User login
Repeal and replace: House bills offer potential road maps
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.
As Republicans dig in to make good on their promise to repeal the Affordable Care Act, hints at what an eventual replacement may look like can be gleaned from legislation previously introduced in the House of Representatives.
One model could be the Empowering Patients First Act (H.R. 2300), sponsored by Rep. Tom Price (R-Ga.), a retired orthopedic surgeon and President-elect Trump’s nominee to head the Health and Human Services department. That legislation offers up a number of the usual GOP proposals related to health care, including refundable tax credits for low-income individuals buying insurance on the individual market; federal grants for states to provide health coverage through a high-risk pool, a reinsurance pool, or other mechanism to help subsidize the purchase of insurance; allowing individuals to purchase insurance through individual member associations; allowing small business owners to purchase insurance for their families and employees across state lines through their trade associations; and allowing insurance companies to sell coverage across state lines.
Another model can be found in House Republican’s health reform plan, called “A Better Way.”
The plan includes a number of provisions similar to those in Dr. Price’s plan, such as expanding consumer-directed health care options, allowing sale of insurance across state lines, expanding opportunities for pooling, and bringing about medical liability reform.
It also increases health insurance portability, helps to preserve the employer-sponsored insurance market, preserves wellness programs, promotes greater use of health savings accounts, and provides for greater opportunities to contribute and use them.
The Better Way plan maintains a few of the popular aspects of the Affordable Care Act, including a ban on coverage denial for preexisting conditions and the ability to keep adult children on parents’ health insurance up to age 26 years, in certain situations.
In addition, the plan would roll back a premium adjustment for older patients. The ACA mandates that premiums for older individuals could be no more than three times that of a younger enrollee. Prior to the ACA, the GOP plan notes that it was generally a limit of five times that of a younger enrollee, and the GOP vision is to bring that limit back.
“The ill-advised three-to-one policy is leading to artificially higher premiums for millions of Americans, especially younger and healthier patients,” according to the GOP plan.
The plan aims to repeal several ACA provisions including the Independent Payment Advisory Board, the Center for Medicare & Medicaid Innovations, and the ban on physician-owned hospitals. It would also extend value-based insurance design to Medicare Advantage, combine Medicare Parts A & B, and reform to uncompensated care.
President-elect Trump also gave hints as to what might be contained in a health reform plan he is formulating. In a Jan. 14 interview with the Washington Post, Mr. Trump said his plan aims to provide “insurance for all” while requiring drug manufacturers to negotiate with Medicare and Medicaid on pricing.
Prolonged work-related stress linked to NHL, other cancers in men

burning building in Quebec
Photo by Sylvain Pedneault
New research suggests that prolonged exposure to work-related stress may increase a man’s risk of several cancers.
The study showed a significant association between work-related stress lasting 15 years or more and non-Hodgkin lymphoma (NHL) as well as lung, colon, rectal, and stomach cancers.
Men who had worked as firefighters, engineers, mechanics, and repair workers were most likely to report work-related stress.
Marie-Élise Parent, PhD, of Institut national de la recherche scientifique (INRS) in Laval, Quebec, Canada, and her colleagues conducted this research and published
the results in Preventive Medicine.
The researchers studied 3103 men with 11 different types of cancer who were diagnosed from 1979 to 1985. The team compared these men to 512 control subjects from the general population.
Both cases and controls were interviewed and asked to describe each job they had during their lifetime, including the occurrence of stress related to a job and the reason for that stress.
The researchers then calculated odds ratios (OR) for the association between perceived workplace stress and its duration, and each cancer site. The analyses were adjusted for lifestyle and occupational factors.
The team found that having at least one stressful job in a lifetime was associated with increased odds of 5 cancers:
- Lung—OR=1.33
- Colon—OR=1.51
- Bladder—OR=1.37
- Rectal—OR=1.52
- Stomach—OR=1.53.
When the researchers looked at the duration of stress, they found no significant association between any of the cancers and work-related stress lasting less than 15 years.
However, there were significant associations for several cancers and work-related stress lasting 15 to 30 years or more than 30 years. These included:
- NHL—15-30 years, OR=1.47; >30 years, OR=1.69 (P=0.02)
- Lung cancer—15-30 years, OR=1.47; >30 years, OR=1.51 (P=0.01)
- Colon cancer—15-30 years, OR=1.32; >30 years, OR=1.64 (P<0.01)
- Rectal cancer—15-30 years, OR=1.84; >30 years, OR=1.48 (P=0.01)
- Stomach cancer—15-30 years, OR=2.15; >30 years, OR=1.48 (P=0.01).
The occupations with the highest prevalence of work-related stress were firefighter (40% of firefighting jobs reported as stressful), industrial and aerospace engineer (31%), and motor vehicle and rail transport mechanic/repair worker (28%).
For the same individual, stress varied depending on the job held.
The study also showed that perceived stress was not limited to high work load and time constraints. Customer service, sales commissions, responsibilities, having an anxious temperament, job insecurity, financial problems, challenging or dangerous work conditions, employee supervision, interpersonal conflict, and a difficult commute were all sources of stress listed by study participants.
The researchers said one of the biggest flaws in previous studies of this kind is that none of them assessed work-related stress over a full working lifetime. The team said this made it impossible to determine how the duration of exposure to work-related stress affects cancer development.
This study, on the other hand, shows the importance of measuring stress at different points in an individual’s working life, the researchers said. They added that their results raise the question of whether chronic psychological stress should be viewed as a public health issue.
However, the team also pointed out that these results are unsubstantiated because they are based on a summary assessment of work-related stress for a given job. There is a need for epidemiological studies based on reliable stress measurements, repeated over time, and that take all sources of stress into account. ![]()

burning building in Quebec
Photo by Sylvain Pedneault
New research suggests that prolonged exposure to work-related stress may increase a man’s risk of several cancers.
The study showed a significant association between work-related stress lasting 15 years or more and non-Hodgkin lymphoma (NHL) as well as lung, colon, rectal, and stomach cancers.
Men who had worked as firefighters, engineers, mechanics, and repair workers were most likely to report work-related stress.
Marie-Élise Parent, PhD, of Institut national de la recherche scientifique (INRS) in Laval, Quebec, Canada, and her colleagues conducted this research and published
the results in Preventive Medicine.
The researchers studied 3103 men with 11 different types of cancer who were diagnosed from 1979 to 1985. The team compared these men to 512 control subjects from the general population.
Both cases and controls were interviewed and asked to describe each job they had during their lifetime, including the occurrence of stress related to a job and the reason for that stress.
The researchers then calculated odds ratios (OR) for the association between perceived workplace stress and its duration, and each cancer site. The analyses were adjusted for lifestyle and occupational factors.
The team found that having at least one stressful job in a lifetime was associated with increased odds of 5 cancers:
- Lung—OR=1.33
- Colon—OR=1.51
- Bladder—OR=1.37
- Rectal—OR=1.52
- Stomach—OR=1.53.
When the researchers looked at the duration of stress, they found no significant association between any of the cancers and work-related stress lasting less than 15 years.
However, there were significant associations for several cancers and work-related stress lasting 15 to 30 years or more than 30 years. These included:
- NHL—15-30 years, OR=1.47; >30 years, OR=1.69 (P=0.02)
- Lung cancer—15-30 years, OR=1.47; >30 years, OR=1.51 (P=0.01)
- Colon cancer—15-30 years, OR=1.32; >30 years, OR=1.64 (P<0.01)
- Rectal cancer—15-30 years, OR=1.84; >30 years, OR=1.48 (P=0.01)
- Stomach cancer—15-30 years, OR=2.15; >30 years, OR=1.48 (P=0.01).
The occupations with the highest prevalence of work-related stress were firefighter (40% of firefighting jobs reported as stressful), industrial and aerospace engineer (31%), and motor vehicle and rail transport mechanic/repair worker (28%).
For the same individual, stress varied depending on the job held.
The study also showed that perceived stress was not limited to high work load and time constraints. Customer service, sales commissions, responsibilities, having an anxious temperament, job insecurity, financial problems, challenging or dangerous work conditions, employee supervision, interpersonal conflict, and a difficult commute were all sources of stress listed by study participants.
The researchers said one of the biggest flaws in previous studies of this kind is that none of them assessed work-related stress over a full working lifetime. The team said this made it impossible to determine how the duration of exposure to work-related stress affects cancer development.
This study, on the other hand, shows the importance of measuring stress at different points in an individual’s working life, the researchers said. They added that their results raise the question of whether chronic psychological stress should be viewed as a public health issue.
However, the team also pointed out that these results are unsubstantiated because they are based on a summary assessment of work-related stress for a given job. There is a need for epidemiological studies based on reliable stress measurements, repeated over time, and that take all sources of stress into account. ![]()

burning building in Quebec
Photo by Sylvain Pedneault
New research suggests that prolonged exposure to work-related stress may increase a man’s risk of several cancers.
The study showed a significant association between work-related stress lasting 15 years or more and non-Hodgkin lymphoma (NHL) as well as lung, colon, rectal, and stomach cancers.
Men who had worked as firefighters, engineers, mechanics, and repair workers were most likely to report work-related stress.
Marie-Élise Parent, PhD, of Institut national de la recherche scientifique (INRS) in Laval, Quebec, Canada, and her colleagues conducted this research and published
the results in Preventive Medicine.
The researchers studied 3103 men with 11 different types of cancer who were diagnosed from 1979 to 1985. The team compared these men to 512 control subjects from the general population.
Both cases and controls were interviewed and asked to describe each job they had during their lifetime, including the occurrence of stress related to a job and the reason for that stress.
The researchers then calculated odds ratios (OR) for the association between perceived workplace stress and its duration, and each cancer site. The analyses were adjusted for lifestyle and occupational factors.
The team found that having at least one stressful job in a lifetime was associated with increased odds of 5 cancers:
- Lung—OR=1.33
- Colon—OR=1.51
- Bladder—OR=1.37
- Rectal—OR=1.52
- Stomach—OR=1.53.
When the researchers looked at the duration of stress, they found no significant association between any of the cancers and work-related stress lasting less than 15 years.
However, there were significant associations for several cancers and work-related stress lasting 15 to 30 years or more than 30 years. These included:
- NHL—15-30 years, OR=1.47; >30 years, OR=1.69 (P=0.02)
- Lung cancer—15-30 years, OR=1.47; >30 years, OR=1.51 (P=0.01)
- Colon cancer—15-30 years, OR=1.32; >30 years, OR=1.64 (P<0.01)
- Rectal cancer—15-30 years, OR=1.84; >30 years, OR=1.48 (P=0.01)
- Stomach cancer—15-30 years, OR=2.15; >30 years, OR=1.48 (P=0.01).
The occupations with the highest prevalence of work-related stress were firefighter (40% of firefighting jobs reported as stressful), industrial and aerospace engineer (31%), and motor vehicle and rail transport mechanic/repair worker (28%).
For the same individual, stress varied depending on the job held.
The study also showed that perceived stress was not limited to high work load and time constraints. Customer service, sales commissions, responsibilities, having an anxious temperament, job insecurity, financial problems, challenging or dangerous work conditions, employee supervision, interpersonal conflict, and a difficult commute were all sources of stress listed by study participants.
The researchers said one of the biggest flaws in previous studies of this kind is that none of them assessed work-related stress over a full working lifetime. The team said this made it impossible to determine how the duration of exposure to work-related stress affects cancer development.
This study, on the other hand, shows the importance of measuring stress at different points in an individual’s working life, the researchers said. They added that their results raise the question of whether chronic psychological stress should be viewed as a public health issue.
However, the team also pointed out that these results are unsubstantiated because they are based on a summary assessment of work-related stress for a given job. There is a need for epidemiological studies based on reliable stress measurements, repeated over time, and that take all sources of stress into account. ![]()
Pharma is gaming the system for orphan drugs, investigation suggests

Photo courtesy of the FDA
An investigation by Kaiser Health News (KHN) suggests some pharmaceutical companies are using the Orphan Drug Act to create monopolies and charge high prices for drugs that are already approved for mass market use in the US.
The US Food and Drug Administration (FDA) grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent conditions that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
However, the KHN investigation showed that some companies have been applying for—and obtaining—orphan designation for drugs already used to treat large populations.
The report states that more than 70 drugs that currently have orphan status were first approved by the FDA for mass market use.
In fact, 7 of the 10 best-selling drugs of 2015 were also orphan drugs. Included on this list are Rituxan (rituximab), Neulasta (pegfilgrastim), and Revlimid (lenalidomide).
The report also states that more than 80 drugs with orphan designation have been approved to treat more than one rare disease. For example, Gleevec (imatinib) has 9 orphan designations.
The KHN investigation revealed that, overall, about a third of orphan designations granted since the Orphan Drug Act was passed in 1983 have been either for repurposed mass market drugs or drugs that received multiple orphan designations. (Roughly 450 orphan drugs have been brought to market since 1983, according to the report.)
For each orphan designation, a drug’s developer qualifies for “a fresh batch of incentives,” the report notes.
The exclusivity incentive means the FDA won’t approve another version of an orphan drug to treat the rare disease(s) in question for 7 years, even if the company’s patent on the brand-name drug has expired.
For example, generic versions of imatinib are being used to treat chronic myeloid leukemia in the US because the patent for Gleevec has expired. However, because of an orphan designation, Novartis still has exclusivity for Gleevec (and will until 2020) as a treatment for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia who are also on chemotherapy.
The KHN report notes that exclusivity can be “a potent pricing tool” due to a lack of competition. And this means orphan drugs may “come with astronomical price tags.”
For instance, there are 33 orphan drugs that cost at least $28,000 for a 30-day supply and 4 orphan drugs that cost more than $70,000 per month.
According to the KHN report, the FDA is planning to investigate this issue.
Gayatri Rao, MD, director of the FDA’s Office of Orphan Products Development, has asked for a review of all orphan designations granted in 2010 and 2015. She said the review will not extend further because the FDA does not have the resources to review all orphan drugs. ![]()

Photo courtesy of the FDA
An investigation by Kaiser Health News (KHN) suggests some pharmaceutical companies are using the Orphan Drug Act to create monopolies and charge high prices for drugs that are already approved for mass market use in the US.
The US Food and Drug Administration (FDA) grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent conditions that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
However, the KHN investigation showed that some companies have been applying for—and obtaining—orphan designation for drugs already used to treat large populations.
The report states that more than 70 drugs that currently have orphan status were first approved by the FDA for mass market use.
In fact, 7 of the 10 best-selling drugs of 2015 were also orphan drugs. Included on this list are Rituxan (rituximab), Neulasta (pegfilgrastim), and Revlimid (lenalidomide).
The report also states that more than 80 drugs with orphan designation have been approved to treat more than one rare disease. For example, Gleevec (imatinib) has 9 orphan designations.
The KHN investigation revealed that, overall, about a third of orphan designations granted since the Orphan Drug Act was passed in 1983 have been either for repurposed mass market drugs or drugs that received multiple orphan designations. (Roughly 450 orphan drugs have been brought to market since 1983, according to the report.)
For each orphan designation, a drug’s developer qualifies for “a fresh batch of incentives,” the report notes.
The exclusivity incentive means the FDA won’t approve another version of an orphan drug to treat the rare disease(s) in question for 7 years, even if the company’s patent on the brand-name drug has expired.
For example, generic versions of imatinib are being used to treat chronic myeloid leukemia in the US because the patent for Gleevec has expired. However, because of an orphan designation, Novartis still has exclusivity for Gleevec (and will until 2020) as a treatment for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia who are also on chemotherapy.
The KHN report notes that exclusivity can be “a potent pricing tool” due to a lack of competition. And this means orphan drugs may “come with astronomical price tags.”
For instance, there are 33 orphan drugs that cost at least $28,000 for a 30-day supply and 4 orphan drugs that cost more than $70,000 per month.
According to the KHN report, the FDA is planning to investigate this issue.
Gayatri Rao, MD, director of the FDA’s Office of Orphan Products Development, has asked for a review of all orphan designations granted in 2010 and 2015. She said the review will not extend further because the FDA does not have the resources to review all orphan drugs. ![]()

Photo courtesy of the FDA
An investigation by Kaiser Health News (KHN) suggests some pharmaceutical companies are using the Orphan Drug Act to create monopolies and charge high prices for drugs that are already approved for mass market use in the US.
The US Food and Drug Administration (FDA) grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent conditions that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
However, the KHN investigation showed that some companies have been applying for—and obtaining—orphan designation for drugs already used to treat large populations.
The report states that more than 70 drugs that currently have orphan status were first approved by the FDA for mass market use.
In fact, 7 of the 10 best-selling drugs of 2015 were also orphan drugs. Included on this list are Rituxan (rituximab), Neulasta (pegfilgrastim), and Revlimid (lenalidomide).
The report also states that more than 80 drugs with orphan designation have been approved to treat more than one rare disease. For example, Gleevec (imatinib) has 9 orphan designations.
The KHN investigation revealed that, overall, about a third of orphan designations granted since the Orphan Drug Act was passed in 1983 have been either for repurposed mass market drugs or drugs that received multiple orphan designations. (Roughly 450 orphan drugs have been brought to market since 1983, according to the report.)
For each orphan designation, a drug’s developer qualifies for “a fresh batch of incentives,” the report notes.
The exclusivity incentive means the FDA won’t approve another version of an orphan drug to treat the rare disease(s) in question for 7 years, even if the company’s patent on the brand-name drug has expired.
For example, generic versions of imatinib are being used to treat chronic myeloid leukemia in the US because the patent for Gleevec has expired. However, because of an orphan designation, Novartis still has exclusivity for Gleevec (and will until 2020) as a treatment for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia who are also on chemotherapy.
The KHN report notes that exclusivity can be “a potent pricing tool” due to a lack of competition. And this means orphan drugs may “come with astronomical price tags.”
For instance, there are 33 orphan drugs that cost at least $28,000 for a 30-day supply and 4 orphan drugs that cost more than $70,000 per month.
According to the KHN report, the FDA is planning to investigate this issue.
Gayatri Rao, MD, director of the FDA’s Office of Orphan Products Development, has asked for a review of all orphan designations granted in 2010 and 2015. She said the review will not extend further because the FDA does not have the resources to review all orphan drugs. ![]()
FDA releases draft guidances on biosimilars, medical communications

Photo by Bill Branson
The US Food and Drug Administration (FDA) has released 3 new draft guidance documents for industry.
One document provides an overview of scientific considerations when attempting to demonstrate that a biosimilar product is interchangeable with a reference product.
The other 2 guidance documents are intended to clarify the FDA’s position regarding communications about medical products.
The first draft guidance, “Considerations in Demonstrating Interchangeability With a Reference Product,” is intended to assist applicants in demonstrating that a proposed therapeutic protein product (eg, monoclonal antibodies) is interchangeable with a reference product under section 351(k) of the Public Health Service Act.
An interchangeable biological product is biosimilar to the reference product and can be expected to produce the same clinical result as the reference product in any given patient.
For a biological product that is administered more than once, the risk in terms of safety or diminished efficacy of alternating or switching between the biological product and the reference product must not be greater than the risk of using the reference product without such alternating/switching.
The approval pathway for biosimilar and interchangeable products was established by the Biologics Price Competition and Innovation Act of 2009, which was enacted as part of the Affordable Care Act in March 2010.
The FDA’s draft guidance on biosimilars contains information on:
- Factors impacting the type and amount of data and information needed to support a demonstration of interchangeability
- The data and information needed to support a demonstration of interchangeability
- Considerations for the design and analysis of a switching study or studies to support a demonstration of interchangeability
- Recommendations regarding the use of US-licensed reference products in a switching study or studies
- Considerations for developing presentations (eg, container closure systems) for proposed interchangeable products.
In addition to soliciting comments on this draft guidance, the FDA is inviting comments on questions posed in the notice of availability about interchangeability in general, the regulation of interchangeable products over their life cycle, and questions about considerations regarding post-approval manufacturing changes.
The FDA also released a Center for Drug Evaluation and Research “From Our Perspective,” by Leah Christl, describing aspects of this draft guidance.
For more information on how to submit comments on the draft guidance and questions posed in the notice of availability, see the Federal Register notice.
Medical communications
The FDA also released 2 draft guidance documents that, the agency says, will each help provide clarity for medical product companies, as well as other interested parties, on the FDA’s current thinking and recommendations for a few different types of communications about medical products.
The first draft guidance, “Drug and Device Manufacturer Communications with Payors, Formulary Committees, and Similar Entities,” explains the FDA’s current thinking and recommendations on firms’ communication of healthcare economic information about approved drugs under section 502(a) of the Federal Food, Drug, and Cosmetic Act, which was recently amended by the 21st Century Cures Act.
The guidance also answers common questions and provides the FDA’s recommendations regarding firms’ communications to payors about investigational drugs and devices that are not yet approved or cleared for any use.
The second draft guidance, “Medical Product Communications That Are Consistent With the FDA-Required Labeling,” explains the FDA’s current thinking about firms’ medical product communications that include data and information that are not contained in their products’ FDA-required labeling, but that concern the approved or cleared uses of their products.
The FDA has opened a public comment period for each draft guidance.
The agency is also asking for stakeholder input on another, distinct topic—communications about unapproved uses of approved or cleared medical products.
The FDA held a Part 15 hearing in November 2016 to hear from a broad range of stakeholders regarding this topic. The agency has now reopened the comment period for the docket opened in connection with that public hearing for an additional 90 days (until April 10, 2017) to allow interested parties an opportunity to review the 2 draft guidances before submitting comments to any of the relevant dockets.
The FDA also added a document to the docket for the public hearing titled, “Memorandum: Public Health Interests and First Amendment Considerations Related to Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products.”
This document provides additional background on the issues the FDA is considering as part of its review of the agency’s rules and policies relating to firm communications regarding unapproved uses of approved or cleared medical products, including a discussion of First Amendment considerations.
The FDA is requesting input on the memorandum as it relates to the questions set forth in the initial notice of public hearing. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has released 3 new draft guidance documents for industry.
One document provides an overview of scientific considerations when attempting to demonstrate that a biosimilar product is interchangeable with a reference product.
The other 2 guidance documents are intended to clarify the FDA’s position regarding communications about medical products.
The first draft guidance, “Considerations in Demonstrating Interchangeability With a Reference Product,” is intended to assist applicants in demonstrating that a proposed therapeutic protein product (eg, monoclonal antibodies) is interchangeable with a reference product under section 351(k) of the Public Health Service Act.
An interchangeable biological product is biosimilar to the reference product and can be expected to produce the same clinical result as the reference product in any given patient.
For a biological product that is administered more than once, the risk in terms of safety or diminished efficacy of alternating or switching between the biological product and the reference product must not be greater than the risk of using the reference product without such alternating/switching.
The approval pathway for biosimilar and interchangeable products was established by the Biologics Price Competition and Innovation Act of 2009, which was enacted as part of the Affordable Care Act in March 2010.
The FDA’s draft guidance on biosimilars contains information on:
- Factors impacting the type and amount of data and information needed to support a demonstration of interchangeability
- The data and information needed to support a demonstration of interchangeability
- Considerations for the design and analysis of a switching study or studies to support a demonstration of interchangeability
- Recommendations regarding the use of US-licensed reference products in a switching study or studies
- Considerations for developing presentations (eg, container closure systems) for proposed interchangeable products.
In addition to soliciting comments on this draft guidance, the FDA is inviting comments on questions posed in the notice of availability about interchangeability in general, the regulation of interchangeable products over their life cycle, and questions about considerations regarding post-approval manufacturing changes.
The FDA also released a Center for Drug Evaluation and Research “From Our Perspective,” by Leah Christl, describing aspects of this draft guidance.
For more information on how to submit comments on the draft guidance and questions posed in the notice of availability, see the Federal Register notice.
Medical communications
The FDA also released 2 draft guidance documents that, the agency says, will each help provide clarity for medical product companies, as well as other interested parties, on the FDA’s current thinking and recommendations for a few different types of communications about medical products.
The first draft guidance, “Drug and Device Manufacturer Communications with Payors, Formulary Committees, and Similar Entities,” explains the FDA’s current thinking and recommendations on firms’ communication of healthcare economic information about approved drugs under section 502(a) of the Federal Food, Drug, and Cosmetic Act, which was recently amended by the 21st Century Cures Act.
The guidance also answers common questions and provides the FDA’s recommendations regarding firms’ communications to payors about investigational drugs and devices that are not yet approved or cleared for any use.
The second draft guidance, “Medical Product Communications That Are Consistent With the FDA-Required Labeling,” explains the FDA’s current thinking about firms’ medical product communications that include data and information that are not contained in their products’ FDA-required labeling, but that concern the approved or cleared uses of their products.
The FDA has opened a public comment period for each draft guidance.
The agency is also asking for stakeholder input on another, distinct topic—communications about unapproved uses of approved or cleared medical products.
The FDA held a Part 15 hearing in November 2016 to hear from a broad range of stakeholders regarding this topic. The agency has now reopened the comment period for the docket opened in connection with that public hearing for an additional 90 days (until April 10, 2017) to allow interested parties an opportunity to review the 2 draft guidances before submitting comments to any of the relevant dockets.
The FDA also added a document to the docket for the public hearing titled, “Memorandum: Public Health Interests and First Amendment Considerations Related to Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products.”
This document provides additional background on the issues the FDA is considering as part of its review of the agency’s rules and policies relating to firm communications regarding unapproved uses of approved or cleared medical products, including a discussion of First Amendment considerations.
The FDA is requesting input on the memorandum as it relates to the questions set forth in the initial notice of public hearing. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has released 3 new draft guidance documents for industry.
One document provides an overview of scientific considerations when attempting to demonstrate that a biosimilar product is interchangeable with a reference product.
The other 2 guidance documents are intended to clarify the FDA’s position regarding communications about medical products.
The first draft guidance, “Considerations in Demonstrating Interchangeability With a Reference Product,” is intended to assist applicants in demonstrating that a proposed therapeutic protein product (eg, monoclonal antibodies) is interchangeable with a reference product under section 351(k) of the Public Health Service Act.
An interchangeable biological product is biosimilar to the reference product and can be expected to produce the same clinical result as the reference product in any given patient.
For a biological product that is administered more than once, the risk in terms of safety or diminished efficacy of alternating or switching between the biological product and the reference product must not be greater than the risk of using the reference product without such alternating/switching.
The approval pathway for biosimilar and interchangeable products was established by the Biologics Price Competition and Innovation Act of 2009, which was enacted as part of the Affordable Care Act in March 2010.
The FDA’s draft guidance on biosimilars contains information on:
- Factors impacting the type and amount of data and information needed to support a demonstration of interchangeability
- The data and information needed to support a demonstration of interchangeability
- Considerations for the design and analysis of a switching study or studies to support a demonstration of interchangeability
- Recommendations regarding the use of US-licensed reference products in a switching study or studies
- Considerations for developing presentations (eg, container closure systems) for proposed interchangeable products.
In addition to soliciting comments on this draft guidance, the FDA is inviting comments on questions posed in the notice of availability about interchangeability in general, the regulation of interchangeable products over their life cycle, and questions about considerations regarding post-approval manufacturing changes.
The FDA also released a Center for Drug Evaluation and Research “From Our Perspective,” by Leah Christl, describing aspects of this draft guidance.
For more information on how to submit comments on the draft guidance and questions posed in the notice of availability, see the Federal Register notice.
Medical communications
The FDA also released 2 draft guidance documents that, the agency says, will each help provide clarity for medical product companies, as well as other interested parties, on the FDA’s current thinking and recommendations for a few different types of communications about medical products.
The first draft guidance, “Drug and Device Manufacturer Communications with Payors, Formulary Committees, and Similar Entities,” explains the FDA’s current thinking and recommendations on firms’ communication of healthcare economic information about approved drugs under section 502(a) of the Federal Food, Drug, and Cosmetic Act, which was recently amended by the 21st Century Cures Act.
The guidance also answers common questions and provides the FDA’s recommendations regarding firms’ communications to payors about investigational drugs and devices that are not yet approved or cleared for any use.
The second draft guidance, “Medical Product Communications That Are Consistent With the FDA-Required Labeling,” explains the FDA’s current thinking about firms’ medical product communications that include data and information that are not contained in their products’ FDA-required labeling, but that concern the approved or cleared uses of their products.
The FDA has opened a public comment period for each draft guidance.
The agency is also asking for stakeholder input on another, distinct topic—communications about unapproved uses of approved or cleared medical products.
The FDA held a Part 15 hearing in November 2016 to hear from a broad range of stakeholders regarding this topic. The agency has now reopened the comment period for the docket opened in connection with that public hearing for an additional 90 days (until April 10, 2017) to allow interested parties an opportunity to review the 2 draft guidances before submitting comments to any of the relevant dockets.
The FDA also added a document to the docket for the public hearing titled, “Memorandum: Public Health Interests and First Amendment Considerations Related to Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products.”
This document provides additional background on the issues the FDA is considering as part of its review of the agency’s rules and policies relating to firm communications regarding unapproved uses of approved or cleared medical products, including a discussion of First Amendment considerations.
The FDA is requesting input on the memorandum as it relates to the questions set forth in the initial notice of public hearing. ![]()
Enzyme derived from yeast kills ALL cells

as it buds before dividing
Image by Carolyn Larabell
L-asparaginase derived from baker’s yeast has shown early promise for treating acute lymphoblastic leukemia (ALL), according to researchers.
The team isolated L-asparaginase from Saccharomyces cerevisiae and found the enzyme could kill ALL cells in vitro, while largely sparing healthy control cells.
Gisele Monteiro de Souza, PhD, of the University of São Paulo in São Paulo, Brazil, and her colleagues detailed these findings in Scientific Reports.
“In this study, we characterized the enzyme L-asparaginase from S cerevisiae,” Dr Souza said. “The results show this protein can efficiently annihilate leukemia cells with low cytotoxicity to healthy cells.”
She and her colleagues conducted this study in search of alternatives to L-asparaginase extracted from bacteria (Escherichia coli and Erwinia chrysanthemi).
“Our goal in this project wasn’t to produce the enzyme, but rather to find a new source of the biodrug in microorganisms for use in patients who develop resistance to the bacterial enzyme,” said study author Marcos Antonio de Oliveira, of São Paulo State University in São Vicente, Brazil.
To this end, the researchers isolated fungi from several different Brazilian environments as well as marine and land environments in Antarctica. According to Oliveira, these organisms often secrete asparaginase into the extracellular medium in response to a shortage of nitrogen.
“This lowers the cost of purifying the molecule for drug production, an important factor from an industrial standpoint,” he said.
The group also used bioinformatics tools to mine information on the genomes of several microorganisms from international databases.
In this way, they identified a gene responsible for producing an enzyme that closely resembles the enzymes found in E coli and E chrysanthemi, but with a number of advantages, in the genome of S cerevisiae.
The gene of interest from L-asparaginase was cloned, and the researchers used genetic engineering to make E coli express large amounts of the enzyme originally found in yeast.
“We were able to obtain the recombinant protein,” said study author Iris Munhoz Costa, of the University of São Paulo.
“We then performed studies to characterize its secondary structure and identify important regions called catalytic sites. Finally, we evaluated its efficacy in vitro.”
The enzyme was tested in 3 different cell lines: ALL cells incapable of producing asparagine at normal levels (MOLT4), another ALL cell line capable of producing asparagine at normal levels (REH), and non-malignant control cells (HUVECs).
These 3 cell lines were subdivided into 2 groups. One was treated with L-asparaginase derived from E coli enzyme, and the other was treated with L-asparaginase from yeast.
“The bacterial enzyme killed about 90% of the MOLT4 human leukemia cells and displayed low toxicity to the healthy HUVEC cells, killing only 10%,” Dr Souza said.
“The yeast enzyme killed between 70% and 80% of the MOLT4 cells and displayed less than 10% toxicity for HUVEC cells. Neither was significantly effective against REH cells.”
In her view, the results are encouraging, in contrast with those of studies performed with the same enzyme in the 1970s. At that time, the tests involved a version of the protein extracted directly from yeast and containing many impurities.
The group’s next step is to perform new in vitro trials with different cell types to evaluate the immune response and toxicity. If the results are positive, the first tests in animals may be next.
The researchers are also studying possible modifications that could be made to the molecule’s structure to increase antitumor activity and extend the enzyme’s half-life. ![]()

as it buds before dividing
Image by Carolyn Larabell
L-asparaginase derived from baker’s yeast has shown early promise for treating acute lymphoblastic leukemia (ALL), according to researchers.
The team isolated L-asparaginase from Saccharomyces cerevisiae and found the enzyme could kill ALL cells in vitro, while largely sparing healthy control cells.
Gisele Monteiro de Souza, PhD, of the University of São Paulo in São Paulo, Brazil, and her colleagues detailed these findings in Scientific Reports.
“In this study, we characterized the enzyme L-asparaginase from S cerevisiae,” Dr Souza said. “The results show this protein can efficiently annihilate leukemia cells with low cytotoxicity to healthy cells.”
She and her colleagues conducted this study in search of alternatives to L-asparaginase extracted from bacteria (Escherichia coli and Erwinia chrysanthemi).
“Our goal in this project wasn’t to produce the enzyme, but rather to find a new source of the biodrug in microorganisms for use in patients who develop resistance to the bacterial enzyme,” said study author Marcos Antonio de Oliveira, of São Paulo State University in São Vicente, Brazil.
To this end, the researchers isolated fungi from several different Brazilian environments as well as marine and land environments in Antarctica. According to Oliveira, these organisms often secrete asparaginase into the extracellular medium in response to a shortage of nitrogen.
“This lowers the cost of purifying the molecule for drug production, an important factor from an industrial standpoint,” he said.
The group also used bioinformatics tools to mine information on the genomes of several microorganisms from international databases.
In this way, they identified a gene responsible for producing an enzyme that closely resembles the enzymes found in E coli and E chrysanthemi, but with a number of advantages, in the genome of S cerevisiae.
The gene of interest from L-asparaginase was cloned, and the researchers used genetic engineering to make E coli express large amounts of the enzyme originally found in yeast.
“We were able to obtain the recombinant protein,” said study author Iris Munhoz Costa, of the University of São Paulo.
“We then performed studies to characterize its secondary structure and identify important regions called catalytic sites. Finally, we evaluated its efficacy in vitro.”
The enzyme was tested in 3 different cell lines: ALL cells incapable of producing asparagine at normal levels (MOLT4), another ALL cell line capable of producing asparagine at normal levels (REH), and non-malignant control cells (HUVECs).
These 3 cell lines were subdivided into 2 groups. One was treated with L-asparaginase derived from E coli enzyme, and the other was treated with L-asparaginase from yeast.
“The bacterial enzyme killed about 90% of the MOLT4 human leukemia cells and displayed low toxicity to the healthy HUVEC cells, killing only 10%,” Dr Souza said.
“The yeast enzyme killed between 70% and 80% of the MOLT4 cells and displayed less than 10% toxicity for HUVEC cells. Neither was significantly effective against REH cells.”
In her view, the results are encouraging, in contrast with those of studies performed with the same enzyme in the 1970s. At that time, the tests involved a version of the protein extracted directly from yeast and containing many impurities.
The group’s next step is to perform new in vitro trials with different cell types to evaluate the immune response and toxicity. If the results are positive, the first tests in animals may be next.
The researchers are also studying possible modifications that could be made to the molecule’s structure to increase antitumor activity and extend the enzyme’s half-life. ![]()

as it buds before dividing
Image by Carolyn Larabell
L-asparaginase derived from baker’s yeast has shown early promise for treating acute lymphoblastic leukemia (ALL), according to researchers.
The team isolated L-asparaginase from Saccharomyces cerevisiae and found the enzyme could kill ALL cells in vitro, while largely sparing healthy control cells.
Gisele Monteiro de Souza, PhD, of the University of São Paulo in São Paulo, Brazil, and her colleagues detailed these findings in Scientific Reports.
“In this study, we characterized the enzyme L-asparaginase from S cerevisiae,” Dr Souza said. “The results show this protein can efficiently annihilate leukemia cells with low cytotoxicity to healthy cells.”
She and her colleagues conducted this study in search of alternatives to L-asparaginase extracted from bacteria (Escherichia coli and Erwinia chrysanthemi).
“Our goal in this project wasn’t to produce the enzyme, but rather to find a new source of the biodrug in microorganisms for use in patients who develop resistance to the bacterial enzyme,” said study author Marcos Antonio de Oliveira, of São Paulo State University in São Vicente, Brazil.
To this end, the researchers isolated fungi from several different Brazilian environments as well as marine and land environments in Antarctica. According to Oliveira, these organisms often secrete asparaginase into the extracellular medium in response to a shortage of nitrogen.
“This lowers the cost of purifying the molecule for drug production, an important factor from an industrial standpoint,” he said.
The group also used bioinformatics tools to mine information on the genomes of several microorganisms from international databases.
In this way, they identified a gene responsible for producing an enzyme that closely resembles the enzymes found in E coli and E chrysanthemi, but with a number of advantages, in the genome of S cerevisiae.
The gene of interest from L-asparaginase was cloned, and the researchers used genetic engineering to make E coli express large amounts of the enzyme originally found in yeast.
“We were able to obtain the recombinant protein,” said study author Iris Munhoz Costa, of the University of São Paulo.
“We then performed studies to characterize its secondary structure and identify important regions called catalytic sites. Finally, we evaluated its efficacy in vitro.”
The enzyme was tested in 3 different cell lines: ALL cells incapable of producing asparagine at normal levels (MOLT4), another ALL cell line capable of producing asparagine at normal levels (REH), and non-malignant control cells (HUVECs).
These 3 cell lines were subdivided into 2 groups. One was treated with L-asparaginase derived from E coli enzyme, and the other was treated with L-asparaginase from yeast.
“The bacterial enzyme killed about 90% of the MOLT4 human leukemia cells and displayed low toxicity to the healthy HUVEC cells, killing only 10%,” Dr Souza said.
“The yeast enzyme killed between 70% and 80% of the MOLT4 cells and displayed less than 10% toxicity for HUVEC cells. Neither was significantly effective against REH cells.”
In her view, the results are encouraging, in contrast with those of studies performed with the same enzyme in the 1970s. At that time, the tests involved a version of the protein extracted directly from yeast and containing many impurities.
The group’s next step is to perform new in vitro trials with different cell types to evaluate the immune response and toxicity. If the results are positive, the first tests in animals may be next.
The researchers are also studying possible modifications that could be made to the molecule’s structure to increase antitumor activity and extend the enzyme’s half-life. ![]()
A New Narrative for Diabetes Management
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
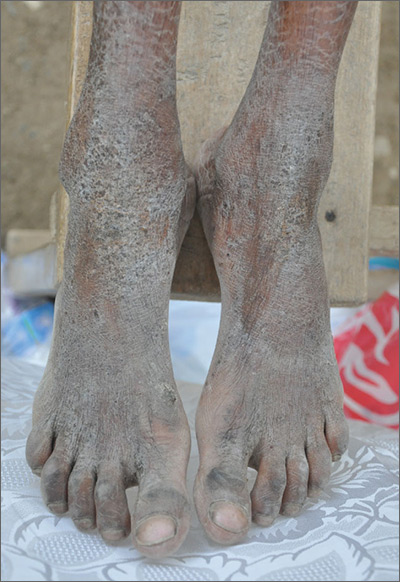
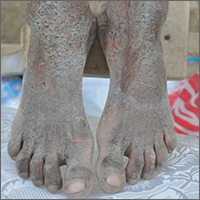
Crusty rash from head to toe
The FP diagnosed crusted scabies based on the patient’s clinical appearance and the distribution of crusting. He happened to have a dermatoscope and was able to see scabies mites moving and creating burrows under the skin. The mites generally appeared as dark triangles with light oval bodies. Crusted scabies is also known as "Norwegian scabies," but the preferred term is crusted scabies. Crusted scabies is more common in individuals who are immunosuppressed, chronically ill (for any reason), or who live in nursing homes.
The child's HIV test was negative and the FP never found out the cause of the cachexia. The diagnosis of crusted scabies was important for everyone involved in the transportation and care of this child. Once the diagnosis was known, everyone was careful to use gloves when caring for her so as to avoid acquiring scabies. The mother was almost certainly infested, along with other family members who’d been in direct contact with the child.
The recommended treatment for crusted scabies is oral ivermectin at a dose of 0.2 mg/kg (maximum dose, 12 mg) once for each infested person to be repeated in 10 days. Fortunately, the global health team had oral ivermectin to give to the child and the family. The FP also told the family to wash all of their clothes and bedclothes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Chanoine P, Smith M. Scabies. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:575-580.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed crusted scabies based on the patient’s clinical appearance and the distribution of crusting. He happened to have a dermatoscope and was able to see scabies mites moving and creating burrows under the skin. The mites generally appeared as dark triangles with light oval bodies. Crusted scabies is also known as "Norwegian scabies," but the preferred term is crusted scabies. Crusted scabies is more common in individuals who are immunosuppressed, chronically ill (for any reason), or who live in nursing homes.
The child's HIV test was negative and the FP never found out the cause of the cachexia. The diagnosis of crusted scabies was important for everyone involved in the transportation and care of this child. Once the diagnosis was known, everyone was careful to use gloves when caring for her so as to avoid acquiring scabies. The mother was almost certainly infested, along with other family members who’d been in direct contact with the child.
The recommended treatment for crusted scabies is oral ivermectin at a dose of 0.2 mg/kg (maximum dose, 12 mg) once for each infested person to be repeated in 10 days. Fortunately, the global health team had oral ivermectin to give to the child and the family. The FP also told the family to wash all of their clothes and bedclothes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Chanoine P, Smith M. Scabies. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:575-580.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed crusted scabies based on the patient’s clinical appearance and the distribution of crusting. He happened to have a dermatoscope and was able to see scabies mites moving and creating burrows under the skin. The mites generally appeared as dark triangles with light oval bodies. Crusted scabies is also known as "Norwegian scabies," but the preferred term is crusted scabies. Crusted scabies is more common in individuals who are immunosuppressed, chronically ill (for any reason), or who live in nursing homes.
The child's HIV test was negative and the FP never found out the cause of the cachexia. The diagnosis of crusted scabies was important for everyone involved in the transportation and care of this child. Once the diagnosis was known, everyone was careful to use gloves when caring for her so as to avoid acquiring scabies. The mother was almost certainly infested, along with other family members who’d been in direct contact with the child.
The recommended treatment for crusted scabies is oral ivermectin at a dose of 0.2 mg/kg (maximum dose, 12 mg) once for each infested person to be repeated in 10 days. Fortunately, the global health team had oral ivermectin to give to the child and the family. The FP also told the family to wash all of their clothes and bedclothes.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Chanoine P, Smith M. Scabies. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:575-580.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
My declining enthusiasm for inpatient work
I’m getting old, and as I age, my desire to do inpatient work seems to diminish each year.
When I started out 20 years ago, I thrived on it. There was excitement in an urgent ED call: the chance to go in and give tissue plasminogen activator, do an emergent lumbar puncture, or break status epilepticus.
Back when I was fresh out of training, I hustled. I walked through the ED to make sure they knew I was around. I hung out in the doctors’ lounge, shaking hands and introducing myself. I was trying to build my practice and get my name out. Other neurologists, closer to retirement than I, were more than happy to let me move in and take the hospital’s late-night and weekend calls.
With time, hospital medicine becomes more of a young person’s game. As I move from being a newly minted attending to an old fogy, I’m happy to leave the work to the next generation.
My hospital work is now down to one-in-3 weekends and no weekdays. I’m not quite ready to give it up entirely and don’t want to turn my back on my call partners of 15 years. I still enjoy the challenge of the cases, the joking around with the nurses, and the family meetings to bring comfort and explain things as best I can.
But I can live without the late night runs, the driving back and forth, and the unpredictable hours. These days, my time at home is more valuable than it was when I started, and I envy those who keep banker’s hours.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m getting old, and as I age, my desire to do inpatient work seems to diminish each year.
When I started out 20 years ago, I thrived on it. There was excitement in an urgent ED call: the chance to go in and give tissue plasminogen activator, do an emergent lumbar puncture, or break status epilepticus.
Back when I was fresh out of training, I hustled. I walked through the ED to make sure they knew I was around. I hung out in the doctors’ lounge, shaking hands and introducing myself. I was trying to build my practice and get my name out. Other neurologists, closer to retirement than I, were more than happy to let me move in and take the hospital’s late-night and weekend calls.
With time, hospital medicine becomes more of a young person’s game. As I move from being a newly minted attending to an old fogy, I’m happy to leave the work to the next generation.
My hospital work is now down to one-in-3 weekends and no weekdays. I’m not quite ready to give it up entirely and don’t want to turn my back on my call partners of 15 years. I still enjoy the challenge of the cases, the joking around with the nurses, and the family meetings to bring comfort and explain things as best I can.
But I can live without the late night runs, the driving back and forth, and the unpredictable hours. These days, my time at home is more valuable than it was when I started, and I envy those who keep banker’s hours.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m getting old, and as I age, my desire to do inpatient work seems to diminish each year.
When I started out 20 years ago, I thrived on it. There was excitement in an urgent ED call: the chance to go in and give tissue plasminogen activator, do an emergent lumbar puncture, or break status epilepticus.
Back when I was fresh out of training, I hustled. I walked through the ED to make sure they knew I was around. I hung out in the doctors’ lounge, shaking hands and introducing myself. I was trying to build my practice and get my name out. Other neurologists, closer to retirement than I, were more than happy to let me move in and take the hospital’s late-night and weekend calls.
With time, hospital medicine becomes more of a young person’s game. As I move from being a newly minted attending to an old fogy, I’m happy to leave the work to the next generation.
My hospital work is now down to one-in-3 weekends and no weekdays. I’m not quite ready to give it up entirely and don’t want to turn my back on my call partners of 15 years. I still enjoy the challenge of the cases, the joking around with the nurses, and the family meetings to bring comfort and explain things as best I can.
But I can live without the late night runs, the driving back and forth, and the unpredictable hours. These days, my time at home is more valuable than it was when I started, and I envy those who keep banker’s hours.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Dr. Price light on ACA replacement details at Senate hearing
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
SHM launches Chapter Development Fund to enhance reach, impact of chapters
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.
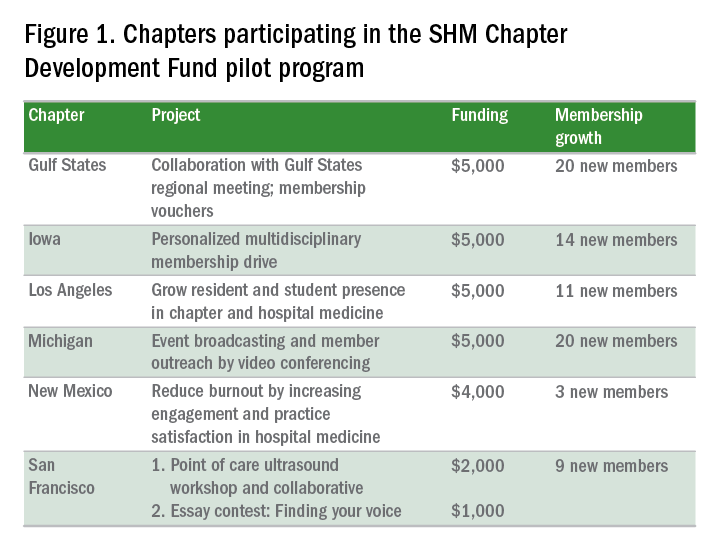
“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.

“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.

“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.