User login
IBD: More patients on vedolizumab vs. anti-TNFs at 2 years
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
AT ECCO 2023
Cannabis tied to lower IBD mortality, hospital costs
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
AT ECCO 2023
NP-PA turf fights: Where the relationship can improve
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
Ovarian cancer risk lower with daily aspirin, despite genetics
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
new research suggests.
The study found that daily or almost daily aspirin use was associated with a 13% reduction in ovarian cancer risk, which was not modified by an individual’s polygenic score (PGS).
“Our findings suggest that frequent use of aspirin is associated with reduced ovarian cancer risk, regardless of whether a woman has lower or higher genetic susceptibility to ovarian cancer, as predicted by a set of known, common risk variants,” said lead author Lauren M. Hurwitz, PhD, MHS, division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md.
The study was published online in JAMA Network Open.
Patients diagnosed with ovarian cancer face difficult survival odds, which make preventive strategies especially important. Evidence suggests that frequent aspirin use can reduce the risk for ovarian cancer by about 13%, but it’s unclear whether genetic factors change those odds.
Although promising for chemoprevention, aspirin use can also come with downsides, including gastric ulcer and hemorrhagic stroke, which is why identifying and targeting individuals at higher risk for ovarian cancer who may benefit from frequent aspirin use is important.
In the current analysis, Dr. Hurwitz and colleagues used a PGS to determine whether the protective effects of daily or near-daily aspirin use for 6 months or more could be modified by genetics.
The study was a pooled analysis of eight case-controlled studies from the Ovarian Cancer Association Consortium conducted in the United Kingdom, the United States, and Australia over a 14-year period. The researchers looked at genetic data and data on frequent aspirin use among 4,476 case patients with nonmucinous ovarian cancer (average age, 57) and 6,659 control participants (average age, 58). Overall, 575 patients (13%) and 1,030 controls (15%) reported frequent aspirin use.
The authors used a PGS previously developed using 22 single-nucleotide variants. Because this PGS was developed for nonmucinous epithelial ovarian cancer, only these patients were included in the analysis.
Consistent with previous evidence, the authors found that frequent aspirin use was associated with a 13% lower risk for nonmucinous ovarian cancer (odds ratio, 0.87). And, notably, this association did not differ by PGS. Risk reductions were greatest for high-grade serous (OR, 0.83) and endometrioid tumors (OR, 0.73), with no evidence that PGS modified this association.
Overall, “we observed consistent protective associations between frequent aspirin use and nonmucinous ovarian cancer across strata of genetic susceptibility to ovarian cancer,” the authors conclude. “This work expands on the evidence base to suggest that chemoprevention programs could target individuals at higher risk of ovarian cancer.”
However, the authors noted that they were unable to test for effect modifications by specific pathogenic variants, such as BRCA1 or BRCA2.
“Our study did not address whether aspirin use is associated with reduced ovarian cancer risk among BRCA or other pathogenic variant carriers, and so our findings should not be used to inform discussions around aspirin use for these specific high-risk groups,” Dr. Hurwitz said. “Women with higher genetic susceptibility to ovarian cancer based on these common risk variants should discuss with their doctor the benefits and harms of taking aspirin for disease prevention.”
This study was supported by a grant from the DoD Ovarian Cancer Research Program. Dr. Hurwitz reports no relevant financial relationships, but several coauthors did report funding and support.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Docs struggle to keep up with the flood of new medical knowledge. Here’s advice
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
Celiac disease appears to double COVID-19 hospitalization risk
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA clinical practice update: Telemedicine in gastroenterology
moving forward, according to a new clinical practice update from the American Gastroenterological Association.
The postpandemic era must balance patient and provider preferences, medical needs, quality of care, regulatory requirements, and reimbursement rules, Ziad Gellad, MD, associate professor of medicine in the gastroenterology division at Duke University, Durham, N.C., and colleagues wrote.
“Spurred by the COVID-19 pandemic, telehealth, and specifically telemedicine, has become an integral part of outpatient gastrointestinal care in the United States,” the authors wrote.
Dr. Gellad and colleagues penned a clinical practice update based on recently published studies and the experiences of the authors, who are active gastroenterologists and hepatologists with extensive experience using telemedicine in clinical practice.
First, the group addressed patient preferences for telemedicine in gastroenterology based on emerging data. During the past 2 years, studies in both the United States and Australia found that most patients voiced ongoing interest and willingness to use video visits, as well as satisfaction with their medical concerns being addressed via telemedicine. They also reported significantly decreased absenteeism, as compared with face-to-face visits.
At the same time, patient preferences may vary based on age, race, and other factors. For instance, younger adults, those with higher incomes, and Hispanic and Latino patients appear to be more likely to prefer video visits than older adults, those with lower incomes, and White or Black patients. In gastroenterology, specific telemedicine studies, especially among patients with inflammatory bowel disease (IBD) or chronic liver disease, older patients, Black patients, and those with Medicaid or Medicare insurance were more likely to complete a phone-based visit rather than a video visit.
Even still, barriers exist for some patients, which should be recognized, the authors wrote. Studies have found racial and socioeconomic disparities in accessing telemedicine, including video visits. When possible, ambulatory practices, institutions, and health systems should provide technical solutions and individual support to help patients overcome these barriers.
So far, telemedicine appears to be better suited for stable chronic conditions rather than acute illnesses, which are more likely to require a follow-up in-person visit or ED care. At the gastrointestinal level, patients being evaluated for liver transplantation via telemedicine had a reduced time from referral to evaluation by a hepatologist and to transplant listing, and liver transplant recipients had lower readmission rates, improved physical function, and better general health. Among studies of IBD patients, telemedicine led to similar quality of care metrics and higher IBD-specific quality of life.
At this time, decisions about using telemedicine for patients with digestive diseases remain nuanced, the authors wrote. In general, those with stable conditions, such as gastroesophageal reflux, irritable bowel syndrome, IBD, chronic constipation, chronic liver disease, and chronic pancreatitis, appear to be good candidates for telemedicine. Patients who are considering a change in therapy and wish to schedule a visit for additional information may also use telemedicine.
In addition, those who live in remote areas could be appropriate candidates for telemedicine as long as they have access, particularly for video visits. Among these patients, studies have shown that telemedicine can be appropriate for patients with IBD and the transition of care from pediatric to adult gastroenterologists. Ultimately, the decision depends on several factors, including the practice setting, geography, and complexity of care.
Many times, the main barrier to virtual care is the regulatory requirement to be licensed in the state where the patient lives. Although these requirements were eased during the COVID-19 pandemic, many restrictions have now returned in most states. Some practices may now support their clinicians in obtaining licenses for surrounding states, but ultimately, some regulatory compromise will be needed to continue multistate telemedicine without additional licensure, the authors wrote.
Reimbursement rules have also remained a barrier. Despite some changes during the pandemic, reimbursement will likely shift in the future, and additional documentation requirements are suggested. For instance, it’s important to document patient consent to telemedicine, the method of telemedicine (whether a secure two-way interactive video or phone call), patient location, provider location, a listing of all clinical participants’ roles and actions, and other individuals (such as trainees) present at the visit.
Finally, the clinical workflow for telemedicine should include a few additional steps, the authors wrote. Office staff should connect with patients before the visit to address any technical issues and ensure a proper connection, set up any assistive services such as an interpreter, complete previsit questionnaires via secure messaging, and conduct standard practices such as medication review. Postvisit instructions should then be sent through a secure portal or mail.
Moving forward, additional studies are needed to verify long-term outcomes associated with telemedicine, as well as the optimal ratio of in-person versus telemedicine visits for various disease states, the authors wrote.
“Telemedicine is accepted by both patients and providers, and is associated with certain key advantages, including reducing patient travel time and cost and work absenteeism,” they wrote. However, “gastroenterology providers need to be cognizant of certain patient and illness barriers to telemedicine and adhere to best practices to ensure high-quality gastrointestinal virtual care.”
The clinical practice update received no funding support. Dr. Gellad disclosed financial relationships with Higgs Boson, Inc.; Merck & Co; and Novo Nordisk. Author Seth Crockett is a consultant for IngenioRx and has received research funding from Freenome, Guardant, and Exact Sciences. Raymond Cross disclosed financial relationships with AbbvVie, BMS, Fzata, Janssen, Magellan Health, Pfizer, and Takeda and has received support from the Crohn's and Colitis Foundation, IBD Education Group, and CorEvitas.
moving forward, according to a new clinical practice update from the American Gastroenterological Association.
The postpandemic era must balance patient and provider preferences, medical needs, quality of care, regulatory requirements, and reimbursement rules, Ziad Gellad, MD, associate professor of medicine in the gastroenterology division at Duke University, Durham, N.C., and colleagues wrote.
“Spurred by the COVID-19 pandemic, telehealth, and specifically telemedicine, has become an integral part of outpatient gastrointestinal care in the United States,” the authors wrote.
Dr. Gellad and colleagues penned a clinical practice update based on recently published studies and the experiences of the authors, who are active gastroenterologists and hepatologists with extensive experience using telemedicine in clinical practice.
First, the group addressed patient preferences for telemedicine in gastroenterology based on emerging data. During the past 2 years, studies in both the United States and Australia found that most patients voiced ongoing interest and willingness to use video visits, as well as satisfaction with their medical concerns being addressed via telemedicine. They also reported significantly decreased absenteeism, as compared with face-to-face visits.
At the same time, patient preferences may vary based on age, race, and other factors. For instance, younger adults, those with higher incomes, and Hispanic and Latino patients appear to be more likely to prefer video visits than older adults, those with lower incomes, and White or Black patients. In gastroenterology, specific telemedicine studies, especially among patients with inflammatory bowel disease (IBD) or chronic liver disease, older patients, Black patients, and those with Medicaid or Medicare insurance were more likely to complete a phone-based visit rather than a video visit.
Even still, barriers exist for some patients, which should be recognized, the authors wrote. Studies have found racial and socioeconomic disparities in accessing telemedicine, including video visits. When possible, ambulatory practices, institutions, and health systems should provide technical solutions and individual support to help patients overcome these barriers.
So far, telemedicine appears to be better suited for stable chronic conditions rather than acute illnesses, which are more likely to require a follow-up in-person visit or ED care. At the gastrointestinal level, patients being evaluated for liver transplantation via telemedicine had a reduced time from referral to evaluation by a hepatologist and to transplant listing, and liver transplant recipients had lower readmission rates, improved physical function, and better general health. Among studies of IBD patients, telemedicine led to similar quality of care metrics and higher IBD-specific quality of life.
At this time, decisions about using telemedicine for patients with digestive diseases remain nuanced, the authors wrote. In general, those with stable conditions, such as gastroesophageal reflux, irritable bowel syndrome, IBD, chronic constipation, chronic liver disease, and chronic pancreatitis, appear to be good candidates for telemedicine. Patients who are considering a change in therapy and wish to schedule a visit for additional information may also use telemedicine.
In addition, those who live in remote areas could be appropriate candidates for telemedicine as long as they have access, particularly for video visits. Among these patients, studies have shown that telemedicine can be appropriate for patients with IBD and the transition of care from pediatric to adult gastroenterologists. Ultimately, the decision depends on several factors, including the practice setting, geography, and complexity of care.
Many times, the main barrier to virtual care is the regulatory requirement to be licensed in the state where the patient lives. Although these requirements were eased during the COVID-19 pandemic, many restrictions have now returned in most states. Some practices may now support their clinicians in obtaining licenses for surrounding states, but ultimately, some regulatory compromise will be needed to continue multistate telemedicine without additional licensure, the authors wrote.
Reimbursement rules have also remained a barrier. Despite some changes during the pandemic, reimbursement will likely shift in the future, and additional documentation requirements are suggested. For instance, it’s important to document patient consent to telemedicine, the method of telemedicine (whether a secure two-way interactive video or phone call), patient location, provider location, a listing of all clinical participants’ roles and actions, and other individuals (such as trainees) present at the visit.
Finally, the clinical workflow for telemedicine should include a few additional steps, the authors wrote. Office staff should connect with patients before the visit to address any technical issues and ensure a proper connection, set up any assistive services such as an interpreter, complete previsit questionnaires via secure messaging, and conduct standard practices such as medication review. Postvisit instructions should then be sent through a secure portal or mail.
Moving forward, additional studies are needed to verify long-term outcomes associated with telemedicine, as well as the optimal ratio of in-person versus telemedicine visits for various disease states, the authors wrote.
“Telemedicine is accepted by both patients and providers, and is associated with certain key advantages, including reducing patient travel time and cost and work absenteeism,” they wrote. However, “gastroenterology providers need to be cognizant of certain patient and illness barriers to telemedicine and adhere to best practices to ensure high-quality gastrointestinal virtual care.”
The clinical practice update received no funding support. Dr. Gellad disclosed financial relationships with Higgs Boson, Inc.; Merck & Co; and Novo Nordisk. Author Seth Crockett is a consultant for IngenioRx and has received research funding from Freenome, Guardant, and Exact Sciences. Raymond Cross disclosed financial relationships with AbbvVie, BMS, Fzata, Janssen, Magellan Health, Pfizer, and Takeda and has received support from the Crohn's and Colitis Foundation, IBD Education Group, and CorEvitas.
moving forward, according to a new clinical practice update from the American Gastroenterological Association.
The postpandemic era must balance patient and provider preferences, medical needs, quality of care, regulatory requirements, and reimbursement rules, Ziad Gellad, MD, associate professor of medicine in the gastroenterology division at Duke University, Durham, N.C., and colleagues wrote.
“Spurred by the COVID-19 pandemic, telehealth, and specifically telemedicine, has become an integral part of outpatient gastrointestinal care in the United States,” the authors wrote.
Dr. Gellad and colleagues penned a clinical practice update based on recently published studies and the experiences of the authors, who are active gastroenterologists and hepatologists with extensive experience using telemedicine in clinical practice.
First, the group addressed patient preferences for telemedicine in gastroenterology based on emerging data. During the past 2 years, studies in both the United States and Australia found that most patients voiced ongoing interest and willingness to use video visits, as well as satisfaction with their medical concerns being addressed via telemedicine. They also reported significantly decreased absenteeism, as compared with face-to-face visits.
At the same time, patient preferences may vary based on age, race, and other factors. For instance, younger adults, those with higher incomes, and Hispanic and Latino patients appear to be more likely to prefer video visits than older adults, those with lower incomes, and White or Black patients. In gastroenterology, specific telemedicine studies, especially among patients with inflammatory bowel disease (IBD) or chronic liver disease, older patients, Black patients, and those with Medicaid or Medicare insurance were more likely to complete a phone-based visit rather than a video visit.
Even still, barriers exist for some patients, which should be recognized, the authors wrote. Studies have found racial and socioeconomic disparities in accessing telemedicine, including video visits. When possible, ambulatory practices, institutions, and health systems should provide technical solutions and individual support to help patients overcome these barriers.
So far, telemedicine appears to be better suited for stable chronic conditions rather than acute illnesses, which are more likely to require a follow-up in-person visit or ED care. At the gastrointestinal level, patients being evaluated for liver transplantation via telemedicine had a reduced time from referral to evaluation by a hepatologist and to transplant listing, and liver transplant recipients had lower readmission rates, improved physical function, and better general health. Among studies of IBD patients, telemedicine led to similar quality of care metrics and higher IBD-specific quality of life.
At this time, decisions about using telemedicine for patients with digestive diseases remain nuanced, the authors wrote. In general, those with stable conditions, such as gastroesophageal reflux, irritable bowel syndrome, IBD, chronic constipation, chronic liver disease, and chronic pancreatitis, appear to be good candidates for telemedicine. Patients who are considering a change in therapy and wish to schedule a visit for additional information may also use telemedicine.
In addition, those who live in remote areas could be appropriate candidates for telemedicine as long as they have access, particularly for video visits. Among these patients, studies have shown that telemedicine can be appropriate for patients with IBD and the transition of care from pediatric to adult gastroenterologists. Ultimately, the decision depends on several factors, including the practice setting, geography, and complexity of care.
Many times, the main barrier to virtual care is the regulatory requirement to be licensed in the state where the patient lives. Although these requirements were eased during the COVID-19 pandemic, many restrictions have now returned in most states. Some practices may now support their clinicians in obtaining licenses for surrounding states, but ultimately, some regulatory compromise will be needed to continue multistate telemedicine without additional licensure, the authors wrote.
Reimbursement rules have also remained a barrier. Despite some changes during the pandemic, reimbursement will likely shift in the future, and additional documentation requirements are suggested. For instance, it’s important to document patient consent to telemedicine, the method of telemedicine (whether a secure two-way interactive video or phone call), patient location, provider location, a listing of all clinical participants’ roles and actions, and other individuals (such as trainees) present at the visit.
Finally, the clinical workflow for telemedicine should include a few additional steps, the authors wrote. Office staff should connect with patients before the visit to address any technical issues and ensure a proper connection, set up any assistive services such as an interpreter, complete previsit questionnaires via secure messaging, and conduct standard practices such as medication review. Postvisit instructions should then be sent through a secure portal or mail.
Moving forward, additional studies are needed to verify long-term outcomes associated with telemedicine, as well as the optimal ratio of in-person versus telemedicine visits for various disease states, the authors wrote.
“Telemedicine is accepted by both patients and providers, and is associated with certain key advantages, including reducing patient travel time and cost and work absenteeism,” they wrote. However, “gastroenterology providers need to be cognizant of certain patient and illness barriers to telemedicine and adhere to best practices to ensure high-quality gastrointestinal virtual care.”
The clinical practice update received no funding support. Dr. Gellad disclosed financial relationships with Higgs Boson, Inc.; Merck & Co; and Novo Nordisk. Author Seth Crockett is a consultant for IngenioRx and has received research funding from Freenome, Guardant, and Exact Sciences. Raymond Cross disclosed financial relationships with AbbvVie, BMS, Fzata, Janssen, Magellan Health, Pfizer, and Takeda and has received support from the Crohn's and Colitis Foundation, IBD Education Group, and CorEvitas.
FROM GASTROENTEROLOGY
Risk of stent infection low, but may be underreported
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
FROM CRT 2023
Integrating intestinal ultrasound into inflammatory bowel disease training and practice in the United States
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)
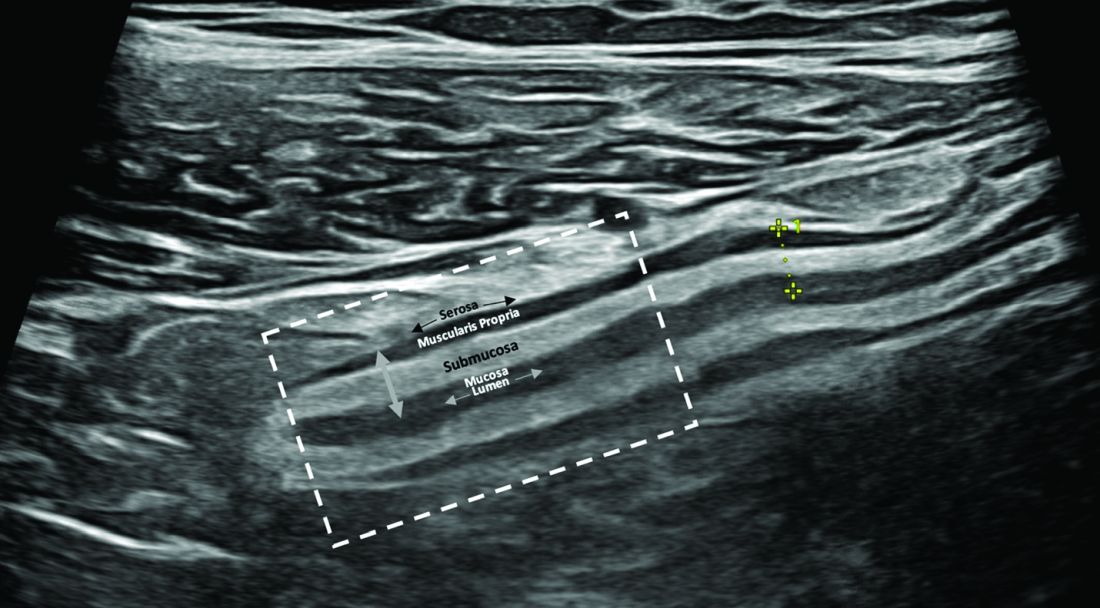
The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)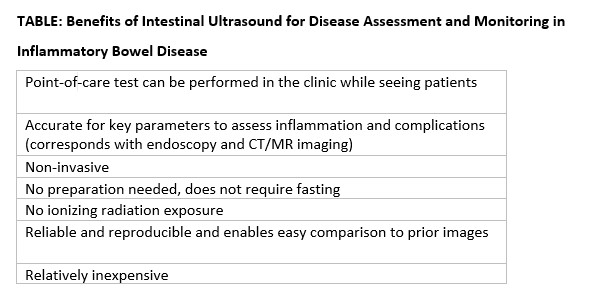
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
A better MS measure?
“When you measure disability, what you really want to know is how things are changing in the patient’s life and not your perception of how they’re changing,” said Mark Gudesblatt, MD, who presented a study comparing the technique, called quantitative gait analysis, to other measures at a poster session during the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The device, called Protokinetics, has been used in clinical studies for Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, stroke, Friederich’s ataxia, and other conditions. The device is a digitized carpet that senses weight change and pressure as the individual walks.
“We can actually measure performance, and the performance is not just how fast you walk 25 feet. We’re measuring things that underlie how you walk: step length, step length variability, velocity, weight shift, how much time you spend on one leg. So it’s like listening to a symphony. We’re not measuring just the trumpets or the violins, we’re measuring everything,” said Dr. Gudesblatt, who is medical director of the Comprehensive MS Center at South Shore Neurologic Associates, Patchogue, N.Y.
Commonly used measures include the Expanded Disability Status Scale (EDSS), the 25-foot time walk (25’TW), and the Timed Up and Go (TUG).
Those measures are useful but don’t really measure up to clinical need, Dr. Gudesblatt said. “What you want is no evidence of disease activity, whether that’s multiple dimensions of thinking or multiple dimensions of walking, or changes on an MRI that are not the radiologist’s impression. Patients always say: ‘Doc, I’m worse.’ And we say: ‘Well, your exam is unchanged, your MRI has not changed. But they are worse for reasons – either their perception or their performance. So you can measure this very granularly, and you can relate it to their fear of falling, their balance confidence. This ups the game,” said Dr. Gudesblatt.
“And here’s where it gets even more interesting. You can use this for signatures of disease,” he added. The data can, for example, suggest that instead of Parkinson’s disease, a patient may have a Parkinson’s variant. “What we’re doing is showing how the 25-foot timed walk and Timed Up and Go are very traditional, conservative measures. They’re equivalent to the Pony Express. They’re good, but not where you want to be.”
Technology provides more sensitive, but more complex data
Digital tools to measure a variety of functions, including gait, cognition, and upper limb function are becoming increasingly common in MS, according to Catherine Larochelle, MD, PhD, who was asked for comment. “They are easily providing measures that are likely more sensitive and diverse and probably more meaningful about the daily functional status of a person than our usual EDSS,” said Dr. Larochelle, who is an associate professor at Université de Montréal.
The next step is to determine how best to use the complex data that such devices generate. “Lots of research is being done to better understand how to use the rich but complex data obtained with these tools to provide useful information to people with MS and their clinical team, to help guide shared clinical decisions, and likely accelerate and improve outcomes in clinical trials. So this is a very exciting new era in terms of clinical neurological assessment,” said Dr. Larochelle.
Granular gait analysis
Dr. Gudesblatt and colleagues analyzed retrospective data from 105 people with MS (69% female; average age, 53.7 years). Participants underwent all tests on the same day. The digital gait analysis captured velocity, double support, cadence, functional ambulation profile, gait variability index, and walk ratio over three trials conducted at preferred walking speed (PWS) and during dual task walking.
There were statistically significant relationships (P ≤ .01) between TUG and 25’TW (R2 = 0.62). There were also significant relationships between 25’TW and digital parameters measured at PWS: velocity (R2 = 0.63); double support (R2 = 0.74); cadence (R2 = 0.56); and gait variability index (R2 = 0.54). During dual task walking, there were relationships between 25’TW and velocity (R2 = 0.53); double support (R2 = 0.30); cadence (R2 = 0.43); and gait variability index (R2 = 0.46).
TUG values were significantly associated with gait parameters during PWS: velocity (R2 = 0.71); double support (R2 = 0.75); cadence (R2 = 0.43); gait variability index (R2 = 0.45); and walk ratio (R2 = 0.06). During dual task walking, TUG values were significantly associated with velocity (R2 = 0.55), double support (R2 = 0.21), cadence (R2 = 0.45), and gait variability index (R2 = 0.39).
“With the availability multiple effective disease modifying therapies and the future potential of restorative or reparative treatments, more granular, validated standardized outcome measures are urgently needed,” said Dr. Gudesblatt. Analysis of gait cycle can provide clinically useful information not adequately captured by the current, more traditional approaches of measuring outcomes in MS.
Dr. Gudesblatt and Dr. Larochelle have no relevant financial disclosures.
“When you measure disability, what you really want to know is how things are changing in the patient’s life and not your perception of how they’re changing,” said Mark Gudesblatt, MD, who presented a study comparing the technique, called quantitative gait analysis, to other measures at a poster session during the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The device, called Protokinetics, has been used in clinical studies for Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, stroke, Friederich’s ataxia, and other conditions. The device is a digitized carpet that senses weight change and pressure as the individual walks.
“We can actually measure performance, and the performance is not just how fast you walk 25 feet. We’re measuring things that underlie how you walk: step length, step length variability, velocity, weight shift, how much time you spend on one leg. So it’s like listening to a symphony. We’re not measuring just the trumpets or the violins, we’re measuring everything,” said Dr. Gudesblatt, who is medical director of the Comprehensive MS Center at South Shore Neurologic Associates, Patchogue, N.Y.
Commonly used measures include the Expanded Disability Status Scale (EDSS), the 25-foot time walk (25’TW), and the Timed Up and Go (TUG).
Those measures are useful but don’t really measure up to clinical need, Dr. Gudesblatt said. “What you want is no evidence of disease activity, whether that’s multiple dimensions of thinking or multiple dimensions of walking, or changes on an MRI that are not the radiologist’s impression. Patients always say: ‘Doc, I’m worse.’ And we say: ‘Well, your exam is unchanged, your MRI has not changed. But they are worse for reasons – either their perception or their performance. So you can measure this very granularly, and you can relate it to their fear of falling, their balance confidence. This ups the game,” said Dr. Gudesblatt.
“And here’s where it gets even more interesting. You can use this for signatures of disease,” he added. The data can, for example, suggest that instead of Parkinson’s disease, a patient may have a Parkinson’s variant. “What we’re doing is showing how the 25-foot timed walk and Timed Up and Go are very traditional, conservative measures. They’re equivalent to the Pony Express. They’re good, but not where you want to be.”
Technology provides more sensitive, but more complex data
Digital tools to measure a variety of functions, including gait, cognition, and upper limb function are becoming increasingly common in MS, according to Catherine Larochelle, MD, PhD, who was asked for comment. “They are easily providing measures that are likely more sensitive and diverse and probably more meaningful about the daily functional status of a person than our usual EDSS,” said Dr. Larochelle, who is an associate professor at Université de Montréal.
The next step is to determine how best to use the complex data that such devices generate. “Lots of research is being done to better understand how to use the rich but complex data obtained with these tools to provide useful information to people with MS and their clinical team, to help guide shared clinical decisions, and likely accelerate and improve outcomes in clinical trials. So this is a very exciting new era in terms of clinical neurological assessment,” said Dr. Larochelle.
Granular gait analysis
Dr. Gudesblatt and colleagues analyzed retrospective data from 105 people with MS (69% female; average age, 53.7 years). Participants underwent all tests on the same day. The digital gait analysis captured velocity, double support, cadence, functional ambulation profile, gait variability index, and walk ratio over three trials conducted at preferred walking speed (PWS) and during dual task walking.
There were statistically significant relationships (P ≤ .01) between TUG and 25’TW (R2 = 0.62). There were also significant relationships between 25’TW and digital parameters measured at PWS: velocity (R2 = 0.63); double support (R2 = 0.74); cadence (R2 = 0.56); and gait variability index (R2 = 0.54). During dual task walking, there were relationships between 25’TW and velocity (R2 = 0.53); double support (R2 = 0.30); cadence (R2 = 0.43); and gait variability index (R2 = 0.46).
TUG values were significantly associated with gait parameters during PWS: velocity (R2 = 0.71); double support (R2 = 0.75); cadence (R2 = 0.43); gait variability index (R2 = 0.45); and walk ratio (R2 = 0.06). During dual task walking, TUG values were significantly associated with velocity (R2 = 0.55), double support (R2 = 0.21), cadence (R2 = 0.45), and gait variability index (R2 = 0.39).
“With the availability multiple effective disease modifying therapies and the future potential of restorative or reparative treatments, more granular, validated standardized outcome measures are urgently needed,” said Dr. Gudesblatt. Analysis of gait cycle can provide clinically useful information not adequately captured by the current, more traditional approaches of measuring outcomes in MS.
Dr. Gudesblatt and Dr. Larochelle have no relevant financial disclosures.
“When you measure disability, what you really want to know is how things are changing in the patient’s life and not your perception of how they’re changing,” said Mark Gudesblatt, MD, who presented a study comparing the technique, called quantitative gait analysis, to other measures at a poster session during the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
The device, called Protokinetics, has been used in clinical studies for Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, stroke, Friederich’s ataxia, and other conditions. The device is a digitized carpet that senses weight change and pressure as the individual walks.
“We can actually measure performance, and the performance is not just how fast you walk 25 feet. We’re measuring things that underlie how you walk: step length, step length variability, velocity, weight shift, how much time you spend on one leg. So it’s like listening to a symphony. We’re not measuring just the trumpets or the violins, we’re measuring everything,” said Dr. Gudesblatt, who is medical director of the Comprehensive MS Center at South Shore Neurologic Associates, Patchogue, N.Y.
Commonly used measures include the Expanded Disability Status Scale (EDSS), the 25-foot time walk (25’TW), and the Timed Up and Go (TUG).
Those measures are useful but don’t really measure up to clinical need, Dr. Gudesblatt said. “What you want is no evidence of disease activity, whether that’s multiple dimensions of thinking or multiple dimensions of walking, or changes on an MRI that are not the radiologist’s impression. Patients always say: ‘Doc, I’m worse.’ And we say: ‘Well, your exam is unchanged, your MRI has not changed. But they are worse for reasons – either their perception or their performance. So you can measure this very granularly, and you can relate it to their fear of falling, their balance confidence. This ups the game,” said Dr. Gudesblatt.
“And here’s where it gets even more interesting. You can use this for signatures of disease,” he added. The data can, for example, suggest that instead of Parkinson’s disease, a patient may have a Parkinson’s variant. “What we’re doing is showing how the 25-foot timed walk and Timed Up and Go are very traditional, conservative measures. They’re equivalent to the Pony Express. They’re good, but not where you want to be.”
Technology provides more sensitive, but more complex data
Digital tools to measure a variety of functions, including gait, cognition, and upper limb function are becoming increasingly common in MS, according to Catherine Larochelle, MD, PhD, who was asked for comment. “They are easily providing measures that are likely more sensitive and diverse and probably more meaningful about the daily functional status of a person than our usual EDSS,” said Dr. Larochelle, who is an associate professor at Université de Montréal.
The next step is to determine how best to use the complex data that such devices generate. “Lots of research is being done to better understand how to use the rich but complex data obtained with these tools to provide useful information to people with MS and their clinical team, to help guide shared clinical decisions, and likely accelerate and improve outcomes in clinical trials. So this is a very exciting new era in terms of clinical neurological assessment,” said Dr. Larochelle.
Granular gait analysis
Dr. Gudesblatt and colleagues analyzed retrospective data from 105 people with MS (69% female; average age, 53.7 years). Participants underwent all tests on the same day. The digital gait analysis captured velocity, double support, cadence, functional ambulation profile, gait variability index, and walk ratio over three trials conducted at preferred walking speed (PWS) and during dual task walking.
There were statistically significant relationships (P ≤ .01) between TUG and 25’TW (R2 = 0.62). There were also significant relationships between 25’TW and digital parameters measured at PWS: velocity (R2 = 0.63); double support (R2 = 0.74); cadence (R2 = 0.56); and gait variability index (R2 = 0.54). During dual task walking, there were relationships between 25’TW and velocity (R2 = 0.53); double support (R2 = 0.30); cadence (R2 = 0.43); and gait variability index (R2 = 0.46).
TUG values were significantly associated with gait parameters during PWS: velocity (R2 = 0.71); double support (R2 = 0.75); cadence (R2 = 0.43); gait variability index (R2 = 0.45); and walk ratio (R2 = 0.06). During dual task walking, TUG values were significantly associated with velocity (R2 = 0.55), double support (R2 = 0.21), cadence (R2 = 0.45), and gait variability index (R2 = 0.39).
“With the availability multiple effective disease modifying therapies and the future potential of restorative or reparative treatments, more granular, validated standardized outcome measures are urgently needed,” said Dr. Gudesblatt. Analysis of gait cycle can provide clinically useful information not adequately captured by the current, more traditional approaches of measuring outcomes in MS.
Dr. Gudesblatt and Dr. Larochelle have no relevant financial disclosures.
FROM ACTRIMS FORUM 2023








