rowan University.
Clinical question: Is therapeutic anticoagulation superior to placebo in patients with symptomatic acute calf deep venous thrombosis (DVT)?
Background: Medical evidence supporting the usage of therapeutic anticoagulation in symptomatic acute isolated calf DVT is lacking. This type of DVT has a low embolic potential. The bleeding risk of anticoagulation might therefore be higher than its benefit.
Study design: Double-blind, placebo-controlled trial.
Setting: Twenty-three centers in Canada, France and Switzerland.
Synopsis: A total of 259 outpatients with a first acute symptomatic objectively confirmed isolated calf DVT were enrolled to receive either a therapeutic dose of the low-molecular weight heparin nadroparin (122 patients), or a placebo (130 patients).
The primary efficacy outcome (a composite endpoint of extension of calf DVT to proximal veins, contralateral proximal DVT and symptomatic pulmonary embolism) was not statistically significant between the two groups (3% in the nadroparin group and 5% in the placebo group, P = .54). The primary safety outcome (the number of patients with major or clinically relevant non-major bleeding) was significantly higher in the nadroparin group (4% in nadroparin group, 0 patients in the placebo group, P = .0255).
The study was limited by the relative low number of patients (goal was 286 patients). The results of the study do not apply to inpatients and to cancer patients as patients with high risk for extension or recurrence of their DVT were excluded.
Bottom line: Therapeutic anticoagulation in low-risk outpatients with isolated calf DVT will likely cause more harm from bleeding than benefit.
Citation: Righini M, Galanaud J, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): A randomised, double-blind, placebo-controlled trial. The Lancet Haematology. 2016;3(12):e556-e562. doi: 10.1016/S2352-3026(16)30131-4.
Dr. Badr is a hospitalist at Cooper University Hospital in Camden, N.J., and an assistant professor of clinical medicine at the Cooper Medical School of Rowan University.
Clinical question: Is therapeutic anticoagulation superior to placebo in patients with symptomatic acute calf deep venous thrombosis (DVT)?
Background: Medical evidence supporting the usage of therapeutic anticoagulation in symptomatic acute isolated calf DVT is lacking. This type of DVT has a low embolic potential. The bleeding risk of anticoagulation might therefore be higher than its benefit.
Study design: Double-blind, placebo-controlled trial.
Setting: Twenty-three centers in Canada, France and Switzerland.
Synopsis: A total of 259 outpatients with a first acute symptomatic objectively confirmed isolated calf DVT were enrolled to receive either a therapeutic dose of the low-molecular weight heparin nadroparin (122 patients), or a placebo (130 patients).
The primary efficacy outcome (a composite endpoint of extension of calf DVT to proximal veins, contralateral proximal DVT and symptomatic pulmonary embolism) was not statistically significant between the two groups (3% in the nadroparin group and 5% in the placebo group, P = .54). The primary safety outcome (the number of patients with major or clinically relevant non-major bleeding) was significantly higher in the nadroparin group (4% in nadroparin group, 0 patients in the placebo group, P = .0255).
The study was limited by the relative low number of patients (goal was 286 patients). The results of the study do not apply to inpatients and to cancer patients as patients with high risk for extension or recurrence of their DVT were excluded.
Bottom line: Therapeutic anticoagulation in low-risk outpatients with isolated calf DVT will likely cause more harm from bleeding than benefit.
Citation: Righini M, Galanaud J, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): A randomised, double-blind, placebo-controlled trial. The Lancet Haematology. 2016;3(12):e556-e562. doi: 10.1016/S2352-3026(16)30131-4.
Dr. Badr is a hospitalist at Cooper University Hospital in Camden, N.J., and an assistant professor of clinical medicine at the Cooper Medical School of Rowan University.
The cost of misdiagnosing cellulitis
Clinical question: What are the national health care costs of misdiagnosing cellulitis?
Background: Lower extremity cellulitis is primarily a clinical diagnosis but many mimickers such as venous stasis, lymphedema, gout, deep venous thrombosis, and contact dermatitis can lead to a misdiagnosis rate of 30%-90%. Between 14% and 17% of emergency department patients with cellulitis are admitted, accounting for 10% of all infectious disease-related hospitalizations. Overdiagnosis leads to antibiotic misuse and increased hospital utilization.
Study design: Retrospective cross-sectional study.
Setting: Emergency department of Massachusetts General Hospital.
Synopsis: Among 259 ED patients identified from all screened (840 patients total) from June 2010 to December 2012, 79 (30.5%) were incorrectly diagnosed with lower extremity cellulitis and 52 of these misdiagnosed patients were admitted primarily for their cellulitis, resulting in 92.3% of this group receiving unnecessary antibiotics and 84.6% unnecessarily hospitalized.
The authors used cost estimates and previously published data from the Medical Expenditure Panel Survey (MEPS) provided by the Agency for Healthcare Research and Quality (AHRQ) 2010 to project that cellulitis misdiagnosis leads to 50,000-130,000 unnecessary hospitalizations and $195-$515 million in avoidable health care expense annually. The estimates include over 44,000 pseudocellulitis patients being exposed to antibiotics annually with an associated 13% readmission rate and medication complications such as rash and gastrointestinal side effects and implications for resistance selection and antimicrobial stewardship efforts. Nationally, the unnecessary antibiotics and hospitalization associated with misdiagnosis were estimated to cause more than 9,000 nosocomial infections, 1,000 to 5,000 Clostridium difficile infections, and two to six cases of anaphylaxis annually.
Bottom line: Misdiagnosis of lower extremity cellulitis is common and leads to unnecessary patient exposures (antibiotics, hospitalization) and excessive health care spending.
Citations: Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2016; doi: 10.1001/jamadermatol.2016.3816.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: What are the national health care costs of misdiagnosing cellulitis?
Background: Lower extremity cellulitis is primarily a clinical diagnosis but many mimickers such as venous stasis, lymphedema, gout, deep venous thrombosis, and contact dermatitis can lead to a misdiagnosis rate of 30%-90%. Between 14% and 17% of emergency department patients with cellulitis are admitted, accounting for 10% of all infectious disease-related hospitalizations. Overdiagnosis leads to antibiotic misuse and increased hospital utilization.
Study design: Retrospective cross-sectional study.
Setting: Emergency department of Massachusetts General Hospital.
Synopsis: Among 259 ED patients identified from all screened (840 patients total) from June 2010 to December 2012, 79 (30.5%) were incorrectly diagnosed with lower extremity cellulitis and 52 of these misdiagnosed patients were admitted primarily for their cellulitis, resulting in 92.3% of this group receiving unnecessary antibiotics and 84.6% unnecessarily hospitalized.
The authors used cost estimates and previously published data from the Medical Expenditure Panel Survey (MEPS) provided by the Agency for Healthcare Research and Quality (AHRQ) 2010 to project that cellulitis misdiagnosis leads to 50,000-130,000 unnecessary hospitalizations and $195-$515 million in avoidable health care expense annually. The estimates include over 44,000 pseudocellulitis patients being exposed to antibiotics annually with an associated 13% readmission rate and medication complications such as rash and gastrointestinal side effects and implications for resistance selection and antimicrobial stewardship efforts. Nationally, the unnecessary antibiotics and hospitalization associated with misdiagnosis were estimated to cause more than 9,000 nosocomial infections, 1,000 to 5,000 Clostridium difficile infections, and two to six cases of anaphylaxis annually.
Bottom line: Misdiagnosis of lower extremity cellulitis is common and leads to unnecessary patient exposures (antibiotics, hospitalization) and excessive health care spending.
Citations: Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2016; doi: 10.1001/jamadermatol.2016.3816.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
Clinical question: What are the national health care costs of misdiagnosing cellulitis?
Background: Lower extremity cellulitis is primarily a clinical diagnosis but many mimickers such as venous stasis, lymphedema, gout, deep venous thrombosis, and contact dermatitis can lead to a misdiagnosis rate of 30%-90%. Between 14% and 17% of emergency department patients with cellulitis are admitted, accounting for 10% of all infectious disease-related hospitalizations. Overdiagnosis leads to antibiotic misuse and increased hospital utilization.
Study design: Retrospective cross-sectional study.
Setting: Emergency department of Massachusetts General Hospital.
Synopsis: Among 259 ED patients identified from all screened (840 patients total) from June 2010 to December 2012, 79 (30.5%) were incorrectly diagnosed with lower extremity cellulitis and 52 of these misdiagnosed patients were admitted primarily for their cellulitis, resulting in 92.3% of this group receiving unnecessary antibiotics and 84.6% unnecessarily hospitalized.
The authors used cost estimates and previously published data from the Medical Expenditure Panel Survey (MEPS) provided by the Agency for Healthcare Research and Quality (AHRQ) 2010 to project that cellulitis misdiagnosis leads to 50,000-130,000 unnecessary hospitalizations and $195-$515 million in avoidable health care expense annually. The estimates include over 44,000 pseudocellulitis patients being exposed to antibiotics annually with an associated 13% readmission rate and medication complications such as rash and gastrointestinal side effects and implications for resistance selection and antimicrobial stewardship efforts. Nationally, the unnecessary antibiotics and hospitalization associated with misdiagnosis were estimated to cause more than 9,000 nosocomial infections, 1,000 to 5,000 Clostridium difficile infections, and two to six cases of anaphylaxis annually.
Bottom line: Misdiagnosis of lower extremity cellulitis is common and leads to unnecessary patient exposures (antibiotics, hospitalization) and excessive health care spending.
Citations: Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2016; doi: 10.1001/jamadermatol.2016.3816.
Dr. Cerceo is an assistant professor in the Division of Hospital Medicine, and associate director of the internal medicine residency program at Cooper Medical School of Rowan University, Camden, N.J.
FDA approves Emflaza for Duchenne muscular dystrophy
Emflaza, a tablet and oral suspension corticosteroid, has been approved by the Food and Drug Administration for the treatment of Duchenne muscular dystrophy in patients aged 5 years and older.
The agency’s Feb. 9 announcement notes that similar corticosteroids have been used around the world to treat Duchenne muscular dystrophy (DMD), but this is the first to gain approval in the United States. Emflaza (deflazacort) works by decreasing inflammation and immune system activity.
DMD is the most common form of muscular dystrophy but is still rare, occurring in about 1 in 3,600 male infants worldwide. One study found that patients taking deflazacort had some improvements in muscle strength at 12 weeks, compared with those taking placebo, and maintained muscle strength stability through 52 weeks. A longer-term study showed that patients who took deflazacort had better average muscle strength than did those taking placebo and suggested that deflazacort helped prolong patients’ ability to walk.
Side effects experienced by patients taking Emflaza are similar to those associated with other corticosteroids, such as facial puffiness (cushingoid appearance), weight gain, increased appetite, upper respiratory tract infection, cough, extraordinary daytime urinary frequency (pollakiuria), unwanted hair growth (hirsutism), and excessive fat around the stomach (central obesity).
In the FDA’s announcement, Billy Dunn, MD, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research, said, “We hope that this treatment option will benefit many patients with DMD.”
Emflaza, a tablet and oral suspension corticosteroid, has been approved by the Food and Drug Administration for the treatment of Duchenne muscular dystrophy in patients aged 5 years and older.
The agency’s Feb. 9 announcement notes that similar corticosteroids have been used around the world to treat Duchenne muscular dystrophy (DMD), but this is the first to gain approval in the United States. Emflaza (deflazacort) works by decreasing inflammation and immune system activity.
DMD is the most common form of muscular dystrophy but is still rare, occurring in about 1 in 3,600 male infants worldwide. One study found that patients taking deflazacort had some improvements in muscle strength at 12 weeks, compared with those taking placebo, and maintained muscle strength stability through 52 weeks. A longer-term study showed that patients who took deflazacort had better average muscle strength than did those taking placebo and suggested that deflazacort helped prolong patients’ ability to walk.
Side effects experienced by patients taking Emflaza are similar to those associated with other corticosteroids, such as facial puffiness (cushingoid appearance), weight gain, increased appetite, upper respiratory tract infection, cough, extraordinary daytime urinary frequency (pollakiuria), unwanted hair growth (hirsutism), and excessive fat around the stomach (central obesity).
In the FDA’s announcement, Billy Dunn, MD, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research, said, “We hope that this treatment option will benefit many patients with DMD.”
Emflaza, a tablet and oral suspension corticosteroid, has been approved by the Food and Drug Administration for the treatment of Duchenne muscular dystrophy in patients aged 5 years and older.
The agency’s Feb. 9 announcement notes that similar corticosteroids have been used around the world to treat Duchenne muscular dystrophy (DMD), but this is the first to gain approval in the United States. Emflaza (deflazacort) works by decreasing inflammation and immune system activity.
DMD is the most common form of muscular dystrophy but is still rare, occurring in about 1 in 3,600 male infants worldwide. One study found that patients taking deflazacort had some improvements in muscle strength at 12 weeks, compared with those taking placebo, and maintained muscle strength stability through 52 weeks. A longer-term study showed that patients who took deflazacort had better average muscle strength than did those taking placebo and suggested that deflazacort helped prolong patients’ ability to walk.
Side effects experienced by patients taking Emflaza are similar to those associated with other corticosteroids, such as facial puffiness (cushingoid appearance), weight gain, increased appetite, upper respiratory tract infection, cough, extraordinary daytime urinary frequency (pollakiuria), unwanted hair growth (hirsutism), and excessive fat around the stomach (central obesity).
In the FDA’s announcement, Billy Dunn, MD, director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research, said, “We hope that this treatment option will benefit many patients with DMD.”
IgG4-related disease can strike any organ system
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Federal judge blocks merger between Anthem and Cigna
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
agallegos@frontlinemedcom.com
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
agallegos@frontlinemedcom.com
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision comes weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer.
“Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement.“If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Cardiovascular Risk Reduction in Patients with Type 2 Diabetes
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
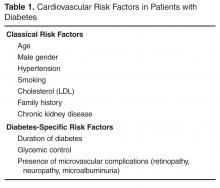
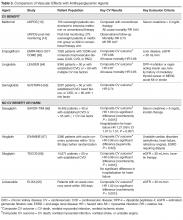
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, bikrampal.sidhu@mail.utoronto.ca.
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, bikrampal.sidhu@mail.utoronto.ca.
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
From the Division of Endocrinology, Department of Medicine, University of Toronto, Ontario, Canada.
Abstract
- Objective: To review the assessment of cardiovascular risk and prevention of vascular disease in patients with type 2 diabetes mellitus (T2DM).
- Methods: Literature and guidelines were reviewed and the evidence is presented around a clinical case.
- Results: T2DM has a high prevalence and confers significant lifetime risk for macrovascular disease, including stroke, heart disease, and peripheral arterial disease. There is strong evidence to support nonpharmacologic interventions, such as smoking cessation and weight loss, and pharmacologic interventions, such as statin therapy, in order to decrease lifetime risk. The effectiveness of an intervention as well as the strength of the evidence supporting an intervention differs depending on the stage of the disease.
- Conclusion: Once a patient is diagnosed with T2DM, it is important to recognize that their lifetime risk for vascular disease is high. Starting at this stage and continuing throughout the disease course, cardiovascular risk should be assessed in an ongoing manner and evidence-based interventions should be implemented whenever they are indicated. Using major guidelines as a framework, we provide an evidence-based approach to the reduction of vascular risk in these patients.
Key words: cardiovascular disease, diabetes, prevention, risk assessment, risk factors.
Type 2 diabetes (T2DM) is considered epidemic in the developed world, and is rapidly increasing in the developing world. Since 1980, there has been a near quadrupling of the number of adults with diabetes worldwide to an estimated 422 million in 2014 [1]. Because diabetes affects the whole body vascular system, there is a significant burden of vascular complications directly attributable to diabetes. Although the rates of diabetes-related complications are declining, the burden of disease remains high due to the increasing prevalence of diabetes [2]. The tremendous burden of diabetes and its complications on the population make it imperative that all health care practitioners understand the vascular effects of diabetes as well as evidence-based interventions that can mitigate them. In this review, we present an approach to the assessment, prevention, and treatment of cardiovascular disease in patients with T2DM.
Case Study
A 38-year-old male presents to his family physician’s office for a routine check-up. He is obese and a smoker, has no other health issues, and is taking no medications. He is sent for routine bloodwork and his A1c and fasting glucose are elevated and are diagnostic for diabetes. He returns to the clinic to discuss his results.
How are cardiovascular risk and risk factors assessed in a patient with diabetes?
There are many risk scores and risk calculators available for assessing cardiovascular risk. The Framingham Risk Score is the most commonly employed and takes into account the most common risk factors for cardiovascular risk, including cholesterol level, age, gender, and smoking status. Unfortunately, because a patient with diabetes may have a high lifetime risk but low or moderate short-term risk, these risk scores tend to underestimate overall risk in the population with diabetes [3,4]. Furthermore, since early intervention can decrease lifetime risk, it is important to recognize the limitations of these risk scores.
What interventions should be used for primary prevention at this stage?
A number of interventions can decrease lifetime risk for cardiovascular disease in persons with diabetes. First, smoking increases risk for all forms of vascular disease, including progression to end-stage renal disease, and is an independent predictor of mortality. Smoking cessation is one of the most effective interventions at decreasing these risks [6]. Second, lifestyle interventions such as diet and exercise are often recommended. The Look AHEAD trial studied the benefits of weight loss and exercise in the treatment of T2DM through a randomized control trial involving more than 5000 overweight patients with T2DM. Patients were randomly assigned to intensive lifestyle interventions targeting weight loss or a support and education group. Although the Action for Health in Diabetes (Look AHEAD) trial did not demonstrate clinical outcome benefit with this intensive intervention, there was improvement in weight, cholesterol level, blood pressure, and glycemic control, and clinical differences may have been related to study power or differences in cardioprotective medication use [7]. Furthermore, at least 1 large randomized trial of dietary intervention in high-risk cardiovascular patients, half of whom had diabetes (Prevención con Dieta Mediterránea [PREDIMED]), showed significant benefits in cardiovascular disease, reducing the incidence of major cardiovascular events [8]. According to most diabetes guidelines, diet and exercise continue to be stressed as initial management for all patients with diabetes [9–12].
In addition, although intensive glucose control decreases microvascular complication rates, it has been more difficult to demonstrate a reduction in cardiovascular disease with more intense glycemic control. However, long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) cohort, a population that was earlier in their diabetes course, clearly demonstrated a reduction in cardiovascular events and mortality with better glycemic control over the long term [13,14]. For those who are later in their diabetes course, meta-analyses of glycemic control trials, along with follow-up studies, have also shown that better glycemic control can reduce cardiovascular events, but not mortality [15–17]. Therefore, glucose lowering should be pursued for cardiovascular risk reduction, in addition to its effects on microvascular complications.
It is well established that a multifactorial approach to cardiovascular risk reduction in patients with type 2 diabetes is effective. In the Steno-2 study, 160 patients with type 2 diabetes were randomly assigned to receive multidisciplinary, multifactorial intensive target-based lifestyle and pharmacologic intervention or standard of care. The intensive therapy group all received smoking cessation counseling, exercise and dietary advice, vitamin supplementation, and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). Acetylsalicylic acid (ASA) was added for all patients with clinical macrovascular disease. Dyslipidemia, hypertension, and hyperglycemia were all treated in a protocolized way if lifestyle interventions did not achieve strict targets. During the mean 7.8 years of follow-up, the adjusted hazard ratio for a composite of cardiovascular death and macrovascular disease was 0.47 (95% confidence interval [CI] 0.22 to 0.74; P = 0.01) [18]. These patients were followed for an additional 5.5 years in an observational study with no further active intervention in both groups. Over the entire period, there was an absolute risk reduction of 20% for death from any cause, resulting in a number needed to treat of 5 for 13 years [19]. As a result of these compelling data, guidelines from around the world support a multifactorial approach, with the Canadian Diabetes Association (CDA) guidelines [20] promoting the use of the “ABCDES” of vascular protection:
A – A1C target
B – Blood pressure target
C – Cholesterol target
D – Drugs for vascular protection
E – Exercise/Eating
S – Smoking cessation
Is any particular dietary pattern recommended?
There is a large and ever-growing number of dietary patterns that are marketed to improve weight and cardiovascular health. Unfortunately, however, few of these interventions have been studied rigorously, and most dietary interventions are found to be unsustainable in the long term. In the case of motivated patients, there are some specific dietary patterns with high-quality evidence to recommend them. The simplest intervention is the implementation of a vegetarian or vegan diet. Over 18 months, this has been shown to improve fasting glucose and cholesterol profile, and promote weight loss [21]. In another study, a calorie-restricted vegetarian diet led to a reduction in diabetes medication in 46% of participants (versus 5% with conventional diet) [22].
A Mediterranean diet is comprised of large amounts of fruits, vegetables, legumes, nuts, and whole grains. In addition, it includes moderate consumption of olive oil, dairy, fish and poultry, with low consumption of red meat. This dietary pattern has been extensively studied, and in a meta-analysis has been shown to improve glycemic control, blood pressure, and lipid profile [23]. The PREDIMED study evaluated the efficacy of 2 versions of the Mediterranean diet, one supplemented with olive oil or mixed nuts, for reducing cardiovascular events. This multicenter randomized control trial of 7447 participants at high cardiovascular risk (48.5% of whom had diabetes) was stopped early due to benefit. Both versions of the diet reduced cardiovascular events by 30% over 5 years of follow-up [8].
The Dietary Approaches to Stop Hypertension (DASH) diet is similar to the Mediterranean diet in focusing on fruits, vegetables, low-fat dairy, whole grains, nuts, fish, and poultry, while avoiding red meat. In addition, it explicitly recommends avoiding sweets and sweetened beverages, as well as dietary fat. In a trial of patients with diabetes matched for moderate sodium intake, the DASH diet has been shown to decrease A1c, blood pressure, and weight and improve lipid profile within 8 weeks [24,25].
In addition to these specific dietary patterns, specific foods have been shown to improve glycemic control and cardiovascular risk profile, including mixed unsalted nuts, almonds, dietary pulses, and low-glycemic versus high-glycemic index carbohydrates [26–31].
In accordance with CDA, American Diabetes Association (ADA), and European Association for the Study of Diabetes (EASD) guidelines, we recognize that a variety of diets can improve the cardiovascular risk profile of a patient [12,32,33]. Therefore, we suggest a tailored approach to dietary changes for each individual patient. This should, whenever possible, be undertaken with a registered dietitian, with emphasis placed on the evidence for vascular protection, improved risk profile, patient preference, and likelihood of long-term sustainability.
Should therapy for weight loss be recommended for this patient?
There are currently a number of effective strategies for achieving weight loss, including lifestyle interventions, pharmacotherapy, and surgery. The evidence base for dietary interventions for diabetes is reviewed above. The Look AHEAD study randomized 5145 overweight or obese patients with T2DM to intensive lifestyle intervention for weight loss through promotion of decreased caloric intake and increased physical activity, or usual diabetes support and education. After a median follow-up of 9.6 years, the study was stopped early on the basis of a futility analysis despite greater weight loss in the intervention group throughout the study. However, other benefits were derived including reduced need for medications, reduced sleep apnea, and improved well-being [7].
Pharmacotherapy agents for weight loss have been approved by various regulatory agencies. None has as yet shown a reduction in cardiovascular events. Therefore, these cannot be recommended as therapies for vascular protection at this time.
Bariatric surgery is an effective option for weight loss in patients with diabetes, with marked and sustained improvements in clinically meaningful outcomes when compared with medical management. The longest study of bariatric surgery is the Swedish Obesity Study, a prospective case-control study of 2010 obese patients who underwent bariatric surgery and 2037 matched controls. After a median of 14.8 years of follow-up, there was a reduction in overall mortality (hazard ratio [HR] 0.71) and decreased incidence of diabetes (HR 0.17), myocardial infarction (HR 0.71), and stroke (HR 0.66). Diabetes remission, defined as normal A1c off of anti-hyperglycemic therapy, was increased at 2 years (odds ratio [OR] 13.3) and sustained at 15 years (OR 6.3) [34–36]. Randomized controlled trials of bariatric surgery have thus far been small and do show some decreases in cardiovascular risk factors [37–40]. However, these have not yet been of sufficient duration or size to demonstrate a decrease in cardiovascular event rate. Although local policies may vary in referral recommendation, the Obesity Society, ADA, and CDA recommend that patients with a body mass index greater than 40 kg/m2, or greater than 35 kg/m2 with an obesity-related comorbidity such as diabetes, should be referred to a center that specializes in bariatric surgery for evaluation [41–43].
Case Continued
After the initial diagnosis, the patient was seen by a registered dietitian and followed a Mediterranean diet for some time but has since stopped. He is seen regularly for follow-up of his diabetes at 3- to 6-month intervals. He initially lost some weight but has unfortunately regained the weight. He tells you proudly that he finally quit smoking. He was started on metformin about 6 months after diagnosis to address his glycemic control. He continues on the metformin now as his only medication.
The patient returns to clinic for his usual follow-up visit approximately 5 years after initial diagnosis. He is feeling well with no new medical issues. He has no clinically apparent retinopathy or macrovascular complications. On examination, his blood pressure is 140/90 mm Hg and the remainder of the exam is unremarkable. His bloodwork shows an A1c of 8% and a low-density lipoprotein cholesterol (LDL-C) level of 124 mg/dL. His albumin-to-creatinine ratio is normal.
How often should cardiovascular risk be reassessed?
When should initiating pharmacotherapy to reduce risk in primary prevention be considered?
In the population with diabetes, statins and renin-angiotensin-aldosterone inhibition are the mainstays of pharmacotherapy for cardiovascular risk reduction. In the presence of clinical macrovascular disease, the standard of care includes both of these therapies. However, there is also a great deal of data that supports the use of these therapies for primary prevention.
Statins
Major studies on the benefits of statin therapy in people with diabetes have consistently shown decreased cardiovascular disease and mortality. The Heart Protection Study included a subgroup of patients with diabetes in which patients over the age of 40 were randomly assigned to simvastatin or placebo. Consistently across all subgroups, there was a relative risk reduction of 22% to 33% for the primary outcome of first cardiovascular event over 5 years. This effect was maintained even in those who did not have elevated LDL-C at randomization [46]. Similarly, the Collaborative Atorvastatin Diabetes Study (CARDS) randomized patients with T2DM, over age 40, with at least 1 other vascular risk factor to atorvastatin 10 mg or placebo. They found a 37% risk reduction in time to first event over 4 years with atorvastatin, with consistent results across all subgroups [47].
Based on these studies, it is recommended that all patients with diabetes be placed on statin therapy to reduce vascular risk at age 40 years (CDA, ADA, American College of Cardiology/American Heart Association [ACC/AHA]) [20,45,48]. If under age 40 years, statin therapy should be considered in the presence of other risk factors (ADA, ACC/AHA) [45,48], or if diabetes duration is more than 15 years and age is greater than 30 years, or there are micro- or macrovascular complications (CDA) [20].
Renin-Angiotensin-Aldosterone Inhibition
Similar to research into statin therapy, a considerable amount of research has been dedicated to renin-angiotensin-aldosterone system (RAAS) blockade for the primary purpose of vascular risk reduction, even in the absence of hypertension, in those with diabetes. In a prespecified substudy of the Heart Outcomes Prevention Evaluation (HOPE) trial, known as MICRO HOPE, patients with diabetes who were older than 55 years of age, with at least 1 other cardiovascular risk factor, were randomized to receive ramipril 10 mg daily or placebo. In this study, ramipril reduced the risk for myocardial infarction (22%), stroke (33%), cardiovascular death (37%), and all-cause mortality (24%) over 4.5 years [49]. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), patients at high risk for cardiovascular disease were randomized to telmisartan 80 mg or ramipril 10 mg. In the diabetes subgroup, there were similar risk reductions and no statistical difference between the groups [50]. A 2012 meta-analysis assessed the benefits of RAAS blockade compared with placebo for primary prevention in high-risk individuals, or secondary prevention in those with established vascular disease. A reduction in cardiovascular death, all-cause mortality, fatal or nonfatal myocardial infarction, and stroke was seen across all subgroups, including those with and without diabetes or hypertension [51].
The CDA currently recommends that an ACE inhibitor or ARB be given to all patients with diabetes who are 55 years of age or older, or have macro- or microvascular disease, for the primary purpose of decreasing risk for vascular disease, even in the absence of hypertension. An agent and dose with proven vascular protective benefit should be chosen when selecting an ACE inhibitor or ARB [20].
Should this patient start ASA therapy?
Whether to start daily low-dose ASA for primary prevention of coronary artery disease has been a long-standing question in patients with diabetes. The benefits of ASA therapy with regards to coronary artery disease have long been known from a secondary prevention standpoint, and given the low risk and long experience, primary prevention seemed reasonable. However, no high-quality randomized controlled trials enrolling large numbers of patients with diabetes have been performed in the current era of medical therapy, specifically in the era of widespread statin use. The initial studies examining ASA use in primary prevention were analyzed in a meta-analysis in 1994 and showed a trend towards benefit for ASA in patients with diabetes [52]. Further trials increased the number of diabetes patient-years studied but did not change the initial result. Five meta-analyses have been conducted on the currently available trials, and all but one do not show a significant reduction in coronary artery disease or stroke in patients with diabetes [53–57]. In addition, ASA is known to cause a small absolute increase in the risk for gastrointestinal hemorrhage that is consistent across all studies, with a number needed to harm of approximately 100 over 2.5 years. Therefore, the possible small absolute benefit that was seen in ASA trials with regards to coronary artery disease in the era before statin therapy must be weighed against the known risk of bleeding. Because of this, the CDA and European Society of Cardiology have recommendations against the routine use of ASA for primary prevention in patients with diabetes [12,20].
A summary of pharmacotherapy for cardiovascular risk reduction is shown in the Figure.
Case Continued
The patient is started on a statin because of his elevated LDL-C level in the context of being over the age of 40 years with T2DM. He is also started on an ACE inhibitor to address the hypertension. In addition, a dipeptidyl peptidase-4 inhibitor is added to his metformin to address the elevated A1c. He continues to follow up every 3 to 6 months.
Six years later, he experiences an episode of retrosternal chest discomfort while exercising. He is brought to hospital and is found to have a non-ST elevation myocardial infarction. He is admitted to hospital, undergoes percutaneous revascularization of a single lesion, and is discharged to a rehabilitation center. He is discharged on aspirin, clopidogrel, an ACE inhibitor, a beta blocker, and a high-intensity statin. His blood pressure is well managed, and he has lost further weight since he was last seen. When he returns to clinic, he wonders if there is anything more he can do to prevent further events.
What secondary prevention of cardiovascular disease is recommended for patients with T2DM?
Optimal secondary prevention following a major vascular event includes a combination of pharmacologic and nonpharmacologic interventions. In the population without diabetes, evidence supports smoking cessation, exercise, cardiac-specific rehabilitation, antiplatelets, RAAS antag-onists, beta-blockade, and statins. Most of the trials that led to this standard suite of interventions had large diabetes subgroups. Therefore, there is no difference in the secondary prevention of cardiovascular disease in the population with diabetes with regard to these interventions.
Have any antihyperglycemic agents been shown to reduce cardiovascular events?
Metformin
A summary of the vascular effects observed during trials of antihyperglycemic agents is shown in Table 3.
Empagliflozin
Many large randomized, controlled cardiovascular outcome trials have been completed or are ongoing looking at the cardiovascular safety of newer antihyperglycemic agents. The majority of the completed trials have shown a neutral effect, suggesting that the agents are safe. However, in September 2015, the first cardiovascular outcome trial of an antihyperglycemic agent with a positive result was published. The Empagliflozin Cardiovascular Outcome Event Trial (EMPA-REG OUTCOME) randomized 7020 patients with T2DM and cardiovascular disease (defined as nonacute myocardial infarction, multivessel coronary artery disease, unstable angina, nonacute stroke, or occlusive peripheral arterial disease) to placebo or 1 of 2 doses of empagliflozin. The primary outcome of cardiovascular mortality, nonfatal myocardial infarction, or stroke was reduced by 14% in the empagliflozin-treated group. Key secondary outcomes of all-cause mortality (HR 0.68) and heart failure hospitalization (HR 0.65) were also statistically different in favor of the empagliflozin arm [63].
On the basis of this trial’s results, empagliflozin should be considered for treatment of all patients with type 2 diabetes and known cardiovascular disease. It is as yet unknown whether this effect will translate to the other members of the sodium-glucose co-transporter 2 (SGLT-2) inhibitor class, although results of studies involving other SGLT-2 inhibitors are expected in the next 2 to 3 years.
Liraglutide
In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial reported results of its cardiovascular safety trial. In this trial, 9340 patients with either established vascular disease or risk factors for vascular disease were randomized to daily liraglutide or placebo injections. The primary composite outcome of cardiovascular death, nonfatal myocardial infarction, or stroke was reduced by 13%. A key secondary outcome of all-cause mortality also showed a significant reduction (HR 0.85). There was no reduction in hospitalization for heart failure [64].
Semaglutide
Most recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was completed, assessing cardiovascular safety of a once-weekly injectable glucagon-like peptide-1 (GLP-1) analogue. This noninferiority trial studied 3297 patients with type 2 diabetes over the age of 50 years with established macrovascular disease, chronic heart failure, or chronic kidney disease (stage III or higher), or over the age of 60 years with at least 1 other cardiovascular risk factor. The patients were randomized to 1 of 2 doses of once-weekly semaglutide or placebo injection. A composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was decreased by 26% in the pooled semaglutide group. This was driven primarily by a reduction in nonfatal stroke, with no statistically significant reduction in nonfatal myocardial infarction or cardiovascular mortality. Significant secondary outcomes showed a reduction in new or worsening nephropathy (HR 0.64), and an unexpected increase in retinopathy (HR 1.76) [65].
All of these trials utilized their respective agents as add-on to existing antihyperglycemic therapy. Therefore, first-line antihyperglycemic therapy in a patient with T2DM remains metformin. For the patient with established vascular disease or who is at high risk for developing vascular disease, add-on therapy using an antihyperglycemic agent with proven cardiovascular benefits, such as empagliflozin or liraglutide, is suggested [9,11]. Semaglutide is not yet available for clinical use. The choice between these agents should be based on patient preference, cost, side effect profile, and absence of contraindications.
Currently, there are more studies underway with similar designs with different agents. As these studies are reported in the upcoming years, it is hoped that the options for reduction of cardiovascular risk will increase, and that we will have multiple antihyperglycemic agents that will provide not only glycemic benefit but also cardiovascular risk reduction.
Case Conclusion
The patient continues to abstain from smoking. He follows up with a dietitian and is enrolled in an exercise program. He remains on his cardiac medications. For glycemic control, he continues on his previous antihyperglycemic therapy and an antihyperglycemic agent with proven cardiovascular benefit is added. With these interventions, he understands that his risk is mitigated, but given his history and previous event, he remains at high risk for future vascular disease.
Conclusion
The care of a patient with diabetes requires a multifactorial approach. All patients are at risk for developing the vascular complications of diabetes, and it is these complications that ultimately result in the nearly doubled risk of mortality in patients with diabetes. Various trials have shown that targeted interventions can and do reduce the risk for cardiovascular disease in a measurable way. Above and beyond targeted interventions, we now know that strict multifactorial interventions can result in a clinically significant reduction in both mortality and cardiovascular disease. This multifactorial approach is supported by guidelines around the world [12,44,45]. A standardized approach to the assessment of risk and the application of interventions is critical. More recent data show that specific antihyperglycemic therapies can also reduce cardiovascular events above and beyond their glycemic effects. The rates of cardiovascular events in patients with diabetes have declined over time, and hopefully this trend will continue as further research supports additional interventions.
Corresponding author: Bikrampal S. Sidhu, MD, Toronto General Hospital, 200 Elizabeth St., 12 EN 242, Toronto, ON, M5G 2C4, bikrampal.sidhu@mail.utoronto.ca.
Financial disclosures: Dr. Cheng has received fees for speaking and/or consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, Novo Nordisk, Sanofi, Servier, and Takeda.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.
2. Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23.
3. Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36.
4. Stevens RJ, Kothari V, Alder A, et al. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–9.
5. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
6. Solberg L, Desai JR, O’Connor PJ, et al. Diabetic patients who smoke: are they different? Diabetes Care 1999;22:1887–98.
7. Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
8. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Pharmacologic management of type 2 diabetes: November 2016 Interim Update. Can J Diabetes 2016;40:484–6.
10. Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes 2013; 37:S61–S68.
11. American Diabetes Association. Pharmacologic approaches to glycemic management. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S64–74.
12. The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013;34:3035–87.
13. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65.
14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
15. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–98.
16. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
17. ACCORD Study Group. Nine-year rffects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39:701–8.
18. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93.
19. Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91.
20. Stone JA, Fitchett D, Grover S, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Vascular protection in people with diabetes. Can J Diabetes 2013;37 (suppl 1):S100–S104.
21. Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588–96.
22. Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–59.
23. Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102.
24. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
25. Azadbakht L, Fard NR, Karimi M, et al. The Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
26. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
27. Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
28. Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
29. Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia 2009;52:1479–95.
30. Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11.
31. Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9.
32. Dworatzek PD, Arcudi K, Gougeon R, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2013;37(suppl 1):S45–55.
33. American Diabetes Association. Lifestyle Management. Sec. 4. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S33–43.
34. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357:741–52.
35. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
36. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–7.
37. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–23.
38. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
39. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
40. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
41. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and
The Obesity Society. Obesity (Silver Spring) 2014;22:S1–S410.
42. Wharton S, Sharma AM, Lau DCW. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Weight management in diabetes. Can J Diabetes 2013;37(suppl 1):S82–6.
43. American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S57–63.
44. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1–S212.
45. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(suppl 1):S1–S135.
46. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet 2003;361:2005–16.
47. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934.
49. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–9.
50. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
51. McAlister FA, Renin Angiotensin System Modulator Meta-Analysis Investigators. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta-analysis of randomized trials. Eur Heart J 2012;33:505–14.
52. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106.
53. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60.
54. Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care 2009;32:2300–6.
55. De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009;339:b4531.
56. Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2010;87:211–8.
57. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395–402.
58. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomised clinical trial. JAMA 2014;312:2510–20.
59. ASCEND Trial Web site. Clinical Trials Service Unit, University of Oxford, and British Heart Foundation. https://ascend.medsci.ox.ac.uk/. Accessed September 20, 2016.
60. De Berardis G, Sacco M, Evangelista V, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 2007;8:21.
61. Maruther NM, Tseng E, Hutfless SS, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
62. Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001204.
63. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
64. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
65. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44
66. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
67. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
68. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
69. Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57.
Effect of PCSK9 Inhibitors on Coronary Artery Disease Progression
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Nicolls SJ, Puri S, Anderson, T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA 2016;316:2372–84.
To Download a PDF of the Full Article:
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
References
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.