User login
CMA report reveals successes and shortcomings

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program. ![]()

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program. ![]()

The European Medicines Agency (EMA) has released a report showing both successes and room for improvement regarding conditional marketing authorizations (CMAs).
CMA is one of the tools available to regulators to support the development of and early access to drugs that address unmet medical needs of patients in the European Union.
Drugs are granted CMA if the public health benefit of their immediate availability is thought to outweigh the risk of an authorization on the basis of less comprehensive data than normally required.
A CMA is valid for 1 year. As part of the authorization, the drug’s developer is obliged to carry out further studies to obtain complete data.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) assesses the data generated by these specific post-authorization obligations at least annually to ensure the balance of benefits and risks of the drug continues to remain positive.
At the end of its assessment, the CHMP issues a recommendation regarding the renewal of the CMA or its conversion into a standard marketing authorization.
Overview
The EMA’s report summarizes the experience with CMAs from the first use of this authorization type in 2006 until June 30, 2016.
During this time, a total of 30 drugs have received a CMA, including several
hematology drugs—Adcetris (brentuximab vedotin),
Arzerra (ofatumumab), Blincyto (blinatumomab), Bosulif (bosutinib), Darzalex (daratumumab), and Pixuvri (pixantrone).
Eleven CMAs have been converted into standard marketing authorizations (including Arzerra’s CMA), 2 have been withdrawn for commercial reasons, and 17 are still conditional authorizations.
None of the drugs that still have CMAs have been authorized for more than 5 years. And none of the CMAs issued since 2006 have had to be revoked or suspended.
Successes
According to the EMA’s analysis, marketing authorization holders comply with the specific obligations imposed by the agency.
More than 90% of completed specific obligations did not result in major changes of scope, and about 70% of specific obligations did not require an extension to the originally specified timelines.
The report shows that it took an average of 4 years to generate the additional data needed and to convert a CMA into a full marketing authorization.
This suggests patients with life-threatening or seriously debilitating conditions had access to promising drugs much earlier than they would have under standard authorization.
Areas for improvement
The EMA’s analysis also revealed room for improvement.
The report showed that, relatively frequently, CMA was first
considered only during the assessment of the drug application, which meant granting a CMA took longer than intended.
Therefore, the EMA recommends that drug developers engage in early dialogue with the EMA
and prospectively plan to apply for a CMA.
The agency said this should support
prompt assessment of such applications and could also facilitate prompt
completion of additional studies and timely availability of
comprehensive data.
The EMA said another area for improvement is engaging other stakeholders involved in bringing drugs to patients—in particular, Health Technology Assessment bodies—to facilitate the generation of all data needed for decision-making through one development program. ![]()
Iron-fortified nutrition bars combat anemia in India

Consuming an iron-fortified nutrition bar daily for a few months can fight anemia without producing side effects, according to research published in the American Journal of Clinical Nutrition.
Anemic
women in India who consumed an iron-fortified nutrition bar each day for 90 days
were much more likely to experience increases in hemoglobin and
hematocrit and to be cured of their anemia than women who did not consume
such bars.
Rajvi Mehta, a medical student at Duke University in Durham, North Carolina, developed the nutrition bars used in this study, known as GudNesS bars.
The bars are made with iron-rich, natural, local (to India), and culturally accepted ingredients. They contain the World Health Organization’s daily recommended dose of iron.
In 2011, Mehta worked with nutritionists and physicians in India to establish a social venture there called Let’s Be Well Red (LBWR) to begin large-scale production of the bars.
The study, conducted from March to August 2014 in Mumbai and Navi Mumbai, India, involved 179 anemic, non-pregnant participants of reproductive age (18-35) at 10 demographically diverse sites.
The sites were randomly placed in either a control group or an intervention group.
Women in the intervention group received 1 iron-fortified nutrition bar (containing 14 mg Fe) daily for 90 days, and women in the control group received nothing. Baseline characteristics were comparable between the groups.
Each group underwent 3 blood tests during the 90-day follow-up period—at 15 days, 45 days, and 90 days—to measure their hemoglobin and hematocrit.
Seventy-six percent of subjects (n=136) completed all follow-up assessments (65 intervention and 71 control subjects).
The primary outcomes were 90-day changes from baseline in hemoglobin concentrations and hematocrit percentages.
The researchers said the mean hemoglobin and hematocrit increases after 90 days were greater for the intervention group than the control group, at 1.4 g/dL and 2.7%, respectively.
And subjects in the intervention group had a much greater decrease in anemia than those in the control group. At 90 days, 29.2% of subjects in the intervention group still had anemia, compared to 98.6% of those in the control group. The odds ratio was 0.007.
The researchers said no side effects were reported.
“We are encouraged by the results of this study, which show a positive connection between consuming an iron-fortified nutrition bar and a reduction in anemia prevalence,” said study author Elizabeth Turner, PhD, of Duke University.
“It appears to be a practical and well-tolerated solution to a significant health challenge in India.”
Let’s Be Well Red is currently operating in 3 locations in India and produces 100,000 bars each year that it distributes throughout the country.
“Anemia is a debilitating condition that can have severe health consequences,” Mehta said. “I am thrilled that my colleagues and I were able to develop a solution that has proven to be effective among a high-risk population. Making an impact in global health has long been a goal of mine.” ![]()

Consuming an iron-fortified nutrition bar daily for a few months can fight anemia without producing side effects, according to research published in the American Journal of Clinical Nutrition.
Anemic
women in India who consumed an iron-fortified nutrition bar each day for 90 days
were much more likely to experience increases in hemoglobin and
hematocrit and to be cured of their anemia than women who did not consume
such bars.
Rajvi Mehta, a medical student at Duke University in Durham, North Carolina, developed the nutrition bars used in this study, known as GudNesS bars.
The bars are made with iron-rich, natural, local (to India), and culturally accepted ingredients. They contain the World Health Organization’s daily recommended dose of iron.
In 2011, Mehta worked with nutritionists and physicians in India to establish a social venture there called Let’s Be Well Red (LBWR) to begin large-scale production of the bars.
The study, conducted from March to August 2014 in Mumbai and Navi Mumbai, India, involved 179 anemic, non-pregnant participants of reproductive age (18-35) at 10 demographically diverse sites.
The sites were randomly placed in either a control group or an intervention group.
Women in the intervention group received 1 iron-fortified nutrition bar (containing 14 mg Fe) daily for 90 days, and women in the control group received nothing. Baseline characteristics were comparable between the groups.
Each group underwent 3 blood tests during the 90-day follow-up period—at 15 days, 45 days, and 90 days—to measure their hemoglobin and hematocrit.
Seventy-six percent of subjects (n=136) completed all follow-up assessments (65 intervention and 71 control subjects).
The primary outcomes were 90-day changes from baseline in hemoglobin concentrations and hematocrit percentages.
The researchers said the mean hemoglobin and hematocrit increases after 90 days were greater for the intervention group than the control group, at 1.4 g/dL and 2.7%, respectively.
And subjects in the intervention group had a much greater decrease in anemia than those in the control group. At 90 days, 29.2% of subjects in the intervention group still had anemia, compared to 98.6% of those in the control group. The odds ratio was 0.007.
The researchers said no side effects were reported.
“We are encouraged by the results of this study, which show a positive connection between consuming an iron-fortified nutrition bar and a reduction in anemia prevalence,” said study author Elizabeth Turner, PhD, of Duke University.
“It appears to be a practical and well-tolerated solution to a significant health challenge in India.”
Let’s Be Well Red is currently operating in 3 locations in India and produces 100,000 bars each year that it distributes throughout the country.
“Anemia is a debilitating condition that can have severe health consequences,” Mehta said. “I am thrilled that my colleagues and I were able to develop a solution that has proven to be effective among a high-risk population. Making an impact in global health has long been a goal of mine.” ![]()

Consuming an iron-fortified nutrition bar daily for a few months can fight anemia without producing side effects, according to research published in the American Journal of Clinical Nutrition.
Anemic
women in India who consumed an iron-fortified nutrition bar each day for 90 days
were much more likely to experience increases in hemoglobin and
hematocrit and to be cured of their anemia than women who did not consume
such bars.
Rajvi Mehta, a medical student at Duke University in Durham, North Carolina, developed the nutrition bars used in this study, known as GudNesS bars.
The bars are made with iron-rich, natural, local (to India), and culturally accepted ingredients. They contain the World Health Organization’s daily recommended dose of iron.
In 2011, Mehta worked with nutritionists and physicians in India to establish a social venture there called Let’s Be Well Red (LBWR) to begin large-scale production of the bars.
The study, conducted from March to August 2014 in Mumbai and Navi Mumbai, India, involved 179 anemic, non-pregnant participants of reproductive age (18-35) at 10 demographically diverse sites.
The sites were randomly placed in either a control group or an intervention group.
Women in the intervention group received 1 iron-fortified nutrition bar (containing 14 mg Fe) daily for 90 days, and women in the control group received nothing. Baseline characteristics were comparable between the groups.
Each group underwent 3 blood tests during the 90-day follow-up period—at 15 days, 45 days, and 90 days—to measure their hemoglobin and hematocrit.
Seventy-six percent of subjects (n=136) completed all follow-up assessments (65 intervention and 71 control subjects).
The primary outcomes were 90-day changes from baseline in hemoglobin concentrations and hematocrit percentages.
The researchers said the mean hemoglobin and hematocrit increases after 90 days were greater for the intervention group than the control group, at 1.4 g/dL and 2.7%, respectively.
And subjects in the intervention group had a much greater decrease in anemia than those in the control group. At 90 days, 29.2% of subjects in the intervention group still had anemia, compared to 98.6% of those in the control group. The odds ratio was 0.007.
The researchers said no side effects were reported.
“We are encouraged by the results of this study, which show a positive connection between consuming an iron-fortified nutrition bar and a reduction in anemia prevalence,” said study author Elizabeth Turner, PhD, of Duke University.
“It appears to be a practical and well-tolerated solution to a significant health challenge in India.”
Let’s Be Well Red is currently operating in 3 locations in India and produces 100,000 bars each year that it distributes throughout the country.
“Anemia is a debilitating condition that can have severe health consequences,” Mehta said. “I am thrilled that my colleagues and I were able to develop a solution that has proven to be effective among a high-risk population. Making an impact in global health has long been a goal of mine.” ![]()
New recommendations for pediatric AMKL
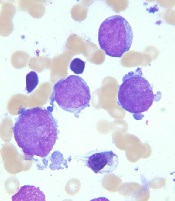
Image courtesy of St. Jude
Children’s Research Hospital
and Tina Motroni
Research has revealed genetic alterations that may prove useful for predicting treatment outcomes in pediatric patients with acute megakaryoblastic leukemia (AMKL) who do not have Down syndrome.
The study suggests that 3
genetic alterations can be used to identify high-risk patients who may benefit
from allogeneic stem cell transplant, and 1 alteration may identify
low-risk patients who require less chemotherapy than their peers.
Researchers said these findings, published in Nature Genetics, support revised diagnostic screening and treatment recommendations for pediatric AMKL.
“Because long-term survival for pediatric AMKL patients without Down syndrome is poor, just 14% to 34%, the standard recommendation by many pediatric oncologists has been to treat all patients with allogeneic stem cell transplantation during their first remission,” said study author Tanja Gruber, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“In this study, we identified several genetic alterations that are important predictors of treatment success. All newly identified pediatric AMKL patients without Down syndrome should be screened for these prognostic indicators at diagnosis. The results will help identify which patients need allogeneic stem cell transplants during their first remission and which do not.”
This study involved next-generation sequencing of the whole exome or RNA of 99 AMKL patients without Down syndrome (75 pediatric and 24 adult patients). Along with the sequencing data, researchers analyzed patients’ gene expression and long-term survival.
Results
The results showed that non-Down syndrome pediatric AMKL can be divided into 7 subgroups based on the underlying genetic alteration, pattern of gene expression, and treatment outcome.
The subgroups include the newly identified HOX subgroup. About 15% of the pediatric patients in this study were in the HOX subgroup, which is characterized by several different HOX fusion genes.
The researchers also identified cooperating mutations that help fuel AMKL in different subgroups. The cooperating mutations include changes in the RB1 gene and recurring mutations in the RAS and JAK pathways.
In addition, the study showed that 3 genetic alterations—CBFA2T3-GLIS2, KMT2A rearrangements, and NUP98-KDM5A—are associated with reduced survival in pediatric AMKL subtypes.
The researchers said patients with these alterations may benefit from allogeneic stem cell transplants, so non-Down syndrome pediatric AMKL patients should be screened for these alterations at diagnosis.
The team also recommended testing AMKL patients for mutations in the GATA1 gene. GATA1 mutations are a hallmark of AMKL in children with Down syndrome, who almost always survive the leukemia. In this study, AMKL patients with GATA1 mutations and no fusion gene had the same favorable outcomes.
“The results raise the possibility that pediatric AMKL patients without Down syndrome who have mutations in GATA1 may benefit from the same reduced chemotherapy used to treat the leukemia in patients with Down syndrome,” Dr Gruber said.
These revised diagnostic screening and treatment recommendations are being implemented at St. Jude.
This study also showed that adults with AMKL lacked recurrent fusion genes. The most common mutations found in the adult patients were in TP53 (21%), cohesin genes (17%), splicing factor genes (17%), ASXL genes (17%), and DNMT3A (13%). About 4% had GATA1 mutations. ![]()

Image courtesy of St. Jude
Children’s Research Hospital
and Tina Motroni
Research has revealed genetic alterations that may prove useful for predicting treatment outcomes in pediatric patients with acute megakaryoblastic leukemia (AMKL) who do not have Down syndrome.
The study suggests that 3
genetic alterations can be used to identify high-risk patients who may benefit
from allogeneic stem cell transplant, and 1 alteration may identify
low-risk patients who require less chemotherapy than their peers.
Researchers said these findings, published in Nature Genetics, support revised diagnostic screening and treatment recommendations for pediatric AMKL.
“Because long-term survival for pediatric AMKL patients without Down syndrome is poor, just 14% to 34%, the standard recommendation by many pediatric oncologists has been to treat all patients with allogeneic stem cell transplantation during their first remission,” said study author Tanja Gruber, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“In this study, we identified several genetic alterations that are important predictors of treatment success. All newly identified pediatric AMKL patients without Down syndrome should be screened for these prognostic indicators at diagnosis. The results will help identify which patients need allogeneic stem cell transplants during their first remission and which do not.”
This study involved next-generation sequencing of the whole exome or RNA of 99 AMKL patients without Down syndrome (75 pediatric and 24 adult patients). Along with the sequencing data, researchers analyzed patients’ gene expression and long-term survival.
Results
The results showed that non-Down syndrome pediatric AMKL can be divided into 7 subgroups based on the underlying genetic alteration, pattern of gene expression, and treatment outcome.
The subgroups include the newly identified HOX subgroup. About 15% of the pediatric patients in this study were in the HOX subgroup, which is characterized by several different HOX fusion genes.
The researchers also identified cooperating mutations that help fuel AMKL in different subgroups. The cooperating mutations include changes in the RB1 gene and recurring mutations in the RAS and JAK pathways.
In addition, the study showed that 3 genetic alterations—CBFA2T3-GLIS2, KMT2A rearrangements, and NUP98-KDM5A—are associated with reduced survival in pediatric AMKL subtypes.
The researchers said patients with these alterations may benefit from allogeneic stem cell transplants, so non-Down syndrome pediatric AMKL patients should be screened for these alterations at diagnosis.
The team also recommended testing AMKL patients for mutations in the GATA1 gene. GATA1 mutations are a hallmark of AMKL in children with Down syndrome, who almost always survive the leukemia. In this study, AMKL patients with GATA1 mutations and no fusion gene had the same favorable outcomes.
“The results raise the possibility that pediatric AMKL patients without Down syndrome who have mutations in GATA1 may benefit from the same reduced chemotherapy used to treat the leukemia in patients with Down syndrome,” Dr Gruber said.
These revised diagnostic screening and treatment recommendations are being implemented at St. Jude.
This study also showed that adults with AMKL lacked recurrent fusion genes. The most common mutations found in the adult patients were in TP53 (21%), cohesin genes (17%), splicing factor genes (17%), ASXL genes (17%), and DNMT3A (13%). About 4% had GATA1 mutations. ![]()

Image courtesy of St. Jude
Children’s Research Hospital
and Tina Motroni
Research has revealed genetic alterations that may prove useful for predicting treatment outcomes in pediatric patients with acute megakaryoblastic leukemia (AMKL) who do not have Down syndrome.
The study suggests that 3
genetic alterations can be used to identify high-risk patients who may benefit
from allogeneic stem cell transplant, and 1 alteration may identify
low-risk patients who require less chemotherapy than their peers.
Researchers said these findings, published in Nature Genetics, support revised diagnostic screening and treatment recommendations for pediatric AMKL.
“Because long-term survival for pediatric AMKL patients without Down syndrome is poor, just 14% to 34%, the standard recommendation by many pediatric oncologists has been to treat all patients with allogeneic stem cell transplantation during their first remission,” said study author Tanja Gruber, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“In this study, we identified several genetic alterations that are important predictors of treatment success. All newly identified pediatric AMKL patients without Down syndrome should be screened for these prognostic indicators at diagnosis. The results will help identify which patients need allogeneic stem cell transplants during their first remission and which do not.”
This study involved next-generation sequencing of the whole exome or RNA of 99 AMKL patients without Down syndrome (75 pediatric and 24 adult patients). Along with the sequencing data, researchers analyzed patients’ gene expression and long-term survival.
Results
The results showed that non-Down syndrome pediatric AMKL can be divided into 7 subgroups based on the underlying genetic alteration, pattern of gene expression, and treatment outcome.
The subgroups include the newly identified HOX subgroup. About 15% of the pediatric patients in this study were in the HOX subgroup, which is characterized by several different HOX fusion genes.
The researchers also identified cooperating mutations that help fuel AMKL in different subgroups. The cooperating mutations include changes in the RB1 gene and recurring mutations in the RAS and JAK pathways.
In addition, the study showed that 3 genetic alterations—CBFA2T3-GLIS2, KMT2A rearrangements, and NUP98-KDM5A—are associated with reduced survival in pediatric AMKL subtypes.
The researchers said patients with these alterations may benefit from allogeneic stem cell transplants, so non-Down syndrome pediatric AMKL patients should be screened for these alterations at diagnosis.
The team also recommended testing AMKL patients for mutations in the GATA1 gene. GATA1 mutations are a hallmark of AMKL in children with Down syndrome, who almost always survive the leukemia. In this study, AMKL patients with GATA1 mutations and no fusion gene had the same favorable outcomes.
“The results raise the possibility that pediatric AMKL patients without Down syndrome who have mutations in GATA1 may benefit from the same reduced chemotherapy used to treat the leukemia in patients with Down syndrome,” Dr Gruber said.
These revised diagnostic screening and treatment recommendations are being implemented at St. Jude.
This study also showed that adults with AMKL lacked recurrent fusion genes. The most common mutations found in the adult patients were in TP53 (21%), cohesin genes (17%), splicing factor genes (17%), ASXL genes (17%), and DNMT3A (13%). About 4% had GATA1 mutations. ![]()
What Do Parasites Have to Do With Leukemia?
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Treat Hypothyroid Conditions
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Finding the Sweet Spot: The Diabetic Kidney
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dabigatran has less bleeding than rivaroxaban in atrial fibrillation
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Unique microbiota mix found in guts of T1D patients
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: Patients with type 1 diabetes (T1D) show signs of unique inflammation and microbiota in the duodenal mucosa, compared with controls and celiac patients.
Major finding: T1D patients had a “peculiar” inflammation signature and a unique microbiota composition, and there’s a sign of a link between inflammation and bacteria levels.
Data source: An analysis of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls.
Disclosures: The study was supported by institutional funds, and the authors report no relevant disclosures.
Experts say don’t SPRINT to adopt low blood pressure target
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.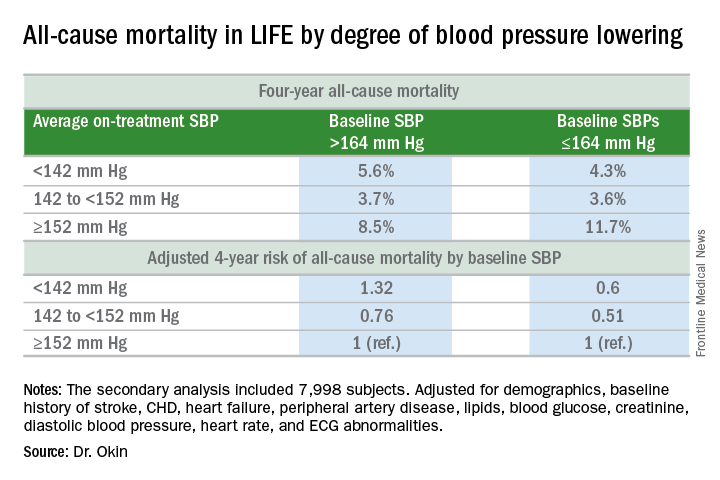
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Deferring RT for brain mets in EGFR-mutated NSCLC shortens survival
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Deferring radiotherapy to administer EGFR tyrosine kinase inhibitors first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer.
Major finding: At 2 years, overall survival rates were 51% with upfront EGFR TKIs, 62% with initial whole-brain radiotherapy, and 78% with stereotactic radiosurgery.
Data source: A retrospective multicenter pooled analysis of survival data for 351 patients with NSCLC brain metastases followed for a median of 22 months.
Disclosures: No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.



