User login
Plantar Hyperpigmentation
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
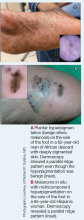
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Use of Hypoglossal Nerve Stimulation for Treating OSA in Military Patient Populations
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
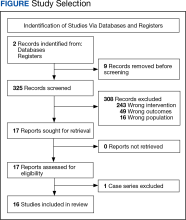
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
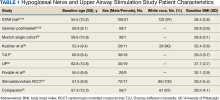
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
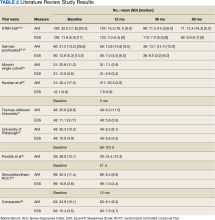
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
Recalcitrant Folliculitis Decalvans Treatment Outcomes With Biologics and Small Molecule Inhibitors
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).
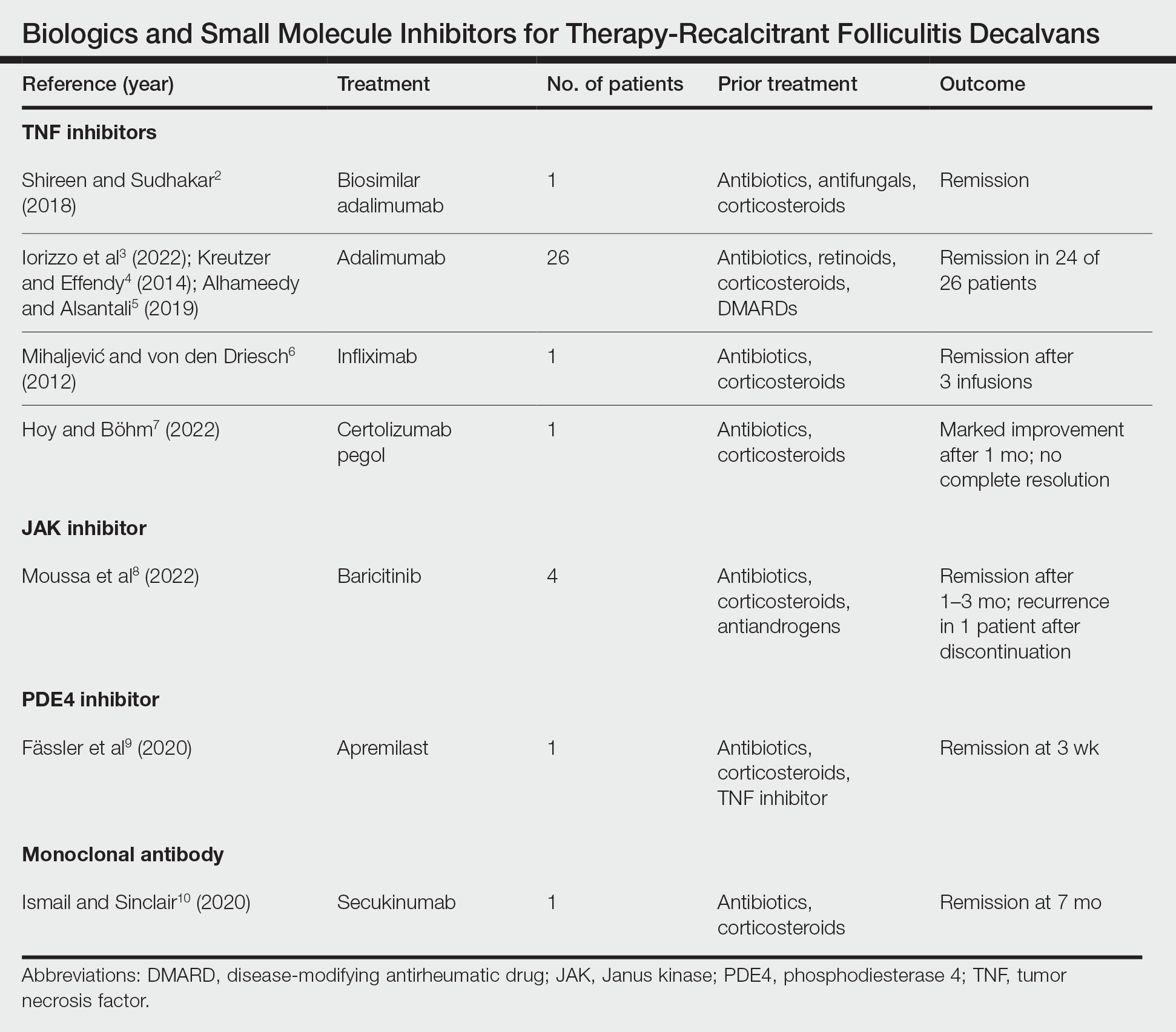
The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Practice Points
- Tumor necrosis factor inhibitors, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and monoclonal antibodies have shown success in the treatment of folliculitis decalvans resistant to traditional therapies.
- The true etiology of folliculitis decalvans is still unknown, but possible factors include Staphylococcus aureus infection and an impaired host immune system, which may benefit from treatment with biologics and small molecule inhibitors.
Exploring Skin Pigmentation Adaptation: A Systematic Review on the Vitamin D Adaptation Hypothesis
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).
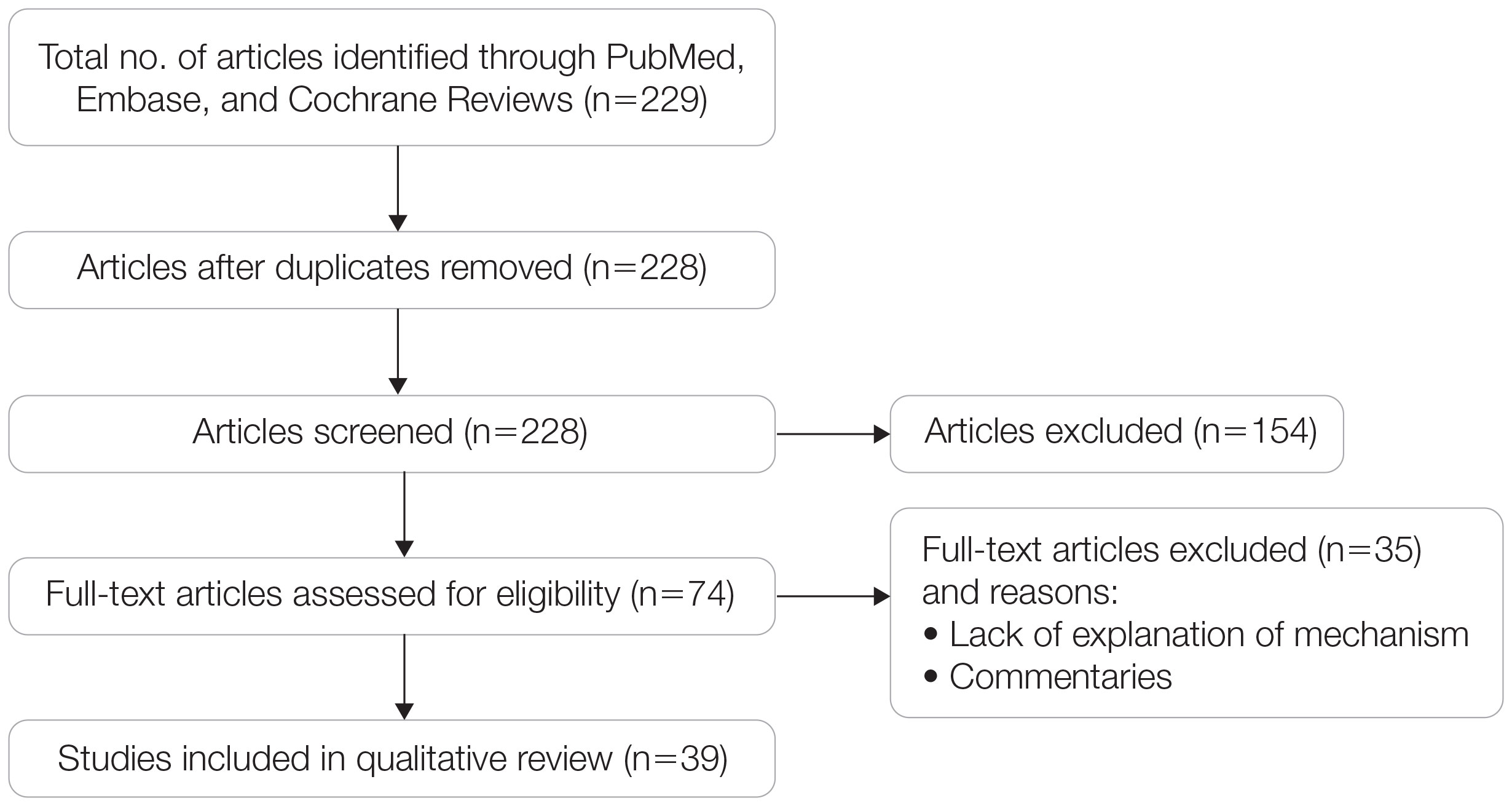
Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6
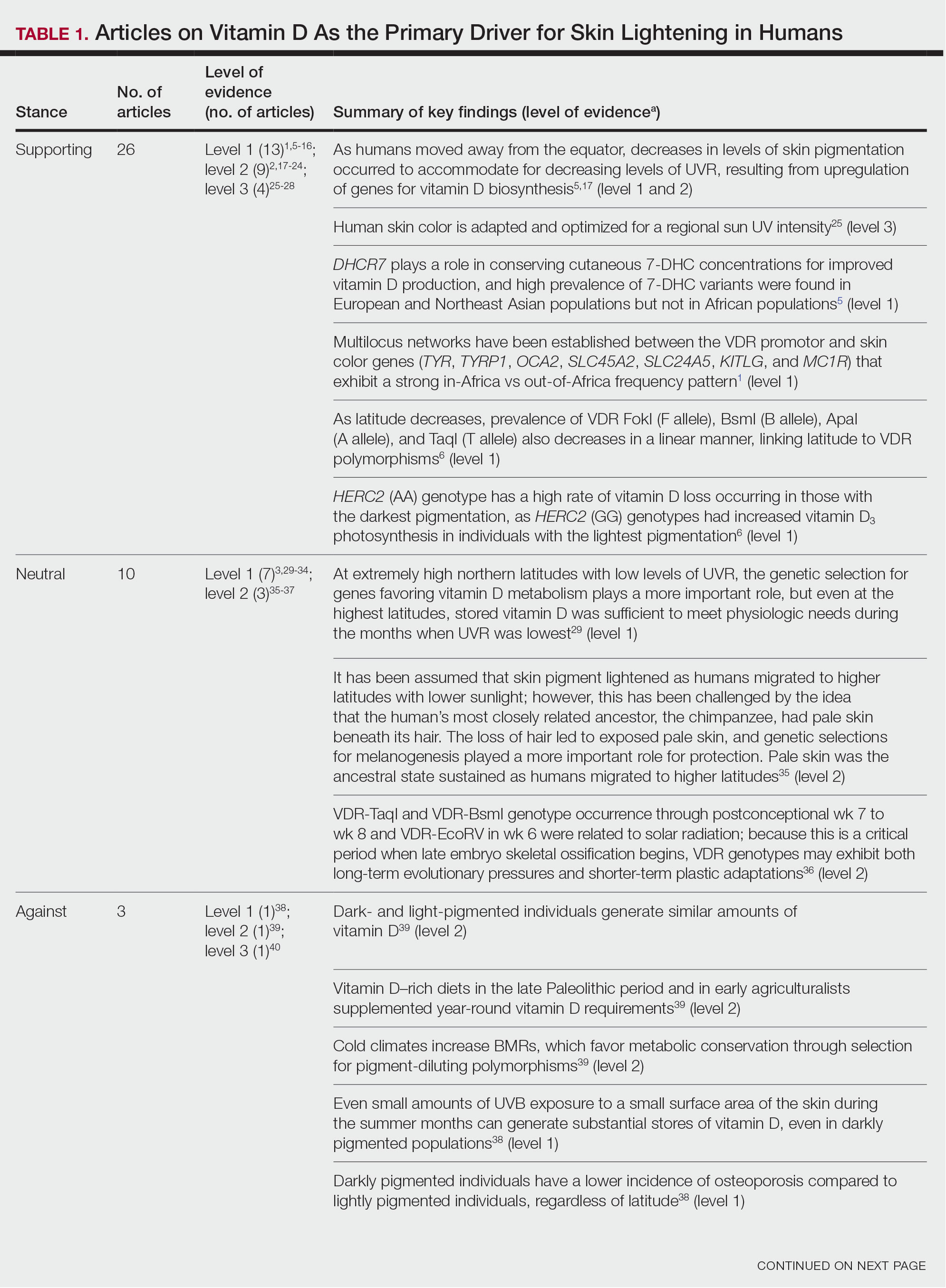
Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.
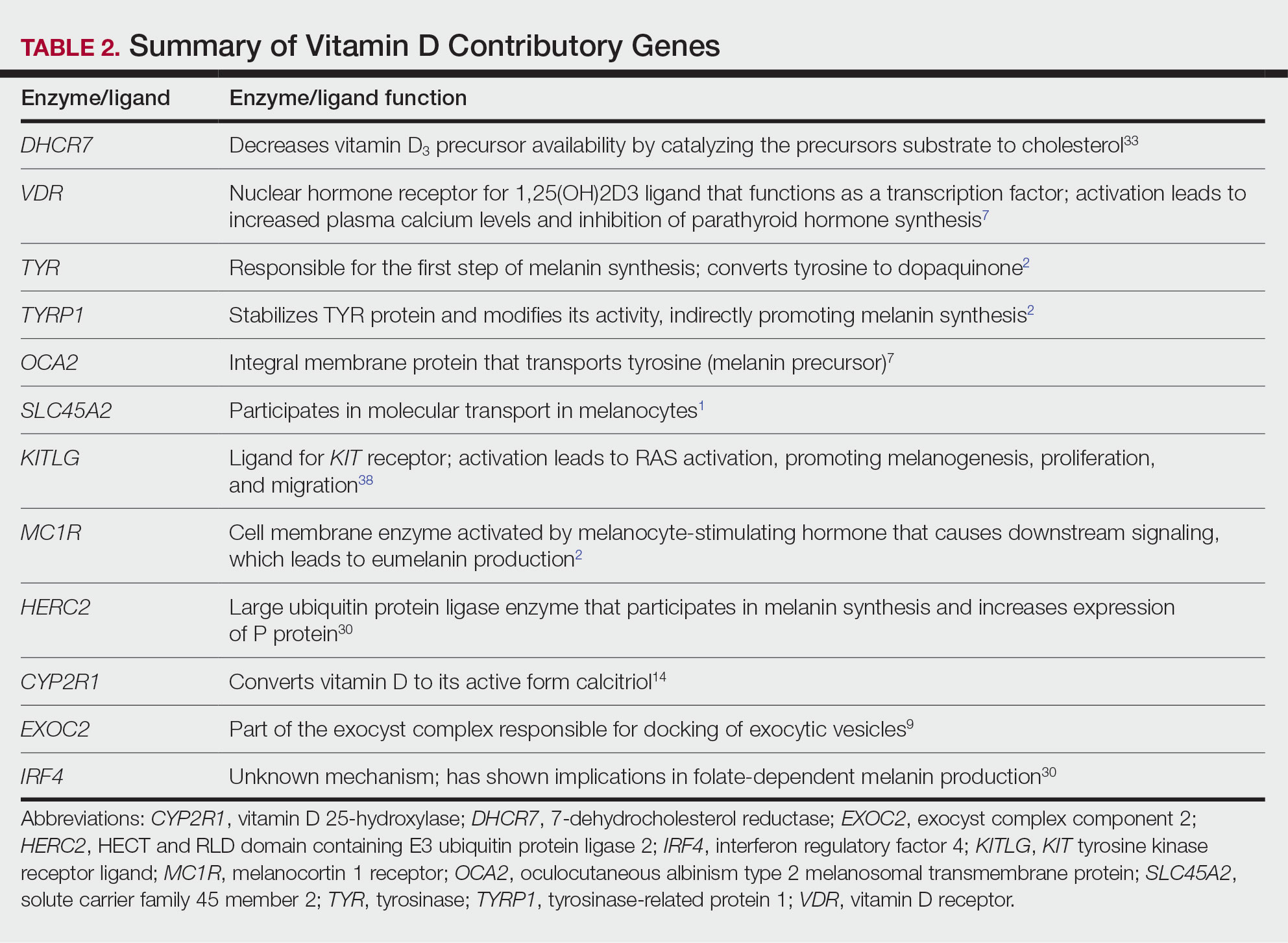
Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
Practice Points
- Sufficient UV radiation exposure is required to synthesize vitamin D, but excess exposure increases skin cancer risk.
- Genes associated with vitamin D production and melanin synthesis form an interconnected network that explains skin tone polymorphisms and their influence on healthy sun behaviors.
- Adaptations in genetics of skin pigmentation and vitamin D metabolism due to anthropologic patterns of migration to northern latitudes may help explain predisposition to dermatologic diseases such as skin cancer.
An Update on Cutaneous Angiosarcoma Diagnosis and Treatment
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2
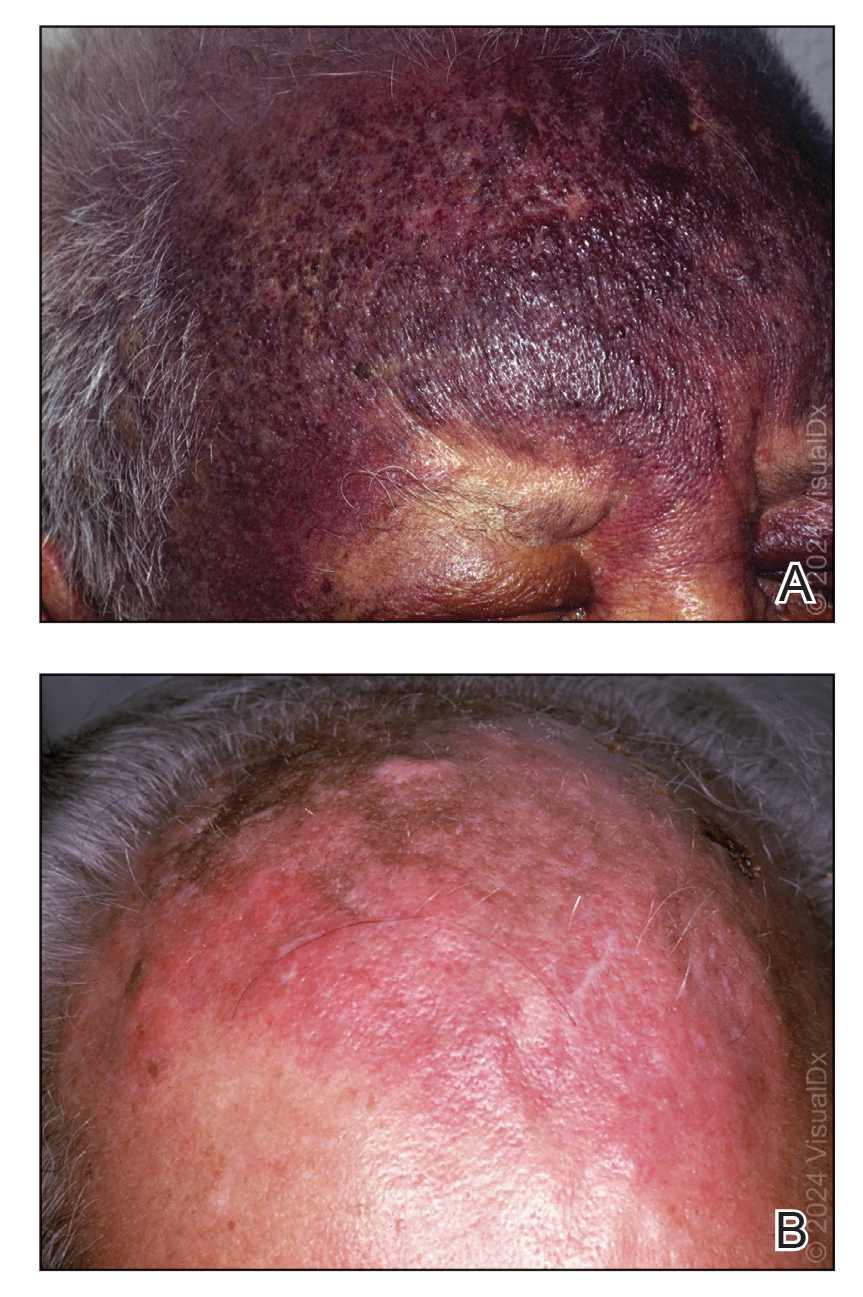
Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24
Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6
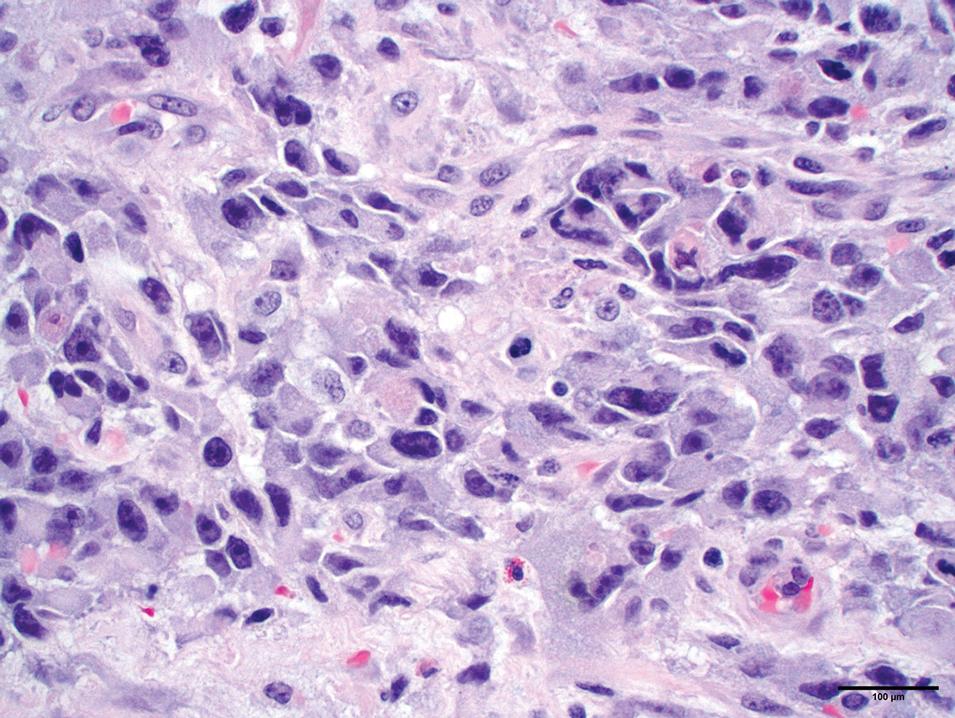
Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
Angiosarcomas are aggressive endothelial cell tumors of vascular origin that account for 1% to 2% of all soft tissue sarcomas in the United States.1,2 They can affect any organ in the body but most commonly affect the skin and soft tissue. Cutaneous angiosarcoma (CAS) is a rare type of skin cancer that can present in 2 forms: primary and secondary.
Dermatologists may be responsible for the initial diagnosis and management of CAS. They must be familiar with its presentation, as this condition can be difficult to diagnose and mimics other diseases. Additionally, dermatologists must understand the role of varying treatment modalities including Mohs micrographic surgery (MMS) in the management of CAS. This review will provide an overview of the epidemiology, presentation, and pathologic features of CAS and will discuss both emerging and existing treatments.
Epidemiology
Cutaneous angiosarcoma may present in various locations in the body, predominantly on the head and neck.4,5 Approximately 85% of cases arise in patients older than 60 years, and most of these patients are White men.1,4,5 The risk factors for the development of CAS include prior radiation exposure; chronic lymphedema (ie, Stewart-Treves syndrome); and familial syndromes including neurofibromatosis 1, BRCA1 or BRCA2 mutations, Maffucci syndrome, and Klippel-Trenaunay syndrome. Exogenous exposure to toxins such as vinyl chloride, thorium dioxide, or anabolic steroids also is associated with angiosarcoma, primarily in the form of visceral disease such as hepatic angiosarcoma.6
The average tumor size is approximately 4 to 5 cm; however, some tumors may grow larger than 10 cm.7,8 Metastasis through hematogenous or lymphatic spread is fairly common, occurring in approximately 16% to 35% of patients. The lungs and liver are the most common sites of metastasis.9,10 The age-adjusted incidence rate of CAS is decreasing for patients younger than 50 years, from 1.30 in 1995 to 2004 to 1.10 in 2005 to 2014, but increasing for individuals older than 70 years, from 2.53 in 1995 to 2004 to 2.87 in 2005 to 2014.4 The incidence of angiosarcoma also has grown in the female population, likely due to the increasing use of radiotherapy for the treatment of breast cancer.11
The high rates of CAS on the head and neck may be explained by the increased vascularity and UV exposure in these locations.12 In a Surveillance, Epidemiology, and End Results population-based study (N=811), 43% of patients with CAS had a history of other malignancies such as breast, prostate, genitourinary, gastrointestinal tract, and respiratory tract cancers.4 Cutaneous angiosarcoma can develop secondary to the primary cancer treatment, as seen in patients who develop CAS following radiation therapy.11
The underlying mechanism of CAS is believed to involve dysregulation of angiogenesis due to the vascular origin of these tumors. Studies have identified overexpression of vascular endothelial growth factor (VEGF), TP53 mutations, and RAS pathway mutations as potential contributing factors to the pathogenesis of angiosarcoma.6 Molecular differences between primary and secondary angiosarcomas are not well documented; however, radiation-associated CAS has been found to have higher expression of LYN and PRKCΘ, while non–radiation-induced lesions express FTL1 and AKT3.2 Chromosomal abnormalities have been identified in a small set of primary CAS patients, but the specific role of these abnormalities in the pathogenesis of CAS remains unclear.7
Prognosis
Cutaneous angiosarcoma has a poor prognosis, with 3-year disease-specific survival rates as low as 40% and 5-year rates as low as 17%.4,5,13,14 Survival rates increased from 1985 to 2014, likely due to earlier diagnoses and more effective treatments.4 Several factors are associated with worse prognosis, including metastatic disease, increasing age, scalp and neck tumor location, tumor size greater than 5 cm, necrosis, multiple skin lesions, and nodular and epithelioid morphology.4,5,10,13-16 Factors including sex, race, and presence of another malignancy do not affect survival.4,5 Prognosis in CAS may be evaluated by TNM tumor staging. The American Joint Committee on Cancer Staging Manual (8th edition) for soft tissue sarcoma (STS) commonly is used; however, CAS is not included in this staging system because it does not share the same behavior and natural history as other types of STS. This staging system provides separate guidelines for STS of the head and neck and STS of the extremities and trunk because of the smaller size but paradoxically higher risk for head and neck tumors.17 Given that there is no agreed-upon staging system for CAS, prognosis and communication among providers may be complicated.
Clinical Presentation
Early CAS typically presents as single or multifocal ill-defined, enlarging, violaceous or dusky red macules or patches (Figure 1). Lesions often rapidly develop into raised nodules and plaques that may bleed and ulcerate. Other common symptoms include pain, edema, neuropathy, anemia, and weight loss; however, it is not uncommon for lesions to be asymptomatic.8,18-20 Nodular lesions are more common on the scalp, and patches are more common on the face and neck.16 Tumors typically extend into the dermis, and aggressive cancers may invade the subcutaneous tissue and fascia.2

Cutaneous angiosarcoma may mimic ecchymosis, hemangioma, lymphangioma, edema, cellulitis, or scarring alopecia. Its nonspecific features make it difficult to recognize without dermoscopy or ultrasonography, which often results in delayed diagnosis and treatment. The median delay typically is 5 to 7 months and up to 1 year for some patients.7,16 Cutaneous angiosarcoma of the scalp tends to have a longer diagnostic delay than other areas of the body, which may be attributable to challenges in tumor identification and visualization by patients.16
Dermoscopy and ultrasonography can aid in the diagnosis of CAS. Dermoscopy may demonstrate a range of colors with yellow, brown, or red areas in a violaceous background. Other reported features include white veils and lines, purple ovals, pink-purple “steamlike” areas, and atypical vessels (Figure 2).21-23 Dermoscopic findings may appear similar to other vascular tumors, such as hemangioma and Kaposi sarcoma, or nonvascular tumors, including amelanotic melanoma, Merkel cell carcinoma, and primary cutaneous B-cell lymphoma. Ultrasonography may show ill-defined, hypoechoic areas with anechoic reticular channels and a hypoechoic subepidermal layer.21 Other radiologic modalities, such as computed tomography, magnetic resonance imaging, or positron emission tomography, are nonspecific and are more useful in evaluating the extent of tumor spread in visceral angiosarcoma. Magnetic resonance imaging in CAS may indicate malignancy with the presence of high T2 and T1 signal intensity and high-flow serpentine vessels.24

Histopathology
Histologically, angiosarcoma is characterized by anastomosing irregular vascular channels lined by a single layer of endothelial cells displaying slight to moderate atypia.25 These vascular channels dissect between collagen bundles and adipocytes. Monocyte infiltration may be observed.6 The neoplastic endothelial cells may present as spindle-shaped, round, polygonal, or epithelioid with eosinophilic cytoplasm. Histologic features differ based on the type of clinical lesion (Figure 3). In a study of CAS in Asian populations, nodular tumors showed solid sheets of pleomorphic spindle cells, many mitotic figures, and widely hemorrhagic spaces, whereas nonnodular tumors showed irregular vascular spaces dissecting collagen.16 Poorly differentiated tumors may present with hyperchromatic nuclei and prominent nucleoli, papillary endothelial formations, mitoses, and possible hemorrhage or necrosis.2,6,8 Histologic specimens also may reveal calcified bodies and hemosiderin particles.19 Angiosarcomas typically are invasive without a clear capsule or border.6

Secondary CAS in the setting of lymphedema and radiation therapy has MYC amplification and is positive for MYC via immunohistochemistry, which is uncommon in primary angiosarcoma.26 Immunohistochemical staining of tumor specimens is helpful to confirm the diagnosis of CAS. These markers include CD31, CD34, CD117, cytokeratin, vimentin, epithelial membrane antigen, factor VIII–related antigen, Ulex europaeus agglutinin-1, von Willebrand factor, and VEGF.6,19,27,28 Notably, advanced angiosarcomas with progressive dedifferentiation often lose these markers.
Treatment
Surgery—The majority of patients treated for CAS undergo surgical resection, as surgery has been shown to have the best prognosis for patients.5,9,10,13,15 Achieving R0 resection (microscopically negative margins) is the most important factor in determining the success of treatment, with incomplete surgical resection resulting in higher rates of systemic and local spread.29 Abraham et al8 found that the median disease-specific survival of patients with microscopically negative margins was 83.7 months; patients with microscopically positive and grossly positive margins had median disease-specific survival of 63.4 and 18.1 months, respectively. In a case series of patients undergoing resection with negative surgical margins, 4 patients demonstrated no evidence of local recurrence or systemic disease at an average of 4.3 years after therapy, and the other 4 patients each had 1 local recurrence but were disease free an average of 4.8 years after removal of the recurrent lesion. In a series of 27 patients with positive surgical margins, there was local recurrence within 2 years for most patients.12
Large tumors invading nearby structures may not be amenable to surgical resection because of extensive local growth, propensity for skip lesions, and localization near vital organs of the head and neck.5,7 The extended delay in diagnosis often seen in CAS allows for advanced local progression, resulting in large areas of resection. In a case series (N=8), the average surgical defect measured 14.3×11.8 cm, necessitating reconstruction with either a tissue flap or split-thickness skin graft in every case because primary closure was not possible. More than 80% of patients in this study still had positive margins after surgery, necessitating the use of additional chemotherapy or radiation to eradicate remaining disease.7 In several studies, multimodality therapy was associated with improved overall survival.7,14,30
Mohs Micrographic Surgery—Mohs micrographic surgery is the standard of care for many aggressive cutaneous malignancies on the head, but its utility for the treatment of CAS is uncertain. Only a few studies have compared the efficacy of MMS vs wide local excision (WLE). There have been reports of recurrence-free follow-up at 12, 16, 18, 20, and 72 months after MMS.31-36 The latter case showed a patient who underwent MMS with a 72-month relapse-free survival, whereas other patients who underwent WLE only survived 5 to 7 months without recurrence.36 In another study, there was a local recurrence rate of 42.9% after a median follow-up of 4 years in 7 patients with CAS treated with complete circumferential peripheral and deep margin assessment, which is less than the reported recurrence rates of 72% to 84% after standard excisional procedures.28,37
Houpe et al38 conducted a systematic review of the use of WLE vs MMS; the median overall survival was longest for WLE in conjunction with chemotherapy, radiotherapy, and immunotherapy at 39.3 months, followed by MMS alone at 37 months. Mohs micrographic surgery in conjunction with chemotherapy and radiotherapy was used in 1 patient, with a median overall survival of 82 months. Wide local excision alone resulted in a median overall survival of 19.8 months. Although these data are promising and suggest that the combination of surgery with adjuvant therapy may be more beneficial than surgery alone, it is important to note that there were only 9 cases treated with MMS compared with 825 cases treated with WLE.38
Several studies have documented that paraffin-embedded sections may be more useful than frozen sections in the determination of margin positivity from a surgical specimen, as frozen sections showed a poor negative predictive value of 33.3%.7,35 Mohs micrographic surgery has been proposed for tumors measuring less than 5 cm; however, the most recent appropriate use criteria for MMS of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery deemed the use of MMS for angiosarcoma uncertain.32,33,37 Further research is necessary to elucidate the role of MMS in the management of CAS.
Radiotherapy—Radiotherapy is a common adjuvant to surgical resection but has been used palliatively in patients with tumors that are unresectable. Improved local control and disease-free survival have been observed with the combination of radiation and surgery. A dose response to radiotherapy has been demonstrated,18,30 with 1 study showing that patients who received more than 5000 cGy of radiotherapy achieved better local control than patients who received 4500 cGy or less.18 Pawlik et al7 showed a decreased chance of death with the addition of adjunctive radiotherapy, and patients who underwent postoperative radiotherapy demonstrated a median survival almost 4-times longer than patients who did not receive radiation. Morrison et al39 reported that radiation therapy administered to patients with no clinically evident disease after surgical resection resulted in improved local control and overall survival vs patients who were irradiated with clinically evident disease.
Complications of radiotherapy for angiosarcoma have been reported, including xerostomia, nonfunctionally significant fibrosis, chronic ulceration/cellulitis of the scalp, necrosis requiring debridement, severe ocular complications, and fibrosis of the eyelids requiring surgical intervention.14 Radiation therapy also poses unique risks to patients with radiation-induced angiosarcoma of the breast, as many of these patients have already received the maximum recommended dose of radiation in the affected areas and additional radiation could exacerbate their CAS.
Chemotherapy—Chemotherapy occasionally is used as an adjunct to surgical resection with positive margins or as palliative care when surgical resection is not possible. Unfortunately, STSs have a response rate of less than 40% to standard chemotherapy.40 Studies in which the use of chemotherapy is evaluated for CAS have mixed results. Mark et al18 reported no significant overall survival benefit when comparing CAS treated with surgery plus radiotherapy with or without chemotherapy. Torres et al41 evaluated radiation-induced angiosarcoma of the breast and found a reduced risk for local recurrence in patients receiving chemotherapy in addition to surgery, indicating that chemotherapy may be useful in this subset of patients when radiation is not recommended.
Cytotoxic chemotherapy agents such as paclitaxel, doxorubicin, or doxorubicin in combination with mesna and ifosfamide (MAI) are common.39 Median progression-free survival is 5.4 months, 4 to 5.6 months, and 3.9 months for MAI, paclitaxel, and doxorubicin, respectively.8,9,42-46 Improved prognosis with MAI may indicate that combination chemotherapy regimens are more effective than single-agent regimens. Cutaneous angiosarcomas may respond better to paclitaxel than doxorubicin, and angiosarcomas of the scalp and face have shown a better response to paclitaxel.47,48
Other Therapies—Although there have not been large-scale studies performed on alternative treatments, there are several case reports on the use of immune modulators, biologics, β-blockers, and various other therapies in the treatment of CAS. The following studies include small sample sizes of patients with metastatic or locally aggressive disease not amenable to surgical resection, which may affect reported outcomes and survival times.49-57 In addition, several studies include patients with visceral angiosarcoma, which may not be generalizable to the CAS population. Even so, these treatment alternatives should not be overlooked because there are few agents that are truly efficacious in the treatment of CAS.
Results on the use of VEGF and tyrosine kinase inhibitors have been disappointing. There have been reports of median progression-free survival of only 3.8 months with sorafenib treatment, 3 months with pazopanib, and 6 months with bevacizumab.49-51 However, one study of patients who were treated with bevacizumab combined with radiation and surgery resulted in a complete response in 2 patients, with no evidence of residual disease at the last follow-up of 8.5 months and 2.1 years.52
Studies on the utility of β-blockers in the treatment of CAS have shown mixed results. Pasquier et al53 evaluated the use of adjunctive therapy with propranolol and vinblastine-based chemotherapy, with a promising median progression-free survival of 11 months compared with an average of 3 to 6 months with conventional chemotherapy regimens. However, in vitro studies reported by Pasquier et al53 indicated that the addition of propranolol to doxorubicin or paclitaxel did not result in increased efficacy. Chow et al54 demonstrated that propranolol monotherapy resulted in a reduction of the proliferative index of scalp angiosarcoma by 34% after only 1 week of treatment. This was followed by combination therapy of propranolol, paclitaxel, and radiation, which resulted in substantial tumor regression and no evidence of metastasis after 8 months of therapy.54
Immune checkpoint inhibitors have been a recent subject of interest in the treatment of angiosarcoma. Two case reports showed improvement in CAS of the face and primary pleural angiosarcoma with a course of pembrolizumab.55,56 In another case series, investigators used immune checkpoint inhibitors in 7 patients with cutaneous, breast, or radiation-associated angiosarcoma and found partial response in several patients treated with pembrolizumab and ipilimumab-nivolumab and complete response in 1 patient treated with anti–cytotoxic T-lymphocyte–associated protein 4 antibodies. The authors of this study hypothesized that treatment response was associated with the mutational profile of tumors, including mutational signatures of UV radiation with a large number of C-to-T substitutions similar to melanomas.57
Conclusion
Cutaneous angiosarcoma is a rare and aggressive tumor with a poor prognosis due to delayed detection. A thorough skin examination and heightened awareness of CAS by dermatologists may result in early biopsy and shortened time to a definitive diagnosis. Until quality evidence allows for the creation of consensus guidelines, care at a cancer center that specializes in rare and difficult-to-treat tumors and employs a multidisciplinary approach is essential to optimizing patient outcomes. Current knowledge supports surgery with negative margins as the mainstay of treatment, with adjuvant radiation, chemotherapy, and targeted therapies as possible additions for extensive disease. The role of MMS is uncertain, and because of the lack of contiguity in CAS, it may not be an optimal treatment.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627.
- Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss’s Soft Tissue Tumors. 7th ed. Elsevier Inc; 2020.
- Arora TK, Terracina KP, Soong J, et al. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014;3:28-34.
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83:809-816.
- Chang C, Wu SP, Hu K, et al. Patterns of care and survival of cutaneous angiosarcoma of the head and neck. Otolaryngol Head Neck Surg. 2020;162:881-887.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Fury MG, Antonescu CR, Van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241-247.
- Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. a retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer. 2013;49:369-376.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Dettenborn T, Wermker K, Schulze HJ, et al. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42:1623-1628.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59:582-589.
- Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. a report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. a review of 99 cases. Cancer. 1995;75:989-996.
- Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036.
- Oranges T, Janowska A, Vitali S, et al. Dermatoscopic and ultra-high frequency ultrasound evaluation in cutaneous postradiation angiosarcoma. J Eur Acad Dermatol Venereol. 2020;34:e741.
- Figueroa-Silva O, Argenziano G, Lallas A, et al. Dermoscopic pattern of radiation-induced angiosarcoma (RIA). J Am Acad Dermatol. 2015;73:E51-E55.
- Cole DW, Huerta T, Andea A, et al. Purpuric plaques-dermoscopic and histopathological correlation of cutaneous angiosarcoma. Dermatol Pract Concept. 2020;10:E2020084. doi:10.5826/dpc.1004a84
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. Vol 2. 4th ed. Elsevier; 2018.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39. doi:10.2353/ajpath.2010.090637
- Ohsawa M, Naka N, Tomita Y, et al. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867-2874.
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850.
- Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16:2502-2509.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Kofler L, Breuninger H, Schulz C, et al. Local recurrence rates of skin tumors after resection with complete circumferential peripheral and deep margin assessment-identification of high-risk entities. Dermatol Surg. 2021;47:E31-E36.
- Houpe JE, Seger EW, Neill BC, et al. Treatment of angiosarcoma of the head and neck: a systematic review. Cutis. 2023;111:247-251. doi:10.12788/cutis.0767
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Gonzalez MJ, Koehler MM, Satter EK. Angiosarcoma of the scalp: a case report and review of current and novel therapeutic regimens. Dermatol Surg. 2009;35:679-684.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269-5274.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523.
- Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178-3186.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361-366.
- Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034-2037.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:2433-2436.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88-92.
- Koontz BF, Miles EF, Rubio MA, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262-266.
- Pasquier E, André N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Wang X, Wei J, Zeng Z, et al. Primary pleural epithelioid angiosarcoma treated successfully with anti-PD-1 therapy: a rare case report. Medicine (Baltimore). 2021;100:E27132.
- Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy—a case report. J Immunother Cancer. 2017;5:58.
- Florou V, Rosenberg AE, Wieder E, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer. 2019;7:213.
PRACTICE POINTS
- Dermatologists should be aware of challenges in diagnosing cutaneous angiosarcoma (CAS) due to its clinical similarity to benign entities such as ecchymosis and hemangioma.
- Surgery with negative margins is the first-line treatment of CAS with the best prognosis.
- Mohs micrographic surgery is useful for well-defined lesions measuring less than 5 cm on the head and neck; however, further studies are needed to determine its use in other areas.
- Paraffin-embedded sections may be more reliable than frozen sections in determining margin clearance.
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.
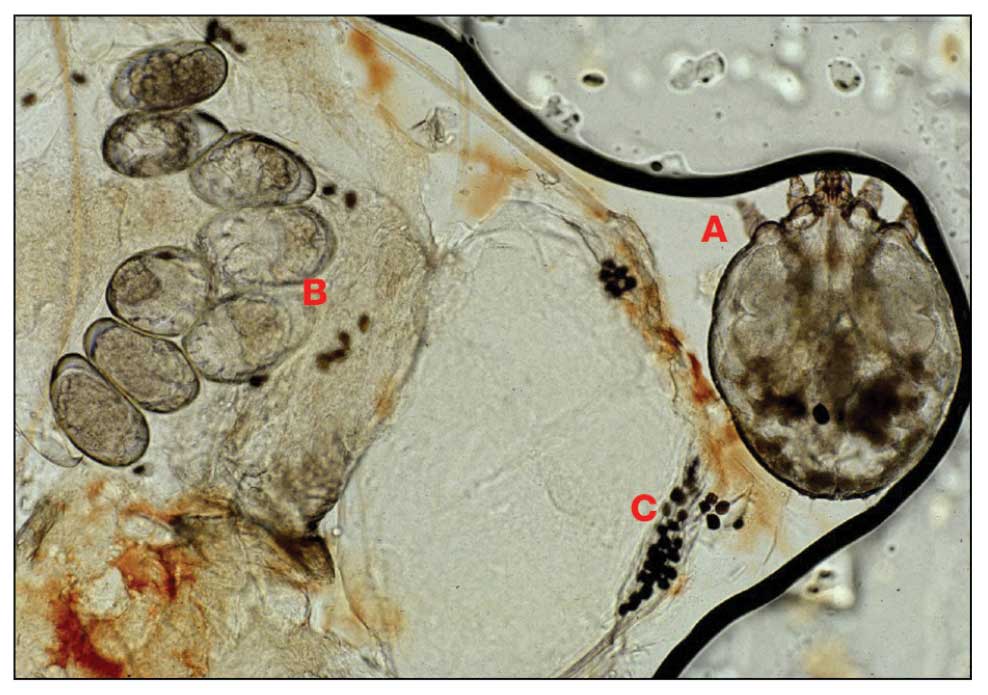
The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27
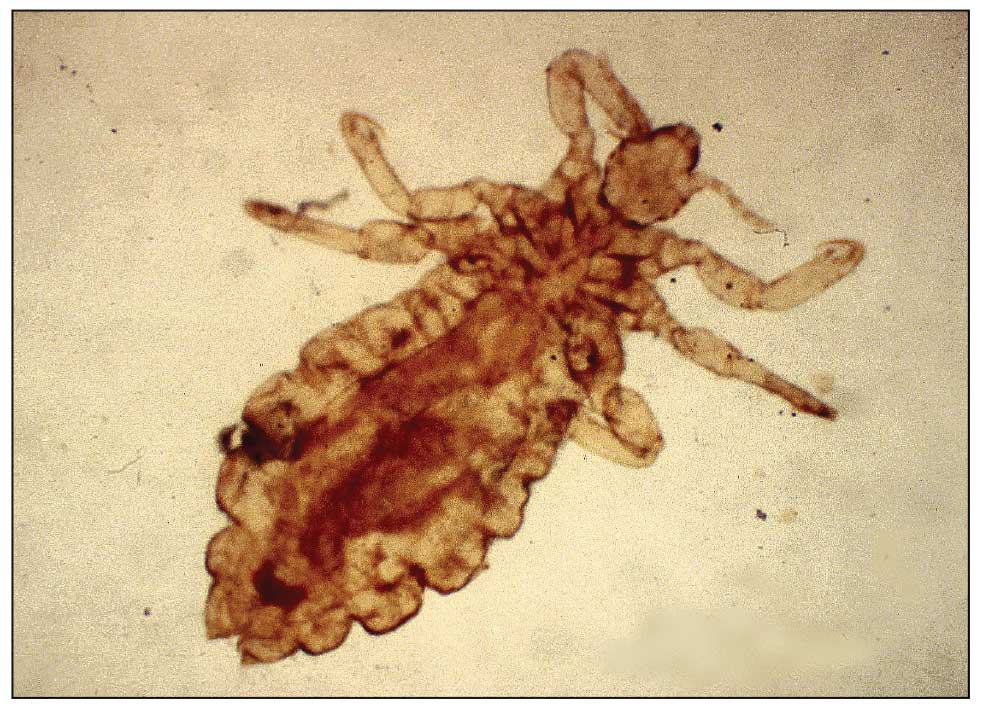
Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32
Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31
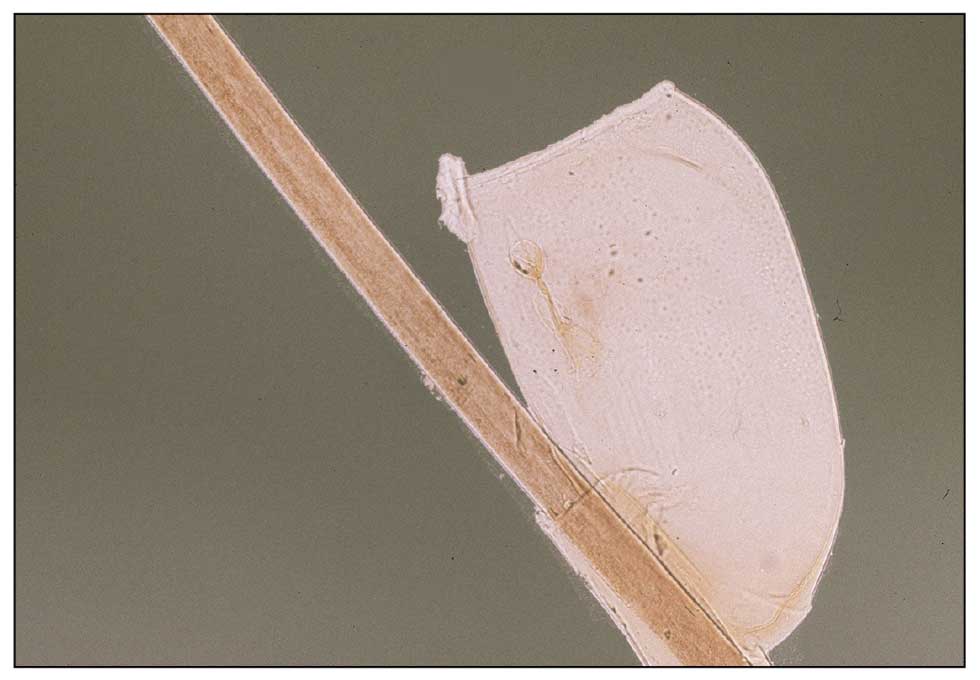
Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.
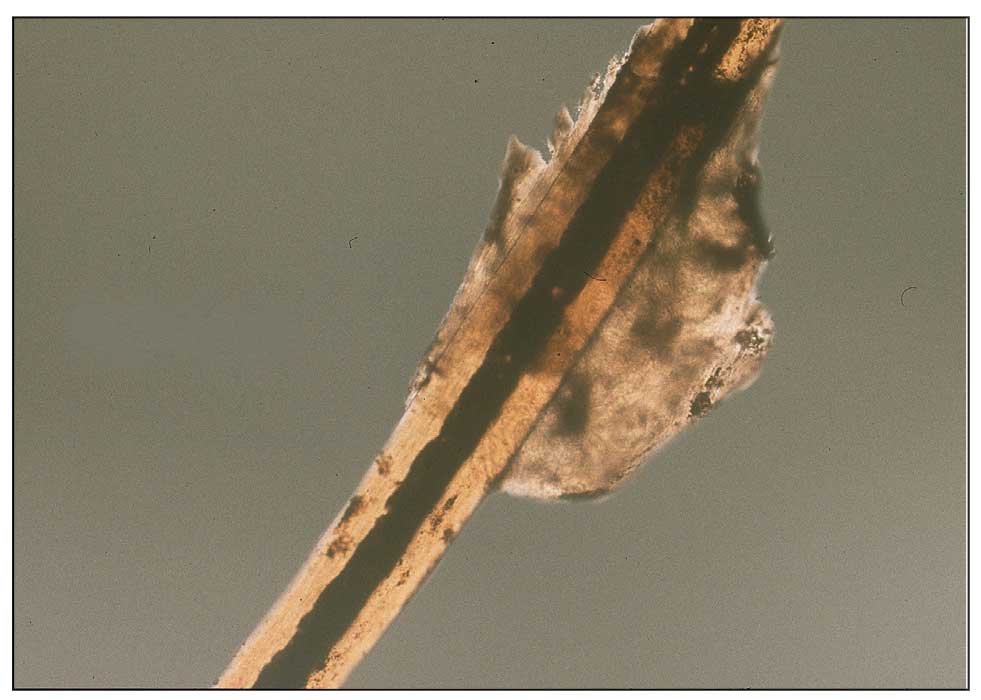
Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Practice Points
- War and natural disasters displace populations and disrupt infrastructure and access to medical care.
- Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.
- Body lice are important disease vectors inrefugee populations.
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.
Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29
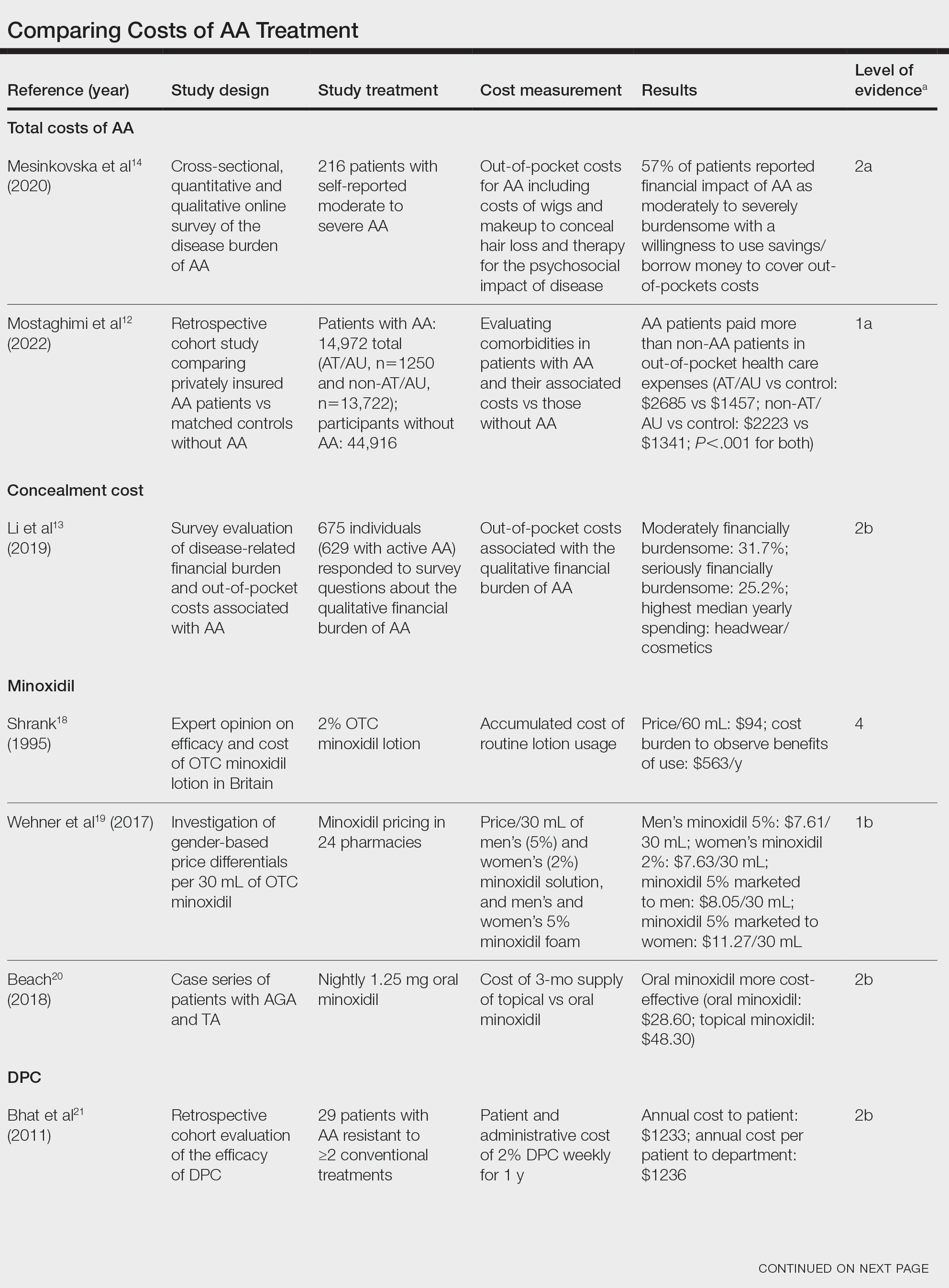
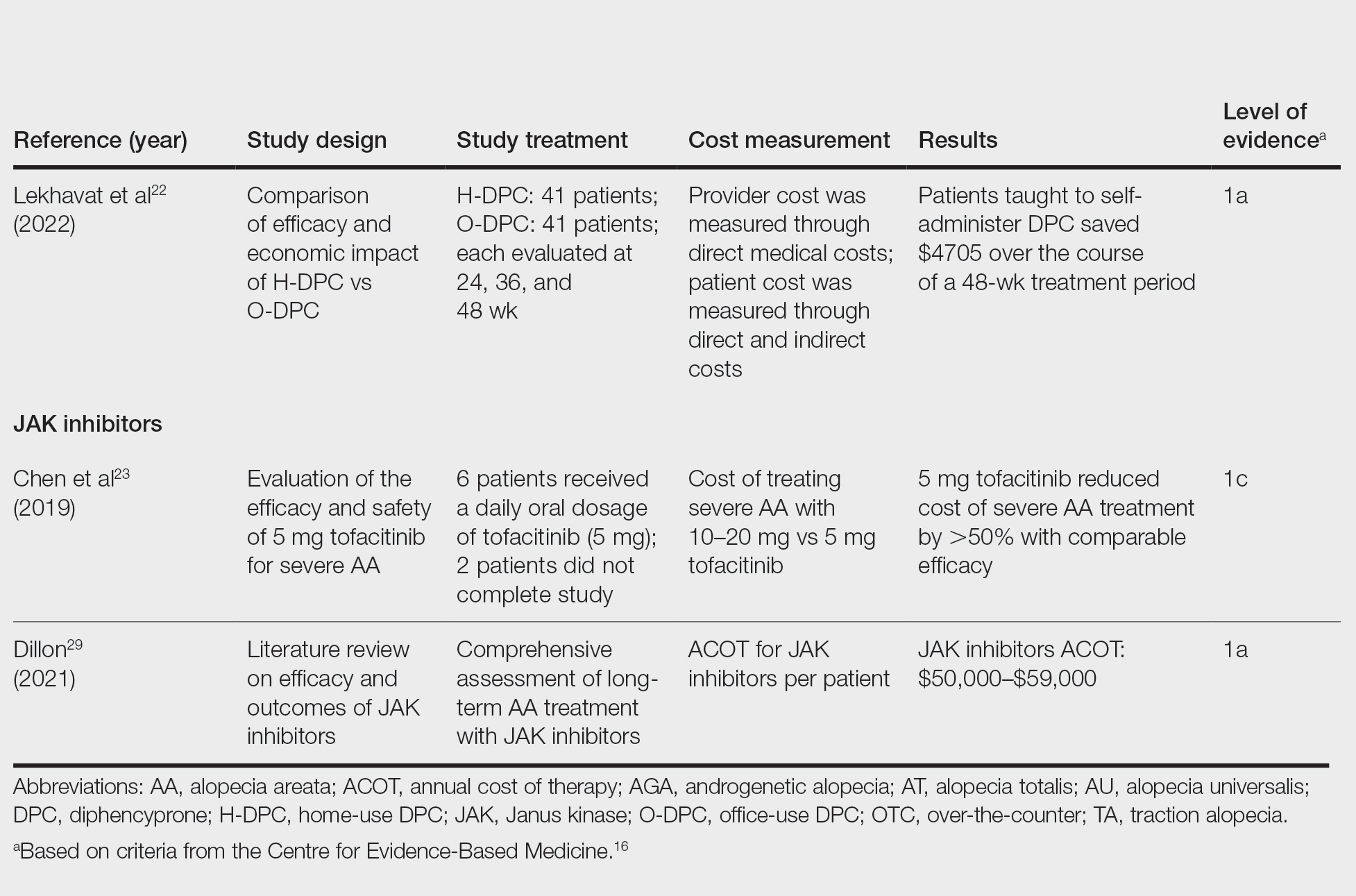
Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Practice Points
- Hair loss treatments and concealment techniques cost the average patient thousands of dollars. Much of this cost burden comes from items not covered by insurance.
- Providers should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective option.
- Self-administering diphencyprone at home is more cost- and time-effective than in-office diphencyprone administration and does not decrease efficacy.
Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28
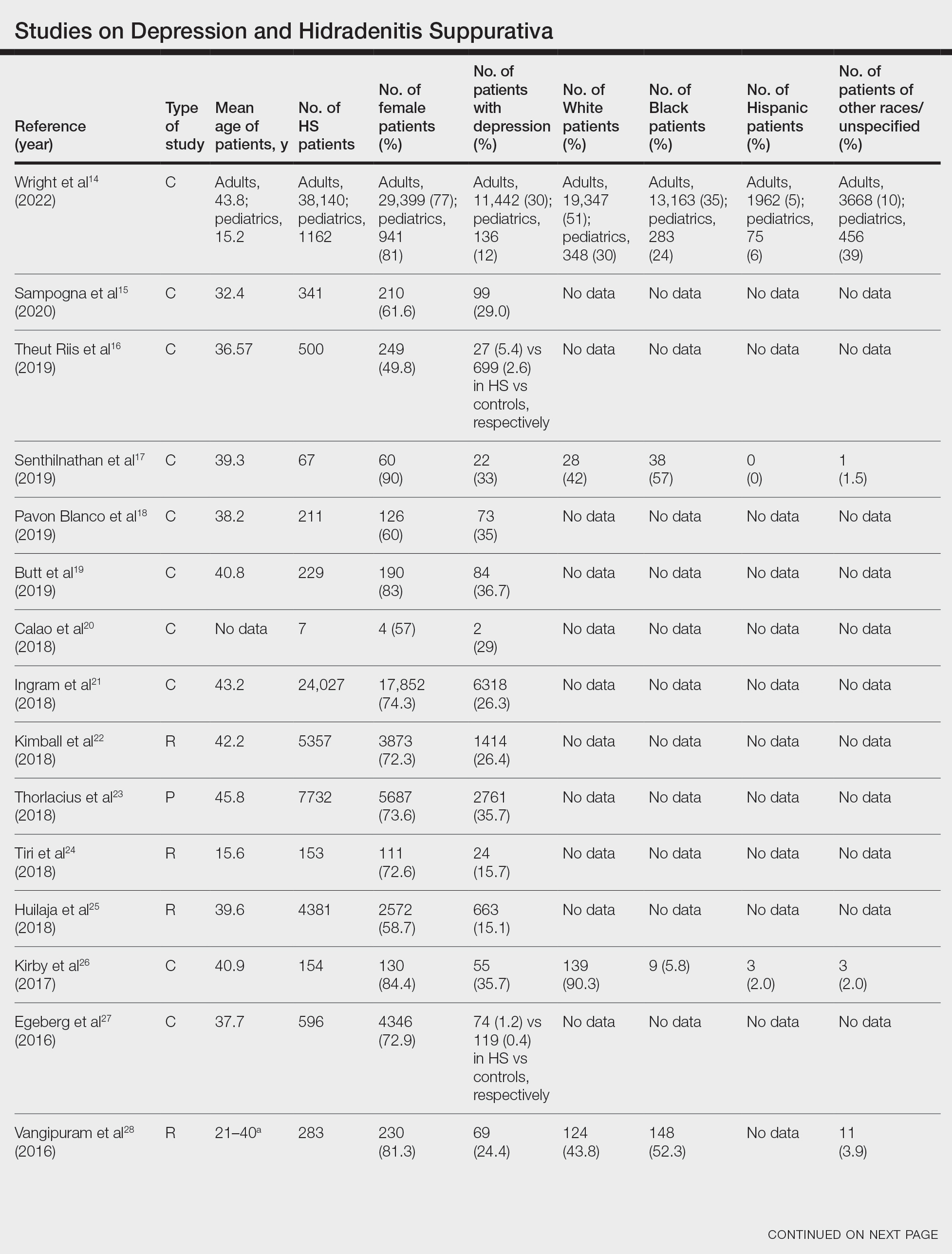
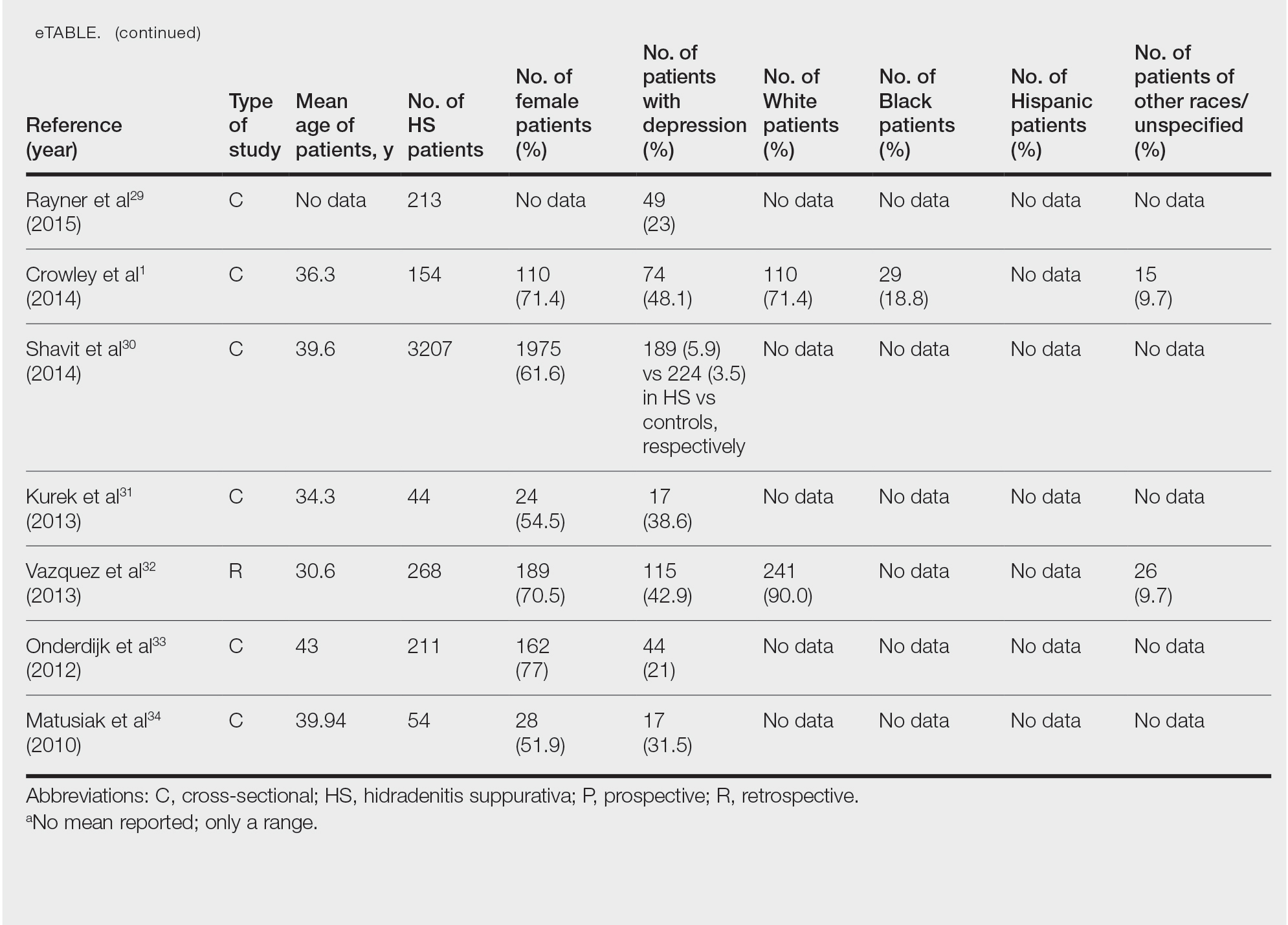
Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Practice Points
- Hidradenitis suppurativa (HS) is known to be associated with systemic inflammation and comorbidities, including depression.
- Depression may be a potential contributing factor to HS in affected patients, and studies on HS with comorbid depression in patients with skin of color are lacking.
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17
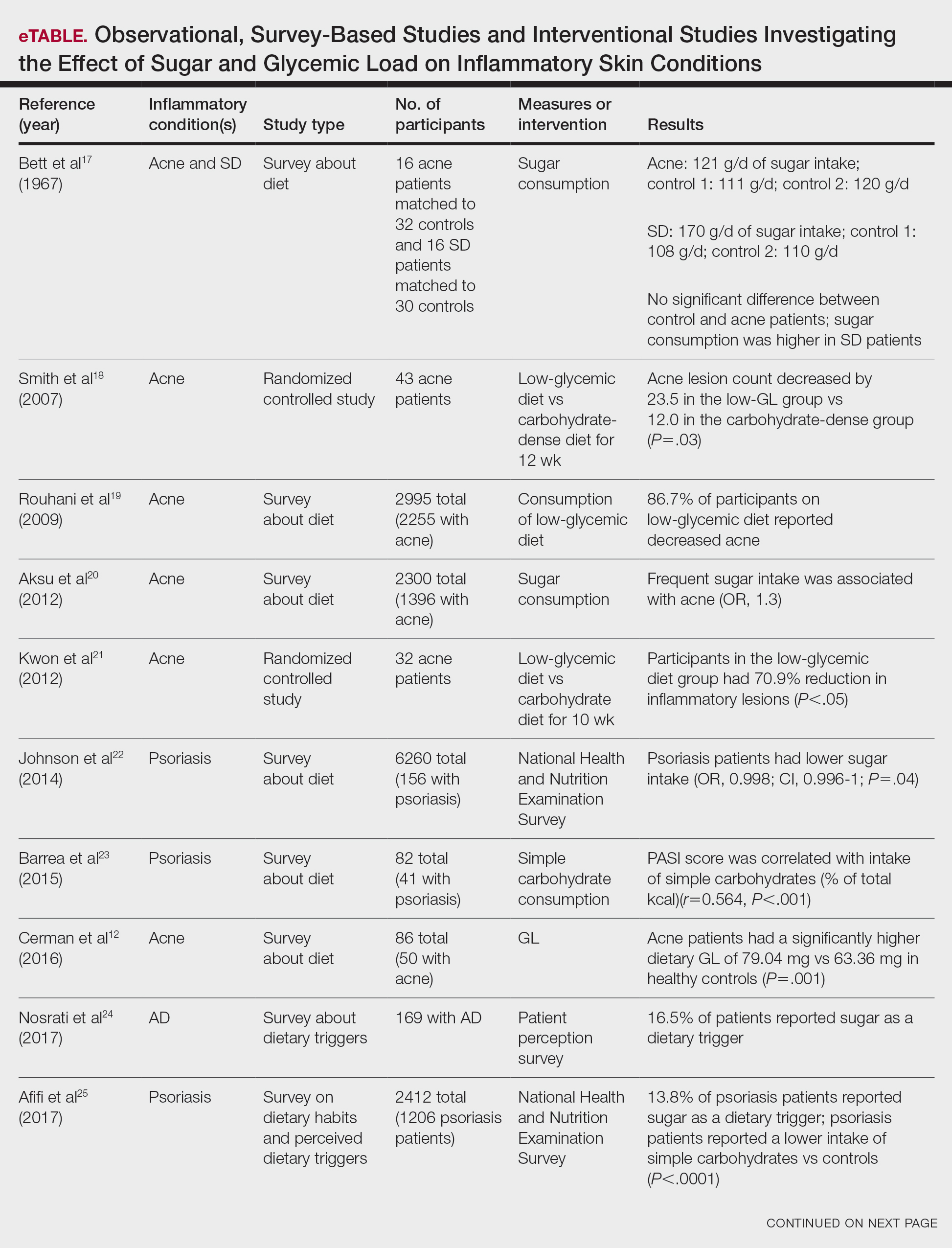

Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
Expanding the Psoriasis Framework: Immunopathogenesis and Treatment Updates
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.
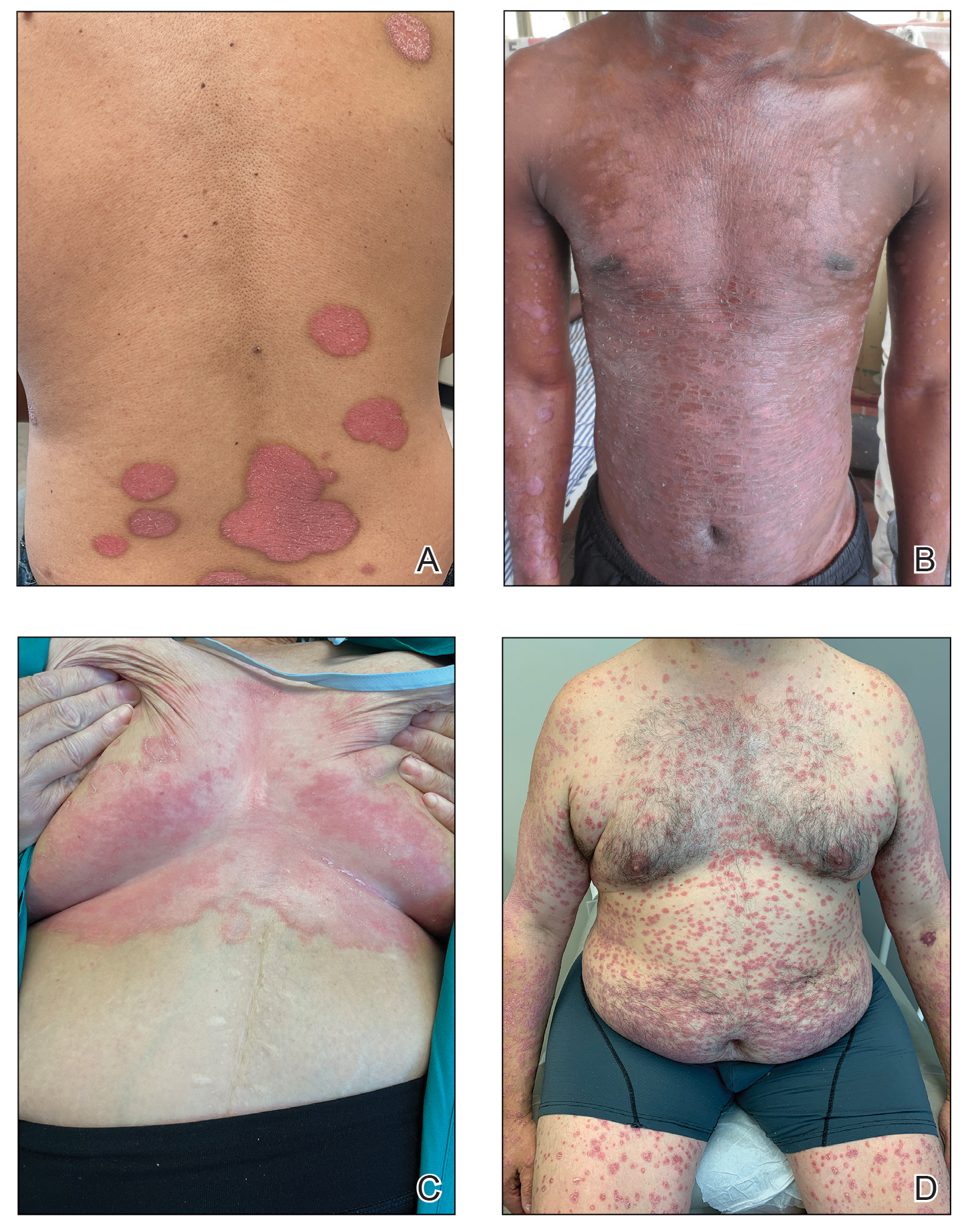
A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.
The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).
Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Practice Points
- Psoriasis is a chronic inflammatory condition characterized by systemic inflammation and dysregulated IL-23/IL-17 signaling.
- Modern discoveries highlight the role of additional immune signals in psoriatic disease such as IL-17C, IL-17F, IL-36, and tyrosine kinase 2, which also contribute to disease development.
- Novel systemic, oral, and topical therapies have become available and add to the rapidly growing armamentarium of safe and effective treatments for psoriatic disease.