User login
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.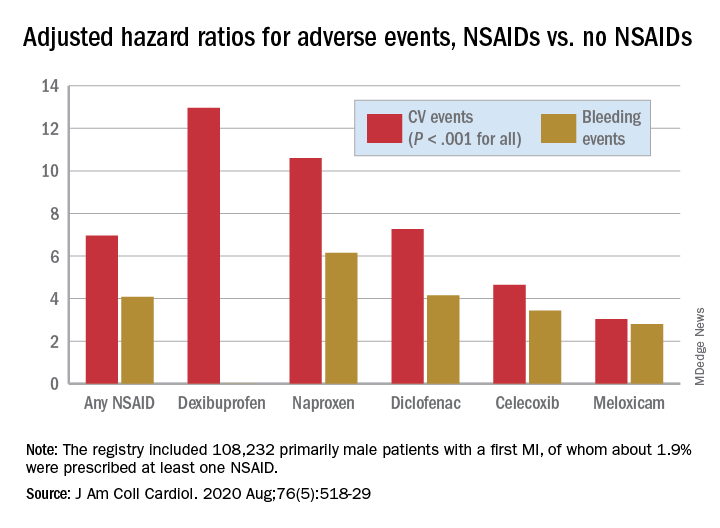
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Value of palliative care shines clearly in a crisis
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: Eytan.Szmuilowicz@nm.org.
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Hospitalists have played a key role
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: Eytan.Szmuilowicz@nm.org.
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: Eytan.Szmuilowicz@nm.org.
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
ACC panel defines, advises on heart failure with ‘recovered’ EF
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hospital vs. outpatient management comparable for elderly syncope patients
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Global study to track COVID-19’s impact on the brain
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
COVID-19 taking financial toll on people in U.S. with diabetes
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
No rise in major hemorrhagic events with antiplatelet therapy after ICH
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Infection ups mortality risk in patients with dementia
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Infection increases mortality risk among patients with dementia, new research suggests. A large, registry-based cohort study showed that
“This is the first study to our knowledge to show that increased mortality is observed across all infection types in people with dementia and that increased mortality is seen both short and long term,” said coinvestigator Janet Janbek, a PhD student at the Danish Dementia Research Center, Rigshospitalet, University of Copenhagen.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference.
Large Danish cohort
The investigators analyzed data from Danish national health registries for nearly 1.5 million individuals aged 65 years and older who had visited the hospital with an infection. There were 575,260 deaths during more than 12.7 million person-years of follow-up.
Patients with dementia who also had a hospital visit for infection died at a 6.5 times higher rate than participants without dementia or an infection. Those with either dementia alone or infection-related contacts alone had a threefold increased rate of death.
The mortality rate was highest within the first 30 days following the hospital visit for infection. However, the rate remained elevated for 10 years after the initial infection-related hospital visit.
Mortality rates from all infections, including major infections, such as sepsis, down to minor ear infections were elevated in patients with dementia, compared with people who did not have dementia or an infection-related hospital visit.
Ms. Janbek said there are several possible explanations for the association of infection and increased mortality risk in those with dementia. “After a hospital contact with a severe infection, people with dementia may become more reliant on external care, become more frail, and have declined functional levels, which might explain the observed association.”
It might also be that patients with dementia have more severe infections than those without dementia at the time of hospital contact, possibly because of delayed diagnosis, which could explain the higher mortality rates, said Ms. Janbek.
“It is also plausible that infections play a role in worsening dementia and subsequently lead to increased mortality,” she noted.
“Clinicians and health care personnel need to pay closer attention to infections of all types in people with dementia, and steps toward better clinical management and improved posthospital care need to be explored and undertaken. We need to identify possible preventive measures and targeted interventions in people with dementia and infections,” Ms. Janbek said.
‘Interesting observation’
Commenting on the study, Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, said it presents “an interesting observation.” However, “we can’t make any direct assumptions from this research per se about infections and dementia and whether they are causative in any way,” noted Dr. Edelmayer, who was not involved with the study.
Instead, the study highlighted the importance of “taking care of our overall health and making sure that individuals that might be vulnerable to infection, like those who are already living with dementia, are getting the best care possible,” she said.
Ms. Janbek and Dr. Edelmayer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
Early palliative care fails to improve QOL in advanced heart failure
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
FROM JAMA INTERNAL MEDICINE
‘Staggering’ increase in COVID-linked depression, anxiety
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.















