User login
Concomitant Herpes Zoster and Herpes Simplex Infection
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
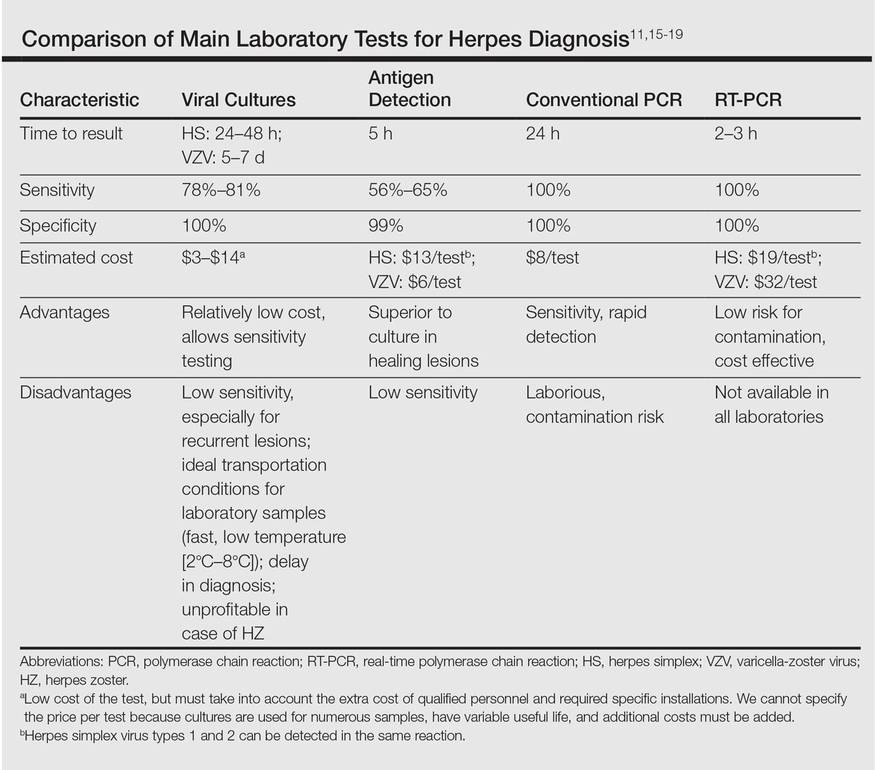
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).
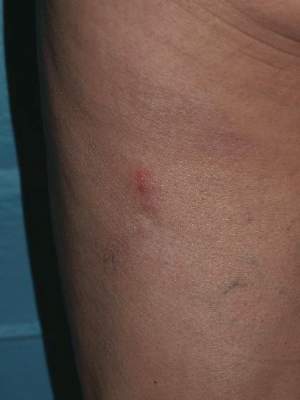
Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.
In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).

Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.
In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).

Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.
In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.
The latest treatments for urinary and fecal incontinence: Which hold water?
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
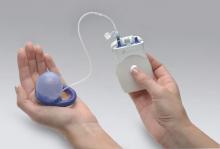
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
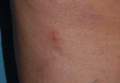
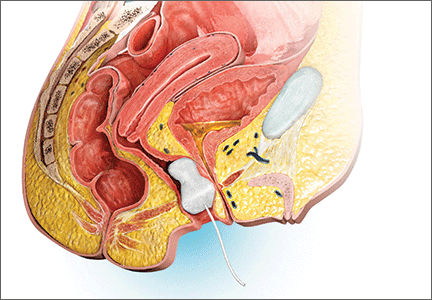
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
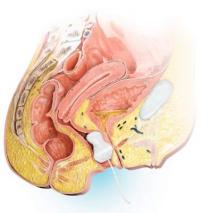
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
In this Article
- New OTC option for SUI
- Second-line OAB treatments
- Promising vaginal insert for fecal incontinence
AATS Graham Foundation Awards Deadline: February 15!
Applications are now open for...
Every Heartbeat Matters Valve Disease Fellowship
Applications for the second award cycle of the Every Heartbeat Matters Valve Fellowship — a joint project of the AATS Graham and Edwards Lifesciences Foundation — are now open. The mission of Every Heartbeat Matters is to impact the global burden of heart valve disease by serving one million people by 2020.
The fellowship’s goal is to enhance the knowledge and skills of practicing cardiothoracic surgeons who are already treating or committed to treating underserved patients worldwide. It provides up to three months of advanced heart valve disease training and education at a host institution.
More information/application
Deadline: February 15, 2016
2016 Graham Surgical Investigator Program
The Graham Surgical Investigator Program supports innovative clinical or translational research for young cardiothoracic surgeons. Topics include, but are not limited to, topics related to outcomes research, as well as robotically assisted surgery, minimally invasive surgery or other applications of new and innovative technologies in cardiothoracic surgery.
Up to four proposals will be funded in 2016.
Thanks to the generosity of Ethicon, Intuitive Surgical, Dr. Mehmet Oz and AATS.
More information/application
Deadline: February 15, 2016
Applications are now open for...
Every Heartbeat Matters Valve Disease Fellowship
Applications for the second award cycle of the Every Heartbeat Matters Valve Fellowship — a joint project of the AATS Graham and Edwards Lifesciences Foundation — are now open. The mission of Every Heartbeat Matters is to impact the global burden of heart valve disease by serving one million people by 2020.
The fellowship’s goal is to enhance the knowledge and skills of practicing cardiothoracic surgeons who are already treating or committed to treating underserved patients worldwide. It provides up to three months of advanced heart valve disease training and education at a host institution.
More information/application
Deadline: February 15, 2016
2016 Graham Surgical Investigator Program
The Graham Surgical Investigator Program supports innovative clinical or translational research for young cardiothoracic surgeons. Topics include, but are not limited to, topics related to outcomes research, as well as robotically assisted surgery, minimally invasive surgery or other applications of new and innovative technologies in cardiothoracic surgery.
Up to four proposals will be funded in 2016.
Thanks to the generosity of Ethicon, Intuitive Surgical, Dr. Mehmet Oz and AATS.
More information/application
Deadline: February 15, 2016
Applications are now open for...
Every Heartbeat Matters Valve Disease Fellowship
Applications for the second award cycle of the Every Heartbeat Matters Valve Fellowship — a joint project of the AATS Graham and Edwards Lifesciences Foundation — are now open. The mission of Every Heartbeat Matters is to impact the global burden of heart valve disease by serving one million people by 2020.
The fellowship’s goal is to enhance the knowledge and skills of practicing cardiothoracic surgeons who are already treating or committed to treating underserved patients worldwide. It provides up to three months of advanced heart valve disease training and education at a host institution.
More information/application
Deadline: February 15, 2016
2016 Graham Surgical Investigator Program
The Graham Surgical Investigator Program supports innovative clinical or translational research for young cardiothoracic surgeons. Topics include, but are not limited to, topics related to outcomes research, as well as robotically assisted surgery, minimally invasive surgery or other applications of new and innovative technologies in cardiothoracic surgery.
Up to four proposals will be funded in 2016.
Thanks to the generosity of Ethicon, Intuitive Surgical, Dr. Mehmet Oz and AATS.
More information/application
Deadline: February 15, 2016
Save the Date: AATS Surgical Patient Safety Course, June 2016
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Improving patient care remains a constant mission for all members of the surgical team. The AATS Surgical Patient Safety Course has been designed to promote a culture of safety and reduce preventable patient harm by engaging, educating, and equipping members of the cardiothoracic surgical team in the multidimensional approach to patient safety.
The course — which features didactic and interactive presentations and focused workshops — will provide attendees with essential patient safety knowledge, skills, and attitudes aimed at improving care in the present and future.
Preliminary Program Now Online
The Patient Safety program includes team training and simulaltion experiences.
Surgical Team Registration
Register three or more members from the same institution and receive one complimentary Healthcare Professional registration. We encourage you to send the entire team to Boston this June for this important course.
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Improving patient care remains a constant mission for all members of the surgical team. The AATS Surgical Patient Safety Course has been designed to promote a culture of safety and reduce preventable patient harm by engaging, educating, and equipping members of the cardiothoracic surgical team in the multidimensional approach to patient safety.
The course — which features didactic and interactive presentations and focused workshops — will provide attendees with essential patient safety knowledge, skills, and attitudes aimed at improving care in the present and future.
Preliminary Program Now Online
The Patient Safety program includes team training and simulaltion experiences.
Surgical Team Registration
Register three or more members from the same institution and receive one complimentary Healthcare Professional registration. We encourage you to send the entire team to Boston this June for this important course.
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Improving patient care remains a constant mission for all members of the surgical team. The AATS Surgical Patient Safety Course has been designed to promote a culture of safety and reduce preventable patient harm by engaging, educating, and equipping members of the cardiothoracic surgical team in the multidimensional approach to patient safety.
The course — which features didactic and interactive presentations and focused workshops — will provide attendees with essential patient safety knowledge, skills, and attitudes aimed at improving care in the present and future.
Preliminary Program Now Online
The Patient Safety program includes team training and simulaltion experiences.
Surgical Team Registration
Register three or more members from the same institution and receive one complimentary Healthcare Professional registration. We encourage you to send the entire team to Boston this June for this important course.
Focus on Thoracic Surgery: Lung and Esophageal Cancer/China This March
March 19-20, 2016
Hilton Shanghai Hongqiao
Shanghai, China
Program Directors
Haiquan Chen
Ke-Neng Chen
G. Alec Patterson
David J. Sugarbaker
Preliminary Program Now Available
Overview
Lung and esophageal disease remain a global concern as two of the deadliest issues facing patients and thoracic surgeons. The 2016 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex nature of treating both lung and esophageal disease while also discussing innovative approaches to enhancing patient care.
The two-day program features a mixture of lectures, relevant case studies, panel discussions, and videos. The faculty includes local and internationally recognized experts in lung and esophageal disease ensuring that attendees will receive insights from a broad spectrum of leaders thoracic surgery field.
March 19-20, 2016
Hilton Shanghai Hongqiao
Shanghai, China
Program Directors
Haiquan Chen
Ke-Neng Chen
G. Alec Patterson
David J. Sugarbaker
Preliminary Program Now Available
Overview
Lung and esophageal disease remain a global concern as two of the deadliest issues facing patients and thoracic surgeons. The 2016 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex nature of treating both lung and esophageal disease while also discussing innovative approaches to enhancing patient care.
The two-day program features a mixture of lectures, relevant case studies, panel discussions, and videos. The faculty includes local and internationally recognized experts in lung and esophageal disease ensuring that attendees will receive insights from a broad spectrum of leaders thoracic surgery field.
March 19-20, 2016
Hilton Shanghai Hongqiao
Shanghai, China
Program Directors
Haiquan Chen
Ke-Neng Chen
G. Alec Patterson
David J. Sugarbaker
Preliminary Program Now Available
Overview
Lung and esophageal disease remain a global concern as two of the deadliest issues facing patients and thoracic surgeons. The 2016 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex nature of treating both lung and esophageal disease while also discussing innovative approaches to enhancing patient care.
The two-day program features a mixture of lectures, relevant case studies, panel discussions, and videos. The faculty includes local and internationally recognized experts in lung and esophageal disease ensuring that attendees will receive insights from a broad spectrum of leaders thoracic surgery field.
Teamwork, Part 1: Should a mental health specialist be on site?
Dr. Lillian M. Beard discusses the challenges of getting reimbursed for providing mental and behavioral health care within her pediatric practice. Addressing her patients’ mental health needs can take three of her regular appointment times. “I am never adequately reimbursed for the time that it really takes,” she says. Having a mental health specialist on site might be one possibility, but is this model feasible? In this video, Dr. Beard, Dr. April Barbour, and Dr. Lorenzo Norris explore ways primary care and psychiatry can work together to provide patients with the best possible care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Lillian M. Beard discusses the challenges of getting reimbursed for providing mental and behavioral health care within her pediatric practice. Addressing her patients’ mental health needs can take three of her regular appointment times. “I am never adequately reimbursed for the time that it really takes,” she says. Having a mental health specialist on site might be one possibility, but is this model feasible? In this video, Dr. Beard, Dr. April Barbour, and Dr. Lorenzo Norris explore ways primary care and psychiatry can work together to provide patients with the best possible care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Lillian M. Beard discusses the challenges of getting reimbursed for providing mental and behavioral health care within her pediatric practice. Addressing her patients’ mental health needs can take three of her regular appointment times. “I am never adequately reimbursed for the time that it really takes,” she says. Having a mental health specialist on site might be one possibility, but is this model feasible? In this video, Dr. Beard, Dr. April Barbour, and Dr. Lorenzo Norris explore ways primary care and psychiatry can work together to provide patients with the best possible care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Teamwork, Part 2: Primary care’s frontline role
The role of primary care physicians in recognizing patients with behavioral and mental health problems is critical, psychiatrist Dr. David Pickar says. “That is what you do for a living. You find out these things. I want to make sure that the primary care physician [who] may be watching this understands he or she is not just the first line, but he or she has good skills at observing what is going on with the patient.” The primary care physician also has a relationship with the patient, psychiatrist Dr. Lorenzo Norris says. “The patient is more inclined to listen to [the primary care physician] than to just some random specialist.” In this video, Dr. Pickar and Dr. Norris discuss the importance of communicating about shared patients – not through electronic medical records, but through true dialogue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The role of primary care physicians in recognizing patients with behavioral and mental health problems is critical, psychiatrist Dr. David Pickar says. “That is what you do for a living. You find out these things. I want to make sure that the primary care physician [who] may be watching this understands he or she is not just the first line, but he or she has good skills at observing what is going on with the patient.” The primary care physician also has a relationship with the patient, psychiatrist Dr. Lorenzo Norris says. “The patient is more inclined to listen to [the primary care physician] than to just some random specialist.” In this video, Dr. Pickar and Dr. Norris discuss the importance of communicating about shared patients – not through electronic medical records, but through true dialogue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The role of primary care physicians in recognizing patients with behavioral and mental health problems is critical, psychiatrist Dr. David Pickar says. “That is what you do for a living. You find out these things. I want to make sure that the primary care physician [who] may be watching this understands he or she is not just the first line, but he or she has good skills at observing what is going on with the patient.” The primary care physician also has a relationship with the patient, psychiatrist Dr. Lorenzo Norris says. “The patient is more inclined to listen to [the primary care physician] than to just some random specialist.” In this video, Dr. Pickar and Dr. Norris discuss the importance of communicating about shared patients – not through electronic medical records, but through true dialogue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Teamwork, Part 3: How much of the burden can primary care physicians shoulder?
Primary care physicians have the potential to detect “distress” diagnoses and mental or behavioral disorders in most patients who come to the office, noted Dr. Lawrence “Bopper” Deyton, senior associate dean for clinical public health and professor of medicine and health policy at George Washington University.
If one embraces the Acceptable Care Act’s priorities of improving quality of care, reducing cost, and improving patient satisfaction, “Aren’t we at the cusp of a reimbursement system that should reward for that?” he asks.
Kennedy Forum policy director Lauren Alfred says there’s only so much that can be done. “Then at some point, we have to talk about collaborative care ... and where we’re going to bring specialists into the equation.”
In this video, Dr. Deyton and Ms. Alfred discuss how much mental health care primary care physicians should be asked to shoulder – and when such care should be more collaborative.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Primary care physicians have the potential to detect “distress” diagnoses and mental or behavioral disorders in most patients who come to the office, noted Dr. Lawrence “Bopper” Deyton, senior associate dean for clinical public health and professor of medicine and health policy at George Washington University.
If one embraces the Acceptable Care Act’s priorities of improving quality of care, reducing cost, and improving patient satisfaction, “Aren’t we at the cusp of a reimbursement system that should reward for that?” he asks.
Kennedy Forum policy director Lauren Alfred says there’s only so much that can be done. “Then at some point, we have to talk about collaborative care ... and where we’re going to bring specialists into the equation.”
In this video, Dr. Deyton and Ms. Alfred discuss how much mental health care primary care physicians should be asked to shoulder – and when such care should be more collaborative.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Primary care physicians have the potential to detect “distress” diagnoses and mental or behavioral disorders in most patients who come to the office, noted Dr. Lawrence “Bopper” Deyton, senior associate dean for clinical public health and professor of medicine and health policy at George Washington University.
If one embraces the Acceptable Care Act’s priorities of improving quality of care, reducing cost, and improving patient satisfaction, “Aren’t we at the cusp of a reimbursement system that should reward for that?” he asks.
Kennedy Forum policy director Lauren Alfred says there’s only so much that can be done. “Then at some point, we have to talk about collaborative care ... and where we’re going to bring specialists into the equation.”
In this video, Dr. Deyton and Ms. Alfred discuss how much mental health care primary care physicians should be asked to shoulder – and when such care should be more collaborative.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Teamwork, Part 4: Obstacles to paying behavioral health partner
Primary care physicians want a collaborative approach to mental and behavioral health care, according to Dr. April Barbour, director of general internal medicine and the primary care residency program at George Washington University, Washington. “That’s a lot of what we teach and train our residents ... and this allows us to truly treat the whole patient.”
Training models are out there, but the question of whether funding is available to keep these models viable is an issue.
“Every professional is going to look to be compensated for his or her time and input,” explains Dr. Lillian M. Beard, physician director of Children’s Pediatricians and Associates, Silver Spring, Md. “We have to look at mind, body, spirit. It’s the whole thing.”
In this video, Dr. Barbour, Dr. Beard, and psychiatrists Dr. Lorenzo Norris and Dr. David Pickar discuss how to make primary care practices responsive to patients in ways that are both holistic and cost effective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Primary care physicians want a collaborative approach to mental and behavioral health care, according to Dr. April Barbour, director of general internal medicine and the primary care residency program at George Washington University, Washington. “That’s a lot of what we teach and train our residents ... and this allows us to truly treat the whole patient.”
Training models are out there, but the question of whether funding is available to keep these models viable is an issue.
“Every professional is going to look to be compensated for his or her time and input,” explains Dr. Lillian M. Beard, physician director of Children’s Pediatricians and Associates, Silver Spring, Md. “We have to look at mind, body, spirit. It’s the whole thing.”
In this video, Dr. Barbour, Dr. Beard, and psychiatrists Dr. Lorenzo Norris and Dr. David Pickar discuss how to make primary care practices responsive to patients in ways that are both holistic and cost effective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Primary care physicians want a collaborative approach to mental and behavioral health care, according to Dr. April Barbour, director of general internal medicine and the primary care residency program at George Washington University, Washington. “That’s a lot of what we teach and train our residents ... and this allows us to truly treat the whole patient.”
Training models are out there, but the question of whether funding is available to keep these models viable is an issue.
“Every professional is going to look to be compensated for his or her time and input,” explains Dr. Lillian M. Beard, physician director of Children’s Pediatricians and Associates, Silver Spring, Md. “We have to look at mind, body, spirit. It’s the whole thing.”
In this video, Dr. Barbour, Dr. Beard, and psychiatrists Dr. Lorenzo Norris and Dr. David Pickar discuss how to make primary care practices responsive to patients in ways that are both holistic and cost effective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In refractory AF, think weight loss before ablation
SNOWMASS, COLO. – Don’t be in a rush to refer a patient with drug-refractory, symptomatic atrial fibrillation (AF) for catheter ablation of the arrhythmia, a prominent electrophysiologist advised at the Annual Cardiovascular Conference at Snowmass.
“AF ablation is not salvation – and that’s coming from somebody who does these procedures. One really needs to be very selective in referring patients for this,” said Dr. N.A. Mark Estes III, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
Misconceptions about AF catheter ablation outcomes abound among nonelectrophysiologists. Results have often been overstated, Dr. Estes continued. And there’s a far more attractive alternative treatment option for those AF patients who are overweight or obese: weight loss.
In the Australian LEGACY study, which he considers practice changing, patients with AF who had a body mass index of at least 27 kg/m2 who participated in a simple structured weight management program and achieved a sustained loss of at least 10% of their body weight had a 65% reduction in their AF burden as objectively documented by repeated 7-day ambulatory monitoring over 5 years of follow-up. Moreover, 46% of patients who maintained that degree of weight reduction were totally free of AF without use of drugs or ablation procedures (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69).
In a related study, the Australian investigators, led by Dr. Rajeev K. Pathak of the University of Adelaide, showed in the same study population that participation in a tailored exercise program paid added dividends on top of the weight loss. Patients who achieved at least a 2-MET increase in cardiorespiratory fitness had a significantly greater rate of freedom from AF than those who didn’t reach that fitness threshold (J Am Coll Cardiol. Sep 1;66[9]:985-96).
“The data are compelling for improved outcomes, including reduced AF burden, with lifestyle modification in obese patients with AF. This is first-line therapy. You can bet it will be in the guidelines soon. It should be in your practice now,” Dr. Estes declared.
“The starting point is weight reduction, even before sending patients to an electrophysiologist for ablation,” he continued. “And if you’ve got patients on drugs who’ve had ablation in whom there continues to be AF, weight reduction – particularly reaching that 10% threshold – results in a dramatic decline in the burden of AF,” he said.
One of the common misconceptions about catheter ablation for AF is that if the pulmonary vein isolation procedure is successful in eliminating the arrhythmia, then the patient can discontinue oral anticoagulant therapy.
“That rationale, while logical, doesn’t really hold up. In many of the ablation trials, including the AFFIRM trial, if you discontinue anticoagulation in patients in sinus rhythm the stroke rate goes back to the same as in patients with AF,” according to the cardiologist.
In a meta-analysis of prospective studies published through 2007, the single-procedure success rate for radiofrequency ablation in achieving sinus rhythm without the use of antiarrhythmic drugs was 57%, climbing to 71% with multiple ablation procedures. In contrast, antiarrhythmic drugs were substantially less successful, with about a 50% success rate as compared with a 25% placebo response (Circ Arrhythm Electrophysiol. 2009 Aug;2[4]:349-61).
“It’s notable that antiarrhythmic drug development has almost been stopped because the drugs don’t work, with the possible exception of amiodarone, which requires an individualized risk/benefit assessment,” according to Dr. Estes.
There is a major caveat regarding the ablation studies: They’ve mainly enrolled patients who are in their 50s, when AF is far less common than in later decades.
“Whether these results are going to hold up long-term in elderly patients who are hypertensive, diabetic, and may have sleep apnea really remains an unanswered question,” Dr. Estes observed.
Also, significant periprocedural complications occur in roughly 1 in 20 patients undergoing radiofrequency catheter ablation, although the safety data for cryoablation look somewhat better, he continued.
Dr. Estes predicted that the future of catheter ablation of AF hangs on three major ongoing rigorous randomized clinical trials comparing it to drug therapy with hard endpoints including all-cause mortality and cardiovascular hospitalizations. These are CASTLE-AF, with 420 patients; CABANA, with 2,200; and the German EAST study, with roughly 3,000 patients. Results are expected in 2018-2019.
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
SNOWMASS, COLO. – Don’t be in a rush to refer a patient with drug-refractory, symptomatic atrial fibrillation (AF) for catheter ablation of the arrhythmia, a prominent electrophysiologist advised at the Annual Cardiovascular Conference at Snowmass.
“AF ablation is not salvation – and that’s coming from somebody who does these procedures. One really needs to be very selective in referring patients for this,” said Dr. N.A. Mark Estes III, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
Misconceptions about AF catheter ablation outcomes abound among nonelectrophysiologists. Results have often been overstated, Dr. Estes continued. And there’s a far more attractive alternative treatment option for those AF patients who are overweight or obese: weight loss.
In the Australian LEGACY study, which he considers practice changing, patients with AF who had a body mass index of at least 27 kg/m2 who participated in a simple structured weight management program and achieved a sustained loss of at least 10% of their body weight had a 65% reduction in their AF burden as objectively documented by repeated 7-day ambulatory monitoring over 5 years of follow-up. Moreover, 46% of patients who maintained that degree of weight reduction were totally free of AF without use of drugs or ablation procedures (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69).
In a related study, the Australian investigators, led by Dr. Rajeev K. Pathak of the University of Adelaide, showed in the same study population that participation in a tailored exercise program paid added dividends on top of the weight loss. Patients who achieved at least a 2-MET increase in cardiorespiratory fitness had a significantly greater rate of freedom from AF than those who didn’t reach that fitness threshold (J Am Coll Cardiol. Sep 1;66[9]:985-96).
“The data are compelling for improved outcomes, including reduced AF burden, with lifestyle modification in obese patients with AF. This is first-line therapy. You can bet it will be in the guidelines soon. It should be in your practice now,” Dr. Estes declared.
“The starting point is weight reduction, even before sending patients to an electrophysiologist for ablation,” he continued. “And if you’ve got patients on drugs who’ve had ablation in whom there continues to be AF, weight reduction – particularly reaching that 10% threshold – results in a dramatic decline in the burden of AF,” he said.
One of the common misconceptions about catheter ablation for AF is that if the pulmonary vein isolation procedure is successful in eliminating the arrhythmia, then the patient can discontinue oral anticoagulant therapy.
“That rationale, while logical, doesn’t really hold up. In many of the ablation trials, including the AFFIRM trial, if you discontinue anticoagulation in patients in sinus rhythm the stroke rate goes back to the same as in patients with AF,” according to the cardiologist.
In a meta-analysis of prospective studies published through 2007, the single-procedure success rate for radiofrequency ablation in achieving sinus rhythm without the use of antiarrhythmic drugs was 57%, climbing to 71% with multiple ablation procedures. In contrast, antiarrhythmic drugs were substantially less successful, with about a 50% success rate as compared with a 25% placebo response (Circ Arrhythm Electrophysiol. 2009 Aug;2[4]:349-61).
“It’s notable that antiarrhythmic drug development has almost been stopped because the drugs don’t work, with the possible exception of amiodarone, which requires an individualized risk/benefit assessment,” according to Dr. Estes.
There is a major caveat regarding the ablation studies: They’ve mainly enrolled patients who are in their 50s, when AF is far less common than in later decades.
“Whether these results are going to hold up long-term in elderly patients who are hypertensive, diabetic, and may have sleep apnea really remains an unanswered question,” Dr. Estes observed.
Also, significant periprocedural complications occur in roughly 1 in 20 patients undergoing radiofrequency catheter ablation, although the safety data for cryoablation look somewhat better, he continued.
Dr. Estes predicted that the future of catheter ablation of AF hangs on three major ongoing rigorous randomized clinical trials comparing it to drug therapy with hard endpoints including all-cause mortality and cardiovascular hospitalizations. These are CASTLE-AF, with 420 patients; CABANA, with 2,200; and the German EAST study, with roughly 3,000 patients. Results are expected in 2018-2019.
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
SNOWMASS, COLO. – Don’t be in a rush to refer a patient with drug-refractory, symptomatic atrial fibrillation (AF) for catheter ablation of the arrhythmia, a prominent electrophysiologist advised at the Annual Cardiovascular Conference at Snowmass.
“AF ablation is not salvation – and that’s coming from somebody who does these procedures. One really needs to be very selective in referring patients for this,” said Dr. N.A. Mark Estes III, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
Misconceptions about AF catheter ablation outcomes abound among nonelectrophysiologists. Results have often been overstated, Dr. Estes continued. And there’s a far more attractive alternative treatment option for those AF patients who are overweight or obese: weight loss.
In the Australian LEGACY study, which he considers practice changing, patients with AF who had a body mass index of at least 27 kg/m2 who participated in a simple structured weight management program and achieved a sustained loss of at least 10% of their body weight had a 65% reduction in their AF burden as objectively documented by repeated 7-day ambulatory monitoring over 5 years of follow-up. Moreover, 46% of patients who maintained that degree of weight reduction were totally free of AF without use of drugs or ablation procedures (J Am Coll Cardiol. 2015 May 26;65[20]:2159-69).
In a related study, the Australian investigators, led by Dr. Rajeev K. Pathak of the University of Adelaide, showed in the same study population that participation in a tailored exercise program paid added dividends on top of the weight loss. Patients who achieved at least a 2-MET increase in cardiorespiratory fitness had a significantly greater rate of freedom from AF than those who didn’t reach that fitness threshold (J Am Coll Cardiol. Sep 1;66[9]:985-96).
“The data are compelling for improved outcomes, including reduced AF burden, with lifestyle modification in obese patients with AF. This is first-line therapy. You can bet it will be in the guidelines soon. It should be in your practice now,” Dr. Estes declared.
“The starting point is weight reduction, even before sending patients to an electrophysiologist for ablation,” he continued. “And if you’ve got patients on drugs who’ve had ablation in whom there continues to be AF, weight reduction – particularly reaching that 10% threshold – results in a dramatic decline in the burden of AF,” he said.
One of the common misconceptions about catheter ablation for AF is that if the pulmonary vein isolation procedure is successful in eliminating the arrhythmia, then the patient can discontinue oral anticoagulant therapy.
“That rationale, while logical, doesn’t really hold up. In many of the ablation trials, including the AFFIRM trial, if you discontinue anticoagulation in patients in sinus rhythm the stroke rate goes back to the same as in patients with AF,” according to the cardiologist.
In a meta-analysis of prospective studies published through 2007, the single-procedure success rate for radiofrequency ablation in achieving sinus rhythm without the use of antiarrhythmic drugs was 57%, climbing to 71% with multiple ablation procedures. In contrast, antiarrhythmic drugs were substantially less successful, with about a 50% success rate as compared with a 25% placebo response (Circ Arrhythm Electrophysiol. 2009 Aug;2[4]:349-61).
“It’s notable that antiarrhythmic drug development has almost been stopped because the drugs don’t work, with the possible exception of amiodarone, which requires an individualized risk/benefit assessment,” according to Dr. Estes.
There is a major caveat regarding the ablation studies: They’ve mainly enrolled patients who are in their 50s, when AF is far less common than in later decades.
“Whether these results are going to hold up long-term in elderly patients who are hypertensive, diabetic, and may have sleep apnea really remains an unanswered question,” Dr. Estes observed.
Also, significant periprocedural complications occur in roughly 1 in 20 patients undergoing radiofrequency catheter ablation, although the safety data for cryoablation look somewhat better, he continued.
Dr. Estes predicted that the future of catheter ablation of AF hangs on three major ongoing rigorous randomized clinical trials comparing it to drug therapy with hard endpoints including all-cause mortality and cardiovascular hospitalizations. These are CASTLE-AF, with 420 patients; CABANA, with 2,200; and the German EAST study, with roughly 3,000 patients. Results are expected in 2018-2019.
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS