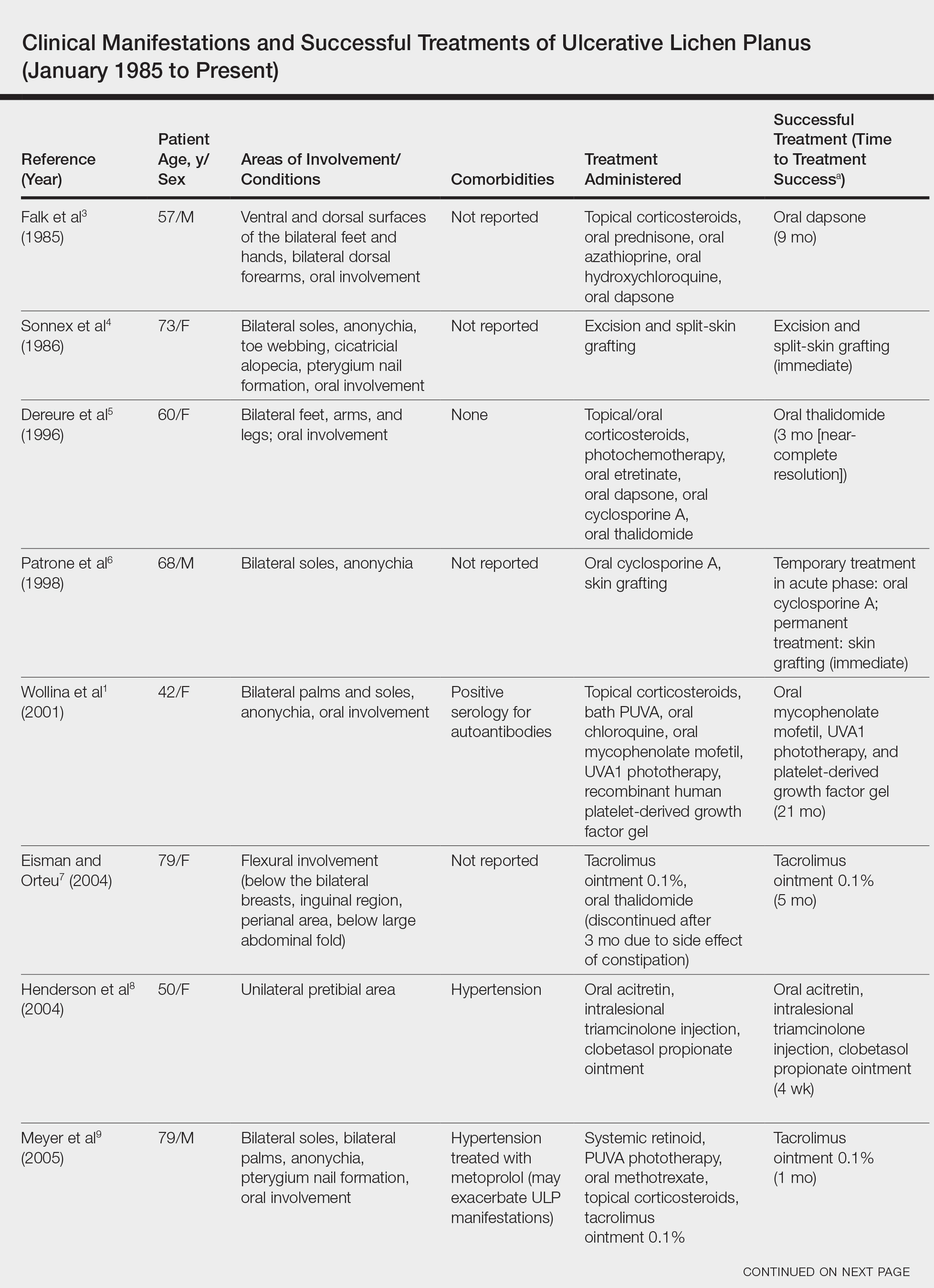
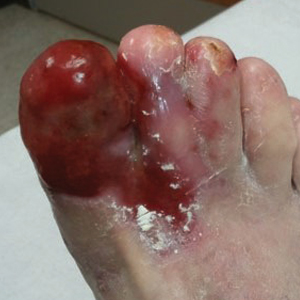
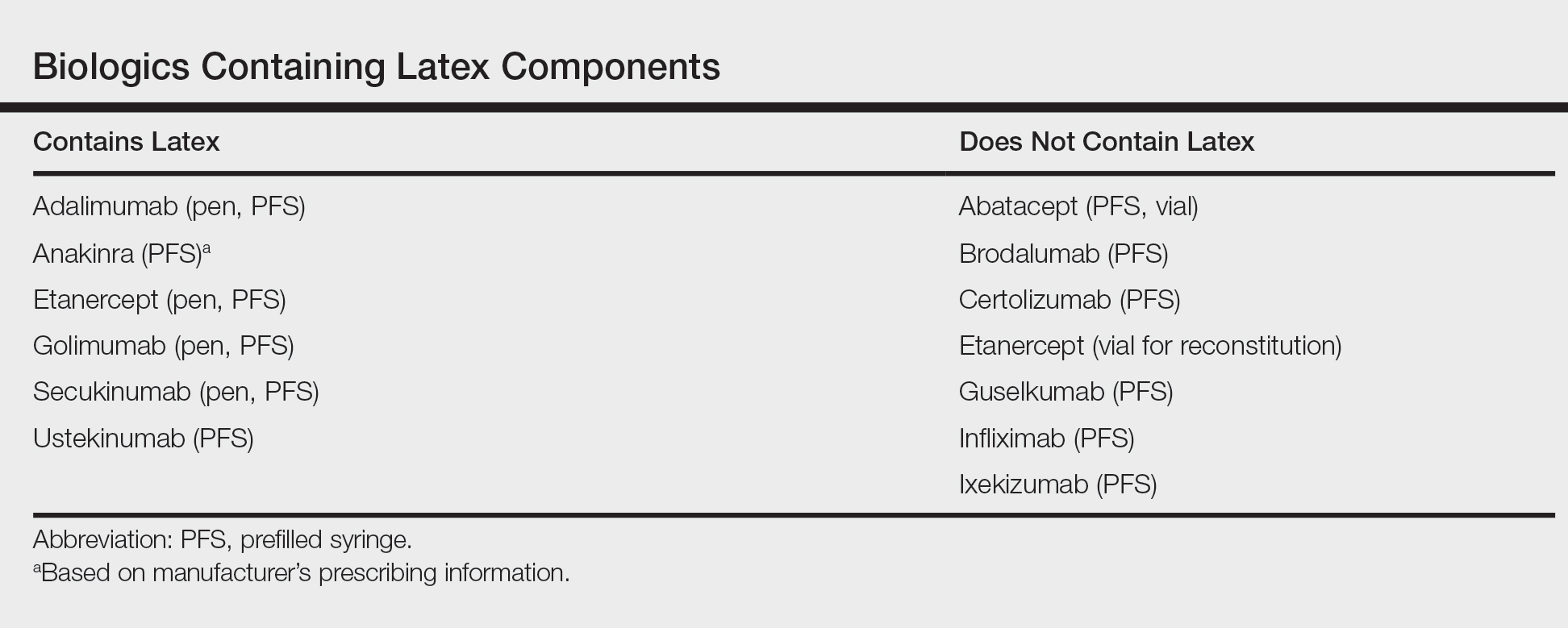
User login
Acute Aortic Occlusion With Spinal Cord Infarction
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
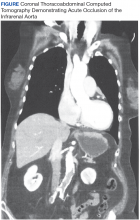
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
Acute aortic occlusion (AAO) is a relatively rare vascular emergency. The actual incidence of AAO is unknown but has been variously reported to be 1% to 4%, and the incidence of AAO secondary to infrarenal abdominal aortic aneurysm is reported to be about 2%.1 Acute aortic occlusion may present with acute onset of neurologic deficits as a consequence of spinal cord ischemia from thrombotic or embolic etiology. Risk factors for thrombosis include hypertension, tobacco smoking, and diabetes mellitus; heart disease and female gender are associated with embolism.2 Spinal cord infarction accounts for only 1% to 2% of all strokes and is characterized by acute onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception. Proprioception and vibratory sense are typically preserved.3 The authors present the case of a patient with acute onset of lower limb paralysis and urinary incontinence who was later found to have AAO due to thrombosis and consequent spinal cord infarction.
Case Presentation
An 80-year-old white woman presented to the emergency department of Jefferson Regional Medical Center in Pine Bluff, Arkansas, with the sudden onset of severe lower back pain, bilateral leg paralysis and paresthesia, and urinary incontinence. The patient stated that she had been watching television when her legs began to tingle and feel numb. Within 10 to 20 minutes she was unable to move her legs and became incontinent of urine. She reported no injury or previous history of back pain. Her medical history was significant for “irregular heartbeat my whole life,” hypertension, hyperlipidemia, and bladder cancer. She was not receiving systemic anticoagulation therapy. She reported a previous 15 pack-year smoking history but reported that she had quit cigarette smoking and usually drank 1 glass of wine daily. She had previously completed 3 rounds of chemotherapy for bladder cancer and received her first radiation treatment earlier that day. The symptoms began about 8 hours later that evening.
On examination the patient was noted to be in acute distress due to pain. Her vital signs in triage were blood pressure (BP) 122/71 mm Hg, pulse 54 beats per min (BPM), respirations 18 breathes per min, temperature 98° F, and pulse oximetry 90% on 2 L/min oxygen via nasal cannula. Laboratory evaluation was remarkable only for serum sodium 132 mEq/L, potassium 2.8 mEq/L, and thrombocytosis with platelets 697 × 103/μL. A neurologic examination showed normal motor function, strength of the upper extremities, and paralysis of the lower extremities, which were insensate to blunt or sharp touch and with decreased skin temperature from the groin distally. Pedal pulses were absent bilaterally. She was incontinent of urine and had anal sphincter laxity.
Magnetic resonance imaging showed bulging lumbar intervertebral discs and foraminal narrowing, which the consulting neurosurgeon did not feel explained her presentation and suggested that a vascular etiology was more likely. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed occlusion of the infrarenal abdominal aorta, bilateral common, and external iliac arteries. The proximal inferior mesenteric artery was occluded, and there was 90% stenosis of the proximal superior mesenteric artery with noncalcified plaque. There was no abdominal aortic aneurysm or dissection demonstrated. A chest CT was unremarkable.
The patient was started on IV heparin 800 U/h and transferred via ambulance to the University of Arkansas for Medical Sciences in Little Rock, Arkansas, for vascular surgery. On arrival her vital signs were BP 108/69 mm Hg, pulse 123 BPM, respirations 18 breathes per min, temperature 98° F, and pulse oximetry 94% on 2 L/min oxygen via nasal cannula. The patient’s electrocardiogram demonstrated atrial fibrillation with rapid ventricular response with premature ventricular complexes. On examination the bilateral lower extremities were cyanotic and cold to the touch. The pedal pulses were nonpalpable, and decreased distal sensation with dense paralysis was noted.
The patient was taken emergently to the operating room (OR) for left axillary bifemoral bypass. Severe atherosclerotic disease was noted at surgery. She was transferred to the surgical intensive care unit (SICU) for postoperative hemodynamic monitoring. Her clinical course became complicated by mesenteric ischemia from chronic superior mesenteric artery (SMA) occlusion. On postoperative day 2, she became progressively more hypotensive, and she was placed on vasopressin 0.04 U/min and amiodarone 0.5 mg/min infusions.
Bedside echocardiography showed diffuse ventricular hypokinesis and a left ventricular ejection fraction (LVEF) of about 30% but no mural thrombus. The patient developed altered mental status and respiratory distress, and her serum lactate increased to 6.1 mg/dL. She was emergently intubated and taken to the OR to attempt recanalization of the SMA occlusion, which was unsuccessful. She was returned to the SICU for continued resuscitation and monitoring. She continued to decline with hypotensive pressures, increasing serum creatinine and lactate, and worsening metabolic acidosis. Management options and goals of care were discussed with the family, and it was decided to honor her do not resuscitate status and pursue comfort care. She was extubated and expired a short time after this was done.
Discussion
Acute aortic occlusion is a rare vascular emergency with a mortality rate that approaches 75%.4-6 It results from numerous etiologies, including saddle embolism at the aortic bifurcation, acute thrombus formation, subsequent to aortic dissection, or other causes related to severe atherosclerotic disease or hypercoagulable states.4,7
A recent retrospective series of 29 cases of AAO found that thrombosis was the cause for 76% of cases, and > 40% of patients had a hypercoagulable state either because of antiphospholipid antibody syndrome (17%) or malignancy (24%).6 The most common presentation of AAO is the abrupt onset of painful bilateral paresis or paraplegia.5,6 While some studies have suggested that the major determinant of mortality is time elapsed until revascularization,7 other studies have reported that the neurologic status of the extremities is more closely related with mortality.2
The anterior spinal artery is the major independent provider of blood flow to the anterior two-thirds of the spinal cord, including the anterior horns, the anterior commissure, the anterior funiculi, and to a variable extent, the lateral funiculi. The largest segmental posterior radicular branch of the anterior spinal artery is the artery of Adamkiewicz, which arises from the T9 to T12 level on the left in 75% of cases and provides perfusion to the lumbar spinal cord and the conus medullaris. Obstruction of blood flow in this region has been implicated in the clinical picture of anterior cord syndrome characterized by abrupt onset of radicular pain, flaccid paresis or paralysis, sphincter dysfunction with urinary and fecal incontinence, and decreased pain and temperature sensation below a sensory level with spared proprioception and vibratory sensation.3, 8-10
Aortography is the gold standard procedure for diagnosis of AAO, but it is a time-consuming procedure, and preoperative testing is controversial. Contrast-enhanced CT is useful for evaluation as it can be quickly accomplished and is more available in general hospitals. Moreover, CT scanning may reveal aortic dissections or aneurysms as the cause of occlusion. Deep Doppler ultrasonography also has demonstrated utility as a noninvasive and rapidly performed diagnostic procedure. Magnetic resonance angiography or CT should be performed for all cases unless the patient’s clinical condition prevents this evaluation. Imaging not only confirms diagnosis, but also is valuable for assessment and planning management.11-14
Once the diagnosis of AAO is made, management with IV fluid hydration, heparin administration, and optimizing cardiac function are essential. However, conservative management with anticoagulation alone is associated with high mortality, and unless the ischemia is irreversible or unless the patient is in a dying state, surgery is appropriate.5,7 Depending on the etiology of the AAO, anatomic considerations, and other patient factors, urgent revascularization with thrombo-embolectomy, direct aortic reconstruction, or anatomic or extra-anatomic bypass procedures may be employed. Aortic reconstruction has been advocated for all patients with infrarenal aortic occlusion given the concern for propagation of thrombosis at the distal aorta proximally to the renal and mesenteric arteries.7 Axillary-bifemoral bypass has been advocated as a rapid revascularization strategy with good patency and less physiologic strain for critically ill AAO patients.6
The patient in this study had a constellation of risk factors for developing AAO due to thrombosis and consequently sustaining spinal cord infarction. Echocardiography ruled out embolism of a mural thrombus. She had cardiac dysfunction due to atrial fibrillation and left ventricular failure causing a low-flow state (LVEF 30%). She also had a hypercoagulable state due to bladder malignancy in addition to severe atherosclerotic disease. She was not on systemic anticoagulation therapy because of her high fall risk. Hence, her risk for thrombosis was quite high. Despite expedient revascularization surgery, her postoperative course was complicated as a result of severe mesenteric ischemia due to chronic SMA occlusion, which caused her death.
Conclusion
Acute aortic occlusion is a rare vascular emergency. The patient presenting with the abrupt onset of bilateral leg pain, neurologic deficits of paresis/paralysis, sensory disturbance, and/or sphincter dysfunction, and stigmata of vascular compromise with lower extremity mottling should alert the physician to AAO. Acute aortic occlusion continues to have high morbidity and mortality, and prompt recognition and appropriate transfer for surgical intervention are essential for improving outcomes.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
1. de Varona Frolov SR, Acosta Silva MP, Volvo Pérez G, Fiuza Pérez MD. Outcomes after treatment of acute aortic occlusion. [Article in English, Spanish] Cir Esp. 2015;93(9):573-579.
2. Dossa CD, Shepard AD, Reddy DJ, et al. Acute aortic occlusion: a 40-year experience. Arch Surg. 1994;129(6):603-608.
3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292.
4. Yamamoto H, Yamamoto F, Tanaka F, et al. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011;17(4):422-427.
5. Zainal AA, Oommen G, Chew LG, Yusha AW. Acute aortic occlusion: the need to be aware. Med J Malaysia. 2000;55(1):29-32.
6. Crawford JD, Perrone KH, Wong VW, et al. A modern series of acute aortic occlusion. J Vasc Surg. 2014;59(4):1044-1050.
7. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion—factors that influence outcome. J Vasc Surg. 1995;21(4):567-572.
8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330.
9. Rosenthal D. Spinal cord ischemia after abdominal aortic operation: is it preventable? J Vasc Surg. 1999;30(3):391-397.
10. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting as flaccid paraplegia. Spine (Phila Pa 1976). 2011;36(15):E1042-E1045.
11. Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15-23.
12. Bollinger B, Strandberg C, Baekgaard N, Mantoni M, Helweg-Larsen S. Diagnosis of acute aortic occlusion by computer tomography. Vasa. 1995;24(2):199-201.
13. Bertucci B, Rotundo A, Perri G, Sessa E, Tamburrini O. Acute thrombotic occlusion of the infrarenal abdominal aorta: its diagnosis with spiral computed tomography in a case [Article in Italian]. Radiol Med. 1997;94(5):541-543.
14. Battaglia S, Danesino GM, Danesino V, Castellani S. Color doppler ultrasonography of the abdominal aorta. J Ultrasound. 2010;13(3):107-117.
An unusual case of primary cardiac prosthetic valve-associated lymphoma
Primary cardiac tumors are extremely rare neoplasms with an incidence of less than 0.4%.1-3 Primary cardiac lymphoma (PCL), the majority of which is non-Hodgkin lymphoma, accounts for around 2% of cardiac tumors and less than 0.5% of extranodal lymphomas.1,4-6 Primary lymphoma involving cardiac valves has been described in few case reports and small case series owing to its rarity.7-10 Most cases of PCL present with manifestations of congestive heart failure or cardiac arrhythmias,11 whereas primary valve-associated lymphoma (PV-AL) is usually diagnosed incidentally during valve repair or replacement. The pathophysiology remains unclear, but a few cases have been associated with Epstein Barr virus (EBV).7 Cases previously described in the literature carried an overall poor prognosis and to date there is no standardized treatment approach. We provide here an unusual case of primary prosthetic valve-associated cardiac large B-cell lymphoma, which was successfully treated with adjuvant chemotherapy after valve repair and which resulted in an excellent long-term outcome.
Case presentation and summary
The patient presented in 2012 as a 65-year-old man with a history of ascending aortic aneurysm with secondary aortic insufficiency who in 2004 had undergone composite valve replacement of the aortic valve (AV) root and ascending aorta with a St Jude Toronto root. In June 2011, he was found to have a right parietal intraparenchymal hemorrhage that was thought to be a thromboembolic hemorrhagic ischemic stroke. In March 2012, he had routine follow-up brain magnetic resonance imaging that incidentally showed a left frontal ischemic stroke with hemorrhagic conversion. In June 2012, he was found to have first degree atrioventricular block with episodic runs of supraventricular tachycardia.
In September 2012, transthoracic echocardiography was done for further evaluation of possible recurrent cryptogenic strokes. The results showed a hypo-echogenic mass within the proximal ascending aortic root, but this was not confirmed on transesophageal echocardiography. A chest computed-tomography (CT) scan was therefore performed, and it showed aneurysmal dilatation of the aortic root with an irregular marginal filling defect just above the AV suggestive of intraluminal thrombus. The patient was placed on full anticoagulation with warfarin and referred for cardiothoracic surgery to consider graft and valve replacement. However, 3 weeks later and before the surgery, the patient developed a third thromboembolic ischemic event (transient ischemic attack). The recurrent strokes were attributed to thromboembolic events secondary to prosthetic AV thrombosis.
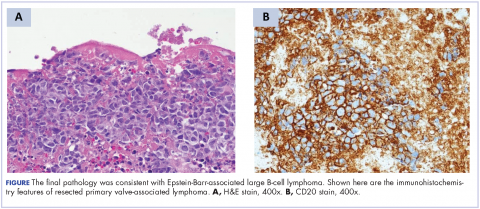
A repeat transthoracic echocardiography was significant for an abnormal AV bioprosthesis with associated thrombus extending to the ascending aorta. Surgical excision and replacement of the AV conduit explant were performed in November 2012. The final pathology was consistent with EBV-associated large B-cell lymphoma (Figure). The initial staging evaluation, including a CT and positron-emission tomography scan and bone marrow biopsy, was negative for any systemic disease. The patient received 4 cycles of R-CHOP-21 (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2 , vincristine 2 mg, and prednisone 100 mg) every 3 weeks in an “adjuvant” setting (because patient had no evidence of disease when given the systemic chemotherapy). The patient tolerated chemotherapy well without significant complications, and he is now over 36 months post-treatment without evidence of recurrent disease.
Discussion
Cardiac lymphoma limited only to prosthetic valves is rare, but it has been reported increasingly over the past few years. Until 2010, only six cases of PV-AL had been reported in the literature.7 Including our case, we identified four additional PubMed-indexed cases (using a PubMed search through February 2015). The patient characteristics and treatments received for all identified cases are described in the accompanying Table. The pathology from all of the cases revealed non-Hodgkin lymphoma of large B-cell subtype. PV-AL predominated among men (60%) and older patients with a median age of 62.5 years at diagnosis (range, 48-80 years). Patients had a median duration of 8 years (range, 4-24 years) from date of prosthesis placement to date of lymphoma diagnosis. The three most common presenting manifestations were valvular dysfunction, stroke, and congestive heart failure. All of the patients had surgical intervention on initial presentation. However, management after surgery was not uniform, with only 3 patients reported to have received systemic chemotherapy (Table). None of the patients received adjuvant radiation therapy. Calculated from date of diagnosis, survival duration ranged from less than a month7 to more than 36 months (as reported in our case).
The pathophysiology of PV-AL is not well understood given the rarity of the condition. Similar to other prosthetic-related neoplasms (metallic implants, breast implants),12-14 it has been hypothesized that chronic inflammation and EBV infection may play an essential role in the pathogenesis of this entity. Further, it has been suggested that Dacron, which is used in composite cardiac valve replacements, is carcinogenic and may play a role in some cases.7,15 PV-AL should be highly considered in the differential diagnosis of a suspicious prosthetic valve mass. Various imaging modalities, including echocardiography, CT, and magnetic resonance imaging have been described to have a role in the preoperative evaluation of cardiac tumors by assessing the cardiac function and defining the location and extent of the cardiac tumors.16-19
Given the rarity of this disease entity, there is no standardized approach for treatment. Surgical resection along with repair or replacement of primary involved prosthetic valve is essential for initial treatment. However, there is no consensus about the best approach for subsequent therapy. We cannot be conclusive about the optimum treatment, because of the limited number of published cases, but based on our reading of those cases, it would seem that early surgical intervention and “adjuvant” systemic therapy may have influenced prognosis. We speculate that poor outcomes in the first 6 months were most likely related to primary cardiopulmonary deterioration, whereas later poor outcomes were more likely to be attributable to recurrent lymphoma, particularly for patients who received suboptimal systemic chemotherapy treatment after surgery. All 3 patients who received chemotherapy had no evidence of recurrent disease at last follow-up. Of the 4 patients who received no chemotherapy and survived longer than 6 months (all except 1 died; Table), 2 had recurrent valve lymphoma, 1 had secondary systemic lymphoma, and 1 died of metastatic breast cancer. Those outcomes are in contrast to the 2 out of 3 patients who received adjuvant chemotherapy and who were reported to be alive at 16 and 36 months after diagnosis.
In conclusion, cardiac PV-AL is an increasingly recognized entity that warrants greater awareness among health care providers for early diagnosis and timely surgical intervention. Most of the cases are large B-cell lymphoma. Similar to patients with limited-stage DLBCL, fit patients should be highly considered for “adjuvant” systemic chemotherapy to optimize long-term outcomes. Reporting of similar cases is highly encouraged to better define this rare iatrogenic malignancy.
1. Hudzik B, Miszalski-Jamka K, Glowacki J, et al. Malignant tumors of the heart. Cancer epidemiol. 2015;39(5):665-672.
2. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press; 2004.
3. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77(1):107.
4. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007;19(10):748-756.
5. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219-228.
6. Burke A, Virmani R. Tumors of the heart and great vessels. In: Atlas of tumor pathology, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology, 1996.
7. Miller DV, Firchau DJ, McClure RF, Kurtin PJ, Feldman AL. Epstein-Barr virus-associated diffuse large B-cell lymphoma arising on cardiac prostheses. Am J Surg Pathol. 2010;34(3):377-384.
8. Albat B, Messner-Pellenc P, Thevenet A. Surgical treatment for primary lymphoma of the heart simulating prosthetic mitral valve thrombosis. J Thoracic Cardiovasc Surg. 1994;108(1):188-189.
9. Bagwan IN, Desai S, Wotherspoon A, Sheppard MN. Unusual presentation of primary cardiac lymphoma. Interact Cardiovasc Thorac Surg. 2009;9(1):127-129.
10. Durrleman NM, El-Hamamsy I, Demaria RG, Carrier M, Perrault LP, Albat B. Cardiac lymphoma following mitral valve replacement. Ann Thorac Surg. 2005;79(3):1040-1042.
11. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117(3):581-589.
12. Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29(6):832-836.
13. Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21(4):455-463.
14. de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030-2035.
15. Durrleman N, El Hamamsy I, Demaria R, Carrier M, Perrault LP, Albat B. Is Dacron carcinogenic? Apropos of a case and review of the literature [In French]. Arch Mal Coeur Vaiss. 2004 Mar;97(3):267-270.16. Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocard. 2006;19(2):230-240.
17. Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27(5):459-469.
18. Krombach GA, Spuentrup E, Buecker A, et al. Heart tumors: magnetic resonance imaging and multislice spiral CT [In German]. RoFo. 2005;177(9):1205-1218.
19. Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64(12):1214-1230.
20. Bonnichsen CR, Dearani JA, Maleszewski JJ, Colgan JP, Williamson EE, Ammash NM. Recurrent Epstein-Barr virus-associated diffuse large B-cell lymphoma in an ascending aorta graft. Circulation. 2013;128(13):1481-1483.
21. Berrio G, Suryadevara A, Singh NK, Wesly OH. Diffuse large B-cell lymphoma in an aortic valve allograft. Tex Heart Inst J. 2010;37(4):492-493.
22. Gruver AM, Huba MA, Dogan A, Hsi ED. Fibrin-associated large B-cell lymphoma: part of the spectrum of cardiac lymphomas. Am J Surg Pathol. 2012;36(10):1527-1537.
23. Farah FJ, Chiles CD. Recurrent primary cardiac lymphoma on aortic valve allograft: implications for therapy. Tex Heart Inst J. 2014;41(5):543-546.
Primary cardiac tumors are extremely rare neoplasms with an incidence of less than 0.4%.1-3 Primary cardiac lymphoma (PCL), the majority of which is non-Hodgkin lymphoma, accounts for around 2% of cardiac tumors and less than 0.5% of extranodal lymphomas.1,4-6 Primary lymphoma involving cardiac valves has been described in few case reports and small case series owing to its rarity.7-10 Most cases of PCL present with manifestations of congestive heart failure or cardiac arrhythmias,11 whereas primary valve-associated lymphoma (PV-AL) is usually diagnosed incidentally during valve repair or replacement. The pathophysiology remains unclear, but a few cases have been associated with Epstein Barr virus (EBV).7 Cases previously described in the literature carried an overall poor prognosis and to date there is no standardized treatment approach. We provide here an unusual case of primary prosthetic valve-associated cardiac large B-cell lymphoma, which was successfully treated with adjuvant chemotherapy after valve repair and which resulted in an excellent long-term outcome.
Case presentation and summary
The patient presented in 2012 as a 65-year-old man with a history of ascending aortic aneurysm with secondary aortic insufficiency who in 2004 had undergone composite valve replacement of the aortic valve (AV) root and ascending aorta with a St Jude Toronto root. In June 2011, he was found to have a right parietal intraparenchymal hemorrhage that was thought to be a thromboembolic hemorrhagic ischemic stroke. In March 2012, he had routine follow-up brain magnetic resonance imaging that incidentally showed a left frontal ischemic stroke with hemorrhagic conversion. In June 2012, he was found to have first degree atrioventricular block with episodic runs of supraventricular tachycardia.
In September 2012, transthoracic echocardiography was done for further evaluation of possible recurrent cryptogenic strokes. The results showed a hypo-echogenic mass within the proximal ascending aortic root, but this was not confirmed on transesophageal echocardiography. A chest computed-tomography (CT) scan was therefore performed, and it showed aneurysmal dilatation of the aortic root with an irregular marginal filling defect just above the AV suggestive of intraluminal thrombus. The patient was placed on full anticoagulation with warfarin and referred for cardiothoracic surgery to consider graft and valve replacement. However, 3 weeks later and before the surgery, the patient developed a third thromboembolic ischemic event (transient ischemic attack). The recurrent strokes were attributed to thromboembolic events secondary to prosthetic AV thrombosis.
A repeat transthoracic echocardiography was significant for an abnormal AV bioprosthesis with associated thrombus extending to the ascending aorta. Surgical excision and replacement of the AV conduit explant were performed in November 2012. The final pathology was consistent with EBV-associated large B-cell lymphoma (Figure). The initial staging evaluation, including a CT and positron-emission tomography scan and bone marrow biopsy, was negative for any systemic disease. The patient received 4 cycles of R-CHOP-21 (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2 , vincristine 2 mg, and prednisone 100 mg) every 3 weeks in an “adjuvant” setting (because patient had no evidence of disease when given the systemic chemotherapy). The patient tolerated chemotherapy well without significant complications, and he is now over 36 months post-treatment without evidence of recurrent disease.
Discussion
Cardiac lymphoma limited only to prosthetic valves is rare, but it has been reported increasingly over the past few years. Until 2010, only six cases of PV-AL had been reported in the literature.7 Including our case, we identified four additional PubMed-indexed cases (using a PubMed search through February 2015). The patient characteristics and treatments received for all identified cases are described in the accompanying Table. The pathology from all of the cases revealed non-Hodgkin lymphoma of large B-cell subtype. PV-AL predominated among men (60%) and older patients with a median age of 62.5 years at diagnosis (range, 48-80 years). Patients had a median duration of 8 years (range, 4-24 years) from date of prosthesis placement to date of lymphoma diagnosis. The three most common presenting manifestations were valvular dysfunction, stroke, and congestive heart failure. All of the patients had surgical intervention on initial presentation. However, management after surgery was not uniform, with only 3 patients reported to have received systemic chemotherapy (Table). None of the patients received adjuvant radiation therapy. Calculated from date of diagnosis, survival duration ranged from less than a month7 to more than 36 months (as reported in our case).
The pathophysiology of PV-AL is not well understood given the rarity of the condition. Similar to other prosthetic-related neoplasms (metallic implants, breast implants),12-14 it has been hypothesized that chronic inflammation and EBV infection may play an essential role in the pathogenesis of this entity. Further, it has been suggested that Dacron, which is used in composite cardiac valve replacements, is carcinogenic and may play a role in some cases.7,15 PV-AL should be highly considered in the differential diagnosis of a suspicious prosthetic valve mass. Various imaging modalities, including echocardiography, CT, and magnetic resonance imaging have been described to have a role in the preoperative evaluation of cardiac tumors by assessing the cardiac function and defining the location and extent of the cardiac tumors.16-19
Given the rarity of this disease entity, there is no standardized approach for treatment. Surgical resection along with repair or replacement of primary involved prosthetic valve is essential for initial treatment. However, there is no consensus about the best approach for subsequent therapy. We cannot be conclusive about the optimum treatment, because of the limited number of published cases, but based on our reading of those cases, it would seem that early surgical intervention and “adjuvant” systemic therapy may have influenced prognosis. We speculate that poor outcomes in the first 6 months were most likely related to primary cardiopulmonary deterioration, whereas later poor outcomes were more likely to be attributable to recurrent lymphoma, particularly for patients who received suboptimal systemic chemotherapy treatment after surgery. All 3 patients who received chemotherapy had no evidence of recurrent disease at last follow-up. Of the 4 patients who received no chemotherapy and survived longer than 6 months (all except 1 died; Table), 2 had recurrent valve lymphoma, 1 had secondary systemic lymphoma, and 1 died of metastatic breast cancer. Those outcomes are in contrast to the 2 out of 3 patients who received adjuvant chemotherapy and who were reported to be alive at 16 and 36 months after diagnosis.
In conclusion, cardiac PV-AL is an increasingly recognized entity that warrants greater awareness among health care providers for early diagnosis and timely surgical intervention. Most of the cases are large B-cell lymphoma. Similar to patients with limited-stage DLBCL, fit patients should be highly considered for “adjuvant” systemic chemotherapy to optimize long-term outcomes. Reporting of similar cases is highly encouraged to better define this rare iatrogenic malignancy.
Primary cardiac tumors are extremely rare neoplasms with an incidence of less than 0.4%.1-3 Primary cardiac lymphoma (PCL), the majority of which is non-Hodgkin lymphoma, accounts for around 2% of cardiac tumors and less than 0.5% of extranodal lymphomas.1,4-6 Primary lymphoma involving cardiac valves has been described in few case reports and small case series owing to its rarity.7-10 Most cases of PCL present with manifestations of congestive heart failure or cardiac arrhythmias,11 whereas primary valve-associated lymphoma (PV-AL) is usually diagnosed incidentally during valve repair or replacement. The pathophysiology remains unclear, but a few cases have been associated with Epstein Barr virus (EBV).7 Cases previously described in the literature carried an overall poor prognosis and to date there is no standardized treatment approach. We provide here an unusual case of primary prosthetic valve-associated cardiac large B-cell lymphoma, which was successfully treated with adjuvant chemotherapy after valve repair and which resulted in an excellent long-term outcome.
Case presentation and summary
The patient presented in 2012 as a 65-year-old man with a history of ascending aortic aneurysm with secondary aortic insufficiency who in 2004 had undergone composite valve replacement of the aortic valve (AV) root and ascending aorta with a St Jude Toronto root. In June 2011, he was found to have a right parietal intraparenchymal hemorrhage that was thought to be a thromboembolic hemorrhagic ischemic stroke. In March 2012, he had routine follow-up brain magnetic resonance imaging that incidentally showed a left frontal ischemic stroke with hemorrhagic conversion. In June 2012, he was found to have first degree atrioventricular block with episodic runs of supraventricular tachycardia.
In September 2012, transthoracic echocardiography was done for further evaluation of possible recurrent cryptogenic strokes. The results showed a hypo-echogenic mass within the proximal ascending aortic root, but this was not confirmed on transesophageal echocardiography. A chest computed-tomography (CT) scan was therefore performed, and it showed aneurysmal dilatation of the aortic root with an irregular marginal filling defect just above the AV suggestive of intraluminal thrombus. The patient was placed on full anticoagulation with warfarin and referred for cardiothoracic surgery to consider graft and valve replacement. However, 3 weeks later and before the surgery, the patient developed a third thromboembolic ischemic event (transient ischemic attack). The recurrent strokes were attributed to thromboembolic events secondary to prosthetic AV thrombosis.
A repeat transthoracic echocardiography was significant for an abnormal AV bioprosthesis with associated thrombus extending to the ascending aorta. Surgical excision and replacement of the AV conduit explant were performed in November 2012. The final pathology was consistent with EBV-associated large B-cell lymphoma (Figure). The initial staging evaluation, including a CT and positron-emission tomography scan and bone marrow biopsy, was negative for any systemic disease. The patient received 4 cycles of R-CHOP-21 (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2 , vincristine 2 mg, and prednisone 100 mg) every 3 weeks in an “adjuvant” setting (because patient had no evidence of disease when given the systemic chemotherapy). The patient tolerated chemotherapy well without significant complications, and he is now over 36 months post-treatment without evidence of recurrent disease.
Discussion
Cardiac lymphoma limited only to prosthetic valves is rare, but it has been reported increasingly over the past few years. Until 2010, only six cases of PV-AL had been reported in the literature.7 Including our case, we identified four additional PubMed-indexed cases (using a PubMed search through February 2015). The patient characteristics and treatments received for all identified cases are described in the accompanying Table. The pathology from all of the cases revealed non-Hodgkin lymphoma of large B-cell subtype. PV-AL predominated among men (60%) and older patients with a median age of 62.5 years at diagnosis (range, 48-80 years). Patients had a median duration of 8 years (range, 4-24 years) from date of prosthesis placement to date of lymphoma diagnosis. The three most common presenting manifestations were valvular dysfunction, stroke, and congestive heart failure. All of the patients had surgical intervention on initial presentation. However, management after surgery was not uniform, with only 3 patients reported to have received systemic chemotherapy (Table). None of the patients received adjuvant radiation therapy. Calculated from date of diagnosis, survival duration ranged from less than a month7 to more than 36 months (as reported in our case).
The pathophysiology of PV-AL is not well understood given the rarity of the condition. Similar to other prosthetic-related neoplasms (metallic implants, breast implants),12-14 it has been hypothesized that chronic inflammation and EBV infection may play an essential role in the pathogenesis of this entity. Further, it has been suggested that Dacron, which is used in composite cardiac valve replacements, is carcinogenic and may play a role in some cases.7,15 PV-AL should be highly considered in the differential diagnosis of a suspicious prosthetic valve mass. Various imaging modalities, including echocardiography, CT, and magnetic resonance imaging have been described to have a role in the preoperative evaluation of cardiac tumors by assessing the cardiac function and defining the location and extent of the cardiac tumors.16-19
Given the rarity of this disease entity, there is no standardized approach for treatment. Surgical resection along with repair or replacement of primary involved prosthetic valve is essential for initial treatment. However, there is no consensus about the best approach for subsequent therapy. We cannot be conclusive about the optimum treatment, because of the limited number of published cases, but based on our reading of those cases, it would seem that early surgical intervention and “adjuvant” systemic therapy may have influenced prognosis. We speculate that poor outcomes in the first 6 months were most likely related to primary cardiopulmonary deterioration, whereas later poor outcomes were more likely to be attributable to recurrent lymphoma, particularly for patients who received suboptimal systemic chemotherapy treatment after surgery. All 3 patients who received chemotherapy had no evidence of recurrent disease at last follow-up. Of the 4 patients who received no chemotherapy and survived longer than 6 months (all except 1 died; Table), 2 had recurrent valve lymphoma, 1 had secondary systemic lymphoma, and 1 died of metastatic breast cancer. Those outcomes are in contrast to the 2 out of 3 patients who received adjuvant chemotherapy and who were reported to be alive at 16 and 36 months after diagnosis.
In conclusion, cardiac PV-AL is an increasingly recognized entity that warrants greater awareness among health care providers for early diagnosis and timely surgical intervention. Most of the cases are large B-cell lymphoma. Similar to patients with limited-stage DLBCL, fit patients should be highly considered for “adjuvant” systemic chemotherapy to optimize long-term outcomes. Reporting of similar cases is highly encouraged to better define this rare iatrogenic malignancy.
1. Hudzik B, Miszalski-Jamka K, Glowacki J, et al. Malignant tumors of the heart. Cancer epidemiol. 2015;39(5):665-672.
2. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press; 2004.
3. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77(1):107.
4. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007;19(10):748-756.
5. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219-228.
6. Burke A, Virmani R. Tumors of the heart and great vessels. In: Atlas of tumor pathology, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology, 1996.
7. Miller DV, Firchau DJ, McClure RF, Kurtin PJ, Feldman AL. Epstein-Barr virus-associated diffuse large B-cell lymphoma arising on cardiac prostheses. Am J Surg Pathol. 2010;34(3):377-384.
8. Albat B, Messner-Pellenc P, Thevenet A. Surgical treatment for primary lymphoma of the heart simulating prosthetic mitral valve thrombosis. J Thoracic Cardiovasc Surg. 1994;108(1):188-189.
9. Bagwan IN, Desai S, Wotherspoon A, Sheppard MN. Unusual presentation of primary cardiac lymphoma. Interact Cardiovasc Thorac Surg. 2009;9(1):127-129.
10. Durrleman NM, El-Hamamsy I, Demaria RG, Carrier M, Perrault LP, Albat B. Cardiac lymphoma following mitral valve replacement. Ann Thorac Surg. 2005;79(3):1040-1042.
11. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117(3):581-589.
12. Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29(6):832-836.
13. Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21(4):455-463.
14. de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030-2035.
15. Durrleman N, El Hamamsy I, Demaria R, Carrier M, Perrault LP, Albat B. Is Dacron carcinogenic? Apropos of a case and review of the literature [In French]. Arch Mal Coeur Vaiss. 2004 Mar;97(3):267-270.16. Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocard. 2006;19(2):230-240.
17. Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27(5):459-469.
18. Krombach GA, Spuentrup E, Buecker A, et al. Heart tumors: magnetic resonance imaging and multislice spiral CT [In German]. RoFo. 2005;177(9):1205-1218.
19. Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64(12):1214-1230.
20. Bonnichsen CR, Dearani JA, Maleszewski JJ, Colgan JP, Williamson EE, Ammash NM. Recurrent Epstein-Barr virus-associated diffuse large B-cell lymphoma in an ascending aorta graft. Circulation. 2013;128(13):1481-1483.
21. Berrio G, Suryadevara A, Singh NK, Wesly OH. Diffuse large B-cell lymphoma in an aortic valve allograft. Tex Heart Inst J. 2010;37(4):492-493.
22. Gruver AM, Huba MA, Dogan A, Hsi ED. Fibrin-associated large B-cell lymphoma: part of the spectrum of cardiac lymphomas. Am J Surg Pathol. 2012;36(10):1527-1537.
23. Farah FJ, Chiles CD. Recurrent primary cardiac lymphoma on aortic valve allograft: implications for therapy. Tex Heart Inst J. 2014;41(5):543-546.
1. Hudzik B, Miszalski-Jamka K, Glowacki J, et al. Malignant tumors of the heart. Cancer epidemiol. 2015;39(5):665-672.
2. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press; 2004.
3. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77(1):107.
4. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007;19(10):748-756.
5. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219-228.
6. Burke A, Virmani R. Tumors of the heart and great vessels. In: Atlas of tumor pathology, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology, 1996.
7. Miller DV, Firchau DJ, McClure RF, Kurtin PJ, Feldman AL. Epstein-Barr virus-associated diffuse large B-cell lymphoma arising on cardiac prostheses. Am J Surg Pathol. 2010;34(3):377-384.
8. Albat B, Messner-Pellenc P, Thevenet A. Surgical treatment for primary lymphoma of the heart simulating prosthetic mitral valve thrombosis. J Thoracic Cardiovasc Surg. 1994;108(1):188-189.
9. Bagwan IN, Desai S, Wotherspoon A, Sheppard MN. Unusual presentation of primary cardiac lymphoma. Interact Cardiovasc Thorac Surg. 2009;9(1):127-129.
10. Durrleman NM, El-Hamamsy I, Demaria RG, Carrier M, Perrault LP, Albat B. Cardiac lymphoma following mitral valve replacement. Ann Thorac Surg. 2005;79(3):1040-1042.
11. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117(3):581-589.
12. Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29(6):832-836.
13. Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21(4):455-463.
14. de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030-2035.
15. Durrleman N, El Hamamsy I, Demaria R, Carrier M, Perrault LP, Albat B. Is Dacron carcinogenic? Apropos of a case and review of the literature [In French]. Arch Mal Coeur Vaiss. 2004 Mar;97(3):267-270.16. Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocard. 2006;19(2):230-240.
17. Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27(5):459-469.
18. Krombach GA, Spuentrup E, Buecker A, et al. Heart tumors: magnetic resonance imaging and multislice spiral CT [In German]. RoFo. 2005;177(9):1205-1218.
19. Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64(12):1214-1230.
20. Bonnichsen CR, Dearani JA, Maleszewski JJ, Colgan JP, Williamson EE, Ammash NM. Recurrent Epstein-Barr virus-associated diffuse large B-cell lymphoma in an ascending aorta graft. Circulation. 2013;128(13):1481-1483.
21. Berrio G, Suryadevara A, Singh NK, Wesly OH. Diffuse large B-cell lymphoma in an aortic valve allograft. Tex Heart Inst J. 2010;37(4):492-493.
22. Gruver AM, Huba MA, Dogan A, Hsi ED. Fibrin-associated large B-cell lymphoma: part of the spectrum of cardiac lymphomas. Am J Surg Pathol. 2012;36(10):1527-1537.
23. Farah FJ, Chiles CD. Recurrent primary cardiac lymphoma on aortic valve allograft: implications for therapy. Tex Heart Inst J. 2014;41(5):543-546.
Durable response to pralatrexate for aggressive PTCL subtypes
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of mature T- and natural killer-cell neoplasms that comprise about 10%-15% of all non-Hodgkin lymphomas in the United States.1,2 The development of effective therapies for PTCL has been challenging because of the rare nature and heterogeneity of these lymphomas. Most therapies are a derivative of aggressive B-cell lymphoma therapies, including CHOP (cyclophosphamide, hydroxydaunorubicin, vinicristine, prednisone) and CHOEP (cyclophosphamide, hydroxydaunorubicin, vinicristine, etoposide, prednisone).1 Many centers use autologous or allogeneic stem cell transplant in this setting,1 but outcomes remain poor and progress in developing effective treatments has been slow.
Pralatrexate is the first drug to have been approved by the US Food and Drug Administration specifically for treating patients with relapsed or refractory PTCL.3 As a folate analog metabolic inhibitor, pralatrexate competitively inhibits dihydrofolate reductase and reduces cellular levels of thymidine monophosphate, which prevents the cell from synthesizing genetic material and triggers it to undergo apoptosis.4 The agency’s approval of pralatrexate was based on results from the PROPEL study, which is possibly the largest prospective study conducted in patients with relapsed or refractory PTCL (109 evaluable patients).2 Findings from the study showed an overall response rate (ORR) of 29%, and a median duration of response (DoR) of 10 months.2
Pralatrexate is administered intravenously at 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. It is generally continued until disease progression or an unacceptable level of toxicity.2 Alternative dosing schedules have been described, including 15 mg/m2 once weekly for 3 weeks of a 4-week treatment cycle for cutaneous T-cell lymphomas.5
In this case series, we examine the outcomes of 2 patients with particularly aggressive subtypes of PTCL who were treated with pralatrexate. The significance of this report is in describing the long duration of response and reporting on a PTCL subtype – subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type – that was underrepresented in the PROPEL study and is underreported in the literature.
Case presentations and summaries
Case 1
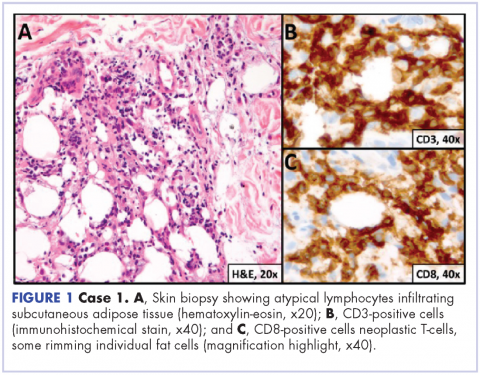
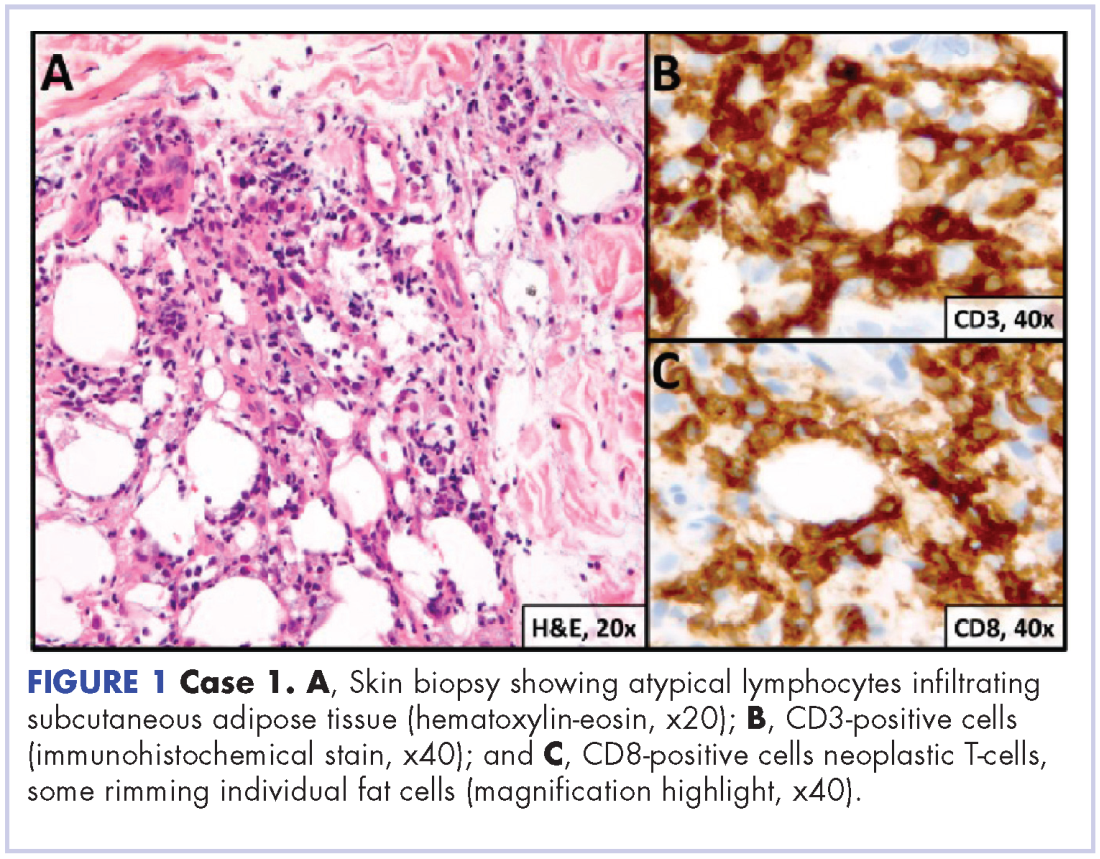
A 23-year-old Asian American man with a medical history of osteogenesis imperfecta presented to Emergency Department at the Hospital of University of Pennsylvania with bilateral lower extremity edema, low-grade fevers, a weight loss of 25 lb, and flat hyperpigmented scaly skin patches across his torso. Symptoms had started manifesting around five months prior to the visit. A punch biopsy of a skin lesion revealed skin tissue with focal infiltrate of small- to medium-sized, atypical lymphocytes infiltrating subcutaneous adipose tissue (panniculitis-like) and adnexa. Immunohistochemical stains showed that the abnormal lymphocytes were positive for CD3, CD8, perforin, granzyme B, TIA-1 (minor subset), and TCR beta; and negative for CD4, CD56, and CD30. Proliferation index (Ki67) was 70%. The findings were consistent with primary subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type (Figure 1). A staging positron-emission tomography–computed tomography (PET–CT) scan demonstrated stage IVB lymphoma with subcutaneous involvement without nodal disease.
He was initially treated with aggressive combination regimens including EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, hydroxydaunorubicin) and ICE (ifosfamide, carboplatin, etoposide), but he had no response and his disease was primary refractory. Because of his osteogenesis imperfecta, he was not a candidate for allogenic stem cell transplant.
He responded to hyperCVAD B combination therapy (methotrexate and cytarabine), but the course was complicated by cytarabine-induced ataxia and dysarthia. He was then treated with 3 months of intravenous alemtuzumab without response. Intravenous methotrexate (2,000 mg/m2) was then used for 3 cycles, but this exacerbated his previous cytarabine-induced neurological symptoms and resulted in only partial response with persistent fluorine-18-deoxyglucose (FDG) avid lesions on a subsequent PET–CT scan.
At that point, the patient was started on pralatrexate at 15 mg/m2 weekly for 3 weeks on a 4-week cycle schedule. This was his fifth line of therapy and at 16 months from his initial diagnosis. This dosage was continued for 6 months, and he tolerated the therapy well. He reported no exacerbations of his dysarthia, and by the second month, he had achieved clinical and radiographic remission with complete resolution of B symptoms (fevers, night sweats, and weight loss). The dosing was modified to 15 mg/m2 every 2 weeks for 3 months. A whole body PET–CT scan showed resolution of previously FDG avid lesions.
The patient was then continued on 15 mg/m2 pralatrexate every 3 weeks for 1 year and he has been maintained on once-a-month dosing for a second and now third year of therapy. He continues to tolerate the therapy and remains disease free at nearly 2 years since starting pralatrexate.
Case 2
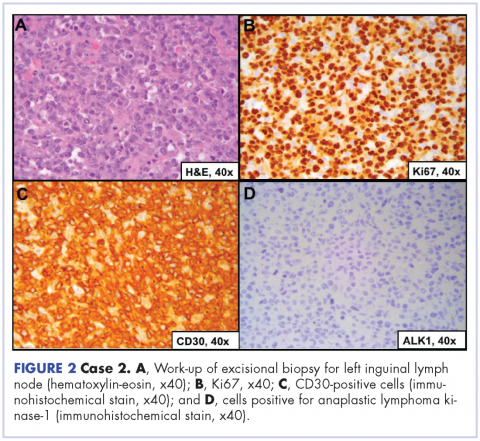
A 64-year-old white man with a medical history of myasthenia gravis (in remission) and invasive thymoma (after thymectomy) presented with diffuse bulky lymphadenopathy and lung lesions to outpatient clinic at the Abramson Cancer Center at the University of Pennsylvania. His LDH was elevated (278 U/L, reference range 98-192 U/L). Excisional biopsy of a left inguinal lymph node revealed sheets of mitotically active large cells with oval to irregular nuclei, clumped chromatin, conspicuous and sometimes multiple nucleoli, and ample eosinophilic cytoplasm. Immunohistochemical staining showed that the neoplastic cells were positive for CD3, CD4, CD30, BCL2 (variable), and MUM1; and negative for ALK 1, CD5, CD8, CD15, CD43, and CD56. Proliferation index (Ki67) was 90% (Figure 2). PET-CT scan showed widespread hypermetabolic lymphoma in the chest, neck, abdomen, and pelvis with pulmonary metastases. Imaging also demonstrated FDG-avid lesions in the gastric and sinus area. The findings were consistent with ALK-negative, anaplastic large cell lymphoma. He was stage IVA; had gastric, lung, and sinus involvement; and disease above and below the diaphragm.
The patient was initially treated with 6 cycles of CHOP and intrathecal methotrexate injections. His post-treatment PET–CT scan showed persistent FDG-avid disease and his LDH level remained elevated. He underwent 1 cycle of ICE and then BCV (busulfan, cyclophosphamide, etoposide) autologous stem cell transplant. Post-transplant PET–CT scan showed improvement from previous 2 scans but still showed several hypermetabolic lymph nodes consistent with persistent disease.
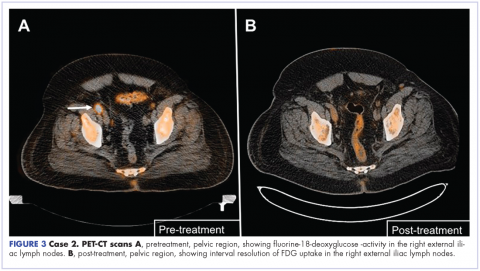
The patient was started on a pralatrexate regimen of 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. After 5 doses, he developed thrombocytopenia and mucositis, which were deemed pralatrexate related. The dosage was reduced to 20 mg/m2 once weekly with variable frequency depending on tolerability. His response assessment with PET–CT scan demonstrated radiographic complete response with resolution of hypermetabolic lesions (Figure 3B).
He then proceeded with pralatrexate for 4 more doses. PET-CT imaging 2 months after the last dose of pralatrexate was consistent with metabolic complete response, and he opted to hold further therapy. His last imaging at 4 years after completion of therapy showed continued remission. At press time, he had been clinically disease free for more than 6 years after his last dose of pralatrexate.
Discussion
PTCL is a rare and heterogeneous lymphoma with poor prognosis. Only 3 agents – pralatrexate, belinostat, and romidespin – have been approved specifically for the treatment of PTCL and all of them have an ORR of less than 30%, based on findings from phase 2 studies.2,6,7 In the PROPEL study, pralatrexate showed an ORR of 29% and a median DoR of 10 months.2 Those results could be considered discouraging, but some PTCL patients may have durable response to pralatrexate monotherapy.
In this case series, each of the patients presented with a particularly aggressive subtype of PTCL, and 1 suffered from a notably rare subtype for which there was scant clinical data to guide treatment. Both patients went through several lines of aggressive treatment that were ineffective and resulted in minimal response. However, both were able to achieve complete resolution of their disease and maintained remission for a significant duration of time after treatment with pralatrexate. In addition, each patient has maintained his remission – one for 6 years after the last dose. These are noteworthy results, and give both patients and clinicians hope that this therapy can be highly effective in some settings.
A better understanding at the molecular level of the oncogenic mechanisms in PTCL patients will be necessary to guide our therapy choices. In these 2 cases, it is likely that the tumor demonstrated superior sensitivity to dihydrofolate reductase inhibition by pralatrexate. In the future, we hope that analysis of the tumor tissue from PTCL patients will allow us to better categorize the tumor sensitivities to particular therapeutic agents. We believe that individualized treatment will lead to better overall outcomes in this challenging group of lymphomas.
1. d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099.
2. O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182-1189.
3. Dondi A, Bari A, Pozzi S, Ferri P, Sacchi S. The potential of pralatrexate as a treatment of peripheral T-cell lymphoma. Expert Opin Investig Drugs. 2014;23(5):711-718.
4. Hui J, Przespo E, Elefante A. Pralatrexate: a novel synthetic antifolate for relapsed or refractory peripheral T-cell lymphoma and other potential uses. J Oncol Pharm Pract. 2012;18(2):275-283.
5. Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012;119(18):4115-4122.
6. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492-2499.
7. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of mature T- and natural killer-cell neoplasms that comprise about 10%-15% of all non-Hodgkin lymphomas in the United States.1,2 The development of effective therapies for PTCL has been challenging because of the rare nature and heterogeneity of these lymphomas. Most therapies are a derivative of aggressive B-cell lymphoma therapies, including CHOP (cyclophosphamide, hydroxydaunorubicin, vinicristine, prednisone) and CHOEP (cyclophosphamide, hydroxydaunorubicin, vinicristine, etoposide, prednisone).1 Many centers use autologous or allogeneic stem cell transplant in this setting,1 but outcomes remain poor and progress in developing effective treatments has been slow.
Pralatrexate is the first drug to have been approved by the US Food and Drug Administration specifically for treating patients with relapsed or refractory PTCL.3 As a folate analog metabolic inhibitor, pralatrexate competitively inhibits dihydrofolate reductase and reduces cellular levels of thymidine monophosphate, which prevents the cell from synthesizing genetic material and triggers it to undergo apoptosis.4 The agency’s approval of pralatrexate was based on results from the PROPEL study, which is possibly the largest prospective study conducted in patients with relapsed or refractory PTCL (109 evaluable patients).2 Findings from the study showed an overall response rate (ORR) of 29%, and a median duration of response (DoR) of 10 months.2
Pralatrexate is administered intravenously at 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. It is generally continued until disease progression or an unacceptable level of toxicity.2 Alternative dosing schedules have been described, including 15 mg/m2 once weekly for 3 weeks of a 4-week treatment cycle for cutaneous T-cell lymphomas.5
In this case series, we examine the outcomes of 2 patients with particularly aggressive subtypes of PTCL who were treated with pralatrexate. The significance of this report is in describing the long duration of response and reporting on a PTCL subtype – subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type – that was underrepresented in the PROPEL study and is underreported in the literature.
Case presentations and summaries
Case 1
A 23-year-old Asian American man with a medical history of osteogenesis imperfecta presented to Emergency Department at the Hospital of University of Pennsylvania with bilateral lower extremity edema, low-grade fevers, a weight loss of 25 lb, and flat hyperpigmented scaly skin patches across his torso. Symptoms had started manifesting around five months prior to the visit. A punch biopsy of a skin lesion revealed skin tissue with focal infiltrate of small- to medium-sized, atypical lymphocytes infiltrating subcutaneous adipose tissue (panniculitis-like) and adnexa. Immunohistochemical stains showed that the abnormal lymphocytes were positive for CD3, CD8, perforin, granzyme B, TIA-1 (minor subset), and TCR beta; and negative for CD4, CD56, and CD30. Proliferation index (Ki67) was 70%. The findings were consistent with primary subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type (Figure 1). A staging positron-emission tomography–computed tomography (PET–CT) scan demonstrated stage IVB lymphoma with subcutaneous involvement without nodal disease.
He was initially treated with aggressive combination regimens including EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, hydroxydaunorubicin) and ICE (ifosfamide, carboplatin, etoposide), but he had no response and his disease was primary refractory. Because of his osteogenesis imperfecta, he was not a candidate for allogenic stem cell transplant.
He responded to hyperCVAD B combination therapy (methotrexate and cytarabine), but the course was complicated by cytarabine-induced ataxia and dysarthia. He was then treated with 3 months of intravenous alemtuzumab without response. Intravenous methotrexate (2,000 mg/m2) was then used for 3 cycles, but this exacerbated his previous cytarabine-induced neurological symptoms and resulted in only partial response with persistent fluorine-18-deoxyglucose (FDG) avid lesions on a subsequent PET–CT scan.
At that point, the patient was started on pralatrexate at 15 mg/m2 weekly for 3 weeks on a 4-week cycle schedule. This was his fifth line of therapy and at 16 months from his initial diagnosis. This dosage was continued for 6 months, and he tolerated the therapy well. He reported no exacerbations of his dysarthia, and by the second month, he had achieved clinical and radiographic remission with complete resolution of B symptoms (fevers, night sweats, and weight loss). The dosing was modified to 15 mg/m2 every 2 weeks for 3 months. A whole body PET–CT scan showed resolution of previously FDG avid lesions.
The patient was then continued on 15 mg/m2 pralatrexate every 3 weeks for 1 year and he has been maintained on once-a-month dosing for a second and now third year of therapy. He continues to tolerate the therapy and remains disease free at nearly 2 years since starting pralatrexate.
Case 2
A 64-year-old white man with a medical history of myasthenia gravis (in remission) and invasive thymoma (after thymectomy) presented with diffuse bulky lymphadenopathy and lung lesions to outpatient clinic at the Abramson Cancer Center at the University of Pennsylvania. His LDH was elevated (278 U/L, reference range 98-192 U/L). Excisional biopsy of a left inguinal lymph node revealed sheets of mitotically active large cells with oval to irregular nuclei, clumped chromatin, conspicuous and sometimes multiple nucleoli, and ample eosinophilic cytoplasm. Immunohistochemical staining showed that the neoplastic cells were positive for CD3, CD4, CD30, BCL2 (variable), and MUM1; and negative for ALK 1, CD5, CD8, CD15, CD43, and CD56. Proliferation index (Ki67) was 90% (Figure 2). PET-CT scan showed widespread hypermetabolic lymphoma in the chest, neck, abdomen, and pelvis with pulmonary metastases. Imaging also demonstrated FDG-avid lesions in the gastric and sinus area. The findings were consistent with ALK-negative, anaplastic large cell lymphoma. He was stage IVA; had gastric, lung, and sinus involvement; and disease above and below the diaphragm.
The patient was initially treated with 6 cycles of CHOP and intrathecal methotrexate injections. His post-treatment PET–CT scan showed persistent FDG-avid disease and his LDH level remained elevated. He underwent 1 cycle of ICE and then BCV (busulfan, cyclophosphamide, etoposide) autologous stem cell transplant. Post-transplant PET–CT scan showed improvement from previous 2 scans but still showed several hypermetabolic lymph nodes consistent with persistent disease.
The patient was started on a pralatrexate regimen of 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. After 5 doses, he developed thrombocytopenia and mucositis, which were deemed pralatrexate related. The dosage was reduced to 20 mg/m2 once weekly with variable frequency depending on tolerability. His response assessment with PET–CT scan demonstrated radiographic complete response with resolution of hypermetabolic lesions (Figure 3B).
He then proceeded with pralatrexate for 4 more doses. PET-CT imaging 2 months after the last dose of pralatrexate was consistent with metabolic complete response, and he opted to hold further therapy. His last imaging at 4 years after completion of therapy showed continued remission. At press time, he had been clinically disease free for more than 6 years after his last dose of pralatrexate.
Discussion
PTCL is a rare and heterogeneous lymphoma with poor prognosis. Only 3 agents – pralatrexate, belinostat, and romidespin – have been approved specifically for the treatment of PTCL and all of them have an ORR of less than 30%, based on findings from phase 2 studies.2,6,7 In the PROPEL study, pralatrexate showed an ORR of 29% and a median DoR of 10 months.2 Those results could be considered discouraging, but some PTCL patients may have durable response to pralatrexate monotherapy.
In this case series, each of the patients presented with a particularly aggressive subtype of PTCL, and 1 suffered from a notably rare subtype for which there was scant clinical data to guide treatment. Both patients went through several lines of aggressive treatment that were ineffective and resulted in minimal response. However, both were able to achieve complete resolution of their disease and maintained remission for a significant duration of time after treatment with pralatrexate. In addition, each patient has maintained his remission – one for 6 years after the last dose. These are noteworthy results, and give both patients and clinicians hope that this therapy can be highly effective in some settings.
A better understanding at the molecular level of the oncogenic mechanisms in PTCL patients will be necessary to guide our therapy choices. In these 2 cases, it is likely that the tumor demonstrated superior sensitivity to dihydrofolate reductase inhibition by pralatrexate. In the future, we hope that analysis of the tumor tissue from PTCL patients will allow us to better categorize the tumor sensitivities to particular therapeutic agents. We believe that individualized treatment will lead to better overall outcomes in this challenging group of lymphomas.
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of mature T- and natural killer-cell neoplasms that comprise about 10%-15% of all non-Hodgkin lymphomas in the United States.1,2 The development of effective therapies for PTCL has been challenging because of the rare nature and heterogeneity of these lymphomas. Most therapies are a derivative of aggressive B-cell lymphoma therapies, including CHOP (cyclophosphamide, hydroxydaunorubicin, vinicristine, prednisone) and CHOEP (cyclophosphamide, hydroxydaunorubicin, vinicristine, etoposide, prednisone).1 Many centers use autologous or allogeneic stem cell transplant in this setting,1 but outcomes remain poor and progress in developing effective treatments has been slow.
Pralatrexate is the first drug to have been approved by the US Food and Drug Administration specifically for treating patients with relapsed or refractory PTCL.3 As a folate analog metabolic inhibitor, pralatrexate competitively inhibits dihydrofolate reductase and reduces cellular levels of thymidine monophosphate, which prevents the cell from synthesizing genetic material and triggers it to undergo apoptosis.4 The agency’s approval of pralatrexate was based on results from the PROPEL study, which is possibly the largest prospective study conducted in patients with relapsed or refractory PTCL (109 evaluable patients).2 Findings from the study showed an overall response rate (ORR) of 29%, and a median duration of response (DoR) of 10 months.2
Pralatrexate is administered intravenously at 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. It is generally continued until disease progression or an unacceptable level of toxicity.2 Alternative dosing schedules have been described, including 15 mg/m2 once weekly for 3 weeks of a 4-week treatment cycle for cutaneous T-cell lymphomas.5
In this case series, we examine the outcomes of 2 patients with particularly aggressive subtypes of PTCL who were treated with pralatrexate. The significance of this report is in describing the long duration of response and reporting on a PTCL subtype – subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type – that was underrepresented in the PROPEL study and is underreported in the literature.
Case presentations and summaries
Case 1
A 23-year-old Asian American man with a medical history of osteogenesis imperfecta presented to Emergency Department at the Hospital of University of Pennsylvania with bilateral lower extremity edema, low-grade fevers, a weight loss of 25 lb, and flat hyperpigmented scaly skin patches across his torso. Symptoms had started manifesting around five months prior to the visit. A punch biopsy of a skin lesion revealed skin tissue with focal infiltrate of small- to medium-sized, atypical lymphocytes infiltrating subcutaneous adipose tissue (panniculitis-like) and adnexa. Immunohistochemical stains showed that the abnormal lymphocytes were positive for CD3, CD8, perforin, granzyme B, TIA-1 (minor subset), and TCR beta; and negative for CD4, CD56, and CD30. Proliferation index (Ki67) was 70%. The findings were consistent with primary subcutaneous panniculitis-like T-cell lymphoma, alpha/beta type (Figure 1). A staging positron-emission tomography–computed tomography (PET–CT) scan demonstrated stage IVB lymphoma with subcutaneous involvement without nodal disease.
He was initially treated with aggressive combination regimens including EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, hydroxydaunorubicin) and ICE (ifosfamide, carboplatin, etoposide), but he had no response and his disease was primary refractory. Because of his osteogenesis imperfecta, he was not a candidate for allogenic stem cell transplant.
He responded to hyperCVAD B combination therapy (methotrexate and cytarabine), but the course was complicated by cytarabine-induced ataxia and dysarthia. He was then treated with 3 months of intravenous alemtuzumab without response. Intravenous methotrexate (2,000 mg/m2) was then used for 3 cycles, but this exacerbated his previous cytarabine-induced neurological symptoms and resulted in only partial response with persistent fluorine-18-deoxyglucose (FDG) avid lesions on a subsequent PET–CT scan.
At that point, the patient was started on pralatrexate at 15 mg/m2 weekly for 3 weeks on a 4-week cycle schedule. This was his fifth line of therapy and at 16 months from his initial diagnosis. This dosage was continued for 6 months, and he tolerated the therapy well. He reported no exacerbations of his dysarthia, and by the second month, he had achieved clinical and radiographic remission with complete resolution of B symptoms (fevers, night sweats, and weight loss). The dosing was modified to 15 mg/m2 every 2 weeks for 3 months. A whole body PET–CT scan showed resolution of previously FDG avid lesions.
The patient was then continued on 15 mg/m2 pralatrexate every 3 weeks for 1 year and he has been maintained on once-a-month dosing for a second and now third year of therapy. He continues to tolerate the therapy and remains disease free at nearly 2 years since starting pralatrexate.
Case 2
A 64-year-old white man with a medical history of myasthenia gravis (in remission) and invasive thymoma (after thymectomy) presented with diffuse bulky lymphadenopathy and lung lesions to outpatient clinic at the Abramson Cancer Center at the University of Pennsylvania. His LDH was elevated (278 U/L, reference range 98-192 U/L). Excisional biopsy of a left inguinal lymph node revealed sheets of mitotically active large cells with oval to irregular nuclei, clumped chromatin, conspicuous and sometimes multiple nucleoli, and ample eosinophilic cytoplasm. Immunohistochemical staining showed that the neoplastic cells were positive for CD3, CD4, CD30, BCL2 (variable), and MUM1; and negative for ALK 1, CD5, CD8, CD15, CD43, and CD56. Proliferation index (Ki67) was 90% (Figure 2). PET-CT scan showed widespread hypermetabolic lymphoma in the chest, neck, abdomen, and pelvis with pulmonary metastases. Imaging also demonstrated FDG-avid lesions in the gastric and sinus area. The findings were consistent with ALK-negative, anaplastic large cell lymphoma. He was stage IVA; had gastric, lung, and sinus involvement; and disease above and below the diaphragm.
The patient was initially treated with 6 cycles of CHOP and intrathecal methotrexate injections. His post-treatment PET–CT scan showed persistent FDG-avid disease and his LDH level remained elevated. He underwent 1 cycle of ICE and then BCV (busulfan, cyclophosphamide, etoposide) autologous stem cell transplant. Post-transplant PET–CT scan showed improvement from previous 2 scans but still showed several hypermetabolic lymph nodes consistent with persistent disease.
The patient was started on a pralatrexate regimen of 30 mg/m2 once weekly for 6 weeks of a 7-week treatment cycle. After 5 doses, he developed thrombocytopenia and mucositis, which were deemed pralatrexate related. The dosage was reduced to 20 mg/m2 once weekly with variable frequency depending on tolerability. His response assessment with PET–CT scan demonstrated radiographic complete response with resolution of hypermetabolic lesions (Figure 3B).
He then proceeded with pralatrexate for 4 more doses. PET-CT imaging 2 months after the last dose of pralatrexate was consistent with metabolic complete response, and he opted to hold further therapy. His last imaging at 4 years after completion of therapy showed continued remission. At press time, he had been clinically disease free for more than 6 years after his last dose of pralatrexate.
Discussion
PTCL is a rare and heterogeneous lymphoma with poor prognosis. Only 3 agents – pralatrexate, belinostat, and romidespin – have been approved specifically for the treatment of PTCL and all of them have an ORR of less than 30%, based on findings from phase 2 studies.2,6,7 In the PROPEL study, pralatrexate showed an ORR of 29% and a median DoR of 10 months.2 Those results could be considered discouraging, but some PTCL patients may have durable response to pralatrexate monotherapy.
In this case series, each of the patients presented with a particularly aggressive subtype of PTCL, and 1 suffered from a notably rare subtype for which there was scant clinical data to guide treatment. Both patients went through several lines of aggressive treatment that were ineffective and resulted in minimal response. However, both were able to achieve complete resolution of their disease and maintained remission for a significant duration of time after treatment with pralatrexate. In addition, each patient has maintained his remission – one for 6 years after the last dose. These are noteworthy results, and give both patients and clinicians hope that this therapy can be highly effective in some settings.
A better understanding at the molecular level of the oncogenic mechanisms in PTCL patients will be necessary to guide our therapy choices. In these 2 cases, it is likely that the tumor demonstrated superior sensitivity to dihydrofolate reductase inhibition by pralatrexate. In the future, we hope that analysis of the tumor tissue from PTCL patients will allow us to better categorize the tumor sensitivities to particular therapeutic agents. We believe that individualized treatment will lead to better overall outcomes in this challenging group of lymphomas.
1. d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099.
2. O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182-1189.
3. Dondi A, Bari A, Pozzi S, Ferri P, Sacchi S. The potential of pralatrexate as a treatment of peripheral T-cell lymphoma. Expert Opin Investig Drugs. 2014;23(5):711-718.
4. Hui J, Przespo E, Elefante A. Pralatrexate: a novel synthetic antifolate for relapsed or refractory peripheral T-cell lymphoma and other potential uses. J Oncol Pharm Pract. 2012;18(2):275-283.
5. Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012;119(18):4115-4122.
6. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492-2499.
7. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
1. d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099.
2. O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182-1189.
3. Dondi A, Bari A, Pozzi S, Ferri P, Sacchi S. The potential of pralatrexate as a treatment of peripheral T-cell lymphoma. Expert Opin Investig Drugs. 2014;23(5):711-718.
4. Hui J, Przespo E, Elefante A. Pralatrexate: a novel synthetic antifolate for relapsed or refractory peripheral T-cell lymphoma and other potential uses. J Oncol Pharm Pract. 2012;18(2):275-283.
5. Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012;119(18):4115-4122.
6. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492-2499.
7. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
A Veteran With Fibromyalgia Presenting With Dyspnea
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
Case Presentation. A 64-year-old US Army veteran with a history of colorectal cancer, melanoma, and fibrinolytic presented with dyspnea to VA Boston Healthcare System (VABHS). Seven years prior to the current presentation, at the time of her diagnosis of colorectal cancer, the patient was found to be HIV negative but to have a positive purified protein derivative (PPD) test. She was treated with isoniazid (INH) therapy for 9 months. Sputum cultures collected prior to initiation of therapy grew Mycobacterium avium complex (MAC) in 1 of 3 samples, with these results reported several months after initiation of therapy. She was a never smoker with no known travel or exposure. At the time of the current presentation, her medications included bupropion, levothyroxine, capsaicin, cyclobenzaprine, ibuprofen, and acetaminophen.
►Lakshmana Swamy, MD, Chief Medical Resident, VABHS and Boston Medical Center. Dr. Monach, this patient is on a variety of pain medications and has a diagnosis of fibromyalgia. This diagnosis often frustrates doctors and patients alike. Can you tell us about fibromyalgia from the rheumatologist’s perspective and what you think of her current treatment regimen?
►Paul A. Monach, MD, PhD, Chief, Section of Rheumatology, VABHS and Associate Professor of Medicine, Boston University School of Medicine. Fibromyalgia is a syndrome of chronic widespread pain without known pathology in the musculoskeletal system. It is thought to be caused by chronic dysfunction of pain-processing pathways in the central nervous system (CNS). It is often accompanied by other somatic symptoms such as chronic fatigue, irritable bowel syndrome, and bladder pain. It is a common condition, affecting up to 5% of otherwise healthy women. It is particularly common in persons with chronic nonrestorative sleep or posttraumatic stress disorder from a wide range of causes. However, it also is more common in persons with autoimmune inflammatory diseases, such as lupus, Sjögren syndrome, or rheumatoid arthritis. Concern for one of these diseases is the main reason to consider referring a patient for evaluation by a rheumatologist. Often rheumatologists participate in the management of fibromyalgia. A patient should be given appropriate expectations by the referring physician.
Effectiveness of treatment varies widely among patients. Nonpharmacologic approaches such as aerobic exercise, cognitive behavioral therapy, and tai chi have support from clinical trials, and yoga and aquatherapy also are widely used.1,2 The classes of drugs used are the same as for neuropathic pain: tricyclics, including cyclobenzaprine; serotonin and norepinephrine reuptake inhibitors (SNRIs); and gabapentinoids. In contrast, nonsteroidal anti-inflammatory drugs and opioids are ineffective unless there is a superimposed mechanical or inflammatory cause in the periphery. The key point is that continuation of any treatment should be based entirely on the patient’s own assessment of benefit.
►Dr. Swamy. Seven years later, the patient returned to her primary care provider, reporting increased dyspnea on exertion as well as significant fatigue. She was referred to the pulmonary department and had repeat computed tomography (CT) scans of the chest, which indicated persistent right middle lobe (RML) bronchiectasis. She then underwent bronchoscopy with a subsequent bronchoalveolar lavage (BAL) culture growing MAC. Dr. Fine, please interpret the baseline and follow-up CT scans and help us understand the significance of the MAC on sputum and BAL cultures.
►Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine. Prior to this presentation, the patient had a pleural-based area of fibrosis with possible associated RML bronchiectasis. This appears to be a postinflammatory process without classic features of malignant or metastatic disease. She then had a sputum, which grew MAC in only 1 of 3 samples and in liquid media only. Importantly, the sputum was not smear positive. All of this suggests a low organism burden. One possibility is that this could reflect colonization with MAC; it is not uncommon for patients with underlying chronic changes in their lung to grow MAC, and it is often difficult to tell whether it is indicative of active disease. Structural lung disease, such as bronchiectasis, predisposes a patient to MAC, but chronic MAC also may cause bronchiectasis. This chicken-and-egg scenario comes up frequently. She may have a MAC infection, but as she is HIV negative and asymptomatic, there is no urgent indication to treat, especially as the burden of therapy is not insignificant.
►Dr. Swamy. Do we need to worry about Mycobacterium tuberculosis (MTB)?
►Dr. Fine. Although she was previously PPD positive, she had already completed 1 year of isoniazid (INH) therapy, making active MTB less likely. From an infection control standpoint, it is important to distinguish MAC from MTB. The former is not contagious, and there is no need for airborne isolation.
►Dr. Swamy. Dr. Fine, where does MAC come from? Does it commonly cause disease?
►Dr. Fine. In the environment, MAC is nearly ubiquitous , especially in water and soil. In one study, 20% of showerheads were positive for MAC; when patients are infected, we may suggest changing/bleaching the showerhead, but there are no definitive recommendations.3 Because MAC is so common in the environment, it is unlikely that measures to target MAC colonization will be clinically meaningful. On the other hand, the incidence of nontuberculous mycobacterial infections is increasing across the US, and it may be a common and frequently underdiagnosed cause of chronic cough, especially in postmenopausal women.
►Dr. Swamy. Four years prior to the current presentation, the patient developed a cough after an upper respiratory tract infection that persisted for more than 2 weeks. Given her history, she underwent a repeat chest CT, which noted a slight increase in nodularity and ground-glass opacity restricted to the RML. She also reported dyspnea on exertion and was referred to the pulmonary medicine department. By the time she arrived, her dyspnea had largely resolved, but she reported persistent fatigue without other systemic symptoms, such as fevers or chills. Dr. Fine, does MAC explain this patient’s dyspnea?
►Dr. Fine. As her pulmonary symptoms resolved in a short period of time with only azithromycin, it is very unlikely that her symptoms were related to her prior disease. The MAC infection is not likely to cause dyspnea on exertion and fatigue and should be worked up more broadly before attributing it to MAC. In view of this, it would not be unreasonable to follow her clinically and see her again in 6 to 8 weeks. In this context, we also should consider the untoward impact of repeated radiation exposure derived from multiple CT scans. When a patient has an abnormality on CT scan, it often leads to further scans even if the symptoms do not match the previous findings, as in this case.
►Dr. Swamy. Given her ongoing fatigue and systemic symptoms (morning stiffness of the shoulders, legs, and thighs, and leg cramps), she was referred to the rheumatology department where the physical examination revealed muscle tenderness in her proximal arms and legs with normal strength, tender points at the elbows and medial side of the bilateral knees, significant tenderness of lower legs, and no synovitis.
Dr. Monach, can you walk us through your approach to this patient? Are we seeing manifestations of fibromyalgia? What diagnoses concerns you and how would you proceed?
►Dr. Monach. The history and exam are most helpful in raising or reducing suspicion for an underlying inflammatory disease. Areas of tenderness described in her case are typical of fibromyalgia, although it can be difficult to interpret symptoms in the hip girdle and shoulder girdle because objective findings are often absent on exam in patients with inflammatory arthritis or bursitis. Similarly, tenderness at sites of tendon insertion (enthuses) without objective abnormalities is common in different forms of spondyloarthritis, so tenderness at the elbow, knee, lateral hip, and low back can be difficult to interpret. What this patient is lacking is prominent subjective or objective findings in the joints most commonly affected in rheumatoid arthritis and lupus: wrists, hands, ankles, and feet.
►Dr. Swamy. Initial laboratory data include an erythrocyte sedimentation rate of 79 with a normal C-reactive protein. A tentative diagnosis of polymyalgia rheumatic is made with consideration of a trial treatment of prednisone.
Dr. Monach, this patient has an indolent infection and is about to be given glucocorticoids. Could you describe the situations in which you feel that glucocorticoids cause a relative immunosuppression?
►Dr. Monach. Glucocorticoids are considered safe in a patient whose infection is not intrinsically dangerous or who has started appropriate antibiotics for that infection. Although all toxicities of glucocorticoids are dose dependent, the long-standing assertion that doses below 10 mg to 15 mg do not increase risk of infection is contradicted by data published in the past 10 to 15 years, with the caveat that these patients were on long-term treatment.
►Dr. Swamy. The patient was started on prednisone 15 mg per day for 15 days. She returned to the clinic after 1 week of prednisone troutment and noted “significant improvement in fatigue, morning stiffness of shoulders, thighs, leg, back is better, leg cramps resolved, shooting pain in many joints resolved.” Further laboratory results were notable for a negative rheumatoid factor, negative antinuclear antibody, and a cyclic citrullinated peptide of 60. A presumptive diagnosis of rheumatoid arthritis (RA) was made and plaquenil 200 mg twice daily was started.
Dr. Monach, can you explain why RA comes up now on serology but was not considered initially? Why does this presentation fit RA, and was her response to treatment typical? How does this fit in with her previous diagnosis of fibromyalgia? Was that just an atypical, indolent presentation of RA?
►Dr. Monach. Though her presentation is atypical for RA, in elderly patients, RA can present with symptoms resembling polymyalgia rheumatica. The question is whether she had RA all along (in which case “elderly onset” would not apply) or had fibromyalgia and developed RA more recently. The response to empiric glucocorticoid therapy is helpful, since fibromyalgia should not improve with prednisone even in a patient with RA unless treatment of RA would allow better sleep and ability to exercise. Rheumatoid arthritis typically responds very well to prednisone in the 5-mg to 15-mg range.
►Dr. Swamy. Given the new diagnosis of an inflammatory arthritis requiring immunosuppression, bronchoscopy with BAL is performed to evaluate for the presence of MAC. These cultures were positive for MAC.
Dr. Fine, does the positive BAL culture indicate an active MAC infection?
►Dr. Fine. Yes, based on these updated data, the patient has an active MAC infection. Active infection is defined as symptoms or imaging consistent with the diagnosis, supporting microbiology data (either 2 sputum or 1 BAL sample growing MAC) and the exclusion of other causes. Previously, this patient grew MAC in just one expectorated sputum; this did not meet the microbiologic criteria. Now sputum has grown in the BAL sample; along with the CT imaging, this is enough to diagnosis active MAC infection.
Treatment for MAC must consider the details of each case. First, this is not an emergency; treatment decisions should be made with the rheumatologist to consider the planned immunosuppression. For example, we must consider potential drug interactions. A specific point should be made of the use of tumor necrosis factor (TNF)-α inhibition, which data indicate can reactivate TB and may inhibit mechanisms that restrain mycobacterial disease. Serious cases of MAC infection have been reported in the literature in the setting of TNF-α inhibition.5,6 Despite these concerns, there is not a contraindication to using these therapies from the perspective of the active MAC disease. All of these decisions will impact the need to commit the patient to MAC therapy.
►Dr. Swamy. Dr. Fine, what do you consider prior to initiating MAC therapy?
► Dr. Fine. The decision to pursue MAC therapy should not be taken lightly. Therapy often entails prolonged multidrug regimens, usually spanning more than a year, with frequent adverse effects. Outside of very specific cases, such as TNF-β inhibition, MAC is rarely a life-threatening disease, so the benefit may be limited. Treatment for MAC is certainly unlikely to be fruitful without a diligent and motivated patient able to handle the high and prolonged pill burden. Of note, it is also important to keep this patient up-to-date with influenza and pneumonia vaccination given her structural lung disease.
►Dr. Swamy. The decision is made to treat MAC with azithromycin, rifampin, and ethambutol. The disease is noted to be nonfibrocavitary. The patient underwent monthly liver function test monitoring and visual acuity testing, which were unremarkable. Dr. Fine, can you describe the phenotypes of nontuberculous mycobacterial (NTM) disease?
►Dr. Fine. There are 3 main phenotypes of NTM.3 First, we see the elderly man with preexisting lung disease—usually chronic obstructive pulmonary disease—with fibrocavitary and/or reticulonodular appearance. Second, we see the slim, elderly woman often without any preexisting lung disease presenting with focal bronchiectasis and nodular lesions in right middle lobe and lingula—the Lady Windermere syndrome. This eponym is derived from Oscar Wilde’s play “Lady Windermere’s Fan, a Play About a Good Woman,” and was first associated with this disease in 1992.7 At the time, it was thought that the voluntary suppression of cough led to poorly draining lung regions, vulnerable to engraftment by atypical mycobacteria. Infection with atypical mycobacteria are associated with this population; however, it is no longer thought to be due to the voluntary suppression of cough.7,8 Third, we do occasionally see atypical presentations, such as focal masses and solitary nodules.
►Dr. Swamy. At 1-year follow-up she successfully completed MAC therapy and noted ongoing control of rheumatoid symptoms.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.
1. Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2018;22(2):242-260.
2. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279.
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417-425.
4. Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23(3):655-670.
5. Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(7):990-997.
6. Lane MA, McDonald JR, Zeringue AL, et al. TNF-α antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore). 2011;90(2):139-145.
7. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
8. Kasthoori JJ, Liam CK, Wastie ML. Lady Windermere syndrome: an inappropriate eponym for an increasingly important condition. Singapore Med J. 2008;49(2):e47-e49.
Osteochondritis Dissecans Lesion of the Radial Head
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).
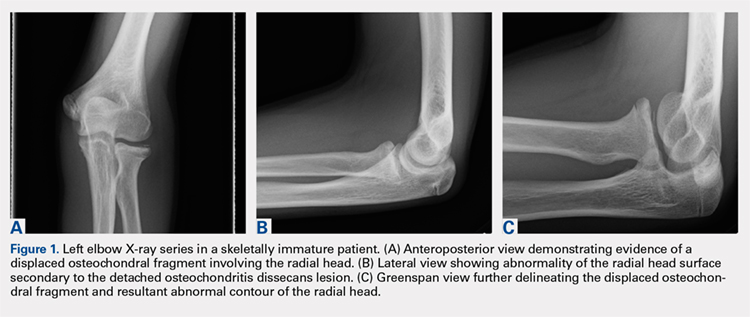
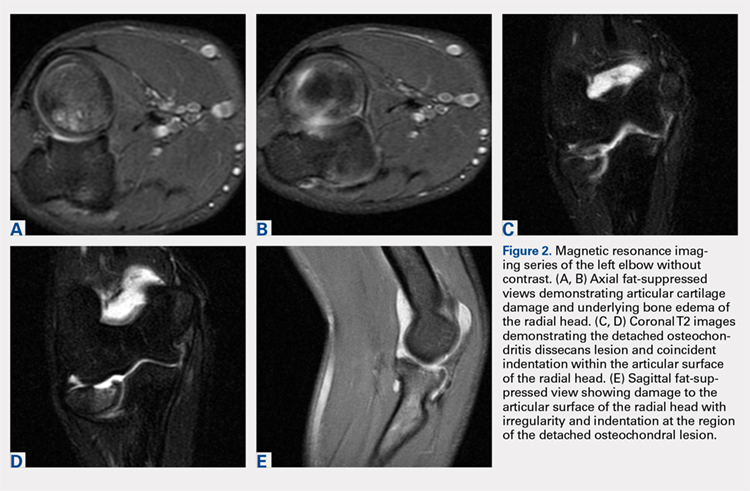
Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).
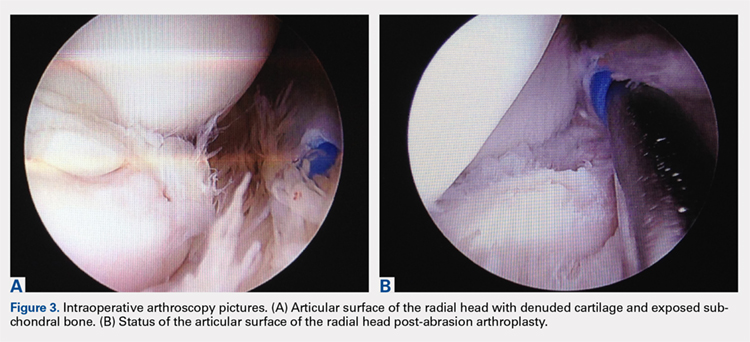
The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
TAKE-HOME POINTS
- Radial Head OCD lesions are uncommon.
- Typically present in athletes that engage in repetitive trauma to elbow (throwers, gymnasts).
- MRI is the best modality for making diagnosis.
- Attempt nonsurgical treatment initially, especially in skeletally immature patients.
- If nonsurgical fails or there is an unstable lesion, consider operative intervention.
Black Esophagus: A Rare Cause of Gastrointestinal Hemorrhage in the Emergency Department
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
In this case presentation of a 65-year-old man who presented to the ED for evaluation of a 1-week history of intermittent coffee-ground emesis and syncope, the authors review the literature about a rare, but potentially fatal diagnosis.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
Case
A 65-year-old man presented to the ED for evaluation of a 1-week history of intermittent, exertional syncope and coffee ground emesis. His medical history was significant for hypertension, peripheral vascular disease, hyperlipidemia, and peptic ulcer disease. Although his social history was positive for alcohol use and abuse, the patient stated that he had not consumed any alcoholic beverages since the onset of nausea and vomiting.
A review of the patient’s systems was positive for lightheadedness upon standing and for palpitations. He had no prior history of melena, hematochezia, or syncope, but did report a previous history of upper gastrointestinal (GI) bleeding due to peptic ulcer disease and alcohol abuse.
The patient’s vital signs at presentation were: blood pressure (BP), 114/74 mm Hg; heart rate, 112 beats/min; respiratory rate, 15 breaths/min; and temperature, 97.7°F. Oxygen saturation was 97% on room air. On examination, the patient was conversant and oriented. He had dried blood around his mouth and chin from vomiting and appeared ill but nontoxic. His mucous membranes were pale. The cardiopulmonary examination was remarkable for tachycardia; however, the patient’s extremities were warm and his capillary refill time was normal. The rectal examination was notable for melenic stool, which was guaiac positive. During the patient’s course in the ED, he passed a large, melenic stool. The remainder of the physical examination was normal.
The chest X-ray was normal, but the electrocardiogram demonstrated sinus tachycardia. Laboratory studies were remarkable for the following:
hemoglobin (Hgb), 12.7 g/dL;
platelet count, 97 x 109/L;
sodium, 122 mmol/L;
chloride, 73 mmol/L;
potassium, 2.9 mmol/L;
blood urea nitrogen, 121 mg/dL;
creatinine, 1.89 mg/dL;
glucose, 297 mg/dL;
calcium, 7.9 mg/dL;
anion gap, 27 mmol/L;
total bilirubin, 1.6 mg/dL (mildly elevated);
direct bilirubin, 0.5 mg/dL;
aspartate aminotransferase, 41 IU/L; and
lactic acid, 5.5 mmol/L (elevated).
The patient’s international normalized ratio and activated partial thromboplastin time were normal. There were no recent prior laboratory studies available for comparison with current findings.
Two large bore intravenous (IV) lines were placed, and the patient was resuscitated with a bolus of 20 mL/kg of isotonic fluids. He was given 1 g of ceftriaxone and 80 mg of pantoprazole IV and was started on an infusion of octreotide. Meanwhile, the patient was consented for blood products and 2 U of packed red blood cells were crossmatched and held in reserve. He received potassium repletion of 60 mEq IV potassium chloride.
The emergency physician (EP) consulted with gastroenterology services. Due to concern for variceal bleeding and to control hemorrhaging, the gastroenterologist recommended emergent upper endoscopy. The upper endoscopy revealed circumferential necrosis of the distal third of the esophagus, which stopped abruptly at the gastroesophageal junction (Figures 1-3). Since no varices were demonstrated on endoscopy, octreotide was discontinued. The gastroenterologist recommended the patient receive nothing orally for 24 hours and that he continue to receive IV proton pump inhibitors (PPIs) and empiric antibiotics. The patient was admitted to the medical intensive care unit (ICU) for further care.
Following admission to the ICU, the patient did not have any additional episodes of hematemesis or melenic or bloody stools. However, his Hgb levels down-trended to 8.6 g/dL and his BP decreased to 84/63 mm Hg. He was transfused a single unit of packed red blood cells, after which BP normalized and Hgb stabilized at 9.5 g/dL. The patient’s diet was advanced on hospital day 1 to clear liquids and then solid foods, and he was discharged home on hospital day 2 with prescriptions for pantoprazole 40 mg twice daily and ranitidine 300 mg nightly and with close primary care and gastroenterology follow-up.
Discussion
Black esophagus, also referred to as acute esophageal necrosis (AEN) or necrotizing esophagitis, is an uncommon, but life-threatening cause of GI bleeding.1 First described by Brennan2 during a patient autopsy in 1967, black esophagus remained a postmortem finding until its first description on endoscopy by Goldenberg et al3 in 1990.With the increased use of endoscopy, black esophagus has been more commonly described in case reports and case series but remains an extremely rare diagnosis, with an incidence of 0.008% to 0.2%.4-7 A single study by Yasuda et al8 demonstrated a surprising incidence of AEN in 6% of patients undergoing upper endoscopy for upper GI hemorrhage.
Patients with black esophagus typically present for evaluation as a result of GI bleeding, which occurs in 65% to 90% of cases.9,10 This condition is more common in elderly patients with a disproportionately higher incidence in men, who represent approximately 80% of cases. A variety of comorbidities are associated with AEN, most commonly diabetes mellitus, malignancy, hypertension, renal insufficiency, heart disease, and duodenal ulcer.5,10 In a recent case series by Gurvits et al,11 tachycardia or hypotension was observed in 90% of cases.
Diagnosis
Black esophagus is defined by diffuse, circumferential necrosis of the esophagus with preferential involvement of the distal third of the esophagus that abruptly stops at the gastroesophageal junction, and in the absence of caustic ingestion.12 The predilection toward involvement of the distal esophagus is thought to be due to its relatively poor perfusion. Blood flow to the distal esophagus is highly variable, but typically occurs through the left gastric and left inferior phrenic arteries. This is believed to result in a “watershed region” that creates a susceptibility to insult.7,13 Histologically, there is necrosis of the mucosa and submucosa, inflammation of the muscle fibers, and occasional thrombosis of blood vessels.4 However, gross findings alone are sufficient for diagnosis, and biopsy is not mandatory.1,14
Etiology
The etiology of acute esophageal necrosis is not well understood. The prevailing theory is that the combination of an ischemic insult and reflux of gastric contents leads to mucosal destruction. The watershed distribution of blood flow to the distal esophagus is thought to predispose patients to ischemia or thrombosis.5,7,10 As previously mentioned, a recent series by Gurvits et al11 demonstrated that 90% of patients with black esophagus also develop tachycardia or hypotension. Further, many of the comorbid conditions noted in cases of AEN are characterized by a tendency toward malperfusion or thrombosis.
Management
The mainstay of managing black esophagus in the ED is aggressive fluid resuscitation, bowel rest, and treatment with IV PPIs. Antibiotics are not indicated unless the patient has an infection, is immunocompromised, continues to decompensate despite adequate IV fluid resuscitation, or has an esophageal perforation.7,11 In practice, the necessity of early antibiotic therapy may be unclear in the ED due to other considerations in the differential diagnosis; therefore, it is prudent to treat the patient empirically until these etiologies can be ruled out. Some clinicians recommend sucralfate due to its ability to bind pepsin and stimulate mucus secretion which theoretically prevents further esophageal injury.4 The initiation of sucralfate should be deferred until after endoscopy.
Esophageal strictures are the most common complication of black esophagus, developing in 16% to 25% of cases. Due to underlying disease, AEN is associated with a high-mortality of 12.5% to 36%.4,11 Mortality as a direct result of esophageal necrosis is less than 6%.10 Complications of black esophagus include perforation and mediastinitis, both of which are indications for emergent surgical intervention.1,15Emergency physicians traditionally manage GI bleeding with conservative measures and early involvement of gastroenterology services. Failure of patients to respond to traditional resuscitative measures may signal mediastinitis and require immediate surgical intervention. This infrequent diagnosis represents a significant deviation from the typical presentations seen by EPs in standard practice; for this reason, EPs should be aware of the signs and symptoms associated with black esophagus and consider it in the differential diagnosis of patients presenting with GI bleeding.
Summary
Emergency physicians are often the first providers to care for patients with an upper GI hemorrhage. While the mainstay of treatment of hematemesis is resuscitation with intravenous fluids and blood products, EPs must be aware of the potential etiologies that may change management. Black esophagus is a rare but important cause of hematemesis—a condition that can lead to esophageal perforation and mediastinitis. In cases wherein patients fail to respond to appropriate resuscitation, subsequently decompensate despite resuscitation, or appear septic, EPs should consider IV broad-spectrum antibiotics and surgical consultation.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.
1. Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: a rare but deadly disease. ACG Case Rep J. 2016;3(2):88-91. doi:10.14309/crj.2016.9.
2. Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581-584.
3. Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493-496.
4. Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y. Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc. 1999;49(4 Pt 1):527-532.
5. Grudell ABM, Mueller PS, Viggiano TR. Black esophagus: report of six cases and review of the literature, 1963-2003. Dis Esophagus. 2006;19(2):105-110. doi:10.1111/j.1442-2050.2006.00549.x.
6. Moretó M, Ojembarrena E, Zaballa M, Tánago JG, Ibánez S. Idiopathic acute esophageal necrosis: not necessarily a terminal event. Endoscopy. 1993;25(8):534-538.
7. Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219-3225.
8. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006;41(3):193-197. doi:10.1007/s00535-005-1741-6.
9. Zacharia GS, Sandesh K, Ramachandran T. Acute esophageal necrosis: an uncommon cause of hematemesis. Oman Med J. 2014;29(4):302-304. doi:10.5001/omj.2014.79.
10. Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42(1):29-38. doi:10.1007/s00535-006-1974-z.
11. Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444-453. doi:10.1007/s10620-014-3382-1.
12. Burtally A, Gregoire P. Acute esophageal necrosis and low-flow state. Can J Gastroenterol. 2007;21(4):245-247.
13. Bear BC, Mathew J, Parker CW III. Acute esophageal necrosis: black esophagus in setting of diabetic ketoacidosis. J Case Rep Images Med. 2015;1:18-21.
14. Altenburger DL, Wagner AS, Li S, Garavaglia J. A case of black esophagus with histopathologic description and characterization. Arch Pathol Lab Med. 2011;135(6):797-798. doi:10.1043/2010-0128-C.1.
15. Hwang J, Weigel TL. Acute esophageal necrosis: “black esophagus.” JSLS. 2007;11(1):165-167.
Asystole Following Nitroglycerin: A Review of Two Cases
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Case reports of a 54-year-old man with angina and a 69-year-old woman demonstrate an underreported, self-limiting side effect associated with nitroglycerin.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
Nitroglycerin (NTG), or glyceryl trinitrate, was first introduced into the medical community by Murrell,1,2 who reported on anecdotal observations of its antianginal properties by workers within manufacturing plants refining the product for its explosive properties. While the route of administration of NTG has changed from this incidental environmental exposure to the now formulated therapies available, its benefit as an outpatient, abortive treatment for stable angina has been validated beyond early subjective observations in the literature.1-3 In fact, its successful use over the years for angina has produced an expansive pharmacopeia, including its use for undifferentiated chest pain and exacerbation of congestive heart failure.3-5
Despite the extensive history of NTG as a proven vasodilator, emerging uses continue to be explored in equal measure with technological advances.2,6 Though morbidity and mortality reductions are dependent on its use within clinical practice, NTG is not an innocuous drug.5 Most of the reported side effects associated with NTG are well established and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.3,6,7 An often forgotten side effect associated with NTG use is asystole. We present the following two cases to highlight both common uses of NTG as well as this underreported side effect.
Case 1: Nitroglycerin for Stable Anginal Chest Pain
A 54-year-old man with a history of hypertension (HTN), hyperlipidemia (HLP), and gastroesophageal reflux disease (GERD) presented to the ED for evaluation of a 3-hour history of intermittent, retrosternal, left-sided, nonradiating chest “pressure and tightness.” The patient stated that the chest discomfort began at rest but was exacerbated by exertion with episodes lasting 10 to 15 minutes. The patient rated the peak pain associated with these episodes as a “7” on a pain scale of 1 to 10. He further noted that his symptoms abated and he became “pain-free” when at rest.
The patient’s vital signs at presentation were: blood pressure (BP), 156/87 mm Hg; heart rate (HR), 68 beats/min; respiratory rate (RR), 18 beats/min; and temperature (T), 98.4°F. Oxygen saturation was 96% on room air.
The patient, who performed regular BP checks at home, noted that his recent BP readings had been very high. A review of the patient’s systems was positive for shortness of breath and diaphoresis; symptoms were otherwise negative, including any prior episodes. His social history was noncontributory and negative for tobacco, alcohol, or drug use. The patient did report that he had taken an uneventful 6-hour car ride the previous week.
On physical examination, the patient was nontoxic and resting comfortably, without signs of acute distress or pain. Cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The abdominal examination was benign and the neurological examination was nonfocal. There was no evidence of peripheral edema or asymmetry of the calves, which were nontender to palpation.
The initial electrocardiogram (ECG) (Figure 1) showed a normal sinus rhythm of 65 beats/min, left axis deviation, and normal intervals; there was no acute ST-segment elevation or depression.
Case 2: Nitroglycerin for Unstable Anginal Chest Pain
A 69-year-old obese woman with a medical history significant for HTN, HLP, and GERD presented to the ED for evaluation of nausea and chest pressure. She described the chest pressure as feeling dull and heavy. She further noted that the discomfort had been occurring intermittently upon exertion, but that this recent episode started while at rest and persisted.
The patient’s vital signs at presentation were: BP, 183/80 mm Hg; HR, 94 beats/min; RR, 20 beats/min; and T, 98.0°F. Oxygen saturation was 92% on room air. On a review of systems, the patient denied any associated symptoms; she likewise denied a history of any recent surgeries, immobilization, active malignancy, or recent travel. Her social history was noncontributory and was negative for tobacco, alcohol, or recreational drug use.
On physical examination the patient was nontoxic and resting comfortably, without signs of acute distress or diaphoresis. The cardiac and pulmonary examinations were normal, and radial pulses were 2+ and symmetric. The patient had trace pedal edema bilaterally, but her calves were symmetric and nontender. The abdomen was benign and the neurological examination was nonfocal. An ECG (Figure 2) showed a normal sinus rhythm with no signs of ischemia (eg, no ST-segment changes or T-wave inversions were present).
Cases 1 and 2: Shared Clinical Course
In both of the two cases presented, ECGs were obtained for the patients upon arrival at the ED. Both patients were placed on telemetry with continuous monitoring, and intravenous (IV) access was obtained. Baseline laboratory evaluation for each of these patients included a complete blood count, basic metabolic panel, and cardiac enzyme measurement. A D-dimer test was also ordered for the patient in Case 1 based on his concerning history and low-pretest probability for a pulmonary embolism (ie, positive pulmonary embolism rule-out criteria). Portable chest X-ray imaging on each of the patients showed no acute pathology, and all of their laboratory results were within normal ranges. Both of the patients in Case 1 and 2 received a 324-mg chewable aspirin and an IV fluid bolus.
Case 1
During evaluation, the patient in Case 1 developed unprovoked chest pain, which he rated as a “7,” for which he was given 400 mcg NTG sublingually (SL). After administration of NTG, the patient reported that his pain reduced to a “4.” Repeat ECG and vital signs remained unchanged. Though Case 1 patient’s pain abated, since it persisted, he was given a second dose of SL NTG. Within 2 minutes of receiving the second dose of NTG, the patient became bradycardic (30 beats/min) with a stable BP and then became unresponsive, converting to asystolic rhythm. Cardiopulmonary resuscitation (CPR) was initiated, with a successful return of vital signs and baseline cognition following 20 seconds of compressions. Despite success following critical interventions, his HR persisted at 30 beats/min with a narrow regular complex, and normal BP. Because of the persistent bradycardia and preceding asystolic rhythm, he was given 0.5 mg of atropine IV, which increased his HR to 80 beats/min. Cardiology service was consulted, and the patient was admitted following an otherwise stable course. Since the cardiologist did not feel emergent cardiac catheterization was indicated, the patient was observed and subsequently discharged home following an uneventful hospitalization, including a normal stress test.
Case 2
The patient in Case 2, had chest pain upon arrival at the ED and was administered SL NTG, with notable improvement in chest pain, but not complete resolution. With serial examinations, including a review of pain scale scores, she was given two subsequent doses of SL NTG. Within 1 minute from receiving the third dose of NTG, the patient complained of lightheadedness and nausea, and became pale and diaphoretic. Telemetry revealed bradycardia, which progressed to junctional escape beats, followed by ventricular escape beats, and then asystole, at which point she became unresponsive and pulseless. Cardiopulmonary resuscitation was initiated, with a return of spontaneous circulation within 15 seconds of intervention; she gradually returned to her baseline with observation. Repeat vital signs were: BP, 155/70 mm Hg; HR, 99 beats/min; RR, 20 breaths/min; and she was afebrile. Oxygen saturation was 99% on 15 liters of oxygen/min, which was weaned prior to hospital admission. A repeat ECG demonstrated a normal sinus rhythm without evidence of ischemia. Cardiology service was consulted and the patient was admitted for further evaluation, including a 3-day inpatient observation, serial cardiac enzymes, thyroid panel, contrast chest computed tomography scan, echocardiogram, and cardiac stress test. All studies were within normal limits, except for an incidental minor pectus excavatum attributed to the quality CPR. In addition, a nuclear medicine perfusion imaging study was obtained, which revealed no evidence of myocardial ischemia or scar, consistent with the patient’s stable course. The patient’s symptoms resolved early in her inpatient stay, and she was discharged home with prescriptions for antihypertensive and antihyperlipidemia agents and instructed to follow-up with her primary care physician.
Discussion
Nitroglycerin is commonly used to treat various symptoms of cardiac origin, namely relief of chest pain due to suspected acute coronary syndromes.2,3The mechanism of action of NTG is predominantly through potent smooth muscle relaxation of the venous and arterial systems, reducing both preload and afterload.2,3 This results in reduced myocardial oxygen demand, potentiating the relief of myocardial ischemia.
Contraindications
Contraindications to NTG include known allergy, pericardial tamponade, restrictive cardiomyopathy, increased intracranial pressure, and concomitant use of phosphodiesterase inhibitors. Moreover, NTG should not be given to treat conditions wherein cardiac output is dependent on venous return, as in the setting of inferior myocardial infarction (MI) with right ventricular involvement. Furthermore, there is no evidence in the literature to support the erroneous use of NTG as a diagnostic therapy, with limited sensitivity yields for conclusive cardiac-associated chest pain.8
Adverse Effects and Events
The common side effects of NTG are well documented and include hypotension, tachycardia, flushing, nausea, vomiting, and headache.7,3,6 Syncope, bradycardia, and cardiac arrest following the administration of NTG are rare events, as evidenced by the paucity of literature describing these complications. Rather, it appears that these side effects are observed only in the setting of myocardial ischemia or MI.3,9-11 Fewer cases of ventricular fibrillation, responsive to defibrillation, and asystole also have been observed.9The exact mechanism for bradycardia without hypotension and subsequent asystole following NTG administration remains elusive, though this response is thought to be associated with the Bezold-Jarisch reflex.
Bezold-Jarisch Reflex
The Bezold-Jarisch reflex is a cardiovascular response consisting of bradycardia and hypotension that is believed to be from stimulation of inhibitory cardiac receptors by stretch, chemical, or pharmacological stimulation.12 The earliest cases of Bezold-Jarisch reflex following NTG occurred in the setting of MI and were attributed to ongoing myocardial ischemia.13 Recent studies have revealed that coronary stenosis without concurrent ischemia is actually not a sensitizing factor, and that bradycardia and asystole following NTG have occurred in patients without evidence of coronary artery disease.9,14 As part of this response, it is theorized that the development of bradycardia is related to vasovagal stimulation, a centrally mediated response to the headache or nausea following NTG administration.10,11,15
Despite these observational studies and after thorough review of the available cases, no unifying factors exist to predict with certainty the patient population in which this response is likely to occur.12,16Based on a literature review, it appears that asystole following NTG is self-limited; however, in most cases, bradycardia was treated with atropine without adverse side effects.12,15,16
Conclusion
The two cases presented involved a middle-aged male patient and an elderly female patient, both of whom had several cardiac risk factors but no evidence of acute ischemia or infarction on ECG or laboratory studies. It is well established that NTG can cause hypotension without bradycardia; however, the development of bradycardia without, or even preceding, hypotension is less recognized. Several mechanisms have been postulated but none fully explain this reaction; moreover, no anticipatory risk factors have been consistently observed. Even though the patients in Case 1 and 2 underwent extensive evaluation, no specific etiology of the observed reaction was identified, though neither patient underwent cardiac catheterization to definitively exclude abnormal coronary artery pathology as a precipitating factor.
These cases illustrate the unpredictable adverse reaction to a common medication used for a ubiquitous complaint. The explanation as to the source for this reaction is lacking, the literature has consistently described the transient and self-limiting effect of asystole following NTG.9,12,14,16Bradycardia, though self-limiting, remains responsive to appropriately dosed atropine when NTG-induced.3,12,16 The authors wish to stress the importance of establishing IV access and being prepared for adverse events whenever administering sublingual nitroglycerin to a patient.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
1. Miura T, Nishinaka T, Terada T, Yonezawa K. Vasodilatory effect of nitroglycerin in Japanese subjects with different aldehyde dehydrogenase 2 (ALDH2) genotypes. Chem Biol Interact. 2017;276:40-45. doi:10.1016/j.cbi.2017.03.012.
2. Noonan PK, Williams RL, Benet LZ. Dose dependent pharmacokinetics of nitroglycerin after multiple intravenous infusions in healthy volunteers. J Pharmacokinet Biopharm. 1985;13(2):143-157.
3. Proulx MH, de Montigny L, Ross D, Vacon C, Juste LE, Segal E. Prehospital nitroglycerin in tachycardic chest pain patients: a risk for hypotension or not? Prehosp Emerg Care. 2017;21(1):68-73. doi:10.1080/10903127.2016.1194929.
4. Huis In ‘t Veld MA, Cullen L, Mahler SA, Backus BE, Dezman ZDW, Mattu A. The fast and the furious: low-risk chest pain and the rapid rule-out protocol. West J Emerg Med. 2017;18(3):474-478. doi:10.5811/westjem.2016.12.32676.
5. Pasupathy S, Tavella R, Grover S, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894-903. doi:10.1161/CIRCULATIONAHA.117.027575.
6. Turan B, Daşlı T, Erkol A, Erden İ. Effectiveness of sublingual nitroglycerin before puncture compared with conventional intra-carterial nitroglycerin in transradial procedures: a randomized trial. Cardiovasc Revasc Med. 2015;16(7):391-396. doi:10.1016/j.carrev.2015.07.006.
7. Nagy-Grócz G, Bohár Z, Fejes-Szabó A, et al. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J Chem Neuroanat. 2017;85:13-20. doi:10.1016/j.jchemneu.2017.06.002.
8. Steele R, McNaughton T, McConahy M, Lam J. Chest pain in emergency department patients: if the pain is relieved by nitroglycerin, is it more likely to be cardiac chest pain? CJEM. 2006;8(3):164-169.
9. Dettorre K, Brywczynski J, McKinney J, Slovis C. Not the nitro? Patient goes into prehospital V-fib arrest following nitroglycerin. JEMS. 2009;34(5):34,36. doi:10.1016/S0197-2510(09)70124-X.
10. Buckley R, Roberts R. Symptomatic bradycardia following the administration of sublingual nitroglycerin. Am J Emerg Med. 1993;11(3):253-255.
11. Takase B, Uehata A, Nishioka T, et al. Different mechanisms of isoproterenol-induced and nitroglycerin-induced syncope during head-up tilt in patients with unexplained syncope: important role of epinephrine in nitroglycerin-induced syncope. J Cardiovasc Electrophysiol. 2001;12(7):791-796.
12. Brandes W, Santiago T, Limacher M. Nitroglycerin-induced hypotension, bradycardia, and asystole: report of a case and review of the literature. Clin Cardiol. 1990;13(10):741-744.
13. Ong EA, Canlas C, Smith W. Nitroglycerin-induced asystole. Arch Intern Med. 1985;145(5):954.
14. Shah SP, Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex Heart Inst J. 2013;40(4):484-486.
15. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90-102.
16. Younas F, Janjua M, Badshah A, DeGregorio M, Patel KC, Cotant JF. Transient complete heart block and isolated ventricular asystole with nitroglycerin. J Cardiovasc Med (Hagerstown). 2012;13(8):533-535. doi:10.2459/JCM.0b013e3283416b8b.
Plantar Ulcerative Lichen Planus: Rapid Improvement With a Novel Triple-Therapy Approach
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.
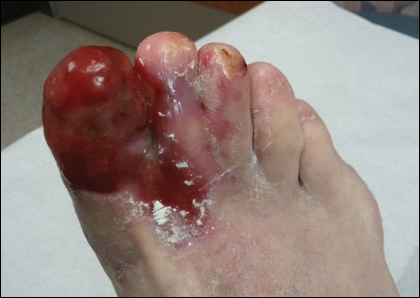

Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.
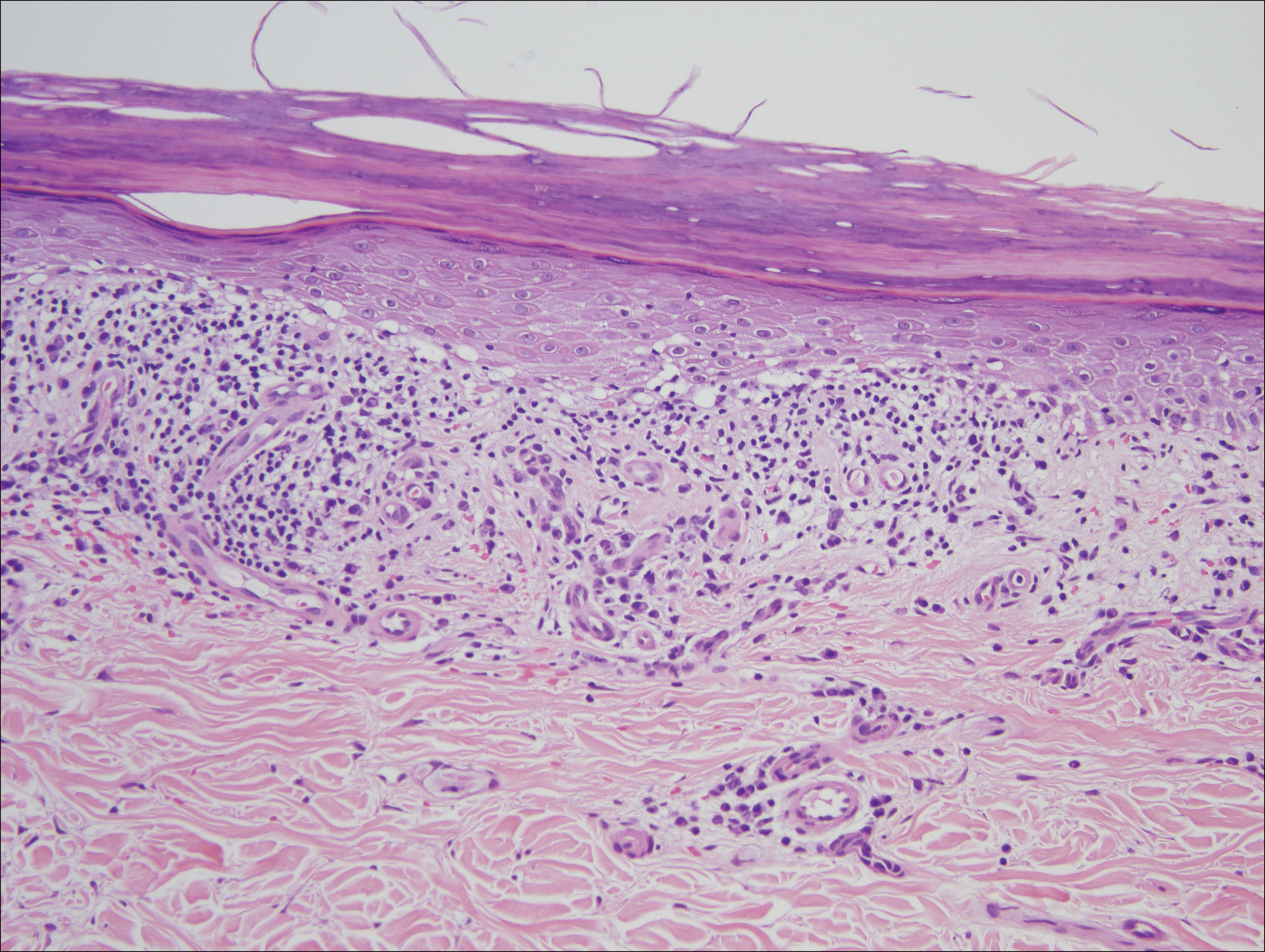
Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.
Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Practice Points
- Consider ulcerative lichen planus (ULP) for chronic wounds on the soles.
- Topical therapeutic options may present a rapidly effective and relatively safe alternative to conventional immunosuppressive agents for long-term management of plantar ULP.
Deer Ked: A Lyme-Carrying Ectoparasite on the Move
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
How to Prevent Mosquito and Tick-Borne Disease
Case Report
A 31-year-old man presented to the dermatology clinic 1 day after mountain biking in the woods in Hartford County, Connecticut. He stated that he found a tick attached to his shirt after riding (Figure). Careful examination of the patient showed no signs of a bite reaction. The insect was identified via microscopy as the deer ked Lipoptena cervi.

Comment
Lipoptena cervi, known as the deer ked, is an ectoparasite of cervids traditionally found in Norway, Sweden, and Finland.1 The deer ked was first reported in American deer in 2 independent sightings in Pennsylvania and New Hampshire in 1907.2 More recently deer keds have been reported in Massachusetts, New York, Pennsylvania, and New Hampshire.3 In the United States, L cervi is thought to be an invasive species transported from Europe in the 1800s.4,5 The main host is thought to be the white-tailed deer (Odocoileus viginianus). Once a suitable host is found, the deer ked sheds its wings and crawls into the fur. After engorging on a blood meal, it deposits prepupae that fall from the host and mature into winged adults during the late summer into the autumn. Adults may exhibit swarming behavior, and it is during this host-seeking activity that they land on humans.3
Following the bite of a deer ked, there are reports of long-lasting dermatitis in both humans and dogs.1,4,6 One case series involving 19 patients following deer ked bites reported pruritic bite papules.4 The reaction appeared to be treatment resistant and lasted from 2 weeks to 12 months. Histologic examination was typical for arthropod assault. Of 11 papules that were biopsied, most (7/11) showed C3 deposition in dermal vessel walls under direct immunofluorescence. Of 19 patients, 57% had elevated serum IgE levels.4
In addition to the associated dermatologic findings, the deer ked is a vector of various infectious agents. Bartonella schoenbuchensis has been isolated from deer ked in Massachusettes.7 A recent study found a 75% prevalence of Bartonella species in 217 deer keds collected from red deer in Poland.5 The first incidence of Borrelia burgdorferi and Anaplasma phagocytophylum in deer keds was reported in the United States in 2016. Of 48 adult deer keds collected from an unknown number of deer, 19 (40%), 14 (29%), and 3 (6%) were positive for B burgdorferi, A phagocytophylum, and both on polymerase chain reaction, respectively.3
A recent study from Europe showed deer keds are now more frequently found in regions where they had not previously been observed.8 It stands to reason that with climate change, L cervi and other disease-carrying vectors are likely to migrate to and inhabit new regions of the country. Even in the current climate, there are more disease-carrying arthropods than are routinely studied in medicine, and all patients who experience an arthropod assault should be monitored for signs of systemic disease.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
- Mysterud A, Madslien K, Herland A, et al. Phenology of deer ked (Lipoptena cervi) host-seeking flight activity and its relationship with prevailing autumn weather. Parasit Vectors. 2016;9:95.
- Bequaert JC. A Monograph of the Melophaginae or Ked-flies of Sheep, Goats, Deer, and Antelopes (Diptera, Hippoboscidae). Brooklyn, NY: Brooklyn Entomological Society; 1942.
- Buss M, Case L, Kearney B, et al. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J Vector Ecol. 2016;41:292-294.
- Rantanen T, Reunala T, Vuojolahti P, et al. Persistent pruritic papules from deer ked bites. Acta Derm Venereol. 1982;62:307-311.
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, et al. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasit Vectors. 2017;10:487.
- Hermosilla C, Pantchev N, Bachmann R, et al. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006;159:286-287.
- Matsumoto K, Berrada ZL, Klinger E, et al. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549-554.
- Sokół R, Gałęcki R. Prevalence of keds on city dogs in central Poland. Med Vet Entomol. 2017;31:114-116.
Practice Points
- There are many more disease-carrying arthropods than are routinely studied by scientists and physicians.
- Even if the insect cannot be identified, it is important to monitor patients who have experienced arthropod assault for signs of clinical diseases.
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.