User login
Donor CAR T cells treat aggressive ALL in infant

person treated with UCART19
Photo courtesy of GOSH
A hospital in the UK has reported success with the first-in-human use of an allogeneic chimeric antigen receptor (CAR) T-cell treatment.
The therapy, UCART19, helped treat an aggressive case of acute lymphoblastic leukemia (ALL) in an infant named Layla Richards.
Chemotherapy, a first transplant, and blinatumomab all failed to treat Layla’s disease. But UCART19 provided a bridge to a second transplant, and Layla
is now free of leukemia.
“We have only used this treatment on one very strong little girl, and we have to be cautious about claiming that this will be a suitable treatment option for all children,” said Waseem Qasim, MBBS, PhD, a professor at University College London’s Institute of Child Health and a consultant immunologist at Great Ormond Street Hospital (GOSH) in London, where Layla was treated.
“But this is a landmark in the use of new gene-engineering technology, and the effects for this child have been staggering. If replicated, it could represent a huge step forward in treating leukemia and other cancers.”
Layla’s history
Layla was born in June 2014 and, at 14 weeks old, was diagnosed with CD19+ ALL (t[11;19] rearrangement). Doctors at GOSH described her leukemia as “one of the most aggressive forms of the disease we have ever seen.”
Layla underwent several rounds of chemotherapy at GOSH and then received a transplant from a mismatched, unrelated donor. Seven weeks later, her leukemia had returned.
A second round of chemotherapy wasn’t an option, so Layla went on to receive blinatumomab. This, too, ultimately failed.
Layla’s family was unwilling to accept palliative care, so her doctors mentioned the possibility of using UCART19.
“The approach was looking incredibly successful in laboratory studies, and so when I heard there were no options left for treating this child’s disease, I thought, ‘Why don’t we use the new UCART19 cells?’” Dr Qasim said.
“The treatment was highly experimental, and we had to get special permissions, but she appeared ideally suited for this type of approach.”
UCART19 treatment
Before Layla received UCART19, investigators from GOSH, University College London, and the biotech company Cellectis had been developing off-the-shelf banks of the cells for use in clinical trials.
UCART19 consists of donor T cells modified using transcription activator-like effector nucleases (TALEN). Like some other CAR T-cell products, UCART19 is designed to target CD19+ cancer cells.
But UCART19 cells are also programmed to be insensitive to alemtuzumab. That way, a patient can receive the drug to prevent rejection of HLA-mismatched cells.
Before receiving UCART19, Layla was given vincristine, dexamethasone, and asparaginase, followed by fludarabine, cyclophosphamide, and alemtuzumab. She then received a single dose (4.5 x 106/kg) of UCART19.
After this, Layla spent several months in isolation to protect her from infections while her immune system was extremely weak. Within weeks of receiving UCART19, Layla demonstrated an immune response in the form of a rash.
The rash worsened, but, aside from that, Layla appeared to be well. To date, she has shown no signs of cytokine release syndrome.
She did, however, show signs of molecular and cytogenetic remission. Once she was deemed leukemia-free, Layla underwent a second transplant. One month later, she was well enough to go home. Today, Layla is still free of ALL.
More details on Layla’s story and UCART19 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 2046). Clinical trials of UCART19 (funded by Cellectis) are set to begin early next year. ![]()

person treated with UCART19
Photo courtesy of GOSH
A hospital in the UK has reported success with the first-in-human use of an allogeneic chimeric antigen receptor (CAR) T-cell treatment.
The therapy, UCART19, helped treat an aggressive case of acute lymphoblastic leukemia (ALL) in an infant named Layla Richards.
Chemotherapy, a first transplant, and blinatumomab all failed to treat Layla’s disease. But UCART19 provided a bridge to a second transplant, and Layla
is now free of leukemia.
“We have only used this treatment on one very strong little girl, and we have to be cautious about claiming that this will be a suitable treatment option for all children,” said Waseem Qasim, MBBS, PhD, a professor at University College London’s Institute of Child Health and a consultant immunologist at Great Ormond Street Hospital (GOSH) in London, where Layla was treated.
“But this is a landmark in the use of new gene-engineering technology, and the effects for this child have been staggering. If replicated, it could represent a huge step forward in treating leukemia and other cancers.”
Layla’s history
Layla was born in June 2014 and, at 14 weeks old, was diagnosed with CD19+ ALL (t[11;19] rearrangement). Doctors at GOSH described her leukemia as “one of the most aggressive forms of the disease we have ever seen.”
Layla underwent several rounds of chemotherapy at GOSH and then received a transplant from a mismatched, unrelated donor. Seven weeks later, her leukemia had returned.
A second round of chemotherapy wasn’t an option, so Layla went on to receive blinatumomab. This, too, ultimately failed.
Layla’s family was unwilling to accept palliative care, so her doctors mentioned the possibility of using UCART19.
“The approach was looking incredibly successful in laboratory studies, and so when I heard there were no options left for treating this child’s disease, I thought, ‘Why don’t we use the new UCART19 cells?’” Dr Qasim said.
“The treatment was highly experimental, and we had to get special permissions, but she appeared ideally suited for this type of approach.”
UCART19 treatment
Before Layla received UCART19, investigators from GOSH, University College London, and the biotech company Cellectis had been developing off-the-shelf banks of the cells for use in clinical trials.
UCART19 consists of donor T cells modified using transcription activator-like effector nucleases (TALEN). Like some other CAR T-cell products, UCART19 is designed to target CD19+ cancer cells.
But UCART19 cells are also programmed to be insensitive to alemtuzumab. That way, a patient can receive the drug to prevent rejection of HLA-mismatched cells.
Before receiving UCART19, Layla was given vincristine, dexamethasone, and asparaginase, followed by fludarabine, cyclophosphamide, and alemtuzumab. She then received a single dose (4.5 x 106/kg) of UCART19.
After this, Layla spent several months in isolation to protect her from infections while her immune system was extremely weak. Within weeks of receiving UCART19, Layla demonstrated an immune response in the form of a rash.
The rash worsened, but, aside from that, Layla appeared to be well. To date, she has shown no signs of cytokine release syndrome.
She did, however, show signs of molecular and cytogenetic remission. Once she was deemed leukemia-free, Layla underwent a second transplant. One month later, she was well enough to go home. Today, Layla is still free of ALL.
More details on Layla’s story and UCART19 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 2046). Clinical trials of UCART19 (funded by Cellectis) are set to begin early next year. ![]()

person treated with UCART19
Photo courtesy of GOSH
A hospital in the UK has reported success with the first-in-human use of an allogeneic chimeric antigen receptor (CAR) T-cell treatment.
The therapy, UCART19, helped treat an aggressive case of acute lymphoblastic leukemia (ALL) in an infant named Layla Richards.
Chemotherapy, a first transplant, and blinatumomab all failed to treat Layla’s disease. But UCART19 provided a bridge to a second transplant, and Layla
is now free of leukemia.
“We have only used this treatment on one very strong little girl, and we have to be cautious about claiming that this will be a suitable treatment option for all children,” said Waseem Qasim, MBBS, PhD, a professor at University College London’s Institute of Child Health and a consultant immunologist at Great Ormond Street Hospital (GOSH) in London, where Layla was treated.
“But this is a landmark in the use of new gene-engineering technology, and the effects for this child have been staggering. If replicated, it could represent a huge step forward in treating leukemia and other cancers.”
Layla’s history
Layla was born in June 2014 and, at 14 weeks old, was diagnosed with CD19+ ALL (t[11;19] rearrangement). Doctors at GOSH described her leukemia as “one of the most aggressive forms of the disease we have ever seen.”
Layla underwent several rounds of chemotherapy at GOSH and then received a transplant from a mismatched, unrelated donor. Seven weeks later, her leukemia had returned.
A second round of chemotherapy wasn’t an option, so Layla went on to receive blinatumomab. This, too, ultimately failed.
Layla’s family was unwilling to accept palliative care, so her doctors mentioned the possibility of using UCART19.
“The approach was looking incredibly successful in laboratory studies, and so when I heard there were no options left for treating this child’s disease, I thought, ‘Why don’t we use the new UCART19 cells?’” Dr Qasim said.
“The treatment was highly experimental, and we had to get special permissions, but she appeared ideally suited for this type of approach.”
UCART19 treatment
Before Layla received UCART19, investigators from GOSH, University College London, and the biotech company Cellectis had been developing off-the-shelf banks of the cells for use in clinical trials.
UCART19 consists of donor T cells modified using transcription activator-like effector nucleases (TALEN). Like some other CAR T-cell products, UCART19 is designed to target CD19+ cancer cells.
But UCART19 cells are also programmed to be insensitive to alemtuzumab. That way, a patient can receive the drug to prevent rejection of HLA-mismatched cells.
Before receiving UCART19, Layla was given vincristine, dexamethasone, and asparaginase, followed by fludarabine, cyclophosphamide, and alemtuzumab. She then received a single dose (4.5 x 106/kg) of UCART19.
After this, Layla spent several months in isolation to protect her from infections while her immune system was extremely weak. Within weeks of receiving UCART19, Layla demonstrated an immune response in the form of a rash.
The rash worsened, but, aside from that, Layla appeared to be well. To date, she has shown no signs of cytokine release syndrome.
She did, however, show signs of molecular and cytogenetic remission. Once she was deemed leukemia-free, Layla underwent a second transplant. One month later, she was well enough to go home. Today, Layla is still free of ALL.
More details on Layla’s story and UCART19 are scheduled to be presented at the 2015 ASH Annual Meeting (abstract 2046). Clinical trials of UCART19 (funded by Cellectis) are set to begin early next year. ![]()
Study challenges ‘textbook’ view of hematopoiesis

University Health Network
Results of a study published in Science challenge traditional ideas about how blood is made.
The findings suggest hematopoiesis does not occur through a gradual process consisting of multipotent, oligopotent, and unilineage progenitor stages.
Rather, hematopoietic stem cells (HSCs) mature into different types of blood cells quickly, and the process differs between early human development (fetal liver HSCs) and adulthood (HSCs from bone marrow).
The research indicates “that the whole classic ‘textbook’ view we thought we knew doesn’t actually even exist,” said study investigator John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
He and his colleagues mapped the lineage potential of nearly 3000 single cells from 33 different populations of HSCs obtained from human blood samples taken at various life stages and ages.
The team’s discoveries build on research published in Science in 2011. In that paper, Dr Dick and his colleagues described isolating an HSC in its purest form—as a single cell capable of regenerating the entire blood system.
“Four years ago, when we isolated the pure stem cell, we realized we had also uncovered populations of stem-cell like ‘daughter’ cells that we thought at the time were other types of stem cells,” Dr Dick said.
“When we burrowed further to study these ‘daughters,’ we discovered they were actually already mature blood lineages. In other words, lineages that had broken off almost immediately from the stem cell compartment and had not developed downstream through the slow, gradual ‘textbook’ process.”
“So in human blood formation, everything begins with the stem cell, which is the executive decision-maker quickly driving the process that replenishes blood at a daily rate that exceeds 300 billion cells.”
The investigators believe this work could help advance the manufacture of blood cells in the lab, and it should aid the study of blood disorders.
“Our discovery means we will be able to understand far better a wide variety of human blood disorders and diseases—from anemia . . . to leukemia,” Dr Dick said. “Think of it as moving from the old world of black-and-white television into the new world of high-definition.” ![]()

University Health Network
Results of a study published in Science challenge traditional ideas about how blood is made.
The findings suggest hematopoiesis does not occur through a gradual process consisting of multipotent, oligopotent, and unilineage progenitor stages.
Rather, hematopoietic stem cells (HSCs) mature into different types of blood cells quickly, and the process differs between early human development (fetal liver HSCs) and adulthood (HSCs from bone marrow).
The research indicates “that the whole classic ‘textbook’ view we thought we knew doesn’t actually even exist,” said study investigator John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
He and his colleagues mapped the lineage potential of nearly 3000 single cells from 33 different populations of HSCs obtained from human blood samples taken at various life stages and ages.
The team’s discoveries build on research published in Science in 2011. In that paper, Dr Dick and his colleagues described isolating an HSC in its purest form—as a single cell capable of regenerating the entire blood system.
“Four years ago, when we isolated the pure stem cell, we realized we had also uncovered populations of stem-cell like ‘daughter’ cells that we thought at the time were other types of stem cells,” Dr Dick said.
“When we burrowed further to study these ‘daughters,’ we discovered they were actually already mature blood lineages. In other words, lineages that had broken off almost immediately from the stem cell compartment and had not developed downstream through the slow, gradual ‘textbook’ process.”
“So in human blood formation, everything begins with the stem cell, which is the executive decision-maker quickly driving the process that replenishes blood at a daily rate that exceeds 300 billion cells.”
The investigators believe this work could help advance the manufacture of blood cells in the lab, and it should aid the study of blood disorders.
“Our discovery means we will be able to understand far better a wide variety of human blood disorders and diseases—from anemia . . . to leukemia,” Dr Dick said. “Think of it as moving from the old world of black-and-white television into the new world of high-definition.” ![]()

University Health Network
Results of a study published in Science challenge traditional ideas about how blood is made.
The findings suggest hematopoiesis does not occur through a gradual process consisting of multipotent, oligopotent, and unilineage progenitor stages.
Rather, hematopoietic stem cells (HSCs) mature into different types of blood cells quickly, and the process differs between early human development (fetal liver HSCs) and adulthood (HSCs from bone marrow).
The research indicates “that the whole classic ‘textbook’ view we thought we knew doesn’t actually even exist,” said study investigator John Dick, PhD, of Princess Margaret Cancer Centre in Toronto, Ontario, Canada.
He and his colleagues mapped the lineage potential of nearly 3000 single cells from 33 different populations of HSCs obtained from human blood samples taken at various life stages and ages.
The team’s discoveries build on research published in Science in 2011. In that paper, Dr Dick and his colleagues described isolating an HSC in its purest form—as a single cell capable of regenerating the entire blood system.
“Four years ago, when we isolated the pure stem cell, we realized we had also uncovered populations of stem-cell like ‘daughter’ cells that we thought at the time were other types of stem cells,” Dr Dick said.
“When we burrowed further to study these ‘daughters,’ we discovered they were actually already mature blood lineages. In other words, lineages that had broken off almost immediately from the stem cell compartment and had not developed downstream through the slow, gradual ‘textbook’ process.”
“So in human blood formation, everything begins with the stem cell, which is the executive decision-maker quickly driving the process that replenishes blood at a daily rate that exceeds 300 billion cells.”
The investigators believe this work could help advance the manufacture of blood cells in the lab, and it should aid the study of blood disorders.
“Our discovery means we will be able to understand far better a wide variety of human blood disorders and diseases—from anemia . . . to leukemia,” Dr Dick said. “Think of it as moving from the old world of black-and-white television into the new world of high-definition.” ![]()
Jury still out on cannabinoid therapy for rheumatic diseases
Only four short-term, randomized trials have addressed the safety, efficacy, and tolerability of cannabinoids for treating rheumatic diseases, and all have methodologic weaknesses and a high risk of bias, according to a qualitative review of data published since 1946.
“There is low-quality evidence suggesting that cannabinoids may be associated with improvements in pain and sleep quality in rheumatoid arthritis and fibromyalgia. Clinical positive effects for the studies assessed in this review must be balanced by the reported adverse events,” wrote Dr. Mary-Ann Fitzcharles of McGill University, Montreal, and her colleagues (Arthritis Care Res. 2015 Nov 9. doi: 10.1002/acr.22727).
The four randomized clinical trials, ranging in duration from 2 to 8 weeks, included one with 58 rheumatoid arthritis patients, two with a total of 71 fibromyalgia patients, and one with 74 osteoarthritis patients. Three trials met their respective primary endpoints and found a statistically significant effect of cannabinoids on pain (in two studies), sleep (in two studies), and improved quality of life (in one study). The investigators noted a high incidence (generally observed in 25%-50% of subjects) of mild to moderate side effects (dizziness, drowsiness, nausea, dry mouth) and many methodologic weaknesses in the evaluated data. The study that tested a fatty acid amide hydrolase inhibitor in osteoarthritis patients was stopped early due to futility. The other three completed studies were associated with an overall high risk of bias.
One of the two fibromyalgia studies was a randomized, double-blind, placebo-controlled trial conducted in 2008 that assessed nabilone in 40 fibromyalgia patients. Nabilone treatment led to significant decreases in the visual analog scale for pain (–2.04, P less than .02) at 4 weeks. A second fibromyalgia trial from 2010 compared amitriptyline to nabilone in 31 subjects and found the latter agent to be superior on the primary endpoint of difference in score on the Insomnia Severity Index (difference = 3.2; 95% confidence interval, 1.2-5.3). A 2006 rheumatoid arthritis trial found that cannabis-based Sativex demonstrated a clinically meaningful advantage over placebo for the primary endpoint of morning pain on movement (difference of –0.95, 95% CI, –1.83 to –0.02; P = .044). No trials assessed herbal cannabis.
“It is not currently possible to recommend this category of treatments as therapy for patients with rheumatic diseases. Any conclusions based on these studies remain tenuous and call for larger, well controlled clinical trials to better understand potential benefits and risks as pertaining to rheumatic conditions,” Dr. Fitzcharles and her coauthors wrote.
No relevant financial disclosures were reported.
rhnews@frontlinemedcom.com
Only four short-term, randomized trials have addressed the safety, efficacy, and tolerability of cannabinoids for treating rheumatic diseases, and all have methodologic weaknesses and a high risk of bias, according to a qualitative review of data published since 1946.
“There is low-quality evidence suggesting that cannabinoids may be associated with improvements in pain and sleep quality in rheumatoid arthritis and fibromyalgia. Clinical positive effects for the studies assessed in this review must be balanced by the reported adverse events,” wrote Dr. Mary-Ann Fitzcharles of McGill University, Montreal, and her colleagues (Arthritis Care Res. 2015 Nov 9. doi: 10.1002/acr.22727).
The four randomized clinical trials, ranging in duration from 2 to 8 weeks, included one with 58 rheumatoid arthritis patients, two with a total of 71 fibromyalgia patients, and one with 74 osteoarthritis patients. Three trials met their respective primary endpoints and found a statistically significant effect of cannabinoids on pain (in two studies), sleep (in two studies), and improved quality of life (in one study). The investigators noted a high incidence (generally observed in 25%-50% of subjects) of mild to moderate side effects (dizziness, drowsiness, nausea, dry mouth) and many methodologic weaknesses in the evaluated data. The study that tested a fatty acid amide hydrolase inhibitor in osteoarthritis patients was stopped early due to futility. The other three completed studies were associated with an overall high risk of bias.
One of the two fibromyalgia studies was a randomized, double-blind, placebo-controlled trial conducted in 2008 that assessed nabilone in 40 fibromyalgia patients. Nabilone treatment led to significant decreases in the visual analog scale for pain (–2.04, P less than .02) at 4 weeks. A second fibromyalgia trial from 2010 compared amitriptyline to nabilone in 31 subjects and found the latter agent to be superior on the primary endpoint of difference in score on the Insomnia Severity Index (difference = 3.2; 95% confidence interval, 1.2-5.3). A 2006 rheumatoid arthritis trial found that cannabis-based Sativex demonstrated a clinically meaningful advantage over placebo for the primary endpoint of morning pain on movement (difference of –0.95, 95% CI, –1.83 to –0.02; P = .044). No trials assessed herbal cannabis.
“It is not currently possible to recommend this category of treatments as therapy for patients with rheumatic diseases. Any conclusions based on these studies remain tenuous and call for larger, well controlled clinical trials to better understand potential benefits and risks as pertaining to rheumatic conditions,” Dr. Fitzcharles and her coauthors wrote.
No relevant financial disclosures were reported.
rhnews@frontlinemedcom.com
Only four short-term, randomized trials have addressed the safety, efficacy, and tolerability of cannabinoids for treating rheumatic diseases, and all have methodologic weaknesses and a high risk of bias, according to a qualitative review of data published since 1946.
“There is low-quality evidence suggesting that cannabinoids may be associated with improvements in pain and sleep quality in rheumatoid arthritis and fibromyalgia. Clinical positive effects for the studies assessed in this review must be balanced by the reported adverse events,” wrote Dr. Mary-Ann Fitzcharles of McGill University, Montreal, and her colleagues (Arthritis Care Res. 2015 Nov 9. doi: 10.1002/acr.22727).
The four randomized clinical trials, ranging in duration from 2 to 8 weeks, included one with 58 rheumatoid arthritis patients, two with a total of 71 fibromyalgia patients, and one with 74 osteoarthritis patients. Three trials met their respective primary endpoints and found a statistically significant effect of cannabinoids on pain (in two studies), sleep (in two studies), and improved quality of life (in one study). The investigators noted a high incidence (generally observed in 25%-50% of subjects) of mild to moderate side effects (dizziness, drowsiness, nausea, dry mouth) and many methodologic weaknesses in the evaluated data. The study that tested a fatty acid amide hydrolase inhibitor in osteoarthritis patients was stopped early due to futility. The other three completed studies were associated with an overall high risk of bias.
One of the two fibromyalgia studies was a randomized, double-blind, placebo-controlled trial conducted in 2008 that assessed nabilone in 40 fibromyalgia patients. Nabilone treatment led to significant decreases in the visual analog scale for pain (–2.04, P less than .02) at 4 weeks. A second fibromyalgia trial from 2010 compared amitriptyline to nabilone in 31 subjects and found the latter agent to be superior on the primary endpoint of difference in score on the Insomnia Severity Index (difference = 3.2; 95% confidence interval, 1.2-5.3). A 2006 rheumatoid arthritis trial found that cannabis-based Sativex demonstrated a clinically meaningful advantage over placebo for the primary endpoint of morning pain on movement (difference of –0.95, 95% CI, –1.83 to –0.02; P = .044). No trials assessed herbal cannabis.
“It is not currently possible to recommend this category of treatments as therapy for patients with rheumatic diseases. Any conclusions based on these studies remain tenuous and call for larger, well controlled clinical trials to better understand potential benefits and risks as pertaining to rheumatic conditions,” Dr. Fitzcharles and her coauthors wrote.
No relevant financial disclosures were reported.
rhnews@frontlinemedcom.com
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Currently, there is insufficient evidence to support cannabinoids as a therapeutic option in rheumatic disease.
Major finding: In two of four reviewed studies, cannabinoids demonstrated a statistically significant effect on pain associated with fibromyalgia and rheumatoid arthritis.
Data source: A qualitative review of four randomized, controlled trials involving 58 patients with rheumatoid arthritis, 71 with fibromyalgia, and 74 with osteoarthritis.
Disclosures: No relevant financial disclosures were reported.
Care Transitions from Hospital to Home
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
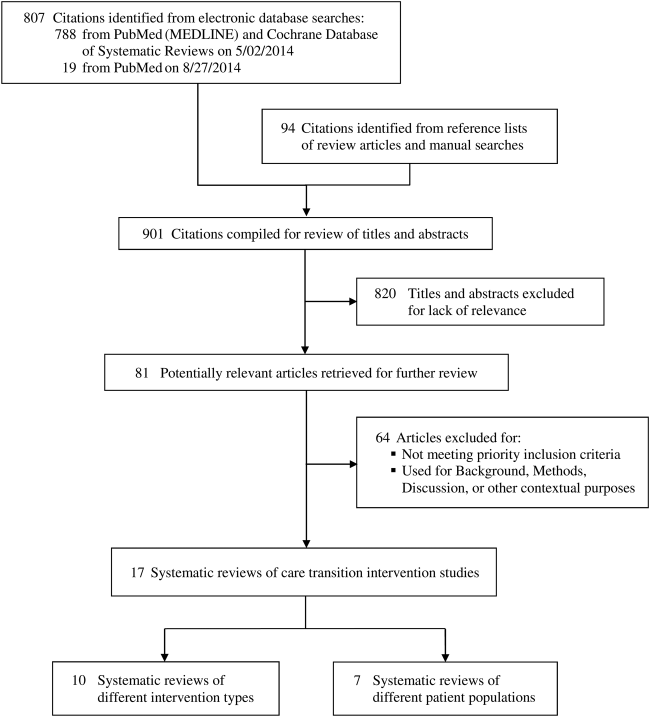
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P < 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
ACR: No long-term benefit for knee OA steroid injections
Regular corticosteroid injections over 2 years were relatively safe for osteoarthritic knees in a trial from Tufts Medical Center in Boston, but they did not slow the progression of joint damage or improve patient outcomes.
The findings come at a time when the role of intra-articular steroid shots is up for debate. They are widely used for knee osteoarthritis (OA) in the hopes of reducing cartilage damage from synovitis, but there’s also concern that they might actually harm cartilage and periarticular bone.
The investigators, led by Dr. Timothy McAlindon, chief of rheumatology at Tufts Medical Center, sought to bring some data to the table. “Our objective was to test the potential for disease modification of synovitic knee OA by triamcinolone hexacetonide [THA] in a clinical trial with comprehensive measurement of effects on cartilage and subchondral bone using MRI and DXA [dual-energy x-ray absorptiometry],” they said.
The investigators randomized 140 patients with symptomatic knee OA (Kellgren-Lawrence grade 2 or 3) and synovitis on ultrasound to either 40 mg THA or saline knee injections every 3 months for 2 years. Randomization was blocked and stratified by gender and Kellgren-Lawrence grade. Patients had clinical assessments at each visit and annual MRIs and DXA knee and hip scans.
There were no significant between-group differences over the course of the study in mean changes on Western Ontario and McMaster Universities Arthritis Index pain (–2.2 for THA vs. –2.8 for placebo; P = .3) and function scores (–7.1 for THA vs. –9.2 for placebo; P = .4), and no differences in chair stand (–1.1 for THA vs. –1.6 for placebo; P = .8) or walk time tests (–0.5 for THA vs. –0.03 for placebo; P = .5).
There were no significant differences on MRI or DXA, except that the steroid group had greater cartilage loss and the placebo group greater progression of fibrillation.
“The greater rate of loss of cartilage thickness ... in the treated group was small in magnitude and of uncertain clinical significance,” said Dr. McAlindon.
The mean body mass index in the study was 31.2 kg/m2. Just over half the subjects were women, and about two-thirds were white. More than 90% of patients in both groups completed the study.
The work was funded by the National Institutes of Health. The investigators have no relevant disclosures.
Regular corticosteroid injections over 2 years were relatively safe for osteoarthritic knees in a trial from Tufts Medical Center in Boston, but they did not slow the progression of joint damage or improve patient outcomes.
The findings come at a time when the role of intra-articular steroid shots is up for debate. They are widely used for knee osteoarthritis (OA) in the hopes of reducing cartilage damage from synovitis, but there’s also concern that they might actually harm cartilage and periarticular bone.
The investigators, led by Dr. Timothy McAlindon, chief of rheumatology at Tufts Medical Center, sought to bring some data to the table. “Our objective was to test the potential for disease modification of synovitic knee OA by triamcinolone hexacetonide [THA] in a clinical trial with comprehensive measurement of effects on cartilage and subchondral bone using MRI and DXA [dual-energy x-ray absorptiometry],” they said.
The investigators randomized 140 patients with symptomatic knee OA (Kellgren-Lawrence grade 2 or 3) and synovitis on ultrasound to either 40 mg THA or saline knee injections every 3 months for 2 years. Randomization was blocked and stratified by gender and Kellgren-Lawrence grade. Patients had clinical assessments at each visit and annual MRIs and DXA knee and hip scans.
There were no significant between-group differences over the course of the study in mean changes on Western Ontario and McMaster Universities Arthritis Index pain (–2.2 for THA vs. –2.8 for placebo; P = .3) and function scores (–7.1 for THA vs. –9.2 for placebo; P = .4), and no differences in chair stand (–1.1 for THA vs. –1.6 for placebo; P = .8) or walk time tests (–0.5 for THA vs. –0.03 for placebo; P = .5).
There were no significant differences on MRI or DXA, except that the steroid group had greater cartilage loss and the placebo group greater progression of fibrillation.
“The greater rate of loss of cartilage thickness ... in the treated group was small in magnitude and of uncertain clinical significance,” said Dr. McAlindon.
The mean body mass index in the study was 31.2 kg/m2. Just over half the subjects were women, and about two-thirds were white. More than 90% of patients in both groups completed the study.
The work was funded by the National Institutes of Health. The investigators have no relevant disclosures.
Regular corticosteroid injections over 2 years were relatively safe for osteoarthritic knees in a trial from Tufts Medical Center in Boston, but they did not slow the progression of joint damage or improve patient outcomes.
The findings come at a time when the role of intra-articular steroid shots is up for debate. They are widely used for knee osteoarthritis (OA) in the hopes of reducing cartilage damage from synovitis, but there’s also concern that they might actually harm cartilage and periarticular bone.
The investigators, led by Dr. Timothy McAlindon, chief of rheumatology at Tufts Medical Center, sought to bring some data to the table. “Our objective was to test the potential for disease modification of synovitic knee OA by triamcinolone hexacetonide [THA] in a clinical trial with comprehensive measurement of effects on cartilage and subchondral bone using MRI and DXA [dual-energy x-ray absorptiometry],” they said.
The investigators randomized 140 patients with symptomatic knee OA (Kellgren-Lawrence grade 2 or 3) and synovitis on ultrasound to either 40 mg THA or saline knee injections every 3 months for 2 years. Randomization was blocked and stratified by gender and Kellgren-Lawrence grade. Patients had clinical assessments at each visit and annual MRIs and DXA knee and hip scans.
There were no significant between-group differences over the course of the study in mean changes on Western Ontario and McMaster Universities Arthritis Index pain (–2.2 for THA vs. –2.8 for placebo; P = .3) and function scores (–7.1 for THA vs. –9.2 for placebo; P = .4), and no differences in chair stand (–1.1 for THA vs. –1.6 for placebo; P = .8) or walk time tests (–0.5 for THA vs. –0.03 for placebo; P = .5).
There were no significant differences on MRI or DXA, except that the steroid group had greater cartilage loss and the placebo group greater progression of fibrillation.
“The greater rate of loss of cartilage thickness ... in the treated group was small in magnitude and of uncertain clinical significance,” said Dr. McAlindon.
The mean body mass index in the study was 31.2 kg/m2. Just over half the subjects were women, and about two-thirds were white. More than 90% of patients in both groups completed the study.
The work was funded by the National Institutes of Health. The investigators have no relevant disclosures.
FROM THE ACR ANNUAL MEETING
Key clinical point: Intra-articular steroid injections do not slow the progression of knee OA or improve patient outcomes.
Major finding: Over 2 years, there were no significant differences between steroid injection and placebo injection patients on Western Ontario and McMaster Universities Arthritis Index pain (–2.2 for THA vs. –2.8 for placebo; P = .3) and function scores (–7.1 for THA vs. –9.2 for placebo; P = .4), and no differences in chair stand (–1.1 for THA vs. –1.6 for placebo; P = .8) or walk time tests (–0.5 for THA vs. –0.03 for placebo; P = .5).
Data source: A randomized, placebo-controlled trial with 140 knee OA patients.
Disclosures: The work was funded by the National Institutes of Health. The investigators have no relevant disclosures.
Fish oil, aspirin didn’t reduce AVF failure in kidney disease
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Three months of fish oil or aspirin intake had no impact in reducing arteriovenous fistula failure at 12 months.
Major finding: Nearly half of patients who received fish oil (47%) had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk, 1.03). Results were similar for patients who received aspirin.
Data source: A study of 567 patients with stage 4 or stage 5 chronic kidney disease who were randomized to receive 12 weeks of fish oil 4 g or placebo (olive oil), or to aspirin 100 mg or matching placebo.
Disclosures: The researchers reported having no financial disclosures.
ACS: No clear answers to black patients’ worse rectal cancer outcomes
CHICAGO – Access to equal care decreases but does not eliminate the survival disparities between black and white patients with rectal cancer, according to investigators from the University of Cincinnati, Ohio.
After rigorous propensity score matching of thousands of black and white patients for patient, disease, and treatment characteristics, median survival was 109.6 months among white patients and 85.8 months among black patients (J Am Coll Surg. 2015 Nov [doi: 10.1016/j.jamcollsurg.2015.07.056]).
“Blacks have worse outcomes, but we don’t have a clear explanation,” explained investigator and surgery resident Dr. Meghan Nolan. For now, the findings suggest that it might be a good idea to start screening black patients before age 50 years, perhaps even as early as age 40 years.
“We are urging people to screen at a lower age in black patients,” Dr. Nolan said at the annual clinical congress of the American College of Surgeons.
Racial disparities in rectal cancer are well known. The investigators wanted to see if they simply are because of unequal access to care, so they analyzed 178,414 white and 18,385 black patients in the National Cancer Data Base from 1998 to 2006.
Mean 5-year survival for blacks was 50.7%, compared with 56.2% for whites, and black patients were more likely to present with stage IV disease. Such findings aren’t new, the investigators noted.
To account for the difference in late-stage presentation, the investigators limited their analysis to patients with stage I-III rectal cancer. But even then, 5-year survival was 66.7% among whites and 58.7% among blacks.
That might have had something to do with the fact 85% of white patients, but only 78% of black patients, had surgery for those potentially curable lower-stage tumors. Among those who didn’t undergo surgery, patient refusal was slightly more common among black patients than white patients, which suggests that “cultural factors may be playing a role” in the surgery disparity, Dr. Nolan said.
The investigators controlled for the different surgery rates by limiting their analysis to stage I-III patients who had appropriate operations. The survival gap narrowed but did not disappear: 65.8% of white patients were alive at 5 years, compared with 61% of black patients. Blacks were also more likely to have positive margins on pathology. “I’m not sure why. That was a surprise,” Dr. Nolan said.
The propensity score matching led the team to conclude that there’s more at work than differences in access to care. A total of 7,569 pairs of black and white patients were matched for age at diagnosis, gender, insurance coverage, income, education, facility type, tumor stage, Charlson-Deyo score, surgical management, margin status, and other potential confounders.
Every matched patient completed their recommended therapy, and there was no statistical difference between matched black and white patients who received appropriate, stage-specific chemoradiation. Still, the survival differences persisted.
Dr. Nolan had no relevant disclosures.
CHICAGO – Access to equal care decreases but does not eliminate the survival disparities between black and white patients with rectal cancer, according to investigators from the University of Cincinnati, Ohio.
After rigorous propensity score matching of thousands of black and white patients for patient, disease, and treatment characteristics, median survival was 109.6 months among white patients and 85.8 months among black patients (J Am Coll Surg. 2015 Nov [doi: 10.1016/j.jamcollsurg.2015.07.056]).
“Blacks have worse outcomes, but we don’t have a clear explanation,” explained investigator and surgery resident Dr. Meghan Nolan. For now, the findings suggest that it might be a good idea to start screening black patients before age 50 years, perhaps even as early as age 40 years.
“We are urging people to screen at a lower age in black patients,” Dr. Nolan said at the annual clinical congress of the American College of Surgeons.
Racial disparities in rectal cancer are well known. The investigators wanted to see if they simply are because of unequal access to care, so they analyzed 178,414 white and 18,385 black patients in the National Cancer Data Base from 1998 to 2006.
Mean 5-year survival for blacks was 50.7%, compared with 56.2% for whites, and black patients were more likely to present with stage IV disease. Such findings aren’t new, the investigators noted.
To account for the difference in late-stage presentation, the investigators limited their analysis to patients with stage I-III rectal cancer. But even then, 5-year survival was 66.7% among whites and 58.7% among blacks.
That might have had something to do with the fact 85% of white patients, but only 78% of black patients, had surgery for those potentially curable lower-stage tumors. Among those who didn’t undergo surgery, patient refusal was slightly more common among black patients than white patients, which suggests that “cultural factors may be playing a role” in the surgery disparity, Dr. Nolan said.
The investigators controlled for the different surgery rates by limiting their analysis to stage I-III patients who had appropriate operations. The survival gap narrowed but did not disappear: 65.8% of white patients were alive at 5 years, compared with 61% of black patients. Blacks were also more likely to have positive margins on pathology. “I’m not sure why. That was a surprise,” Dr. Nolan said.
The propensity score matching led the team to conclude that there’s more at work than differences in access to care. A total of 7,569 pairs of black and white patients were matched for age at diagnosis, gender, insurance coverage, income, education, facility type, tumor stage, Charlson-Deyo score, surgical management, margin status, and other potential confounders.
Every matched patient completed their recommended therapy, and there was no statistical difference between matched black and white patients who received appropriate, stage-specific chemoradiation. Still, the survival differences persisted.
Dr. Nolan had no relevant disclosures.
CHICAGO – Access to equal care decreases but does not eliminate the survival disparities between black and white patients with rectal cancer, according to investigators from the University of Cincinnati, Ohio.
After rigorous propensity score matching of thousands of black and white patients for patient, disease, and treatment characteristics, median survival was 109.6 months among white patients and 85.8 months among black patients (J Am Coll Surg. 2015 Nov [doi: 10.1016/j.jamcollsurg.2015.07.056]).
“Blacks have worse outcomes, but we don’t have a clear explanation,” explained investigator and surgery resident Dr. Meghan Nolan. For now, the findings suggest that it might be a good idea to start screening black patients before age 50 years, perhaps even as early as age 40 years.
“We are urging people to screen at a lower age in black patients,” Dr. Nolan said at the annual clinical congress of the American College of Surgeons.
Racial disparities in rectal cancer are well known. The investigators wanted to see if they simply are because of unequal access to care, so they analyzed 178,414 white and 18,385 black patients in the National Cancer Data Base from 1998 to 2006.
Mean 5-year survival for blacks was 50.7%, compared with 56.2% for whites, and black patients were more likely to present with stage IV disease. Such findings aren’t new, the investigators noted.
To account for the difference in late-stage presentation, the investigators limited their analysis to patients with stage I-III rectal cancer. But even then, 5-year survival was 66.7% among whites and 58.7% among blacks.
That might have had something to do with the fact 85% of white patients, but only 78% of black patients, had surgery for those potentially curable lower-stage tumors. Among those who didn’t undergo surgery, patient refusal was slightly more common among black patients than white patients, which suggests that “cultural factors may be playing a role” in the surgery disparity, Dr. Nolan said.
The investigators controlled for the different surgery rates by limiting their analysis to stage I-III patients who had appropriate operations. The survival gap narrowed but did not disappear: 65.8% of white patients were alive at 5 years, compared with 61% of black patients. Blacks were also more likely to have positive margins on pathology. “I’m not sure why. That was a surprise,” Dr. Nolan said.
The propensity score matching led the team to conclude that there’s more at work than differences in access to care. A total of 7,569 pairs of black and white patients were matched for age at diagnosis, gender, insurance coverage, income, education, facility type, tumor stage, Charlson-Deyo score, surgical management, margin status, and other potential confounders.
Every matched patient completed their recommended therapy, and there was no statistical difference between matched black and white patients who received appropriate, stage-specific chemoradiation. Still, the survival differences persisted.
Dr. Nolan had no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Screen black patients for rectal cancer before the age of 50.
Major finding: Even with rigorous propensity score matching, median survival was 109.6 months among white patients and 85.8 months among black patients with rectal cancer.
Data source: Review of almost 200,000 rectal cancer patients in the National Cancer Data Base.
Disclosures: Dr. Nolan had no relevant disclosures.
Survivors of out-of-hospital cardiac arrest usually had intact brain function
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr. Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, N.C., and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Dr. Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation.
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr. Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, N.C., and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Dr. Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation.
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr. Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, N.C., and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Dr. Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, regardless of duration of CPR in the field.
Major finding: Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Data source: A retrospective observational study of 3,814 adults who had an out-of-hospital cardiac arrest between 2005 and 2014.
Disclosures: Dr. Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation.
FDA: Long-term clopidogrel doesn’t affect risk of death

A review conducted by the US Food and Drug Administration (FDA) indicates that long-term use of the antiplatelet drug clopidogrel (Plavix) does not increase or decrease the overall risk of death in patients with, or at risk for, heart disease.
In addition, the FDA said there is no evidence to suggest that clopidogrel increases the risk of cancer or death from cancer.
These findings contradict those of the Dual Antiplatelet Therapy (DAPT) trial, the study that prompted the FDA’s review.
In this trial, researchers compared 12 months of DAPT (either clopidogrel or prasugrel plus aspirin) to 30 months of DAPT in patients who had a drug-eluting coronary stent.
Compared to patients taking clopidogrel for 12 months, patients who received clopidogrel for 30 months had lower rates of heart attacks and stent thrombosis but higher rates of death, primarily from cancer or trauma.
To investigate these findings, the FDA examined data from the DAPT trial and performed trial-level meta-analyses of other large, long-term trials.
The trials had a clopidogrel-plus-aspirin arm (long-term treatment was 12 months or longer) and a comparator arm of either aspirin alone or short-term clopidogrel plus aspirin (6 months or less). They also had a planned follow-up of at least 1 year.
All-cause death
In the DAPT trial, extended use of clopidogrel plus aspirin was associated with a significantly increased risk of death (2.2% for 30 months vs 1.5% for 12 months). But there was no increased risk for prasugrel plus aspirin (1.6% for 30 months vs 1.6% for 12 months).
The FDA’s initial meta-analysis on mortality included 12 trials (56,799 patients). The incidence of all-cause mortality was 6.7% for the long-term clopidogrel-plus-aspirin arm and 6.6% for the comparator arm, resulting in a Mantel Haenszel Risk Difference (MH RD) of 0.04% (95% CI, -0.35%-0.44%).
The FDA also conducted a meta-analysis of 9 of the 12 trials (45,374 patients), which enrolled patients with coronary artery disease or patients at risk of coronary artery disease. This analysis showed no difference in the risk of all-cause mortality—MH RD of -0.07% (95% CI, -0.43%-0.29%).
Cancer and death in DAPT
In the DAPT trial, the risk of cancer reported as an adverse event was not different between the 30-month (2.4% ) and 12-month (2.3%) groups receiving clopidogrel, when considering cancers reported after enrollment (from month 0 to 33 of the study).
The FDA performed several analyses of the cancer adverse event data, and the relative risk of cancer for the 30-month vs 12-month arm in the clopidogrel group ranged from 0.95 to 1.2, depending on the analysis.
Similar analyses for the prasugrel group revealed relative risks of cancer-related adverse events ranging from 1.4 to 1.6.
The FDA noted that, although there was no increase in the risk of cancer-related adverse events for clopidogrel in the 30-month arm, the risk of cancer-related death was higher than in the 12-month arm—0.7% and 0.2%, respectively.
For prasugrel, there was a trend toward a higher risk of cancer adverse events in the 30-month arm than in the 12-month arm, but the risk of cancer death was identical in both arms—0.4% vs 0.4%.
The FDA said these findings are difficult to reconcile.
Meta-analyses of cancer and death
The FDA performed 2 trial-level meta-analyses to investigate the role of clopidogrel in cancer and cancer death.
The first was an analysis of cancer-related adverse events from 4 trials (37,835 patients) that compared long-term clopidogrel and aspirin to either aspirin alone or short-term clopidogrel plus aspirin.
The incidence of cancer adverse events was 4.2% for the long-term clopidogrel-plus-aspirin arm and 4.0% for the comparator arm (MH RD of 0.19%; 95% CI, -0.2%-0.59%).
The FDA conducted the second meta-analysis to assess cancer-related death. The analysis included 5 trials (40,855 patients).
The incidence of cancer death was 0.9% for the long-term clopidogrel-plus-aspirin arm and 1.1% for the comparator arm (MH RD of -0.14%; 95% CI, -0.33%-0.06%).
The FDA said that, taken together, the results of this review do not suggest an increased risk of cancer adverse events, cancer-related death, or all-cause mortality with long-term clopidogrel therapy.
More details can be found in the FDA’s Drug Safety Communication on this topic. ![]()

A review conducted by the US Food and Drug Administration (FDA) indicates that long-term use of the antiplatelet drug clopidogrel (Plavix) does not increase or decrease the overall risk of death in patients with, or at risk for, heart disease.
In addition, the FDA said there is no evidence to suggest that clopidogrel increases the risk of cancer or death from cancer.
These findings contradict those of the Dual Antiplatelet Therapy (DAPT) trial, the study that prompted the FDA’s review.
In this trial, researchers compared 12 months of DAPT (either clopidogrel or prasugrel plus aspirin) to 30 months of DAPT in patients who had a drug-eluting coronary stent.
Compared to patients taking clopidogrel for 12 months, patients who received clopidogrel for 30 months had lower rates of heart attacks and stent thrombosis but higher rates of death, primarily from cancer or trauma.
To investigate these findings, the FDA examined data from the DAPT trial and performed trial-level meta-analyses of other large, long-term trials.
The trials had a clopidogrel-plus-aspirin arm (long-term treatment was 12 months or longer) and a comparator arm of either aspirin alone or short-term clopidogrel plus aspirin (6 months or less). They also had a planned follow-up of at least 1 year.
All-cause death
In the DAPT trial, extended use of clopidogrel plus aspirin was associated with a significantly increased risk of death (2.2% for 30 months vs 1.5% for 12 months). But there was no increased risk for prasugrel plus aspirin (1.6% for 30 months vs 1.6% for 12 months).
The FDA’s initial meta-analysis on mortality included 12 trials (56,799 patients). The incidence of all-cause mortality was 6.7% for the long-term clopidogrel-plus-aspirin arm and 6.6% for the comparator arm, resulting in a Mantel Haenszel Risk Difference (MH RD) of 0.04% (95% CI, -0.35%-0.44%).
The FDA also conducted a meta-analysis of 9 of the 12 trials (45,374 patients), which enrolled patients with coronary artery disease or patients at risk of coronary artery disease. This analysis showed no difference in the risk of all-cause mortality—MH RD of -0.07% (95% CI, -0.43%-0.29%).
Cancer and death in DAPT
In the DAPT trial, the risk of cancer reported as an adverse event was not different between the 30-month (2.4% ) and 12-month (2.3%) groups receiving clopidogrel, when considering cancers reported after enrollment (from month 0 to 33 of the study).
The FDA performed several analyses of the cancer adverse event data, and the relative risk of cancer for the 30-month vs 12-month arm in the clopidogrel group ranged from 0.95 to 1.2, depending on the analysis.
Similar analyses for the prasugrel group revealed relative risks of cancer-related adverse events ranging from 1.4 to 1.6.
The FDA noted that, although there was no increase in the risk of cancer-related adverse events for clopidogrel in the 30-month arm, the risk of cancer-related death was higher than in the 12-month arm—0.7% and 0.2%, respectively.
For prasugrel, there was a trend toward a higher risk of cancer adverse events in the 30-month arm than in the 12-month arm, but the risk of cancer death was identical in both arms—0.4% vs 0.4%.
The FDA said these findings are difficult to reconcile.
Meta-analyses of cancer and death
The FDA performed 2 trial-level meta-analyses to investigate the role of clopidogrel in cancer and cancer death.
The first was an analysis of cancer-related adverse events from 4 trials (37,835 patients) that compared long-term clopidogrel and aspirin to either aspirin alone or short-term clopidogrel plus aspirin.
The incidence of cancer adverse events was 4.2% for the long-term clopidogrel-plus-aspirin arm and 4.0% for the comparator arm (MH RD of 0.19%; 95% CI, -0.2%-0.59%).
The FDA conducted the second meta-analysis to assess cancer-related death. The analysis included 5 trials (40,855 patients).
The incidence of cancer death was 0.9% for the long-term clopidogrel-plus-aspirin arm and 1.1% for the comparator arm (MH RD of -0.14%; 95% CI, -0.33%-0.06%).
The FDA said that, taken together, the results of this review do not suggest an increased risk of cancer adverse events, cancer-related death, or all-cause mortality with long-term clopidogrel therapy.
More details can be found in the FDA’s Drug Safety Communication on this topic. ![]()

A review conducted by the US Food and Drug Administration (FDA) indicates that long-term use of the antiplatelet drug clopidogrel (Plavix) does not increase or decrease the overall risk of death in patients with, or at risk for, heart disease.
In addition, the FDA said there is no evidence to suggest that clopidogrel increases the risk of cancer or death from cancer.
These findings contradict those of the Dual Antiplatelet Therapy (DAPT) trial, the study that prompted the FDA’s review.
In this trial, researchers compared 12 months of DAPT (either clopidogrel or prasugrel plus aspirin) to 30 months of DAPT in patients who had a drug-eluting coronary stent.
Compared to patients taking clopidogrel for 12 months, patients who received clopidogrel for 30 months had lower rates of heart attacks and stent thrombosis but higher rates of death, primarily from cancer or trauma.
To investigate these findings, the FDA examined data from the DAPT trial and performed trial-level meta-analyses of other large, long-term trials.
The trials had a clopidogrel-plus-aspirin arm (long-term treatment was 12 months or longer) and a comparator arm of either aspirin alone or short-term clopidogrel plus aspirin (6 months or less). They also had a planned follow-up of at least 1 year.
All-cause death
In the DAPT trial, extended use of clopidogrel plus aspirin was associated with a significantly increased risk of death (2.2% for 30 months vs 1.5% for 12 months). But there was no increased risk for prasugrel plus aspirin (1.6% for 30 months vs 1.6% for 12 months).
The FDA’s initial meta-analysis on mortality included 12 trials (56,799 patients). The incidence of all-cause mortality was 6.7% for the long-term clopidogrel-plus-aspirin arm and 6.6% for the comparator arm, resulting in a Mantel Haenszel Risk Difference (MH RD) of 0.04% (95% CI, -0.35%-0.44%).
The FDA also conducted a meta-analysis of 9 of the 12 trials (45,374 patients), which enrolled patients with coronary artery disease or patients at risk of coronary artery disease. This analysis showed no difference in the risk of all-cause mortality—MH RD of -0.07% (95% CI, -0.43%-0.29%).
Cancer and death in DAPT
In the DAPT trial, the risk of cancer reported as an adverse event was not different between the 30-month (2.4% ) and 12-month (2.3%) groups receiving clopidogrel, when considering cancers reported after enrollment (from month 0 to 33 of the study).
The FDA performed several analyses of the cancer adverse event data, and the relative risk of cancer for the 30-month vs 12-month arm in the clopidogrel group ranged from 0.95 to 1.2, depending on the analysis.
Similar analyses for the prasugrel group revealed relative risks of cancer-related adverse events ranging from 1.4 to 1.6.
The FDA noted that, although there was no increase in the risk of cancer-related adverse events for clopidogrel in the 30-month arm, the risk of cancer-related death was higher than in the 12-month arm—0.7% and 0.2%, respectively.
For prasugrel, there was a trend toward a higher risk of cancer adverse events in the 30-month arm than in the 12-month arm, but the risk of cancer death was identical in both arms—0.4% vs 0.4%.
The FDA said these findings are difficult to reconcile.
Meta-analyses of cancer and death
The FDA performed 2 trial-level meta-analyses to investigate the role of clopidogrel in cancer and cancer death.
The first was an analysis of cancer-related adverse events from 4 trials (37,835 patients) that compared long-term clopidogrel and aspirin to either aspirin alone or short-term clopidogrel plus aspirin.
The incidence of cancer adverse events was 4.2% for the long-term clopidogrel-plus-aspirin arm and 4.0% for the comparator arm (MH RD of 0.19%; 95% CI, -0.2%-0.59%).
The FDA conducted the second meta-analysis to assess cancer-related death. The analysis included 5 trials (40,855 patients).
The incidence of cancer death was 0.9% for the long-term clopidogrel-plus-aspirin arm and 1.1% for the comparator arm (MH RD of -0.14%; 95% CI, -0.33%-0.06%).
The FDA said that, taken together, the results of this review do not suggest an increased risk of cancer adverse events, cancer-related death, or all-cause mortality with long-term clopidogrel therapy.
More details can be found in the FDA’s Drug Safety Communication on this topic. ![]()
Topicals, PDT offer field treatment options for actinic keratoses
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR