User login
Pandemic strains blood supply for COVID-19 and noninfected patients
The COVID-19 pandemic is putting a strain on the blood supply and could be putting people – including those who normally get transfusions, such as patients with sickle cell disease and cancer – at risk.
“Around the beginning of March, the hematology community got wind of what was going on because the blood banks were saying think about your patients and begin to restrict blood usage because we are expecting an increase in usage for COVID-positive ICU patients,” Ifeyinwa (Ify) Osunkwo, MD, a specialist in hematology and sickle cell disease at Levine Cancer Institute in Charlotte, N.C., said in an interview.
“I think that was the first call to arms around hematology ... you don’t want to shortchange somebody who is well and who is being sustained by life-giving transfusions and cut out their transfusion therapy because you are hoping to use the blood for people who are coming in with COVID-19,” she continued. “That is an ethical dilemma that no doctor wants to have to go through. But the reality is we have to do something to make it work for everybody.”
And the timing of the social restrictions due to the pandemic has added additional strain on the blood supply.
“Over the winter, traditionally, blood drives slow down because of the flu and different viruses,” she noted. “The spring and the summer are when we see the biggest recruitment and uptake of blood donation. COVID-19 hit [and] a lot of the blood drives that were traditionally scheduled to supply blood for the country have been canceled because of the new guidance for social distancing.”
Another big source of blood are health care professionals themselves and they may not be able to donate because of the extra hours being worked because of the pandemic.
In speaking about the needs for traditional patients such as those who are dealing with cancer or leukemia or sickle cell diseases as well as those who are being treated for COVID-19 in North Carolina, “we are not at the critical point, but I am a little bit nervous that we may get there because they are not going to up the usual blood drives anytime this summer. We project [sometime] in the fall, but maybe not even then. So there needs to be a significant call-out for people to make every effort to donate blood,” said Dr. Osunkwo. She added that in places such as New York City that are hot spots for the COVID-19 outbreak, the need is likely a lot greater.
She recalled a recent incident at a New York hospital that highlighted how those managing blood supplies are being restrictive and how this could be harming patients.
“A sickle cell patient came in with COVID-19 and the treatment recommendation was do a red blood cell exchange but the blood bank was nervous about getting enough blood to supply for that exchange transfusion,” she said, noting that the doctor still went to bat for that patient to get the needed treatment. “We gave her the supporting evidence that when you are on treatment for sickle cell disease, you tend to do better if you get COVID-19 or any other viral infection. The symptoms of COVID-19 in sickle cell disease is acute chest syndrome, for which the treatment is red blood cell exchange. Not doing that for [these patients] is really not giving them the optimal way of managing their disease, and managing their disease in the setting of COVID-19.”
To that end, Dr. Osunkwo stressed that doctors need to be doing all they can to get the word out that blood is needed and that the American Red Cross and other donation organizations are making it safe for people to donate. She has been using social media to highlight when her fellow doctors and others make donations as a way to motivate individuals.
“Everybody can do something during this pandemic,” she said. “Don’t feel like you are not working, that you are not a frontline worker, that you have nothing to contribute. You can donate blood. Your cousin can donate blood. You can tell your friends, your neighbors, your relatives, your enemies to go donate. We will take every kind of blood we can get because people are needing it more now. Even though we canceled elective surgeries, my patients when they get COVID-19, they need more blood ... than they usually do during their regular sickle cell admission. It is going to be the same for people who have other blood disorders like cancer and leukemia. We can’t stop life-saving treatments just because we have the COVID pandemic.”
Dr. Osunkwo also praised recent actions taken by the Food and Drug Administration to lessen some of the deferral periods for when an individual can donate.
The FDA on April 2 issued three sets of revised recommendations aimed at getting more people eligible to donate blood. All of the revised recommendations will remain in effect after the COVID-19 health emergency is declared over.
The first revised recommendation makes changes to December 2015 guidance.
For male blood donors who would have been deferred for having sex with another male partner, the deferral period has been reduced from 12 months to 3 months. That deferral period change also applies to female donors who had sex with a man who had sex with another man as well as for those with recent tattoos and piercings.
The second recommendation revises guidance from August 2013 and relates to the risk of transfusion-transmitted malaria.
Under the new recommendations, for those who traveled to malaria-endemic areas (and are residents of malaria non-endemic countries), the FDA is lowering the recommended deferral period from 12 months to 3 months, and also provides notices of an alternate procedure that permits donations without a deferral period provided the blood components are pathogen-reduced using an FDA-approved pathogen reduction device.
The third recommendation finalizes draft guidance from January that eliminates the referral period for donors who spent time in certain European countries or were on military bases in Europe and were previously considered to have been exposed to a potential risk of transmission of Creutzfeldt-Jakob Disease or Variant Creutzfeldt-Jakob Disease.
Dr. Osunkwo reports consultancy and being on the speakers bureau and participating in the advisory board for Novartis, and relationships with a variety of other pharmaceutical companies. She is the editor-in-chief for Hematology News.
The COVID-19 pandemic is putting a strain on the blood supply and could be putting people – including those who normally get transfusions, such as patients with sickle cell disease and cancer – at risk.
“Around the beginning of March, the hematology community got wind of what was going on because the blood banks were saying think about your patients and begin to restrict blood usage because we are expecting an increase in usage for COVID-positive ICU patients,” Ifeyinwa (Ify) Osunkwo, MD, a specialist in hematology and sickle cell disease at Levine Cancer Institute in Charlotte, N.C., said in an interview.
“I think that was the first call to arms around hematology ... you don’t want to shortchange somebody who is well and who is being sustained by life-giving transfusions and cut out their transfusion therapy because you are hoping to use the blood for people who are coming in with COVID-19,” she continued. “That is an ethical dilemma that no doctor wants to have to go through. But the reality is we have to do something to make it work for everybody.”
And the timing of the social restrictions due to the pandemic has added additional strain on the blood supply.
“Over the winter, traditionally, blood drives slow down because of the flu and different viruses,” she noted. “The spring and the summer are when we see the biggest recruitment and uptake of blood donation. COVID-19 hit [and] a lot of the blood drives that were traditionally scheduled to supply blood for the country have been canceled because of the new guidance for social distancing.”
Another big source of blood are health care professionals themselves and they may not be able to donate because of the extra hours being worked because of the pandemic.
In speaking about the needs for traditional patients such as those who are dealing with cancer or leukemia or sickle cell diseases as well as those who are being treated for COVID-19 in North Carolina, “we are not at the critical point, but I am a little bit nervous that we may get there because they are not going to up the usual blood drives anytime this summer. We project [sometime] in the fall, but maybe not even then. So there needs to be a significant call-out for people to make every effort to donate blood,” said Dr. Osunkwo. She added that in places such as New York City that are hot spots for the COVID-19 outbreak, the need is likely a lot greater.
She recalled a recent incident at a New York hospital that highlighted how those managing blood supplies are being restrictive and how this could be harming patients.
“A sickle cell patient came in with COVID-19 and the treatment recommendation was do a red blood cell exchange but the blood bank was nervous about getting enough blood to supply for that exchange transfusion,” she said, noting that the doctor still went to bat for that patient to get the needed treatment. “We gave her the supporting evidence that when you are on treatment for sickle cell disease, you tend to do better if you get COVID-19 or any other viral infection. The symptoms of COVID-19 in sickle cell disease is acute chest syndrome, for which the treatment is red blood cell exchange. Not doing that for [these patients] is really not giving them the optimal way of managing their disease, and managing their disease in the setting of COVID-19.”
To that end, Dr. Osunkwo stressed that doctors need to be doing all they can to get the word out that blood is needed and that the American Red Cross and other donation organizations are making it safe for people to donate. She has been using social media to highlight when her fellow doctors and others make donations as a way to motivate individuals.
“Everybody can do something during this pandemic,” she said. “Don’t feel like you are not working, that you are not a frontline worker, that you have nothing to contribute. You can donate blood. Your cousin can donate blood. You can tell your friends, your neighbors, your relatives, your enemies to go donate. We will take every kind of blood we can get because people are needing it more now. Even though we canceled elective surgeries, my patients when they get COVID-19, they need more blood ... than they usually do during their regular sickle cell admission. It is going to be the same for people who have other blood disorders like cancer and leukemia. We can’t stop life-saving treatments just because we have the COVID pandemic.”
Dr. Osunkwo also praised recent actions taken by the Food and Drug Administration to lessen some of the deferral periods for when an individual can donate.
The FDA on April 2 issued three sets of revised recommendations aimed at getting more people eligible to donate blood. All of the revised recommendations will remain in effect after the COVID-19 health emergency is declared over.
The first revised recommendation makes changes to December 2015 guidance.
For male blood donors who would have been deferred for having sex with another male partner, the deferral period has been reduced from 12 months to 3 months. That deferral period change also applies to female donors who had sex with a man who had sex with another man as well as for those with recent tattoos and piercings.
The second recommendation revises guidance from August 2013 and relates to the risk of transfusion-transmitted malaria.
Under the new recommendations, for those who traveled to malaria-endemic areas (and are residents of malaria non-endemic countries), the FDA is lowering the recommended deferral period from 12 months to 3 months, and also provides notices of an alternate procedure that permits donations without a deferral period provided the blood components are pathogen-reduced using an FDA-approved pathogen reduction device.
The third recommendation finalizes draft guidance from January that eliminates the referral period for donors who spent time in certain European countries or were on military bases in Europe and were previously considered to have been exposed to a potential risk of transmission of Creutzfeldt-Jakob Disease or Variant Creutzfeldt-Jakob Disease.
Dr. Osunkwo reports consultancy and being on the speakers bureau and participating in the advisory board for Novartis, and relationships with a variety of other pharmaceutical companies. She is the editor-in-chief for Hematology News.
The COVID-19 pandemic is putting a strain on the blood supply and could be putting people – including those who normally get transfusions, such as patients with sickle cell disease and cancer – at risk.
“Around the beginning of March, the hematology community got wind of what was going on because the blood banks were saying think about your patients and begin to restrict blood usage because we are expecting an increase in usage for COVID-positive ICU patients,” Ifeyinwa (Ify) Osunkwo, MD, a specialist in hematology and sickle cell disease at Levine Cancer Institute in Charlotte, N.C., said in an interview.
“I think that was the first call to arms around hematology ... you don’t want to shortchange somebody who is well and who is being sustained by life-giving transfusions and cut out their transfusion therapy because you are hoping to use the blood for people who are coming in with COVID-19,” she continued. “That is an ethical dilemma that no doctor wants to have to go through. But the reality is we have to do something to make it work for everybody.”
And the timing of the social restrictions due to the pandemic has added additional strain on the blood supply.
“Over the winter, traditionally, blood drives slow down because of the flu and different viruses,” she noted. “The spring and the summer are when we see the biggest recruitment and uptake of blood donation. COVID-19 hit [and] a lot of the blood drives that were traditionally scheduled to supply blood for the country have been canceled because of the new guidance for social distancing.”
Another big source of blood are health care professionals themselves and they may not be able to donate because of the extra hours being worked because of the pandemic.
In speaking about the needs for traditional patients such as those who are dealing with cancer or leukemia or sickle cell diseases as well as those who are being treated for COVID-19 in North Carolina, “we are not at the critical point, but I am a little bit nervous that we may get there because they are not going to up the usual blood drives anytime this summer. We project [sometime] in the fall, but maybe not even then. So there needs to be a significant call-out for people to make every effort to donate blood,” said Dr. Osunkwo. She added that in places such as New York City that are hot spots for the COVID-19 outbreak, the need is likely a lot greater.
She recalled a recent incident at a New York hospital that highlighted how those managing blood supplies are being restrictive and how this could be harming patients.
“A sickle cell patient came in with COVID-19 and the treatment recommendation was do a red blood cell exchange but the blood bank was nervous about getting enough blood to supply for that exchange transfusion,” she said, noting that the doctor still went to bat for that patient to get the needed treatment. “We gave her the supporting evidence that when you are on treatment for sickle cell disease, you tend to do better if you get COVID-19 or any other viral infection. The symptoms of COVID-19 in sickle cell disease is acute chest syndrome, for which the treatment is red blood cell exchange. Not doing that for [these patients] is really not giving them the optimal way of managing their disease, and managing their disease in the setting of COVID-19.”
To that end, Dr. Osunkwo stressed that doctors need to be doing all they can to get the word out that blood is needed and that the American Red Cross and other donation organizations are making it safe for people to donate. She has been using social media to highlight when her fellow doctors and others make donations as a way to motivate individuals.
“Everybody can do something during this pandemic,” she said. “Don’t feel like you are not working, that you are not a frontline worker, that you have nothing to contribute. You can donate blood. Your cousin can donate blood. You can tell your friends, your neighbors, your relatives, your enemies to go donate. We will take every kind of blood we can get because people are needing it more now. Even though we canceled elective surgeries, my patients when they get COVID-19, they need more blood ... than they usually do during their regular sickle cell admission. It is going to be the same for people who have other blood disorders like cancer and leukemia. We can’t stop life-saving treatments just because we have the COVID pandemic.”
Dr. Osunkwo also praised recent actions taken by the Food and Drug Administration to lessen some of the deferral periods for when an individual can donate.
The FDA on April 2 issued three sets of revised recommendations aimed at getting more people eligible to donate blood. All of the revised recommendations will remain in effect after the COVID-19 health emergency is declared over.
The first revised recommendation makes changes to December 2015 guidance.
For male blood donors who would have been deferred for having sex with another male partner, the deferral period has been reduced from 12 months to 3 months. That deferral period change also applies to female donors who had sex with a man who had sex with another man as well as for those with recent tattoos and piercings.
The second recommendation revises guidance from August 2013 and relates to the risk of transfusion-transmitted malaria.
Under the new recommendations, for those who traveled to malaria-endemic areas (and are residents of malaria non-endemic countries), the FDA is lowering the recommended deferral period from 12 months to 3 months, and also provides notices of an alternate procedure that permits donations without a deferral period provided the blood components are pathogen-reduced using an FDA-approved pathogen reduction device.
The third recommendation finalizes draft guidance from January that eliminates the referral period for donors who spent time in certain European countries or were on military bases in Europe and were previously considered to have been exposed to a potential risk of transmission of Creutzfeldt-Jakob Disease or Variant Creutzfeldt-Jakob Disease.
Dr. Osunkwo reports consultancy and being on the speakers bureau and participating in the advisory board for Novartis, and relationships with a variety of other pharmaceutical companies. She is the editor-in-chief for Hematology News.
COVID-19 PPE-related skin effects described in survey of Chinese doctors, nurses
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Severe COVID-19 may lower hemoglobin levels
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
FROM HEMATOLOGY, TRANSFUSION AND CELL THERAPY
COVID-19: When health care personnel become patients
according to the Centers for Disease Control and Prevention.
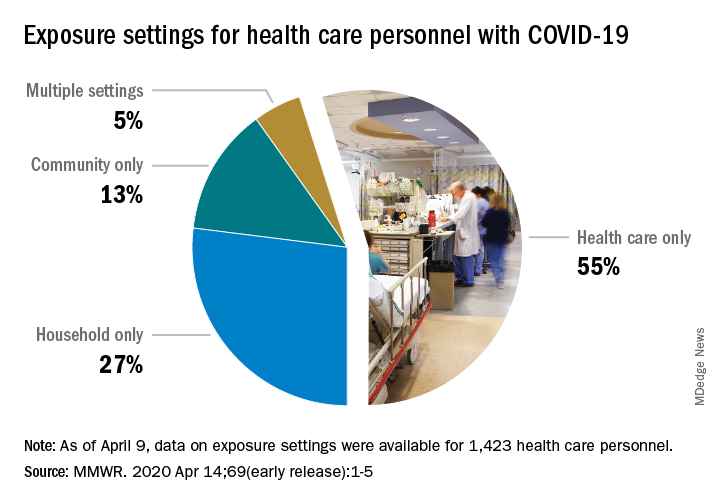
That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
according to the Centers for Disease Control and Prevention.

That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
according to the Centers for Disease Control and Prevention.

That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
FROM THE MMWR
FDA approves emergency use of saliva test to detect COVID-19
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
Social distancing comes to the medicine wards
As the coronavirus pandemic has swept across America, so have advisories for social distancing. As of April 2, stay-at-home orders had been given in 38 states and parts of 7 more, affecting about 300 million people. Most of these people have been asked to maintain 6 feet of separation to anyone outside their immediate family and to avoid all avoidable contacts.
Typical hospital medicine patients at an academic hospital, however, traditionally receive visits from their hospitalist, an intern, a resident, and sometimes several medical students, pharmacists, and case managers. At University of California, San Diego, Health, many of these visits would occur during Focused Interdisciplinary Team rounds, with providers moving together in close proximity.
Asymptomatic and presymptomatic spread of coronavirus have been documented, which means distancing is a good idea for everyone. The risks of traditional patient visits during the coronavirus pandemic include spread to both patients (at high risk of complications) and staff (taken out of the workforce during surge times). Even if coronavirus were not a risk, visits to isolation rooms consume PPE, which is in short supply.
In response to the pandemic, UCSD Hospital Medicine drafted guidelines for the reduction of patient contacts. Our slide presentations and written guidelines were then distributed to physicians, nurses, pharmacists, and other staff by our pandemic response command center. Key points include the following:
- Target one in-person MD visit per day for stable patients. This means that attending reexaminations of patients seen by residents, nurse practitioners, physician assistants, and so on would not be done for billing or teaching purposes, only when clinically necessary.
- Use phone or video conferencing for follow-up discussions unless direct patient contact is needed.
- Consider skipping daily exams on patients who do not require them, such as patients awaiting placement or stably receiving long courses of antibiotics. Interview them remotely or from the door instead.
- Conduct team rounds, patient discussions, and handoffs with all members 6 feet apart or by telephone or video. Avoid shared work rooms. Substitute video conferences for in-person meetings. Use EMR embedded messaging to reduce face-to-face discussions.
- Check if a patient is ready for a visit before donning PPE to avoid waste.
- Explain to patients that distancing is being conducted to protect them. In our experience, when patients are asked about distancing, they welcome the changes.
We have also considered that most patient visits are generated by nurses and assistants. To increase distancing and reduce PPE waste, we have encouraged nurses and pharmacists to maximize their use of remote communication with patients and to suggest changes to care plans and come up with creative solutions to reduce traffic. We specifically suggested the following changes to routine care:
- Reduce frequency of taking vital signs, such as just daily or as needed, in stable patients (for example, those awaiting placement).
- Reduce checks for alcohol withdrawal and neurologic status as soon as possible, and stop fingersticks in patients with well-controlled diabetes not receiving insulin.
- Substitute less frequently administered medications where appropriate if doing so would reduce room traffic (such as enoxaparin for heparin, ceftriaxone for cefazolin, naproxen for ibuprofen, or patient-controlled analgesia for as needed morphine).
- Place intravenous pumps in halls if needed – luckily, our situation has not required these measures in San Diego.
- Explore the possibility of increased patient self-management (self-dosed insulin or inhalers) where medically appropriate.
- Eliminate food service and janitorial trips to isolation rooms unless requested by registered nurse.
There are clear downsides to medical distancing for hospital medicine patients. Patients might have delayed diagnosis of new conditions or inadequate management of conditions requiring frequent assessment, such as alcohol withdrawal. Opportunities for miscommunication (either patient-provider or provider-provider) may be increased with distancing. Isolation also comes with emotional costs such as stress and feelings of isolation or abandonment. Given the dynamic nature of the pandemic response, we are continually reevaluating our distancing guidelines to administer the safest and most effective hospital care possible as we approach California’s expected peak coronavirus infection period.
Dr. Jenkins is professor and chair of the Patient Safety Committee in the Division of Hospital Medicine at UCSD. Dr. Seymann is clinical professor and vice chief for academic affairs, UCSD division of hospital medicine. Dr. Horman and Dr. Bell are hospitalists and associate professors of medicine at UC San Diego Health.
As the coronavirus pandemic has swept across America, so have advisories for social distancing. As of April 2, stay-at-home orders had been given in 38 states and parts of 7 more, affecting about 300 million people. Most of these people have been asked to maintain 6 feet of separation to anyone outside their immediate family and to avoid all avoidable contacts.
Typical hospital medicine patients at an academic hospital, however, traditionally receive visits from their hospitalist, an intern, a resident, and sometimes several medical students, pharmacists, and case managers. At University of California, San Diego, Health, many of these visits would occur during Focused Interdisciplinary Team rounds, with providers moving together in close proximity.
Asymptomatic and presymptomatic spread of coronavirus have been documented, which means distancing is a good idea for everyone. The risks of traditional patient visits during the coronavirus pandemic include spread to both patients (at high risk of complications) and staff (taken out of the workforce during surge times). Even if coronavirus were not a risk, visits to isolation rooms consume PPE, which is in short supply.
In response to the pandemic, UCSD Hospital Medicine drafted guidelines for the reduction of patient contacts. Our slide presentations and written guidelines were then distributed to physicians, nurses, pharmacists, and other staff by our pandemic response command center. Key points include the following:
- Target one in-person MD visit per day for stable patients. This means that attending reexaminations of patients seen by residents, nurse practitioners, physician assistants, and so on would not be done for billing or teaching purposes, only when clinically necessary.
- Use phone or video conferencing for follow-up discussions unless direct patient contact is needed.
- Consider skipping daily exams on patients who do not require them, such as patients awaiting placement or stably receiving long courses of antibiotics. Interview them remotely or from the door instead.
- Conduct team rounds, patient discussions, and handoffs with all members 6 feet apart or by telephone or video. Avoid shared work rooms. Substitute video conferences for in-person meetings. Use EMR embedded messaging to reduce face-to-face discussions.
- Check if a patient is ready for a visit before donning PPE to avoid waste.
- Explain to patients that distancing is being conducted to protect them. In our experience, when patients are asked about distancing, they welcome the changes.
We have also considered that most patient visits are generated by nurses and assistants. To increase distancing and reduce PPE waste, we have encouraged nurses and pharmacists to maximize their use of remote communication with patients and to suggest changes to care plans and come up with creative solutions to reduce traffic. We specifically suggested the following changes to routine care:
- Reduce frequency of taking vital signs, such as just daily or as needed, in stable patients (for example, those awaiting placement).
- Reduce checks for alcohol withdrawal and neurologic status as soon as possible, and stop fingersticks in patients with well-controlled diabetes not receiving insulin.
- Substitute less frequently administered medications where appropriate if doing so would reduce room traffic (such as enoxaparin for heparin, ceftriaxone for cefazolin, naproxen for ibuprofen, or patient-controlled analgesia for as needed morphine).
- Place intravenous pumps in halls if needed – luckily, our situation has not required these measures in San Diego.
- Explore the possibility of increased patient self-management (self-dosed insulin or inhalers) where medically appropriate.
- Eliminate food service and janitorial trips to isolation rooms unless requested by registered nurse.
There are clear downsides to medical distancing for hospital medicine patients. Patients might have delayed diagnosis of new conditions or inadequate management of conditions requiring frequent assessment, such as alcohol withdrawal. Opportunities for miscommunication (either patient-provider or provider-provider) may be increased with distancing. Isolation also comes with emotional costs such as stress and feelings of isolation or abandonment. Given the dynamic nature of the pandemic response, we are continually reevaluating our distancing guidelines to administer the safest and most effective hospital care possible as we approach California’s expected peak coronavirus infection period.
Dr. Jenkins is professor and chair of the Patient Safety Committee in the Division of Hospital Medicine at UCSD. Dr. Seymann is clinical professor and vice chief for academic affairs, UCSD division of hospital medicine. Dr. Horman and Dr. Bell are hospitalists and associate professors of medicine at UC San Diego Health.
As the coronavirus pandemic has swept across America, so have advisories for social distancing. As of April 2, stay-at-home orders had been given in 38 states and parts of 7 more, affecting about 300 million people. Most of these people have been asked to maintain 6 feet of separation to anyone outside their immediate family and to avoid all avoidable contacts.
Typical hospital medicine patients at an academic hospital, however, traditionally receive visits from their hospitalist, an intern, a resident, and sometimes several medical students, pharmacists, and case managers. At University of California, San Diego, Health, many of these visits would occur during Focused Interdisciplinary Team rounds, with providers moving together in close proximity.
Asymptomatic and presymptomatic spread of coronavirus have been documented, which means distancing is a good idea for everyone. The risks of traditional patient visits during the coronavirus pandemic include spread to both patients (at high risk of complications) and staff (taken out of the workforce during surge times). Even if coronavirus were not a risk, visits to isolation rooms consume PPE, which is in short supply.
In response to the pandemic, UCSD Hospital Medicine drafted guidelines for the reduction of patient contacts. Our slide presentations and written guidelines were then distributed to physicians, nurses, pharmacists, and other staff by our pandemic response command center. Key points include the following:
- Target one in-person MD visit per day for stable patients. This means that attending reexaminations of patients seen by residents, nurse practitioners, physician assistants, and so on would not be done for billing or teaching purposes, only when clinically necessary.
- Use phone or video conferencing for follow-up discussions unless direct patient contact is needed.
- Consider skipping daily exams on patients who do not require them, such as patients awaiting placement or stably receiving long courses of antibiotics. Interview them remotely or from the door instead.
- Conduct team rounds, patient discussions, and handoffs with all members 6 feet apart or by telephone or video. Avoid shared work rooms. Substitute video conferences for in-person meetings. Use EMR embedded messaging to reduce face-to-face discussions.
- Check if a patient is ready for a visit before donning PPE to avoid waste.
- Explain to patients that distancing is being conducted to protect them. In our experience, when patients are asked about distancing, they welcome the changes.
We have also considered that most patient visits are generated by nurses and assistants. To increase distancing and reduce PPE waste, we have encouraged nurses and pharmacists to maximize their use of remote communication with patients and to suggest changes to care plans and come up with creative solutions to reduce traffic. We specifically suggested the following changes to routine care:
- Reduce frequency of taking vital signs, such as just daily or as needed, in stable patients (for example, those awaiting placement).
- Reduce checks for alcohol withdrawal and neurologic status as soon as possible, and stop fingersticks in patients with well-controlled diabetes not receiving insulin.
- Substitute less frequently administered medications where appropriate if doing so would reduce room traffic (such as enoxaparin for heparin, ceftriaxone for cefazolin, naproxen for ibuprofen, or patient-controlled analgesia for as needed morphine).
- Place intravenous pumps in halls if needed – luckily, our situation has not required these measures in San Diego.
- Explore the possibility of increased patient self-management (self-dosed insulin or inhalers) where medically appropriate.
- Eliminate food service and janitorial trips to isolation rooms unless requested by registered nurse.
There are clear downsides to medical distancing for hospital medicine patients. Patients might have delayed diagnosis of new conditions or inadequate management of conditions requiring frequent assessment, such as alcohol withdrawal. Opportunities for miscommunication (either patient-provider or provider-provider) may be increased with distancing. Isolation also comes with emotional costs such as stress and feelings of isolation or abandonment. Given the dynamic nature of the pandemic response, we are continually reevaluating our distancing guidelines to administer the safest and most effective hospital care possible as we approach California’s expected peak coronavirus infection period.
Dr. Jenkins is professor and chair of the Patient Safety Committee in the Division of Hospital Medicine at UCSD. Dr. Seymann is clinical professor and vice chief for academic affairs, UCSD division of hospital medicine. Dr. Horman and Dr. Bell are hospitalists and associate professors of medicine at UC San Diego Health.
Inflammatory markers may explain COVID-19, diabetes dynamic
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
FROM DIABETES/METABOLISM RESEARCH AND REVIEWS
SARS-CoV-2 may confound seasons, persist in warmer months, report shows
Although conflicting, the available data indicate that SARS-CoV-2 could continue to spread in warmer spring and summer months in the US, according to a new report from the National Academies of Science, Engineering, and Medicine (NAS).
Current data suggest that the novel coronavirus may be transmitted less efficiently in higher temperatures and humidity, but the studies are not conclusive because of poor data quality, confounding factors, and the relatively short existence of the pandemic, which makes it difficult to determine its true course, writes David A. Relman, MD, a member of the NAS’ Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, in a rapid expert consultation letter to the White House Office of Science and Technology Policy on April 7.
A number of factors could influence whether SARS-CoV-2 follows the same seasonal pattern as the influenza virus and other seasonal coronaviruses, which wane during warmer months, writes Relman, a professor of microbiology and immunology at Stanford University in California.
But he pointed out that previous coronavirus strains that have caused serious illness – SARS-CoV and MERS-CoV – “have not demonstrated any evidence of seasonality following their emergence.”
Relman cites an example from the current outbreak: “Given that countries currently in ‘summer’ climates, such as Australia and Iran, are experiencing rapid virus spread, a decrease in cases with increases in humidity and temperature elsewhere should not be assumed…Additional studies as the pandemic unfolds could shed more light on the effects of climate on transmission,” he writes.
And even if SARS-CoV-2 turns out to be less infectious in warmer months, “given the lack of host immunity globally, this reduction in transmission efficiency may not lead to a significant reduction in disease spread without the concomitant adoption of major public health interventions,” writes Relman.
Conflicting Data
Relman cites a handful of studies indicating that, on the one hand, SARS-CoV-2 has declined with increasing humidity and temperatures, but that conversely, infectivity has increased in warmer, more humid climates.
A recent study in China, published on the repository and international journal site SSRN, found that while increased temperatures and humidity decreased the infectivity, “the average R0 (R naught) was still close to 2 at maximum temperatures and humidity in their data set, suggesting that the virus will still spread exponentially at higher temperatures and humidity,” said Relman.
Several other studies found higher growth rates in temperate regions. One study, still in preprint on MedRxiv, looked at 310 geographic regions across 116 countries, and shows an inverse relationship between temperature and humidity and the incidence of COVID-19.
All the available studies so far have significant limitations, including limitation in time and location, confounding factors having to do with geography, access to and the quality of public health and health care systems, human behavior, and the availability of testing, said Relman.
However, he said, “it is useful to note that pandemic influenza strains have not exhibited the typical seasonal pattern of endemic/epidemic strains,” and, regardless of whether they started in a warmer or a cooler month, “all had a peak second wave approximately six months after the emergence in the human population.”
Worrisome Persistence on Masks
Seasonality can also be potentially gauged in the laboratory. Most of the studies on environmental persistence of SARS-CoV-2 have been conducted using virus grown in tissue culture. But that, too, is an imperfect method.
Virus disseminated into the environment from naturally infected humans likely has different survival properties than virus grown in culture, said Relman.
In addition, many labs cannot, or fail to, control and vary relative humidity, the committee letter noted. The aerosol studies so far have used humidity levels of 50% to 65%, which is more favorable to decay, while respiratory fluid is more likely to protect against infectivity, and the 20%-to-40% wintertime indoor humidity in temperate regions is more favorable for virus survival.
Even with these caveats, the committee cited worrisome studies on SARS-CoV-2 survival.
In a study published April 2 online in The Lancet, Hong Kong researchers reported significant reductions in virus in culture starting with temperatures at 37°C (98.6°F) or above.
On surfaces at a room temperature of 22°C (71.6°F) with a relative humidity of 65%, there was no infectious virus on printing paper or tissue papers after just 3 hours. It took 4 days for an infectious level to break down on glass and money, and 7 days for stainless steel and plastic. But after 7 days, investigators found 0.1% of the original inoculum on the outside of a surgical mask.
“The persistence of infectious virus on PPE is concerning,” writes Relman, noting that more studies are needed to guide healthcare workers, especially on what might be used to disinfect personal protective equipment “when they cannot be discarded after single use.”
Chad Roy, PhD, a researcher from Tulane University National Primate Research Center in New Orleans, Louisiana, told Relman by phone that in experiments where the virus was suspended as an aerosol at a temperature of 23°C (73.4° F) and about 50% humidity, SARS-CoV-2 had a longer half-life than the influenza virus, SARS-CoV-1, monkeypox virus, and Mycobacterium tuberculosis.
“This result is also concerning, but quite preliminary,” writes Relman.
This article first appeared on Medscape.com.
Although conflicting, the available data indicate that SARS-CoV-2 could continue to spread in warmer spring and summer months in the US, according to a new report from the National Academies of Science, Engineering, and Medicine (NAS).
Current data suggest that the novel coronavirus may be transmitted less efficiently in higher temperatures and humidity, but the studies are not conclusive because of poor data quality, confounding factors, and the relatively short existence of the pandemic, which makes it difficult to determine its true course, writes David A. Relman, MD, a member of the NAS’ Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, in a rapid expert consultation letter to the White House Office of Science and Technology Policy on April 7.
A number of factors could influence whether SARS-CoV-2 follows the same seasonal pattern as the influenza virus and other seasonal coronaviruses, which wane during warmer months, writes Relman, a professor of microbiology and immunology at Stanford University in California.
But he pointed out that previous coronavirus strains that have caused serious illness – SARS-CoV and MERS-CoV – “have not demonstrated any evidence of seasonality following their emergence.”
Relman cites an example from the current outbreak: “Given that countries currently in ‘summer’ climates, such as Australia and Iran, are experiencing rapid virus spread, a decrease in cases with increases in humidity and temperature elsewhere should not be assumed…Additional studies as the pandemic unfolds could shed more light on the effects of climate on transmission,” he writes.
And even if SARS-CoV-2 turns out to be less infectious in warmer months, “given the lack of host immunity globally, this reduction in transmission efficiency may not lead to a significant reduction in disease spread without the concomitant adoption of major public health interventions,” writes Relman.
Conflicting Data
Relman cites a handful of studies indicating that, on the one hand, SARS-CoV-2 has declined with increasing humidity and temperatures, but that conversely, infectivity has increased in warmer, more humid climates.
A recent study in China, published on the repository and international journal site SSRN, found that while increased temperatures and humidity decreased the infectivity, “the average R0 (R naught) was still close to 2 at maximum temperatures and humidity in their data set, suggesting that the virus will still spread exponentially at higher temperatures and humidity,” said Relman.
Several other studies found higher growth rates in temperate regions. One study, still in preprint on MedRxiv, looked at 310 geographic regions across 116 countries, and shows an inverse relationship between temperature and humidity and the incidence of COVID-19.
All the available studies so far have significant limitations, including limitation in time and location, confounding factors having to do with geography, access to and the quality of public health and health care systems, human behavior, and the availability of testing, said Relman.
However, he said, “it is useful to note that pandemic influenza strains have not exhibited the typical seasonal pattern of endemic/epidemic strains,” and, regardless of whether they started in a warmer or a cooler month, “all had a peak second wave approximately six months after the emergence in the human population.”
Worrisome Persistence on Masks
Seasonality can also be potentially gauged in the laboratory. Most of the studies on environmental persistence of SARS-CoV-2 have been conducted using virus grown in tissue culture. But that, too, is an imperfect method.
Virus disseminated into the environment from naturally infected humans likely has different survival properties than virus grown in culture, said Relman.
In addition, many labs cannot, or fail to, control and vary relative humidity, the committee letter noted. The aerosol studies so far have used humidity levels of 50% to 65%, which is more favorable to decay, while respiratory fluid is more likely to protect against infectivity, and the 20%-to-40% wintertime indoor humidity in temperate regions is more favorable for virus survival.
Even with these caveats, the committee cited worrisome studies on SARS-CoV-2 survival.
In a study published April 2 online in The Lancet, Hong Kong researchers reported significant reductions in virus in culture starting with temperatures at 37°C (98.6°F) or above.
On surfaces at a room temperature of 22°C (71.6°F) with a relative humidity of 65%, there was no infectious virus on printing paper or tissue papers after just 3 hours. It took 4 days for an infectious level to break down on glass and money, and 7 days for stainless steel and plastic. But after 7 days, investigators found 0.1% of the original inoculum on the outside of a surgical mask.
“The persistence of infectious virus on PPE is concerning,” writes Relman, noting that more studies are needed to guide healthcare workers, especially on what might be used to disinfect personal protective equipment “when they cannot be discarded after single use.”
Chad Roy, PhD, a researcher from Tulane University National Primate Research Center in New Orleans, Louisiana, told Relman by phone that in experiments where the virus was suspended as an aerosol at a temperature of 23°C (73.4° F) and about 50% humidity, SARS-CoV-2 had a longer half-life than the influenza virus, SARS-CoV-1, monkeypox virus, and Mycobacterium tuberculosis.
“This result is also concerning, but quite preliminary,” writes Relman.
This article first appeared on Medscape.com.
Although conflicting, the available data indicate that SARS-CoV-2 could continue to spread in warmer spring and summer months in the US, according to a new report from the National Academies of Science, Engineering, and Medicine (NAS).
Current data suggest that the novel coronavirus may be transmitted less efficiently in higher temperatures and humidity, but the studies are not conclusive because of poor data quality, confounding factors, and the relatively short existence of the pandemic, which makes it difficult to determine its true course, writes David A. Relman, MD, a member of the NAS’ Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, in a rapid expert consultation letter to the White House Office of Science and Technology Policy on April 7.
A number of factors could influence whether SARS-CoV-2 follows the same seasonal pattern as the influenza virus and other seasonal coronaviruses, which wane during warmer months, writes Relman, a professor of microbiology and immunology at Stanford University in California.
But he pointed out that previous coronavirus strains that have caused serious illness – SARS-CoV and MERS-CoV – “have not demonstrated any evidence of seasonality following their emergence.”
Relman cites an example from the current outbreak: “Given that countries currently in ‘summer’ climates, such as Australia and Iran, are experiencing rapid virus spread, a decrease in cases with increases in humidity and temperature elsewhere should not be assumed…Additional studies as the pandemic unfolds could shed more light on the effects of climate on transmission,” he writes.
And even if SARS-CoV-2 turns out to be less infectious in warmer months, “given the lack of host immunity globally, this reduction in transmission efficiency may not lead to a significant reduction in disease spread without the concomitant adoption of major public health interventions,” writes Relman.
Conflicting Data
Relman cites a handful of studies indicating that, on the one hand, SARS-CoV-2 has declined with increasing humidity and temperatures, but that conversely, infectivity has increased in warmer, more humid climates.
A recent study in China, published on the repository and international journal site SSRN, found that while increased temperatures and humidity decreased the infectivity, “the average R0 (R naught) was still close to 2 at maximum temperatures and humidity in their data set, suggesting that the virus will still spread exponentially at higher temperatures and humidity,” said Relman.
Several other studies found higher growth rates in temperate regions. One study, still in preprint on MedRxiv, looked at 310 geographic regions across 116 countries, and shows an inverse relationship between temperature and humidity and the incidence of COVID-19.
All the available studies so far have significant limitations, including limitation in time and location, confounding factors having to do with geography, access to and the quality of public health and health care systems, human behavior, and the availability of testing, said Relman.
However, he said, “it is useful to note that pandemic influenza strains have not exhibited the typical seasonal pattern of endemic/epidemic strains,” and, regardless of whether they started in a warmer or a cooler month, “all had a peak second wave approximately six months after the emergence in the human population.”
Worrisome Persistence on Masks
Seasonality can also be potentially gauged in the laboratory. Most of the studies on environmental persistence of SARS-CoV-2 have been conducted using virus grown in tissue culture. But that, too, is an imperfect method.
Virus disseminated into the environment from naturally infected humans likely has different survival properties than virus grown in culture, said Relman.
In addition, many labs cannot, or fail to, control and vary relative humidity, the committee letter noted. The aerosol studies so far have used humidity levels of 50% to 65%, which is more favorable to decay, while respiratory fluid is more likely to protect against infectivity, and the 20%-to-40% wintertime indoor humidity in temperate regions is more favorable for virus survival.
Even with these caveats, the committee cited worrisome studies on SARS-CoV-2 survival.
In a study published April 2 online in The Lancet, Hong Kong researchers reported significant reductions in virus in culture starting with temperatures at 37°C (98.6°F) or above.
On surfaces at a room temperature of 22°C (71.6°F) with a relative humidity of 65%, there was no infectious virus on printing paper or tissue papers after just 3 hours. It took 4 days for an infectious level to break down on glass and money, and 7 days for stainless steel and plastic. But after 7 days, investigators found 0.1% of the original inoculum on the outside of a surgical mask.
“The persistence of infectious virus on PPE is concerning,” writes Relman, noting that more studies are needed to guide healthcare workers, especially on what might be used to disinfect personal protective equipment “when they cannot be discarded after single use.”
Chad Roy, PhD, a researcher from Tulane University National Primate Research Center in New Orleans, Louisiana, told Relman by phone that in experiments where the virus was suspended as an aerosol at a temperature of 23°C (73.4° F) and about 50% humidity, SARS-CoV-2 had a longer half-life than the influenza virus, SARS-CoV-1, monkeypox virus, and Mycobacterium tuberculosis.
“This result is also concerning, but quite preliminary,” writes Relman.
This article first appeared on Medscape.com.
Remdesivir tops list of promising COVID-19 treatments in review of nearly 300 trials
, according to authors of a recent review covering nearly 300 active clinical treatment trials underway for the disease.
Remdesivir, which has potent in vitro activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not approved by the Food and Drug Administration and is currently being tested in randomized trials, according to the review authors, led by James M. Sanders, PhD, of the department of pharmacy at University of Texas Southwestern Medical Center in Dallas.
By contrast, oseltamivir has not demonstrated efficacy against the virus, corticosteroids are not recommended, and promising data from a small French hydroxychloroquine study are balanced by “several major limitations” including small sample size and exclusion of early dropouts from the analysis, among others, Dr. Sanders and coauthors said in their report.
“These limitations coupled with concerns of additive cardiotoxicity with combination therapy [i.e., hydroxychloroquine with azithromycin] do not support adoption of this regimen without additional studies,” the researchers wrote. Their report is in JAMA.
Dr. Sanders and colleagues identified 291 COVID-19–specific studies listed in ClinicalTrials.gov through April 2, including 29 placebo-controlled trials.
This might represent just a sliver of the treatments that could combat COVID-19, according to the researchers, who said more than 3,000 small-molecule drug candidates with potential activity against human coronaviruses have been identified.
“This large amount of potential agents will hopefully yield more candidate therapeutics in the race to find effective treatments or preventive strategies against COVID-19,” said Dr. Sanders and coauthors.
Remdesivir for COVID-19
Remdesivir, an investigational nucleotide analog, is one promising agent because of its broad-spectrum and potent activity against SARS-CoV-2 and other novel coronaviruses, they said, adding that phase 1 trials demonstrated the drug was well tolerated without observed liver or kidney toxicity.
There have been “successful” case reports of remdesivir use in COVID-19, and at least five ongoing clinical trials are evaluating the drug’s safety and antiviral activity in this disease. Among those studies is a National Institutes of Health–sponsored adaptive, randomized, placebo-controlled trial that will provide data on the use of remdesivir versus supportive care.
“As the results from randomized controlled trials are anticipated, inclusion of this agent for treatment of COVID-19 may be considered,” Dr. Sanders and colleagues wrote in their report. To date, remdesivir remains investigational and needs to be obtained via compassionate use, through expanded access, or by participating in a clinical trial, they added.
Hydroxychloroquine and chloroquine
Among the published hydroxychloroquine studies is a “promising” 36-patient open-label nonrandomized French study, in which the antimalarial agent given every 8 hours improved virologic clearance by day 6 versus controls (70% vs. 12.5%, respectively), the review authors said. Moreover, viral clearance was 100% for 6 patients who received hydroxychloroquine plus azithromycin, compared to 57% (8 of 14) for patients treated with hydroxychloroquine alone. However, that study had several important limitations, including the small sample size, variable viral loads at baseline between groups, and a lack of safety and clinical outcomes reporting, according to the investigators. Moreover, six patients in the hydroxychloroquine group were taken out of the analysis because of early treatment stoppage due to medical intolerance or critical illness, the authors noted.
One prospective study including 30 patients in China demonstrated no difference in virologic outcomes for patients randomized to hydroxychloroquine plus standard of care versus standard of care alone, they added. There is also a case series of more than 100 patients with COVID-19 that reportedly improved viral clearance and reduced disease progression, though they said results haven’t been published or presented beyond a news briefing in China.
Randomized, controlled trials of chloroquine and hydroxychloroquine for COVID-19 treatment are underway, and studies are planned or enrolling to look at chloroquine prophylaxis in health care personnel and hydroxychloroquine for postexposure prophylaxis, authors said.
In results from one of those randomized trials, just reported, a higher dose of chloroquine was associated with a cardiac adverse event and an increased mortality risk, leading to the closure of that study arm. In the parallel, double-blinded, phase IIb clinical trial, patients in Brazil with SARS-CoV-2 infection received low or high doses of chloroquine plus ceftriaxone and azithromycin. According to the preprint publication, a higher rate of heart rate–corrected QT interval (QTc) prolongation and a “trend toward higher lethality” was observed in the high-dose group, leading investigators to “strongly recommend” the higher dose be abandoned.
“No apparent benefit of chloroquine was seen regarding lethality in our patients so far, but we will still enroll patients in the low chloroquine dose group to complete the originally planned sample size,” said investigators of the study, which at the time of the report had enrolled 81 out of an anticipated 440 patients.
Other COVID-19 pharmacologic therapies under study
Treatments of note in the review included the following:
- Tocilizumab. This monoclonal antibody IL-6 receptor antagonist, approved by the FDA for treatment of rheumatoid arthritis and for cytokine release syndrome related to chimeric antigen receptor (CAR) T-cell therapy, has yielded success in small series of patients with severe cases of COVID-19, according to authors. In one 21-patient report, 91% had clinical improvement, usually after a single dose. In China, tocilizumab is included in COVID-19 treatment guidelines, and several randomized clinical trials are underway in China including patients with COVID-19 with severe pneumonia.
- Immunoglobulin therapy. Antibodies from recovered COVID-19 patients could help with free virus and infected cell immune clearance, the authors said, adding that further studies are warranted beyond a few small published case series that suggest promise. Furthermore, on March 24 the FDA released guidance for screening donors for COVID-19 convalescent plasma and on emergency investigational new drug applications based on this modality.
- Lopinavir/ritonavir. Despite demonstrated in vitro activity against other novel coronaviruses, there is no published in vitro data for lopinavir/ritonavir in SARS-CoV-2, and likely a “limited role” for this combination anticipated in treating COVID-19, according to the review authors. In an open-label randomized clinical trial published in the New England Journal of Medicine (2020 Mar 18. doi: 10.1056/NEJMoa2001282), there were no differences in clinical improvement, viral clearance, or mortality for antiviral treatment versus standard care. Delayed treatment initiation may explain the ineffectiveness, though a subgroup analysis didn’t show a shorter time to clinical improvement for those who got the treatment earlier.
- Ribavirin. Likewise, this antiviral medication has efficacy and safety data suggesting “limited value” for treatment of COVID-19. Treatment of SARS yielded “inconclusive results” for ribavirin, which was also associated with substantial toxicity that included hemolytic anemia in 60% of SARS patients.
- Oseltamivir. While it may treat influenza, it has no documented activity against SARS-CoV-2 in vitro: “This agent has no role in the management of COVID-19 once influenza has been excluded,” said Dr. Sanders and coauthors.
- Corticosteroids. They could decrease inflammatory responses in the lung, but they could also lead to delays in viral clearance and increases in secondary infection risk. Guidelines for COVID-19 say to avoid corticosteroids, and the authors of the review concur, saying that potential harms and lack of proven benefit mean they usually should not be used outside of a randomized clinical trial setting.
- Vaccines. Clearly, vaccines represent the “most effective long-term strategy” to prevent future COVID-19 outbreaks, though at least 12-18 months would be required until vaccines can be widely deployed, authors said.
Dr. Sanders reported no potential conflicts. Senior author James B. Cutrell, MD, also of the University of Texas Southwestern Medical Center, reported nonfinancial support from Gilead and Regeneron outside of the study. No other authors reported disclosures.
, according to authors of a recent review covering nearly 300 active clinical treatment trials underway for the disease.
Remdesivir, which has potent in vitro activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not approved by the Food and Drug Administration and is currently being tested in randomized trials, according to the review authors, led by James M. Sanders, PhD, of the department of pharmacy at University of Texas Southwestern Medical Center in Dallas.
By contrast, oseltamivir has not demonstrated efficacy against the virus, corticosteroids are not recommended, and promising data from a small French hydroxychloroquine study are balanced by “several major limitations” including small sample size and exclusion of early dropouts from the analysis, among others, Dr. Sanders and coauthors said in their report.
“These limitations coupled with concerns of additive cardiotoxicity with combination therapy [i.e., hydroxychloroquine with azithromycin] do not support adoption of this regimen without additional studies,” the researchers wrote. Their report is in JAMA.
Dr. Sanders and colleagues identified 291 COVID-19–specific studies listed in ClinicalTrials.gov through April 2, including 29 placebo-controlled trials.
This might represent just a sliver of the treatments that could combat COVID-19, according to the researchers, who said more than 3,000 small-molecule drug candidates with potential activity against human coronaviruses have been identified.
“This large amount of potential agents will hopefully yield more candidate therapeutics in the race to find effective treatments or preventive strategies against COVID-19,” said Dr. Sanders and coauthors.
Remdesivir for COVID-19
Remdesivir, an investigational nucleotide analog, is one promising agent because of its broad-spectrum and potent activity against SARS-CoV-2 and other novel coronaviruses, they said, adding that phase 1 trials demonstrated the drug was well tolerated without observed liver or kidney toxicity.
There have been “successful” case reports of remdesivir use in COVID-19, and at least five ongoing clinical trials are evaluating the drug’s safety and antiviral activity in this disease. Among those studies is a National Institutes of Health–sponsored adaptive, randomized, placebo-controlled trial that will provide data on the use of remdesivir versus supportive care.
“As the results from randomized controlled trials are anticipated, inclusion of this agent for treatment of COVID-19 may be considered,” Dr. Sanders and colleagues wrote in their report. To date, remdesivir remains investigational and needs to be obtained via compassionate use, through expanded access, or by participating in a clinical trial, they added.
Hydroxychloroquine and chloroquine
Among the published hydroxychloroquine studies is a “promising” 36-patient open-label nonrandomized French study, in which the antimalarial agent given every 8 hours improved virologic clearance by day 6 versus controls (70% vs. 12.5%, respectively), the review authors said. Moreover, viral clearance was 100% for 6 patients who received hydroxychloroquine plus azithromycin, compared to 57% (8 of 14) for patients treated with hydroxychloroquine alone. However, that study had several important limitations, including the small sample size, variable viral loads at baseline between groups, and a lack of safety and clinical outcomes reporting, according to the investigators. Moreover, six patients in the hydroxychloroquine group were taken out of the analysis because of early treatment stoppage due to medical intolerance or critical illness, the authors noted.
One prospective study including 30 patients in China demonstrated no difference in virologic outcomes for patients randomized to hydroxychloroquine plus standard of care versus standard of care alone, they added. There is also a case series of more than 100 patients with COVID-19 that reportedly improved viral clearance and reduced disease progression, though they said results haven’t been published or presented beyond a news briefing in China.
Randomized, controlled trials of chloroquine and hydroxychloroquine for COVID-19 treatment are underway, and studies are planned or enrolling to look at chloroquine prophylaxis in health care personnel and hydroxychloroquine for postexposure prophylaxis, authors said.
In results from one of those randomized trials, just reported, a higher dose of chloroquine was associated with a cardiac adverse event and an increased mortality risk, leading to the closure of that study arm. In the parallel, double-blinded, phase IIb clinical trial, patients in Brazil with SARS-CoV-2 infection received low or high doses of chloroquine plus ceftriaxone and azithromycin. According to the preprint publication, a higher rate of heart rate–corrected QT interval (QTc) prolongation and a “trend toward higher lethality” was observed in the high-dose group, leading investigators to “strongly recommend” the higher dose be abandoned.
“No apparent benefit of chloroquine was seen regarding lethality in our patients so far, but we will still enroll patients in the low chloroquine dose group to complete the originally planned sample size,” said investigators of the study, which at the time of the report had enrolled 81 out of an anticipated 440 patients.
Other COVID-19 pharmacologic therapies under study
Treatments of note in the review included the following:
- Tocilizumab. This monoclonal antibody IL-6 receptor antagonist, approved by the FDA for treatment of rheumatoid arthritis and for cytokine release syndrome related to chimeric antigen receptor (CAR) T-cell therapy, has yielded success in small series of patients with severe cases of COVID-19, according to authors. In one 21-patient report, 91% had clinical improvement, usually after a single dose. In China, tocilizumab is included in COVID-19 treatment guidelines, and several randomized clinical trials are underway in China including patients with COVID-19 with severe pneumonia.
- Immunoglobulin therapy. Antibodies from recovered COVID-19 patients could help with free virus and infected cell immune clearance, the authors said, adding that further studies are warranted beyond a few small published case series that suggest promise. Furthermore, on March 24 the FDA released guidance for screening donors for COVID-19 convalescent plasma and on emergency investigational new drug applications based on this modality.
- Lopinavir/ritonavir. Despite demonstrated in vitro activity against other novel coronaviruses, there is no published in vitro data for lopinavir/ritonavir in SARS-CoV-2, and likely a “limited role” for this combination anticipated in treating COVID-19, according to the review authors. In an open-label randomized clinical trial published in the New England Journal of Medicine (2020 Mar 18. doi: 10.1056/NEJMoa2001282), there were no differences in clinical improvement, viral clearance, or mortality for antiviral treatment versus standard care. Delayed treatment initiation may explain the ineffectiveness, though a subgroup analysis didn’t show a shorter time to clinical improvement for those who got the treatment earlier.
- Ribavirin. Likewise, this antiviral medication has efficacy and safety data suggesting “limited value” for treatment of COVID-19. Treatment of SARS yielded “inconclusive results” for ribavirin, which was also associated with substantial toxicity that included hemolytic anemia in 60% of SARS patients.
- Oseltamivir. While it may treat influenza, it has no documented activity against SARS-CoV-2 in vitro: “This agent has no role in the management of COVID-19 once influenza has been excluded,” said Dr. Sanders and coauthors.
- Corticosteroids. They could decrease inflammatory responses in the lung, but they could also lead to delays in viral clearance and increases in secondary infection risk. Guidelines for COVID-19 say to avoid corticosteroids, and the authors of the review concur, saying that potential harms and lack of proven benefit mean they usually should not be used outside of a randomized clinical trial setting.
- Vaccines. Clearly, vaccines represent the “most effective long-term strategy” to prevent future COVID-19 outbreaks, though at least 12-18 months would be required until vaccines can be widely deployed, authors said.
Dr. Sanders reported no potential conflicts. Senior author James B. Cutrell, MD, also of the University of Texas Southwestern Medical Center, reported nonfinancial support from Gilead and Regeneron outside of the study. No other authors reported disclosures.
, according to authors of a recent review covering nearly 300 active clinical treatment trials underway for the disease.
Remdesivir, which has potent in vitro activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not approved by the Food and Drug Administration and is currently being tested in randomized trials, according to the review authors, led by James M. Sanders, PhD, of the department of pharmacy at University of Texas Southwestern Medical Center in Dallas.
By contrast, oseltamivir has not demonstrated efficacy against the virus, corticosteroids are not recommended, and promising data from a small French hydroxychloroquine study are balanced by “several major limitations” including small sample size and exclusion of early dropouts from the analysis, among others, Dr. Sanders and coauthors said in their report.
“These limitations coupled with concerns of additive cardiotoxicity with combination therapy [i.e., hydroxychloroquine with azithromycin] do not support adoption of this regimen without additional studies,” the researchers wrote. Their report is in JAMA.
Dr. Sanders and colleagues identified 291 COVID-19–specific studies listed in ClinicalTrials.gov through April 2, including 29 placebo-controlled trials.
This might represent just a sliver of the treatments that could combat COVID-19, according to the researchers, who said more than 3,000 small-molecule drug candidates with potential activity against human coronaviruses have been identified.
“This large amount of potential agents will hopefully yield more candidate therapeutics in the race to find effective treatments or preventive strategies against COVID-19,” said Dr. Sanders and coauthors.
Remdesivir for COVID-19
Remdesivir, an investigational nucleotide analog, is one promising agent because of its broad-spectrum and potent activity against SARS-CoV-2 and other novel coronaviruses, they said, adding that phase 1 trials demonstrated the drug was well tolerated without observed liver or kidney toxicity.
There have been “successful” case reports of remdesivir use in COVID-19, and at least five ongoing clinical trials are evaluating the drug’s safety and antiviral activity in this disease. Among those studies is a National Institutes of Health–sponsored adaptive, randomized, placebo-controlled trial that will provide data on the use of remdesivir versus supportive care.
“As the results from randomized controlled trials are anticipated, inclusion of this agent for treatment of COVID-19 may be considered,” Dr. Sanders and colleagues wrote in their report. To date, remdesivir remains investigational and needs to be obtained via compassionate use, through expanded access, or by participating in a clinical trial, they added.
Hydroxychloroquine and chloroquine
Among the published hydroxychloroquine studies is a “promising” 36-patient open-label nonrandomized French study, in which the antimalarial agent given every 8 hours improved virologic clearance by day 6 versus controls (70% vs. 12.5%, respectively), the review authors said. Moreover, viral clearance was 100% for 6 patients who received hydroxychloroquine plus azithromycin, compared to 57% (8 of 14) for patients treated with hydroxychloroquine alone. However, that study had several important limitations, including the small sample size, variable viral loads at baseline between groups, and a lack of safety and clinical outcomes reporting, according to the investigators. Moreover, six patients in the hydroxychloroquine group were taken out of the analysis because of early treatment stoppage due to medical intolerance or critical illness, the authors noted.
One prospective study including 30 patients in China demonstrated no difference in virologic outcomes for patients randomized to hydroxychloroquine plus standard of care versus standard of care alone, they added. There is also a case series of more than 100 patients with COVID-19 that reportedly improved viral clearance and reduced disease progression, though they said results haven’t been published or presented beyond a news briefing in China.
Randomized, controlled trials of chloroquine and hydroxychloroquine for COVID-19 treatment are underway, and studies are planned or enrolling to look at chloroquine prophylaxis in health care personnel and hydroxychloroquine for postexposure prophylaxis, authors said.
In results from one of those randomized trials, just reported, a higher dose of chloroquine was associated with a cardiac adverse event and an increased mortality risk, leading to the closure of that study arm. In the parallel, double-blinded, phase IIb clinical trial, patients in Brazil with SARS-CoV-2 infection received low or high doses of chloroquine plus ceftriaxone and azithromycin. According to the preprint publication, a higher rate of heart rate–corrected QT interval (QTc) prolongation and a “trend toward higher lethality” was observed in the high-dose group, leading investigators to “strongly recommend” the higher dose be abandoned.
“No apparent benefit of chloroquine was seen regarding lethality in our patients so far, but we will still enroll patients in the low chloroquine dose group to complete the originally planned sample size,” said investigators of the study, which at the time of the report had enrolled 81 out of an anticipated 440 patients.
Other COVID-19 pharmacologic therapies under study
Treatments of note in the review included the following:
- Tocilizumab. This monoclonal antibody IL-6 receptor antagonist, approved by the FDA for treatment of rheumatoid arthritis and for cytokine release syndrome related to chimeric antigen receptor (CAR) T-cell therapy, has yielded success in small series of patients with severe cases of COVID-19, according to authors. In one 21-patient report, 91% had clinical improvement, usually after a single dose. In China, tocilizumab is included in COVID-19 treatment guidelines, and several randomized clinical trials are underway in China including patients with COVID-19 with severe pneumonia.
- Immunoglobulin therapy. Antibodies from recovered COVID-19 patients could help with free virus and infected cell immune clearance, the authors said, adding that further studies are warranted beyond a few small published case series that suggest promise. Furthermore, on March 24 the FDA released guidance for screening donors for COVID-19 convalescent plasma and on emergency investigational new drug applications based on this modality.
- Lopinavir/ritonavir. Despite demonstrated in vitro activity against other novel coronaviruses, there is no published in vitro data for lopinavir/ritonavir in SARS-CoV-2, and likely a “limited role” for this combination anticipated in treating COVID-19, according to the review authors. In an open-label randomized clinical trial published in the New England Journal of Medicine (2020 Mar 18. doi: 10.1056/NEJMoa2001282), there were no differences in clinical improvement, viral clearance, or mortality for antiviral treatment versus standard care. Delayed treatment initiation may explain the ineffectiveness, though a subgroup analysis didn’t show a shorter time to clinical improvement for those who got the treatment earlier.
- Ribavirin. Likewise, this antiviral medication has efficacy and safety data suggesting “limited value” for treatment of COVID-19. Treatment of SARS yielded “inconclusive results” for ribavirin, which was also associated with substantial toxicity that included hemolytic anemia in 60% of SARS patients.
- Oseltamivir. While it may treat influenza, it has no documented activity against SARS-CoV-2 in vitro: “This agent has no role in the management of COVID-19 once influenza has been excluded,” said Dr. Sanders and coauthors.
- Corticosteroids. They could decrease inflammatory responses in the lung, but they could also lead to delays in viral clearance and increases in secondary infection risk. Guidelines for COVID-19 say to avoid corticosteroids, and the authors of the review concur, saying that potential harms and lack of proven benefit mean they usually should not be used outside of a randomized clinical trial setting.
- Vaccines. Clearly, vaccines represent the “most effective long-term strategy” to prevent future COVID-19 outbreaks, though at least 12-18 months would be required until vaccines can be widely deployed, authors said.
Dr. Sanders reported no potential conflicts. Senior author James B. Cutrell, MD, also of the University of Texas Southwestern Medical Center, reported nonfinancial support from Gilead and Regeneron outside of the study. No other authors reported disclosures.
FROM JAMA
Troponins touted as ‘ally’ in COVID-19 triage, but message is nuanced
, cardiologists in the United Kingdom advise in a recently published viewpoint.
The tests can be used to “inform the triage of patients to critical care, guide the use of supportive treatments, and facilitate targeted cardiac investigations in those most likely to benefit,” Nicholas Mills, MD, PhD, University of Edinburgh, United Kingdom, told theheart.org | Medscape Cardiology. He is senior author on the viewpoint published online April 6 in the journal Circulation.
Older adults and those with a history of underlying cardiovascular disease appear to be at greatest risk of dying from COVID-19. “From early reports it is clear that elevated cardiac troponin concentrations predict in-hospital mortality,” said Mills.
In a recent report on hospitalized patients with COVID-19 in Wuhan, China, for example, cardiac injury (hs-cTn above the 99th-percentile upper reference limit) was seen in 1 in 5 patients and was an independent predictor of dying in the hospital. Mortality was 10-fold higher in those with cardiac injury on presentation.
Elevated cardiac troponin in the setting of COVID-19, Mills said, “may reflect illness severity with myocardial injury arising due to myocardial oxygen supply–demand imbalance. Or it may be due to direct cardiac involvement through viral myocarditis or stress cardiomyopathy, or where the prothrombotic and proinflammatory state is precipitating acute coronary syndromes.”
In their viewpoint, the authors note that circulating cTn is a marker of myocardial injury, “including but not limited to myocardial infarction or myocarditis, and the clinical relevance of this distinction has never been so clear.”
Therefore, the consequence of not measuring cardiac troponin may be to “ignore the plethora of ischemic and nonischemic causes” of myocardial injury related to COVID-19. “Clinicians who have used troponin measurement as a binary test for myocardial infarction independent of clinical context and those who consider an elevated cardiac troponin concentration to be a mandate for invasive coronary angiography must recalibrate,” they write.
“Rather than encouraging avoidance of troponin testing, we must harness the unheralded engagement from the cardiovascular community due to COVID-19 to better understand the utility of this essential biomarker and to educate clinicians on its interpretation and implications for prognosis and clinical decision making.”
Based on “same logic” as recent ACC guidance
The viewpoint was to some extent a response to a recent informal guidance from the American College of Cardiology (ACC) that advised caution in use of troponin and natriuretic peptide tests in patients with COVID-19.
Even so, that ACC guidance and the new viewpoint in Circulation are based on the “same logic,” James Januzzi Jr, MD, Massachusetts General Hospital, Boston, told theheart.org | Medscape Cardiology. Both documents:
- Point out that troponins are frequently abnormal in patients with severe cases of COVID-19
- Caution that clinicians should not equate an abnormal hs-cTn with acute myocardial infarction
- Note that, in most cases, hs-cTn elevations are a result of noncoronary mechanisms
- Recognize the potential risk to caregivers and the continued unchecked spread of SARS-CoV-2 related to downstream testing that might not be needed
“The Circulation opinion piece states that clinicians often use troponin as a binary test for myocardial infarction and a mandate for downstream testing, suggesting clinicians will need to recalibrate that approach, something I agree with and which is the central message of the ACC position,” Januzzi said.
Probably the biggest difference between the two documents, he said, is in the Circulation authors’ apparent enthusiasm to use hs-cTn as a tool to judge disease severity in patients with COVID-19.
It’s been known for more than a decade that myocardial injury is “an important risk predictor” in critical illness, Januzzi explained. “So the link between cardiac injury and outcomes in critical illness is nothing new. The difference is the fact we are seeing so many patients with COVID-19 all at once, and the authors suggest that using troponin might help in triage decision making.”
“There may be [such] a role here, but the data have not been systematically collected, and whether troponin truly adds something beyond information already available at the bedside — for example, does it add anything not already obvious at the bedside? — has not yet been conclusively proven,” Januzzi cautioned.
“As well, there are no prospective data supporting troponin as a trigger for ICU triage or for deciding on specific treatments.”
Positive cTn status “common” in COVID-19 patients
In his experience, Barry Cohen, MD, Morristown Medical Center, New Jersey, told theheart.org | Medscape Cardiology, that positive cTn status is “common in COVID-19 patients and appears to have prognostic value, not only in type 1 MI due to atherothrombotic disease (related to a proinflammatory and prothrombotic state), but more frequently type 2 MI (supply–demand mismatch), viral myocarditis, coronary microvascular ischemia, stress cardiomyopathy or tachyarrhythmias.”
Moreover, Cohen said, hs-cTn “has identified patients at increased risk for ventilation support (invasive and noninvasive), acute respiratory distress syndrome, acute kidney injury, and mortality.”
Echoing both the ACC document and the Circulation report, Cohen also said hs-cTn measurements “appear to help risk stratify COVID-19 patients, but clearly do not mean that a troponin-positive patient needs to go to the cath lab and be treated as having acute coronary syndrome. Only a minority of these patients require this intervention.”
Mills discloses receiving honoraria from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. Januzzi has previously disclosed receiving personal fees from the American College of Cardiology, Pfizer, Merck, AbbVie, Amgen, Boehringer Ingelheim, and Takeda; grants and personal fees from Novartis, Roche, Abbott, and Janssen; and grants from Singulex and Prevencio. Cohen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, cardiologists in the United Kingdom advise in a recently published viewpoint.
The tests can be used to “inform the triage of patients to critical care, guide the use of supportive treatments, and facilitate targeted cardiac investigations in those most likely to benefit,” Nicholas Mills, MD, PhD, University of Edinburgh, United Kingdom, told theheart.org | Medscape Cardiology. He is senior author on the viewpoint published online April 6 in the journal Circulation.
Older adults and those with a history of underlying cardiovascular disease appear to be at greatest risk of dying from COVID-19. “From early reports it is clear that elevated cardiac troponin concentrations predict in-hospital mortality,” said Mills.
In a recent report on hospitalized patients with COVID-19 in Wuhan, China, for example, cardiac injury (hs-cTn above the 99th-percentile upper reference limit) was seen in 1 in 5 patients and was an independent predictor of dying in the hospital. Mortality was 10-fold higher in those with cardiac injury on presentation.
Elevated cardiac troponin in the setting of COVID-19, Mills said, “may reflect illness severity with myocardial injury arising due to myocardial oxygen supply–demand imbalance. Or it may be due to direct cardiac involvement through viral myocarditis or stress cardiomyopathy, or where the prothrombotic and proinflammatory state is precipitating acute coronary syndromes.”
In their viewpoint, the authors note that circulating cTn is a marker of myocardial injury, “including but not limited to myocardial infarction or myocarditis, and the clinical relevance of this distinction has never been so clear.”
Therefore, the consequence of not measuring cardiac troponin may be to “ignore the plethora of ischemic and nonischemic causes” of myocardial injury related to COVID-19. “Clinicians who have used troponin measurement as a binary test for myocardial infarction independent of clinical context and those who consider an elevated cardiac troponin concentration to be a mandate for invasive coronary angiography must recalibrate,” they write.
“Rather than encouraging avoidance of troponin testing, we must harness the unheralded engagement from the cardiovascular community due to COVID-19 to better understand the utility of this essential biomarker and to educate clinicians on its interpretation and implications for prognosis and clinical decision making.”
Based on “same logic” as recent ACC guidance
The viewpoint was to some extent a response to a recent informal guidance from the American College of Cardiology (ACC) that advised caution in use of troponin and natriuretic peptide tests in patients with COVID-19.
Even so, that ACC guidance and the new viewpoint in Circulation are based on the “same logic,” James Januzzi Jr, MD, Massachusetts General Hospital, Boston, told theheart.org | Medscape Cardiology. Both documents:
- Point out that troponins are frequently abnormal in patients with severe cases of COVID-19
- Caution that clinicians should not equate an abnormal hs-cTn with acute myocardial infarction
- Note that, in most cases, hs-cTn elevations are a result of noncoronary mechanisms
- Recognize the potential risk to caregivers and the continued unchecked spread of SARS-CoV-2 related to downstream testing that might not be needed
“The Circulation opinion piece states that clinicians often use troponin as a binary test for myocardial infarction and a mandate for downstream testing, suggesting clinicians will need to recalibrate that approach, something I agree with and which is the central message of the ACC position,” Januzzi said.
Probably the biggest difference between the two documents, he said, is in the Circulation authors’ apparent enthusiasm to use hs-cTn as a tool to judge disease severity in patients with COVID-19.
It’s been known for more than a decade that myocardial injury is “an important risk predictor” in critical illness, Januzzi explained. “So the link between cardiac injury and outcomes in critical illness is nothing new. The difference is the fact we are seeing so many patients with COVID-19 all at once, and the authors suggest that using troponin might help in triage decision making.”
“There may be [such] a role here, but the data have not been systematically collected, and whether troponin truly adds something beyond information already available at the bedside — for example, does it add anything not already obvious at the bedside? — has not yet been conclusively proven,” Januzzi cautioned.
“As well, there are no prospective data supporting troponin as a trigger for ICU triage or for deciding on specific treatments.”
Positive cTn status “common” in COVID-19 patients
In his experience, Barry Cohen, MD, Morristown Medical Center, New Jersey, told theheart.org | Medscape Cardiology, that positive cTn status is “common in COVID-19 patients and appears to have prognostic value, not only in type 1 MI due to atherothrombotic disease (related to a proinflammatory and prothrombotic state), but more frequently type 2 MI (supply–demand mismatch), viral myocarditis, coronary microvascular ischemia, stress cardiomyopathy or tachyarrhythmias.”
Moreover, Cohen said, hs-cTn “has identified patients at increased risk for ventilation support (invasive and noninvasive), acute respiratory distress syndrome, acute kidney injury, and mortality.”
Echoing both the ACC document and the Circulation report, Cohen also said hs-cTn measurements “appear to help risk stratify COVID-19 patients, but clearly do not mean that a troponin-positive patient needs to go to the cath lab and be treated as having acute coronary syndrome. Only a minority of these patients require this intervention.”
Mills discloses receiving honoraria from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. Januzzi has previously disclosed receiving personal fees from the American College of Cardiology, Pfizer, Merck, AbbVie, Amgen, Boehringer Ingelheim, and Takeda; grants and personal fees from Novartis, Roche, Abbott, and Janssen; and grants from Singulex and Prevencio. Cohen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, cardiologists in the United Kingdom advise in a recently published viewpoint.
The tests can be used to “inform the triage of patients to critical care, guide the use of supportive treatments, and facilitate targeted cardiac investigations in those most likely to benefit,” Nicholas Mills, MD, PhD, University of Edinburgh, United Kingdom, told theheart.org | Medscape Cardiology. He is senior author on the viewpoint published online April 6 in the journal Circulation.
Older adults and those with a history of underlying cardiovascular disease appear to be at greatest risk of dying from COVID-19. “From early reports it is clear that elevated cardiac troponin concentrations predict in-hospital mortality,” said Mills.
In a recent report on hospitalized patients with COVID-19 in Wuhan, China, for example, cardiac injury (hs-cTn above the 99th-percentile upper reference limit) was seen in 1 in 5 patients and was an independent predictor of dying in the hospital. Mortality was 10-fold higher in those with cardiac injury on presentation.
Elevated cardiac troponin in the setting of COVID-19, Mills said, “may reflect illness severity with myocardial injury arising due to myocardial oxygen supply–demand imbalance. Or it may be due to direct cardiac involvement through viral myocarditis or stress cardiomyopathy, or where the prothrombotic and proinflammatory state is precipitating acute coronary syndromes.”
In their viewpoint, the authors note that circulating cTn is a marker of myocardial injury, “including but not limited to myocardial infarction or myocarditis, and the clinical relevance of this distinction has never been so clear.”
Therefore, the consequence of not measuring cardiac troponin may be to “ignore the plethora of ischemic and nonischemic causes” of myocardial injury related to COVID-19. “Clinicians who have used troponin measurement as a binary test for myocardial infarction independent of clinical context and those who consider an elevated cardiac troponin concentration to be a mandate for invasive coronary angiography must recalibrate,” they write.
“Rather than encouraging avoidance of troponin testing, we must harness the unheralded engagement from the cardiovascular community due to COVID-19 to better understand the utility of this essential biomarker and to educate clinicians on its interpretation and implications for prognosis and clinical decision making.”
Based on “same logic” as recent ACC guidance
The viewpoint was to some extent a response to a recent informal guidance from the American College of Cardiology (ACC) that advised caution in use of troponin and natriuretic peptide tests in patients with COVID-19.
Even so, that ACC guidance and the new viewpoint in Circulation are based on the “same logic,” James Januzzi Jr, MD, Massachusetts General Hospital, Boston, told theheart.org | Medscape Cardiology. Both documents:
- Point out that troponins are frequently abnormal in patients with severe cases of COVID-19
- Caution that clinicians should not equate an abnormal hs-cTn with acute myocardial infarction
- Note that, in most cases, hs-cTn elevations are a result of noncoronary mechanisms
- Recognize the potential risk to caregivers and the continued unchecked spread of SARS-CoV-2 related to downstream testing that might not be needed
“The Circulation opinion piece states that clinicians often use troponin as a binary test for myocardial infarction and a mandate for downstream testing, suggesting clinicians will need to recalibrate that approach, something I agree with and which is the central message of the ACC position,” Januzzi said.
Probably the biggest difference between the two documents, he said, is in the Circulation authors’ apparent enthusiasm to use hs-cTn as a tool to judge disease severity in patients with COVID-19.
It’s been known for more than a decade that myocardial injury is “an important risk predictor” in critical illness, Januzzi explained. “So the link between cardiac injury and outcomes in critical illness is nothing new. The difference is the fact we are seeing so many patients with COVID-19 all at once, and the authors suggest that using troponin might help in triage decision making.”
“There may be [such] a role here, but the data have not been systematically collected, and whether troponin truly adds something beyond information already available at the bedside — for example, does it add anything not already obvious at the bedside? — has not yet been conclusively proven,” Januzzi cautioned.
“As well, there are no prospective data supporting troponin as a trigger for ICU triage or for deciding on specific treatments.”
Positive cTn status “common” in COVID-19 patients
In his experience, Barry Cohen, MD, Morristown Medical Center, New Jersey, told theheart.org | Medscape Cardiology, that positive cTn status is “common in COVID-19 patients and appears to have prognostic value, not only in type 1 MI due to atherothrombotic disease (related to a proinflammatory and prothrombotic state), but more frequently type 2 MI (supply–demand mismatch), viral myocarditis, coronary microvascular ischemia, stress cardiomyopathy or tachyarrhythmias.”
Moreover, Cohen said, hs-cTn “has identified patients at increased risk for ventilation support (invasive and noninvasive), acute respiratory distress syndrome, acute kidney injury, and mortality.”
Echoing both the ACC document and the Circulation report, Cohen also said hs-cTn measurements “appear to help risk stratify COVID-19 patients, but clearly do not mean that a troponin-positive patient needs to go to the cath lab and be treated as having acute coronary syndrome. Only a minority of these patients require this intervention.”
Mills discloses receiving honoraria from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. Januzzi has previously disclosed receiving personal fees from the American College of Cardiology, Pfizer, Merck, AbbVie, Amgen, Boehringer Ingelheim, and Takeda; grants and personal fees from Novartis, Roche, Abbott, and Janssen; and grants from Singulex and Prevencio. Cohen has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.