User login
School-based CBT program reduces depression, suicidality
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
FROM PLOS ONE
Key clinical point: A school-based cognitive-behavioral therapy program was tied to substantial declines in adolescent depression and suicidality.
Major finding: At 12-week follow-up, the number of students who were actively suicidal was 73% lower than at baseline.
Data source: Multicenter Canadian prospective study of 3,244 students aged 11-18 years.
Disclosures: Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Cutaneous Infection With Mycobacterium kansasii in a Patient With Myelodysplastic Syndrome and Sweet Syndrome
To the Editor:
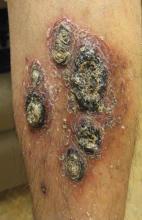
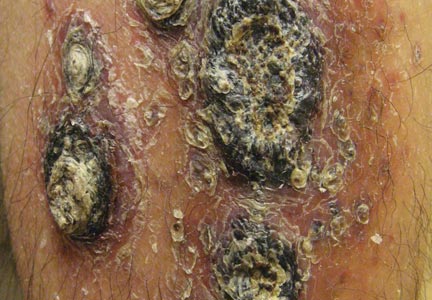
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
Women with ACS showed improved outcomes, but remain underrepresented in trials
Though acute coronary syndromes are the leading cause of death in U.S. women, an analysis of clinical trials showed that enrollment among women remained disproportionately low from 1994 to 2010, reported Dr. Kristian Kragholm and coauthors at Duke Clinical Research Institute.
An analysis of data in 76,148 non–ST-segment elevation acute coronary syndrome patients from 11 phase III clinical trials found that women comprised just 33.3% of participants, which did not change significantly over the 17-year period. Women consistently had higher incidence of diabetes, hypertension, and heart failure, the authors reported.
Use of ACE inhibitors/angiotensin II receptor blockers, thienopyridines, beta-blockers, and lipid-lowering drugs significantly increased over time for both sexes, as did use of coronary angiography and percutaneous coronary intervention. Observed in-hospital, 30-day, and 6-month mortality decreased significantly in both men and women, Dr. Kragholm and colleagues said.
The findings suggest that “current efforts to representatively enroll women in NSTE ACS trials are insufficient,” the authors wrote. “Because safety and efficacy findings may differ according to sex, this disparity could undermine generalizability of clinical trial results to treatment of the overall NSTE ACS population.”
Read the full article here.
Though acute coronary syndromes are the leading cause of death in U.S. women, an analysis of clinical trials showed that enrollment among women remained disproportionately low from 1994 to 2010, reported Dr. Kristian Kragholm and coauthors at Duke Clinical Research Institute.
An analysis of data in 76,148 non–ST-segment elevation acute coronary syndrome patients from 11 phase III clinical trials found that women comprised just 33.3% of participants, which did not change significantly over the 17-year period. Women consistently had higher incidence of diabetes, hypertension, and heart failure, the authors reported.
Use of ACE inhibitors/angiotensin II receptor blockers, thienopyridines, beta-blockers, and lipid-lowering drugs significantly increased over time for both sexes, as did use of coronary angiography and percutaneous coronary intervention. Observed in-hospital, 30-day, and 6-month mortality decreased significantly in both men and women, Dr. Kragholm and colleagues said.
The findings suggest that “current efforts to representatively enroll women in NSTE ACS trials are insufficient,” the authors wrote. “Because safety and efficacy findings may differ according to sex, this disparity could undermine generalizability of clinical trial results to treatment of the overall NSTE ACS population.”
Read the full article here.
Though acute coronary syndromes are the leading cause of death in U.S. women, an analysis of clinical trials showed that enrollment among women remained disproportionately low from 1994 to 2010, reported Dr. Kristian Kragholm and coauthors at Duke Clinical Research Institute.
An analysis of data in 76,148 non–ST-segment elevation acute coronary syndrome patients from 11 phase III clinical trials found that women comprised just 33.3% of participants, which did not change significantly over the 17-year period. Women consistently had higher incidence of diabetes, hypertension, and heart failure, the authors reported.
Use of ACE inhibitors/angiotensin II receptor blockers, thienopyridines, beta-blockers, and lipid-lowering drugs significantly increased over time for both sexes, as did use of coronary angiography and percutaneous coronary intervention. Observed in-hospital, 30-day, and 6-month mortality decreased significantly in both men and women, Dr. Kragholm and colleagues said.
The findings suggest that “current efforts to representatively enroll women in NSTE ACS trials are insufficient,” the authors wrote. “Because safety and efficacy findings may differ according to sex, this disparity could undermine generalizability of clinical trial results to treatment of the overall NSTE ACS population.”
Read the full article here.
Three Cheers for B3?
At the recent American Society of Clinical Oncology Annual Meeting, Martin et al presented data from the Australian Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) study. This prospective double-blind, randomized, controlled trial examined 386 immunocompetent patients with 2 or more nonmelanoma skin cancers (NMSCs) in the last 5 years (average, 8). The patients were randomized to receive oral nicotinamide 500 mg twice daily or placebo for 1 year, resulting in significant reduction of new NMSCs (average rate 1.77 vs 2.42; relative rate reduction, 23%; P=.02), with similar results for basal and squamous cell carcinomas. Actinic keratosis counts were reduced throughout the year by up to 20%, peaking at 9 months. No differences in adverse events were noted between the treatment and placebo groups.
What’s the issue?
High-risk NMSC patients present a challenge to dermatologists, as their need for constant surveillance, field therapy for actinic keratoses, and revolving visits between skin examinations and procedural modalities such as Mohs micrographic surgery can be staggering. Chemopreventive strategies pose difficulties, especially for elderly patients, due to tolerability and adherence, skin irritation and cosmetic limitations of topical therapies such as 5-fluorouracil, and inadequacy or financial inaccessibility of oral therapies such as acitretin.
Nicotinamide is a confusing supplement, as it is also called niacinamide. One of the 2 forms of vitamin B3, nicotinic acid (or niacin) is the other form and can be converted to nicotinamide in the body. It has cholesterol and vasodilatory/flushing effects that nicotinamide itself does not. Therefore, these supplement subtypes are not generally interchangeable.
Nicotinamide is postulated to enhance DNA repair and reverse UV immunosuppression in NMSC patients and is a well-tolerated and inexpensive supplement (approximately $10 a month for the dosage in this study). Although the decrease in skin cancer number per year seems modest in this study, my patients would likely welcome at least 1 fewer surgery per year and much less cryotherapy or 5-fluorouracil cream, especially if it is as simple as buying the supplement at the grocery store as they do for their fish oil capsules and probiotics. Does vitamin B3 hold promise for your high-risk NMSC patients?
At the recent American Society of Clinical Oncology Annual Meeting, Martin et al presented data from the Australian Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) study. This prospective double-blind, randomized, controlled trial examined 386 immunocompetent patients with 2 or more nonmelanoma skin cancers (NMSCs) in the last 5 years (average, 8). The patients were randomized to receive oral nicotinamide 500 mg twice daily or placebo for 1 year, resulting in significant reduction of new NMSCs (average rate 1.77 vs 2.42; relative rate reduction, 23%; P=.02), with similar results for basal and squamous cell carcinomas. Actinic keratosis counts were reduced throughout the year by up to 20%, peaking at 9 months. No differences in adverse events were noted between the treatment and placebo groups.
What’s the issue?
High-risk NMSC patients present a challenge to dermatologists, as their need for constant surveillance, field therapy for actinic keratoses, and revolving visits between skin examinations and procedural modalities such as Mohs micrographic surgery can be staggering. Chemopreventive strategies pose difficulties, especially for elderly patients, due to tolerability and adherence, skin irritation and cosmetic limitations of topical therapies such as 5-fluorouracil, and inadequacy or financial inaccessibility of oral therapies such as acitretin.
Nicotinamide is a confusing supplement, as it is also called niacinamide. One of the 2 forms of vitamin B3, nicotinic acid (or niacin) is the other form and can be converted to nicotinamide in the body. It has cholesterol and vasodilatory/flushing effects that nicotinamide itself does not. Therefore, these supplement subtypes are not generally interchangeable.
Nicotinamide is postulated to enhance DNA repair and reverse UV immunosuppression in NMSC patients and is a well-tolerated and inexpensive supplement (approximately $10 a month for the dosage in this study). Although the decrease in skin cancer number per year seems modest in this study, my patients would likely welcome at least 1 fewer surgery per year and much less cryotherapy or 5-fluorouracil cream, especially if it is as simple as buying the supplement at the grocery store as they do for their fish oil capsules and probiotics. Does vitamin B3 hold promise for your high-risk NMSC patients?
At the recent American Society of Clinical Oncology Annual Meeting, Martin et al presented data from the Australian Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) study. This prospective double-blind, randomized, controlled trial examined 386 immunocompetent patients with 2 or more nonmelanoma skin cancers (NMSCs) in the last 5 years (average, 8). The patients were randomized to receive oral nicotinamide 500 mg twice daily or placebo for 1 year, resulting in significant reduction of new NMSCs (average rate 1.77 vs 2.42; relative rate reduction, 23%; P=.02), with similar results for basal and squamous cell carcinomas. Actinic keratosis counts were reduced throughout the year by up to 20%, peaking at 9 months. No differences in adverse events were noted between the treatment and placebo groups.
What’s the issue?
High-risk NMSC patients present a challenge to dermatologists, as their need for constant surveillance, field therapy for actinic keratoses, and revolving visits between skin examinations and procedural modalities such as Mohs micrographic surgery can be staggering. Chemopreventive strategies pose difficulties, especially for elderly patients, due to tolerability and adherence, skin irritation and cosmetic limitations of topical therapies such as 5-fluorouracil, and inadequacy or financial inaccessibility of oral therapies such as acitretin.
Nicotinamide is a confusing supplement, as it is also called niacinamide. One of the 2 forms of vitamin B3, nicotinic acid (or niacin) is the other form and can be converted to nicotinamide in the body. It has cholesterol and vasodilatory/flushing effects that nicotinamide itself does not. Therefore, these supplement subtypes are not generally interchangeable.
Nicotinamide is postulated to enhance DNA repair and reverse UV immunosuppression in NMSC patients and is a well-tolerated and inexpensive supplement (approximately $10 a month for the dosage in this study). Although the decrease in skin cancer number per year seems modest in this study, my patients would likely welcome at least 1 fewer surgery per year and much less cryotherapy or 5-fluorouracil cream, especially if it is as simple as buying the supplement at the grocery store as they do for their fish oil capsules and probiotics. Does vitamin B3 hold promise for your high-risk NMSC patients?
Heroin use up across demographic groups from 2002 to 2013
Heroin use is rising across all demographic groups in the United States, and is gaining traction among groups that previously have been associated with lower use, doubling among women and more than doubling among non-Hispanic whites, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said in a July 7 telebriefing.
Between 2002 and 2013, the rate of heroin-related overdose deaths nearly quadrupled, and more than 8,200 people died in 2013, according to the latest Vital Signs report, a combined project from the CDC and the Food and Drug Administration that analyzed data from the 2002-2013 National Survey on Drug Use and Health.
In addition, the gaps between men and women, low and higher incomes, and people with Medicaid and private insurance have narrowed in the past decade, although the most at-risk groups are still non-Hispanic whites, men, 18- to 25-year-olds, people with an annual household incomes of less than $20,000, Medicaid recipients, and the uninsured.
Although people who are addicted to prescription opioid painkillers were 40 times more likely to abuse heroin, the general idea that people gravitating to heroin abuse are doing so because opiates have become harder to get is not true, Dr. Frieden noted. The study identified two factors that were likely responsible for the increase in heroin users – the combination of the increased supply of heroin and higher demand, as well as the number of people already addicted to opioids who are “primed” for a heroin addiction.
However, state agencies have a central role to play in curbing heroin abuse, and will need to increase support for drug monitoring and surveillance programs to make tracking opiate abusers easier and more efficient, Dr. Frieden said.
The CDC is addressing the epidemic by helping to create federal guidelines for pain management, and supporting research and development for less addictive pain medications, he said.
“Improving prescribing practices is part of the solution, not part of the cause,” Dr. Frieden said.
Individual health care providers can help by following best practices for responsible painkiller prescribing to reduce opioid painkiller addiction, and by providing training for ways to adequately and comprehensively address pain beyond simply prescribing painkillers.
“Opiates are very good at curbing severe pain ... But for chronic, noncancer pain, you really need to look at the risks and benefits,” said Christopher M. Jones, Pharm.D., the study’s coauthor and a senior adviser at the FDA.
Heroin use is rising across all demographic groups in the United States, and is gaining traction among groups that previously have been associated with lower use, doubling among women and more than doubling among non-Hispanic whites, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said in a July 7 telebriefing.
Between 2002 and 2013, the rate of heroin-related overdose deaths nearly quadrupled, and more than 8,200 people died in 2013, according to the latest Vital Signs report, a combined project from the CDC and the Food and Drug Administration that analyzed data from the 2002-2013 National Survey on Drug Use and Health.
In addition, the gaps between men and women, low and higher incomes, and people with Medicaid and private insurance have narrowed in the past decade, although the most at-risk groups are still non-Hispanic whites, men, 18- to 25-year-olds, people with an annual household incomes of less than $20,000, Medicaid recipients, and the uninsured.
Although people who are addicted to prescription opioid painkillers were 40 times more likely to abuse heroin, the general idea that people gravitating to heroin abuse are doing so because opiates have become harder to get is not true, Dr. Frieden noted. The study identified two factors that were likely responsible for the increase in heroin users – the combination of the increased supply of heroin and higher demand, as well as the number of people already addicted to opioids who are “primed” for a heroin addiction.
However, state agencies have a central role to play in curbing heroin abuse, and will need to increase support for drug monitoring and surveillance programs to make tracking opiate abusers easier and more efficient, Dr. Frieden said.
The CDC is addressing the epidemic by helping to create federal guidelines for pain management, and supporting research and development for less addictive pain medications, he said.
“Improving prescribing practices is part of the solution, not part of the cause,” Dr. Frieden said.
Individual health care providers can help by following best practices for responsible painkiller prescribing to reduce opioid painkiller addiction, and by providing training for ways to adequately and comprehensively address pain beyond simply prescribing painkillers.
“Opiates are very good at curbing severe pain ... But for chronic, noncancer pain, you really need to look at the risks and benefits,” said Christopher M. Jones, Pharm.D., the study’s coauthor and a senior adviser at the FDA.
Heroin use is rising across all demographic groups in the United States, and is gaining traction among groups that previously have been associated with lower use, doubling among women and more than doubling among non-Hispanic whites, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, said in a July 7 telebriefing.
Between 2002 and 2013, the rate of heroin-related overdose deaths nearly quadrupled, and more than 8,200 people died in 2013, according to the latest Vital Signs report, a combined project from the CDC and the Food and Drug Administration that analyzed data from the 2002-2013 National Survey on Drug Use and Health.
In addition, the gaps between men and women, low and higher incomes, and people with Medicaid and private insurance have narrowed in the past decade, although the most at-risk groups are still non-Hispanic whites, men, 18- to 25-year-olds, people with an annual household incomes of less than $20,000, Medicaid recipients, and the uninsured.
Although people who are addicted to prescription opioid painkillers were 40 times more likely to abuse heroin, the general idea that people gravitating to heroin abuse are doing so because opiates have become harder to get is not true, Dr. Frieden noted. The study identified two factors that were likely responsible for the increase in heroin users – the combination of the increased supply of heroin and higher demand, as well as the number of people already addicted to opioids who are “primed” for a heroin addiction.
However, state agencies have a central role to play in curbing heroin abuse, and will need to increase support for drug monitoring and surveillance programs to make tracking opiate abusers easier and more efficient, Dr. Frieden said.
The CDC is addressing the epidemic by helping to create federal guidelines for pain management, and supporting research and development for less addictive pain medications, he said.
“Improving prescribing practices is part of the solution, not part of the cause,” Dr. Frieden said.
Individual health care providers can help by following best practices for responsible painkiller prescribing to reduce opioid painkiller addiction, and by providing training for ways to adequately and comprehensively address pain beyond simply prescribing painkillers.
“Opiates are very good at curbing severe pain ... But for chronic, noncancer pain, you really need to look at the risks and benefits,” said Christopher M. Jones, Pharm.D., the study’s coauthor and a senior adviser at the FDA.
FROM A CDC TELEBRIEFING
Extended warfarin delays return of unprovoked pulmonary embolism
Adding an extra 18 months of warfarin therapy to the standard 6 months of anticoagulation delays the recurrence of venous thrombosis in patients who have a first episode of unprovoked pulmonary embolism – but the risk of recurrence resumes as soon as the warfarin is discontinued, according to a report published online July 7 in JAMA.
“Our results suggest that patients such as those who participated in our study require long-term secondary prophylaxis measures. Whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or be tailored according to patient risk factors (including elevated D-dimer levels) needs further investigation,” said Dr. Francis Couturaud of the department of internal medicine and chest diseases, University of Brest (France) Hospital, and his associates (JAMA 2015;314:31-40).
Adults with a first episode of unprovoked VT are at much greater risk of recurrence when the standard 6 months of anticoagulation runs out, compared with those whose VT is provoked by a known, transient risk factor such as lengthy surgery, trauma with immobilization of the lower limbs, or bed rest extending longer than 72 hours.
Some experts have advocated extending anticoagulation further in such patients; but whether this is actually beneficial remains uncertain, the investigators said, because most studies have not pursued follow-up beyond the end of treatment.
The researchers performed a multicenter, double-blind trial in which 371 consecutive patients with a first episode of unprovoked PE completed 6 months of anticoagulation and then were randomly assigned to a further 18 months on either warfarin or matching placebo.
During this 18-month treatment period, the primary outcome – a composite of recurrent VT (including PE) and major bleeding – occurred in 3.3% of the warfarin group and 13.5% of the placebo group. That significant difference translated to a 78% reduction in favor of warfarin (hazard ratio, 0.22), Dr. Couturaud and his associates said.
However, after the treatment period ended, the composite outcome occurred in 17.7% of the warfarin group and 10.3% of the placebo group. Thus, the risk of recurrence returned to its normal high level once warfarin was discontinued, the study authors noted.
The study was supported by the Programme Hospitalier de Recherche Clinique (the French Department of Health) and the University Hospital of Brest (France). Dr. Couturaud reported receiving research grants, honoraria, and travel pay from Actelion, AstraZeneca, Bayer, Daiichi Sankyo, Intermune, Leo Pharma, and Pfizer, and his associates reported ties to numerous industry sources.
Related Information
- Computed tomographic pulmonary angiography (CTPA) may be useful in the diagnosis of suspected PE, wrote Dr. Gregoire Le Gal and co-authors from the University of Ottawa. Alternately, a V/Q scan may be performed. The complete accompanying article on diagnostic testing methods for suspected pulmonary embolism can be found here.
- The recently approved anticoagulant edoxaban is similar to warfarin in its ability to treat acute VTE, according to a report published in the Medical Letter on Drugs and Therapeutics in the same issue. However, further study is needed to evaluate its safety and efficacy compared with dabigatran, rivaroxaban, and apixaban, the three other oral anticoagulant drugs currently FDA-approved for acute VTE.
- A meta-analysis of 3,716 patients with VTE found that long-term treatment with Vitamin K antagonists was associated with lower rates of thromboembolic events (relative risk = 0.20) and higher rates of bleeding complications (RR = 3.44), compared with short-term therapy, Dr. Saskia Middeldorp and Dr. Barbara A. Hutten of the University of Amsterdam reported in the same issue. There was no difference in mortality between the two groups.
- Currently, recommended treatment duration for PE can range from three months to lifelong treatment, wrote Dr. Jill Jin in a clinical synopsis for patients published with the study.
- Read the full article and listen to the related podcast: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.7046
Madhu Rajaraman contributed to this report.
Adding an extra 18 months of warfarin therapy to the standard 6 months of anticoagulation delays the recurrence of venous thrombosis in patients who have a first episode of unprovoked pulmonary embolism – but the risk of recurrence resumes as soon as the warfarin is discontinued, according to a report published online July 7 in JAMA.
“Our results suggest that patients such as those who participated in our study require long-term secondary prophylaxis measures. Whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or be tailored according to patient risk factors (including elevated D-dimer levels) needs further investigation,” said Dr. Francis Couturaud of the department of internal medicine and chest diseases, University of Brest (France) Hospital, and his associates (JAMA 2015;314:31-40).
Adults with a first episode of unprovoked VT are at much greater risk of recurrence when the standard 6 months of anticoagulation runs out, compared with those whose VT is provoked by a known, transient risk factor such as lengthy surgery, trauma with immobilization of the lower limbs, or bed rest extending longer than 72 hours.
Some experts have advocated extending anticoagulation further in such patients; but whether this is actually beneficial remains uncertain, the investigators said, because most studies have not pursued follow-up beyond the end of treatment.
The researchers performed a multicenter, double-blind trial in which 371 consecutive patients with a first episode of unprovoked PE completed 6 months of anticoagulation and then were randomly assigned to a further 18 months on either warfarin or matching placebo.
During this 18-month treatment period, the primary outcome – a composite of recurrent VT (including PE) and major bleeding – occurred in 3.3% of the warfarin group and 13.5% of the placebo group. That significant difference translated to a 78% reduction in favor of warfarin (hazard ratio, 0.22), Dr. Couturaud and his associates said.
However, after the treatment period ended, the composite outcome occurred in 17.7% of the warfarin group and 10.3% of the placebo group. Thus, the risk of recurrence returned to its normal high level once warfarin was discontinued, the study authors noted.
The study was supported by the Programme Hospitalier de Recherche Clinique (the French Department of Health) and the University Hospital of Brest (France). Dr. Couturaud reported receiving research grants, honoraria, and travel pay from Actelion, AstraZeneca, Bayer, Daiichi Sankyo, Intermune, Leo Pharma, and Pfizer, and his associates reported ties to numerous industry sources.
Related Information
- Computed tomographic pulmonary angiography (CTPA) may be useful in the diagnosis of suspected PE, wrote Dr. Gregoire Le Gal and co-authors from the University of Ottawa. Alternately, a V/Q scan may be performed. The complete accompanying article on diagnostic testing methods for suspected pulmonary embolism can be found here.
- The recently approved anticoagulant edoxaban is similar to warfarin in its ability to treat acute VTE, according to a report published in the Medical Letter on Drugs and Therapeutics in the same issue. However, further study is needed to evaluate its safety and efficacy compared with dabigatran, rivaroxaban, and apixaban, the three other oral anticoagulant drugs currently FDA-approved for acute VTE.
- A meta-analysis of 3,716 patients with VTE found that long-term treatment with Vitamin K antagonists was associated with lower rates of thromboembolic events (relative risk = 0.20) and higher rates of bleeding complications (RR = 3.44), compared with short-term therapy, Dr. Saskia Middeldorp and Dr. Barbara A. Hutten of the University of Amsterdam reported in the same issue. There was no difference in mortality between the two groups.
- Currently, recommended treatment duration for PE can range from three months to lifelong treatment, wrote Dr. Jill Jin in a clinical synopsis for patients published with the study.
- Read the full article and listen to the related podcast: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.7046
Madhu Rajaraman contributed to this report.
Adding an extra 18 months of warfarin therapy to the standard 6 months of anticoagulation delays the recurrence of venous thrombosis in patients who have a first episode of unprovoked pulmonary embolism – but the risk of recurrence resumes as soon as the warfarin is discontinued, according to a report published online July 7 in JAMA.
“Our results suggest that patients such as those who participated in our study require long-term secondary prophylaxis measures. Whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or be tailored according to patient risk factors (including elevated D-dimer levels) needs further investigation,” said Dr. Francis Couturaud of the department of internal medicine and chest diseases, University of Brest (France) Hospital, and his associates (JAMA 2015;314:31-40).
Adults with a first episode of unprovoked VT are at much greater risk of recurrence when the standard 6 months of anticoagulation runs out, compared with those whose VT is provoked by a known, transient risk factor such as lengthy surgery, trauma with immobilization of the lower limbs, or bed rest extending longer than 72 hours.
Some experts have advocated extending anticoagulation further in such patients; but whether this is actually beneficial remains uncertain, the investigators said, because most studies have not pursued follow-up beyond the end of treatment.
The researchers performed a multicenter, double-blind trial in which 371 consecutive patients with a first episode of unprovoked PE completed 6 months of anticoagulation and then were randomly assigned to a further 18 months on either warfarin or matching placebo.
During this 18-month treatment period, the primary outcome – a composite of recurrent VT (including PE) and major bleeding – occurred in 3.3% of the warfarin group and 13.5% of the placebo group. That significant difference translated to a 78% reduction in favor of warfarin (hazard ratio, 0.22), Dr. Couturaud and his associates said.
However, after the treatment period ended, the composite outcome occurred in 17.7% of the warfarin group and 10.3% of the placebo group. Thus, the risk of recurrence returned to its normal high level once warfarin was discontinued, the study authors noted.
The study was supported by the Programme Hospitalier de Recherche Clinique (the French Department of Health) and the University Hospital of Brest (France). Dr. Couturaud reported receiving research grants, honoraria, and travel pay from Actelion, AstraZeneca, Bayer, Daiichi Sankyo, Intermune, Leo Pharma, and Pfizer, and his associates reported ties to numerous industry sources.
Related Information
- Computed tomographic pulmonary angiography (CTPA) may be useful in the diagnosis of suspected PE, wrote Dr. Gregoire Le Gal and co-authors from the University of Ottawa. Alternately, a V/Q scan may be performed. The complete accompanying article on diagnostic testing methods for suspected pulmonary embolism can be found here.
- The recently approved anticoagulant edoxaban is similar to warfarin in its ability to treat acute VTE, according to a report published in the Medical Letter on Drugs and Therapeutics in the same issue. However, further study is needed to evaluate its safety and efficacy compared with dabigatran, rivaroxaban, and apixaban, the three other oral anticoagulant drugs currently FDA-approved for acute VTE.
- A meta-analysis of 3,716 patients with VTE found that long-term treatment with Vitamin K antagonists was associated with lower rates of thromboembolic events (relative risk = 0.20) and higher rates of bleeding complications (RR = 3.44), compared with short-term therapy, Dr. Saskia Middeldorp and Dr. Barbara A. Hutten of the University of Amsterdam reported in the same issue. There was no difference in mortality between the two groups.
- Currently, recommended treatment duration for PE can range from three months to lifelong treatment, wrote Dr. Jill Jin in a clinical synopsis for patients published with the study.
- Read the full article and listen to the related podcast: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.7046
Madhu Rajaraman contributed to this report.
FROM JAMA
Key clinical point: Eighteen additional months of warfarin therapy delays the recurrence of unprovoked pulmonary embolism.
Major finding: During treatment, the primary outcome – a composite of recurrent venous thromboembolism and major bleeding – occurred in 3.3% of the warfarin group and 13.5% of the placebo group, a significant difference that translated to a 78% reduction in favor of warfarin (hazard ratio, 0.22).
Data source: A multicenter, randomized, double-blind, placebo-controlled clinical trial involving 371 patients followed for a mean of 41 months.
Disclosures: This study was supported by the Programme Hospitalier de Recherche Clinique (the French Department of Health) and the University Hospital of Brest (France). Dr. Couturaud reported receiving research grants, honoraria, and travel pay from Actelion, AstraZeneca, Bayer, Daiichi Sankyo, Intermune, Leo Pharma, and Pfizer, and his associates reported ties to numerous industry sources.
Madelung Deformity and Extensor Tendon Rupture
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
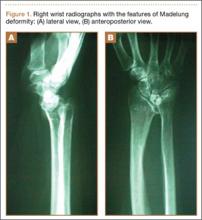
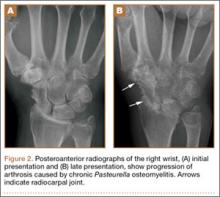
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
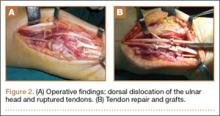
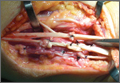
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
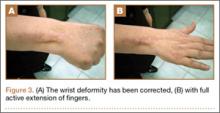
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
Septic Arthritis and Osteomyelitis Caused by Pasteurella multocida
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
Interim PET provides limited prognostic value for diffuse large B-cell lymphoma
Results from positron emission tomography after 2 and 4 weeks of standardized, dose-dense chemotherapy in patients with diffuse large B-cell lymphoma (DLBCL) failed to provide sufficient prognostic accuracy to guide therapeutic decisions, according to a report published online in the Journal of Clinical Oncology.
“Our data demonstrate that interim PET-CT fails to deliver a PPV [positive predictive value] or NPV [negative predictive value] that reflects the clinical outcome for patients with DLBCL treated with R-CHOP-14 with sufficient accuracy to guide therapeutic decisions such as treatment intensification/deintensification at the present stage,” wrote Dr. Chrisoph Mamot of the division of haematology/oncology, Cantonal Hospital of Aarau, Switzerland, and colleagues (J. Clin. Onc. 2015 July 6 [doi:10.1200/JCO.2014.58.9846]).
Although the likelihood of 2-year event-free survival (EFS) was significantly lower among patients who had positive PET results after 2 weeks of therapy (PET-2-positive), compared with PET-2-negative patients (48% vs. 74%; P = .004), a large proportion of interim PET-positive patients had favorable outcomes. This may be due to the dose-dense regimen, Dr. Mamot and colleagues said.
Patients underwent six cycles of R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/m2 for 5 days) every 14 days, followed by two cycles of rituximab.
Patients who had a positive PET-2 result received another PET scan after completion of four chemotherapy cycles. The 2-year EFS for PET-4-positive and PET-4-negative patients were not significantly different.
“Our original hypothesis that an interim PET/CT at a later time point might be able to better separate patients with good or poor prognosis was not confirmed,” the authors wrote. The end-of-treatment PET provided the best prediction of good vs. poor prognosis, but this time point is too late to adapt treatment. The authors concluded that interim PET/CT in patients with DLBCL presently is not accurate enough to guide treatment decisions in individual patients.
The multicenter, prospective study evaluated 138 patients, median age 58.5 years, who had untreated DLBCL, Ann Arbor stage I (12%), II (34%), III (23%), or IV (30%).
The study was supported by grants from Amgen (Switzerland) and OncoSuisse. Dr. Christoph Mamot reported consulting or advisory roles with Roche, Novartis, Amgen, and Boehringer-Ingelheim. Several of his coauthors reported ties to industry sources.
Results from positron emission tomography after 2 and 4 weeks of standardized, dose-dense chemotherapy in patients with diffuse large B-cell lymphoma (DLBCL) failed to provide sufficient prognostic accuracy to guide therapeutic decisions, according to a report published online in the Journal of Clinical Oncology.
“Our data demonstrate that interim PET-CT fails to deliver a PPV [positive predictive value] or NPV [negative predictive value] that reflects the clinical outcome for patients with DLBCL treated with R-CHOP-14 with sufficient accuracy to guide therapeutic decisions such as treatment intensification/deintensification at the present stage,” wrote Dr. Chrisoph Mamot of the division of haematology/oncology, Cantonal Hospital of Aarau, Switzerland, and colleagues (J. Clin. Onc. 2015 July 6 [doi:10.1200/JCO.2014.58.9846]).
Although the likelihood of 2-year event-free survival (EFS) was significantly lower among patients who had positive PET results after 2 weeks of therapy (PET-2-positive), compared with PET-2-negative patients (48% vs. 74%; P = .004), a large proportion of interim PET-positive patients had favorable outcomes. This may be due to the dose-dense regimen, Dr. Mamot and colleagues said.
Patients underwent six cycles of R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/m2 for 5 days) every 14 days, followed by two cycles of rituximab.
Patients who had a positive PET-2 result received another PET scan after completion of four chemotherapy cycles. The 2-year EFS for PET-4-positive and PET-4-negative patients were not significantly different.
“Our original hypothesis that an interim PET/CT at a later time point might be able to better separate patients with good or poor prognosis was not confirmed,” the authors wrote. The end-of-treatment PET provided the best prediction of good vs. poor prognosis, but this time point is too late to adapt treatment. The authors concluded that interim PET/CT in patients with DLBCL presently is not accurate enough to guide treatment decisions in individual patients.
The multicenter, prospective study evaluated 138 patients, median age 58.5 years, who had untreated DLBCL, Ann Arbor stage I (12%), II (34%), III (23%), or IV (30%).
The study was supported by grants from Amgen (Switzerland) and OncoSuisse. Dr. Christoph Mamot reported consulting or advisory roles with Roche, Novartis, Amgen, and Boehringer-Ingelheim. Several of his coauthors reported ties to industry sources.
Results from positron emission tomography after 2 and 4 weeks of standardized, dose-dense chemotherapy in patients with diffuse large B-cell lymphoma (DLBCL) failed to provide sufficient prognostic accuracy to guide therapeutic decisions, according to a report published online in the Journal of Clinical Oncology.
“Our data demonstrate that interim PET-CT fails to deliver a PPV [positive predictive value] or NPV [negative predictive value] that reflects the clinical outcome for patients with DLBCL treated with R-CHOP-14 with sufficient accuracy to guide therapeutic decisions such as treatment intensification/deintensification at the present stage,” wrote Dr. Chrisoph Mamot of the division of haematology/oncology, Cantonal Hospital of Aarau, Switzerland, and colleagues (J. Clin. Onc. 2015 July 6 [doi:10.1200/JCO.2014.58.9846]).
Although the likelihood of 2-year event-free survival (EFS) was significantly lower among patients who had positive PET results after 2 weeks of therapy (PET-2-positive), compared with PET-2-negative patients (48% vs. 74%; P = .004), a large proportion of interim PET-positive patients had favorable outcomes. This may be due to the dose-dense regimen, Dr. Mamot and colleagues said.
Patients underwent six cycles of R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/m2 for 5 days) every 14 days, followed by two cycles of rituximab.
Patients who had a positive PET-2 result received another PET scan after completion of four chemotherapy cycles. The 2-year EFS for PET-4-positive and PET-4-negative patients were not significantly different.
“Our original hypothesis that an interim PET/CT at a later time point might be able to better separate patients with good or poor prognosis was not confirmed,” the authors wrote. The end-of-treatment PET provided the best prediction of good vs. poor prognosis, but this time point is too late to adapt treatment. The authors concluded that interim PET/CT in patients with DLBCL presently is not accurate enough to guide treatment decisions in individual patients.
The multicenter, prospective study evaluated 138 patients, median age 58.5 years, who had untreated DLBCL, Ann Arbor stage I (12%), II (34%), III (23%), or IV (30%).
The study was supported by grants from Amgen (Switzerland) and OncoSuisse. Dr. Christoph Mamot reported consulting or advisory roles with Roche, Novartis, Amgen, and Boehringer-Ingelheim. Several of his coauthors reported ties to industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: After two cycles of standardized, dose-dense chemotherapy in patients with diffuse large B-cell lymphoma (DLBCL), positive results from PET signaled worse 2-year event-free survival (EFS) than negative interim PET results, but overall survival was not significantly different.
Major finding: After two cycles of chemotherapy, 2-year EFS for PET-positive, compared with PET-negative patients was 48.2% vs. 74.2% (P = .004). Overall survival was 87.7% vs. 90.6% (P = .6).
Data source: The multicenter, prospective study evaluated 138 patients, median age 58.5 years, who had untreated DLBCL, Ann Arbor stage I (12%), II (34%), III (23%), or IV (30%).
Disclosures: The study was supported by grants from Amgen (Switzerland) and OncoSuisse. Dr. Christoph Mamot reported consulting or advisory roles with Roche, Novartis, Amgen, and Boehringer-Ingelheim. Several of his coauthors reported ties to industry sources.
Enoxaparin and Warfarin for Venous Thromboembolism Prophylaxis in Total Hip Arthroplasty: To Bridge or Not to Bridge?
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.