User login
Vitamin E acetate confirmed as likely source of EVALI
Vitamin E acetate was found in fluid from the lungs of 94% of patients with electronic cigarette, or vaping, product use–associated lung injury, data from a convenience sample of 51 patients indicate. The findings were published in the New England Journal of Medicine.
Cases of electronic cigarette, or vaping, product use–associated lung injury (EVALI) were reported to the Centers for Disease Control and Prevention starting in early 2019, and numbers rose throughout the year, “which suggests new or increased exposure to one or more toxicants from the use of e-cigarette products,” wrote Benjamin C. Blount, PhD, of the National Center for Environmental Health at the CDC, and colleagues.
To further investigate potential toxins in patients with EVALI, the researchers examined bronchoalveolar-lavage (BAL) fluid from 51 EVALI patients and 99 healthy controls.
After the researchers used isotope dilution mass spectrometry on the samples, 48 of the 51 patients (94%) showed vitamin E acetate in their BAL samples. No other potential toxins – including plant oils, medium-chain triglyceride oil, petroleum distillates, and diluent terpenes – were identified. The samples of one patient each showed coconut oil and limonene.
A total of 47 of 51 patients for whom complete laboratory data were available either reported vaping tetrahydrocannabinol products within 90 days of becoming ill, or showed tetrahydrocannabinol or its metabolites in their BAL fluid. In addition, 30 of 47 patients showed nicotine or nicotine metabolites in their BAL fluid.
The average age of the patients was 23 years, 69% were male. Overall, 25 were confirmed EVALI cases and 26 were probable cases, and probable cases included the three patients who showed no vitamin E acetate.
The safety of inhaling vitamin E acetate, which is a common ingredient in dietary supplements and skin care creams, has not been well studied. It could contribute to lung injury when heated in e-cigarette products by splitting the acetate to create the reactive compound and potential lung irritant ketene, the researchers said.
The study findings were limited by several factors including the possibility that vitamin E acetate is a marker for exposure to other toxicants, a lack of data on the impact of heating vitamin e acetate, and the inability to assess the timing of the vitamin E acetate exposure compared to BAL sample collection, the researchers noted.
However, the results suggest that vitamin E acetate may play a role in EVALI because of the high detection rate in patients from across the United States, the biologically possible potential for lung injury from vitamin e acetate, and the timing of the rise of EVALI and the use of vitamin E acetate in vaping products, they concluded.
The research was supported by the National Cancer Institute, the FDA Center for Tobacco Products, and The Ohio State University Pelotonia intramural research program. The authors had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2019 Dec 20. doi: 10.1056/NEJMoa1916433.
Vitamin E acetate was found in fluid from the lungs of 94% of patients with electronic cigarette, or vaping, product use–associated lung injury, data from a convenience sample of 51 patients indicate. The findings were published in the New England Journal of Medicine.
Cases of electronic cigarette, or vaping, product use–associated lung injury (EVALI) were reported to the Centers for Disease Control and Prevention starting in early 2019, and numbers rose throughout the year, “which suggests new or increased exposure to one or more toxicants from the use of e-cigarette products,” wrote Benjamin C. Blount, PhD, of the National Center for Environmental Health at the CDC, and colleagues.
To further investigate potential toxins in patients with EVALI, the researchers examined bronchoalveolar-lavage (BAL) fluid from 51 EVALI patients and 99 healthy controls.
After the researchers used isotope dilution mass spectrometry on the samples, 48 of the 51 patients (94%) showed vitamin E acetate in their BAL samples. No other potential toxins – including plant oils, medium-chain triglyceride oil, petroleum distillates, and diluent terpenes – were identified. The samples of one patient each showed coconut oil and limonene.
A total of 47 of 51 patients for whom complete laboratory data were available either reported vaping tetrahydrocannabinol products within 90 days of becoming ill, or showed tetrahydrocannabinol or its metabolites in their BAL fluid. In addition, 30 of 47 patients showed nicotine or nicotine metabolites in their BAL fluid.
The average age of the patients was 23 years, 69% were male. Overall, 25 were confirmed EVALI cases and 26 were probable cases, and probable cases included the three patients who showed no vitamin E acetate.
The safety of inhaling vitamin E acetate, which is a common ingredient in dietary supplements and skin care creams, has not been well studied. It could contribute to lung injury when heated in e-cigarette products by splitting the acetate to create the reactive compound and potential lung irritant ketene, the researchers said.
The study findings were limited by several factors including the possibility that vitamin E acetate is a marker for exposure to other toxicants, a lack of data on the impact of heating vitamin e acetate, and the inability to assess the timing of the vitamin E acetate exposure compared to BAL sample collection, the researchers noted.
However, the results suggest that vitamin E acetate may play a role in EVALI because of the high detection rate in patients from across the United States, the biologically possible potential for lung injury from vitamin e acetate, and the timing of the rise of EVALI and the use of vitamin E acetate in vaping products, they concluded.
The research was supported by the National Cancer Institute, the FDA Center for Tobacco Products, and The Ohio State University Pelotonia intramural research program. The authors had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2019 Dec 20. doi: 10.1056/NEJMoa1916433.
Vitamin E acetate was found in fluid from the lungs of 94% of patients with electronic cigarette, or vaping, product use–associated lung injury, data from a convenience sample of 51 patients indicate. The findings were published in the New England Journal of Medicine.
Cases of electronic cigarette, or vaping, product use–associated lung injury (EVALI) were reported to the Centers for Disease Control and Prevention starting in early 2019, and numbers rose throughout the year, “which suggests new or increased exposure to one or more toxicants from the use of e-cigarette products,” wrote Benjamin C. Blount, PhD, of the National Center for Environmental Health at the CDC, and colleagues.
To further investigate potential toxins in patients with EVALI, the researchers examined bronchoalveolar-lavage (BAL) fluid from 51 EVALI patients and 99 healthy controls.
After the researchers used isotope dilution mass spectrometry on the samples, 48 of the 51 patients (94%) showed vitamin E acetate in their BAL samples. No other potential toxins – including plant oils, medium-chain triglyceride oil, petroleum distillates, and diluent terpenes – were identified. The samples of one patient each showed coconut oil and limonene.
A total of 47 of 51 patients for whom complete laboratory data were available either reported vaping tetrahydrocannabinol products within 90 days of becoming ill, or showed tetrahydrocannabinol or its metabolites in their BAL fluid. In addition, 30 of 47 patients showed nicotine or nicotine metabolites in their BAL fluid.
The average age of the patients was 23 years, 69% were male. Overall, 25 were confirmed EVALI cases and 26 were probable cases, and probable cases included the three patients who showed no vitamin E acetate.
The safety of inhaling vitamin E acetate, which is a common ingredient in dietary supplements and skin care creams, has not been well studied. It could contribute to lung injury when heated in e-cigarette products by splitting the acetate to create the reactive compound and potential lung irritant ketene, the researchers said.
The study findings were limited by several factors including the possibility that vitamin E acetate is a marker for exposure to other toxicants, a lack of data on the impact of heating vitamin e acetate, and the inability to assess the timing of the vitamin E acetate exposure compared to BAL sample collection, the researchers noted.
However, the results suggest that vitamin E acetate may play a role in EVALI because of the high detection rate in patients from across the United States, the biologically possible potential for lung injury from vitamin e acetate, and the timing of the rise of EVALI and the use of vitamin E acetate in vaping products, they concluded.
The research was supported by the National Cancer Institute, the FDA Center for Tobacco Products, and The Ohio State University Pelotonia intramural research program. The authors had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2019 Dec 20. doi: 10.1056/NEJMoa1916433.
FROM NEW ENGLAND JOURNAL OF MEDICINE
A novel communication framework for inpatient pain management
Introducing the VIEW Framework
Case
A 55-year-old male with a history of diabetes mellitus, lumbar degenerative disc disease, and chronic low back pain was admitted overnight with right lower extremity cellulitis. He reported taking oral hydromorphone for chronic pain, but review of the Prescription Drug Monitoring Program (PDMP) revealed multiple short-term prescriptions from various ED providers, as well as monthly prescriptions from a variety of primary care providers.
Throughout the EHR, he is described as manipulative and narcotic-seeking with notation of multiple ED visits for pain. Multiple discharges against medical advice were noted. He was given two doses of IV hydromorphone in the ED and requested that this be continued. He was admitted for IV antibiotics for severe leg pain that he rated 15/10.
Background
The Society of Hospital Medicine published a consensus statement in the Journal of Hospital Medicine in 2018 that included 16 clinical recommendations on the safe use of opioids for the treatment of acute pain in hospitalized adults.1 In regard to communication about pain, clinicians are encouraged to set realistic goals and expectations of opioid therapy, closely monitor response to opioid therapy, and provide education about the side effects and potential risks of opioid therapy for patients and their families.
However, even when these strategies are employed, the social and behavioral complexities of individual patients can contribute to unsatisfactory interactions with health care staff. Because difficult encounters have been linked to provider burnout, enhanced communication strategies can benefit both the patient and physician.2
SHM’s Patient Experience Committee saw an opportunity to provide complementary evidence-based best-practice tips for communication about pain. Specifically, the committee worked collectively to develop a framework that can be applied to more challenging encounters.
The VIEW Framework
VISIT the patient’s chart and your own mental state.
First, visit the patient’s chart to review information relevant to the patient’s pain history. The EHR can be leveraged through filters and search functions to identify encounters, consultations, and notes relevant to pain management.
Look at the prior to admission medication list and active medication list and see if there are discrepancies. The medication administration record (MAR) can help identify adjunctive medications that the patient may be refusing. PDMP data should be screened for signs of aberrant use, including multiple pharmacies, multiple prescribers, short intervals between prescriptions, and serially prescribed, multiple, low-quantity prescriptions.
While documented pain scores can be a marker of patient distress, objective aspects of the patient’s functional status can shed light on how much his/her discomfort impairs day-to-day living. Examples of these measures include nutritional intake, sleep cycle, out of bed activity, and participation with therapy. Lastly, assess for opioid-related side-effects including constipation, decreased respiratory rate, and any notation of over sedation in narrative documentation from ancillary services.
Once this information has been accrued, it is important to take a moment of mindfulness before meeting with the patient. Take steps to minimize interruptions with electronic devices by silencing your pager/cell phone and disengaging from computers/tablets. Some examples of mindfulness-based practices include taking cycles of deep breathing, going for a short walk to appreciate hospital artwork or view points, or focusing on the sensory aspects of washing your hands prior to seeing the patient. Self-reflection on prior meaningful encounters can also help reset your state of mind. These activities can help clear prior subconscious thoughts and frustrations and prepare for the task ahead of you.3
Intense focus and awareness can enhance your recognition of patient distress, increase your ability to engage in active listening, and enable you to be more receptive to verbal and nonverbal cues.2 Additionally, mindful behaviors have been shown to contribute to decreased burnout and improved empathy.4,5
INTERVIEW the patient.
Once you enter the room, introduce yourself to the patient and others who are present. Interview the patient by eliciting subjective information. Use open-ended and nonjudgmental language, and take moments to summarize the patient’s perspective.
Inquire about the patient’s home baseline pain scores and past levels of acceptable function. Further explore the patient’s performance goals related to activities of daily living and quality of life. Ask about any prior history of addiction to any substance, and if needed, discuss your specific concerns related to substance misuse and abuse.
EMPATHIZE with the patient.
Integrate empathy into your interview by validating any frustrations and experience of pain. Identifying with loss of function and quality of life can help you connect with the patient and initiate a therapeutic relationship. Observe both verbal and nonverbal behaviors that reveal signs of emotional discomfort.6 Use open-ended questions to create space and trust for patients to share their feelings.
Pause to summarize the patient’s perspective while acknowledging and validating emotions that he or she may be experiencing such as anxiety, fear, frustration and anger.6 Statements such as “ I know it is frustrating to ... ” or “I can’t imagine what it must feel like to ... ” can help convey empathy. Multiple studies have suggested that enhanced provider empathy and positive messaging can also reduce patient pain and anxiety and increase quality of life.7,8 Empathic responses to negative emotional expressions from patients have also been associated with higher ratings of communication.9
WRAP UP.
Finally, wrap up by aligning expectations with the patient for pain control and summarize your management recommendations. Educate the patient and his/her family on the risks and benefits of recommended therapy as well as the expected course of recovery. Setting shared goals for functionality relevant to the patient’s personal values and quality of life can build connection between you and your patient.
While handing over the patient to the next provider, refrain from using stereotypical language such as “narcotic-seeking patient.” Clearly communicate the management plan and milestones to other team members, such as nurses, physical therapists, and oncoming hospitalists, to maintain consistency. This will help align patients and their care team and may stave off maladaptive patient behaviors such as splitting.
Applying the VIEW framework to the case
Visit
Upon visiting the medical chart, the physician realized that the patient’s opioid use began in his 20s when he injured his back in a traumatic motor vehicle accident. His successful athletic career came to a halt after this injury and opioid dependence ensued.
While reviewing past notes and prescription data via the PDMP, the physician noted that the patient had been visiting many different providers in order to get more pain medications. The most recent prescription was for oral hydromorphone 4 mg every 4 hours as needed, filled 1 week prior to this presentation.
She reviewed his vital signs and found that he had been persistently hypertensive and tachycardic. His nurse mentioned that he appeared to be in severe pain because of facial grimacing with standing and walking.
Prior to entering the patient’s room, the physician took a moment of mindfulness to become aware of her emotional state because she recognized that she was worried this could be a difficult encounter. She considered how hard his life has been and how much emotional and physical pain he might be experiencing. She took a deep breath, silenced her phone, and entered the room.
Interview
The physician sat at the bedside and interviewed the patient using a calm and nonjudgmental tone. It was quickly obvious to her that he was experiencing real pain. His cellulitis appeared severe and was tender to even minimal palpation. She learned that the pain in his leg had been worsening over the past week to the point that it was becoming difficult to ambulate, sleep and perform his daily hygiene routine. He was taking 4 mg tablets of hydromorphone every 2 hours, and he had run out a few days ago. He added that his mood was increasingly depressed, and he had even admitted to occasional suicidal thoughts because the pain was so unbearable.
When asked directly, he admitted that he was worried he was addicted to hydromorphone. He had first received it for low back pain after the motor vehicle accident, and it been refilled multiple times for ongoing pain over the course of a year. Importantly, she also learned that he felt he was often treated as an addict by medical professionals and felt that doctors no longer listened to him or believed him.
Empathize
As the conversation went on, the physician offered empathetic statements, recognizing the way it might feel to have your pain ignored or minimized by doctors. She expressed how frustrating it is to not be able to perform basic functions and how difficult it must be to constantly live in pain.
She said, “I don’t want you to suffer in pain. I care about you and my goal is to treat your pain so that you can return to doing the things in life that you find meaningful.” She also recognized the severity of his depression and discussed with him the role and importance of psychiatric consultation.
Wrap Up
The physician wrapped up the encounter by summarizing her plan to treat the infection and work together with him to treat his pain with the goal that he could ambulate and perform activities of daily living.
She reviewed the side effects of both acute and long-term use of opioids and discussed the risks and benefits. Given the fact that patient was on chronic baseline opioids and also had objective signs of acute pain, she started an initial regimen of hydromorphone 6 mg tablets every 4 hours as needed (a 50% increase over his home dose) and added acetaminophen 1000 mg every 6 hours and ibuprofen 600 mg every 8 hours.
She informed him that she would check on him in the afternoon and that the ultimate plan would be to taper down on his hydromorphone dose each day as his cellulitis improved. She also communicated that bidirectional respect between the patient and care team members was critical to a successful pain management.
Finally, she explained that there was going to be a different doctor covering at night and major changes to the prescription regimen would be deferred to daytime hours.
When she left the room, she summarized the plan with the patient’s nurse and shared a few details about the patient’s difficult past. At the end of the shift, the physician signed out to the overnight team that the patient had objective signs of pain and recommended a visit to the bedside if the patient’s symptoms were reported as worsening.
During his hospital stay, she monitored the patient’s nonverbal responses to movement, participation in physical therapy, and ability to sleep. She tapered the hydromorphone down each day as the patient’s cellulitis improved. At discharge, he was prescribed a 3-day supply of his home dose of hydromorphone and the same acetaminophen and ibuprofen regimen he had been on in the hospital with instructions for tapering. Finally, after coming to an agreement with the patient, she arranged for follow-up in the opioid taper clinic and communicated the plan with the patient’s primary care provider.
Dr. Horman is a hospitalist and assistant professor of medicine at UC San Diego Health. Dr. Richards is a hospitalist and assistant professor of medicine at the University of Nebraska Medical Center in Omaha. Dr. Horman and Dr. Richards note that they wrote this article in collaboration with the Society of Hospital Medicine Patient Experience Committee.
Key points
- Spend adequate time to fully visit patients’ history as it relates to their current pain complaints.
- Review notes and prescription data to better understand past and current pain regimen.
- Be vigilant about taking a mindful moment to visit your thoughts and potential biases.
- Interview patients using a calm tone and nonjudgmental, reassuring words.
- Empathize with patients and validate any frustrations and experience of pain.
- Wrap-up by summarizing your recommendations with patients, their families, the care team, and subsequent providers.
References
1. Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: A Systematic Review of Existing Guidelines. J Hosp Med. 2018;13(4):256-62.
2. An PG et al. (MEM Investigators). Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169(4):410-4.
3. Sanyer O, Fortenberry K. Using Mindfulness Techniques to improve difficult clinical encounters. Am Fam Physician. 2013;87(6):402.
4. Beckman HB et al. The impact of a program in mindful communication on primary care physicians. Acad Med. 2012;87(6):815-8.
5. Krasner MS et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12):1284-93.
6. Dean M, Street R. A 3-Stage model of patient centered communication for addressing cancer patients’ emotional distress. Patient Educ Couns. 2014;94(2):143-8.
7. Howick J et al. Effects of empathic and positive communication in healthcare consultations: A systematic review and meta-analysis. J R Soc Med. 2018;111(7):240-52.
8. Mistiaen P et al. The effect of patient-practitioner communication on pain: A systematic review. Eur J Pain. 2016;20:675-88.
9. Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
Introducing the VIEW Framework
Introducing the VIEW Framework
Case
A 55-year-old male with a history of diabetes mellitus, lumbar degenerative disc disease, and chronic low back pain was admitted overnight with right lower extremity cellulitis. He reported taking oral hydromorphone for chronic pain, but review of the Prescription Drug Monitoring Program (PDMP) revealed multiple short-term prescriptions from various ED providers, as well as monthly prescriptions from a variety of primary care providers.
Throughout the EHR, he is described as manipulative and narcotic-seeking with notation of multiple ED visits for pain. Multiple discharges against medical advice were noted. He was given two doses of IV hydromorphone in the ED and requested that this be continued. He was admitted for IV antibiotics for severe leg pain that he rated 15/10.
Background
The Society of Hospital Medicine published a consensus statement in the Journal of Hospital Medicine in 2018 that included 16 clinical recommendations on the safe use of opioids for the treatment of acute pain in hospitalized adults.1 In regard to communication about pain, clinicians are encouraged to set realistic goals and expectations of opioid therapy, closely monitor response to opioid therapy, and provide education about the side effects and potential risks of opioid therapy for patients and their families.
However, even when these strategies are employed, the social and behavioral complexities of individual patients can contribute to unsatisfactory interactions with health care staff. Because difficult encounters have been linked to provider burnout, enhanced communication strategies can benefit both the patient and physician.2
SHM’s Patient Experience Committee saw an opportunity to provide complementary evidence-based best-practice tips for communication about pain. Specifically, the committee worked collectively to develop a framework that can be applied to more challenging encounters.
The VIEW Framework
VISIT the patient’s chart and your own mental state.
First, visit the patient’s chart to review information relevant to the patient’s pain history. The EHR can be leveraged through filters and search functions to identify encounters, consultations, and notes relevant to pain management.
Look at the prior to admission medication list and active medication list and see if there are discrepancies. The medication administration record (MAR) can help identify adjunctive medications that the patient may be refusing. PDMP data should be screened for signs of aberrant use, including multiple pharmacies, multiple prescribers, short intervals between prescriptions, and serially prescribed, multiple, low-quantity prescriptions.
While documented pain scores can be a marker of patient distress, objective aspects of the patient’s functional status can shed light on how much his/her discomfort impairs day-to-day living. Examples of these measures include nutritional intake, sleep cycle, out of bed activity, and participation with therapy. Lastly, assess for opioid-related side-effects including constipation, decreased respiratory rate, and any notation of over sedation in narrative documentation from ancillary services.
Once this information has been accrued, it is important to take a moment of mindfulness before meeting with the patient. Take steps to minimize interruptions with electronic devices by silencing your pager/cell phone and disengaging from computers/tablets. Some examples of mindfulness-based practices include taking cycles of deep breathing, going for a short walk to appreciate hospital artwork or view points, or focusing on the sensory aspects of washing your hands prior to seeing the patient. Self-reflection on prior meaningful encounters can also help reset your state of mind. These activities can help clear prior subconscious thoughts and frustrations and prepare for the task ahead of you.3
Intense focus and awareness can enhance your recognition of patient distress, increase your ability to engage in active listening, and enable you to be more receptive to verbal and nonverbal cues.2 Additionally, mindful behaviors have been shown to contribute to decreased burnout and improved empathy.4,5
INTERVIEW the patient.
Once you enter the room, introduce yourself to the patient and others who are present. Interview the patient by eliciting subjective information. Use open-ended and nonjudgmental language, and take moments to summarize the patient’s perspective.
Inquire about the patient’s home baseline pain scores and past levels of acceptable function. Further explore the patient’s performance goals related to activities of daily living and quality of life. Ask about any prior history of addiction to any substance, and if needed, discuss your specific concerns related to substance misuse and abuse.
EMPATHIZE with the patient.
Integrate empathy into your interview by validating any frustrations and experience of pain. Identifying with loss of function and quality of life can help you connect with the patient and initiate a therapeutic relationship. Observe both verbal and nonverbal behaviors that reveal signs of emotional discomfort.6 Use open-ended questions to create space and trust for patients to share their feelings.
Pause to summarize the patient’s perspective while acknowledging and validating emotions that he or she may be experiencing such as anxiety, fear, frustration and anger.6 Statements such as “ I know it is frustrating to ... ” or “I can’t imagine what it must feel like to ... ” can help convey empathy. Multiple studies have suggested that enhanced provider empathy and positive messaging can also reduce patient pain and anxiety and increase quality of life.7,8 Empathic responses to negative emotional expressions from patients have also been associated with higher ratings of communication.9
WRAP UP.
Finally, wrap up by aligning expectations with the patient for pain control and summarize your management recommendations. Educate the patient and his/her family on the risks and benefits of recommended therapy as well as the expected course of recovery. Setting shared goals for functionality relevant to the patient’s personal values and quality of life can build connection between you and your patient.
While handing over the patient to the next provider, refrain from using stereotypical language such as “narcotic-seeking patient.” Clearly communicate the management plan and milestones to other team members, such as nurses, physical therapists, and oncoming hospitalists, to maintain consistency. This will help align patients and their care team and may stave off maladaptive patient behaviors such as splitting.
Applying the VIEW framework to the case
Visit
Upon visiting the medical chart, the physician realized that the patient’s opioid use began in his 20s when he injured his back in a traumatic motor vehicle accident. His successful athletic career came to a halt after this injury and opioid dependence ensued.
While reviewing past notes and prescription data via the PDMP, the physician noted that the patient had been visiting many different providers in order to get more pain medications. The most recent prescription was for oral hydromorphone 4 mg every 4 hours as needed, filled 1 week prior to this presentation.
She reviewed his vital signs and found that he had been persistently hypertensive and tachycardic. His nurse mentioned that he appeared to be in severe pain because of facial grimacing with standing and walking.
Prior to entering the patient’s room, the physician took a moment of mindfulness to become aware of her emotional state because she recognized that she was worried this could be a difficult encounter. She considered how hard his life has been and how much emotional and physical pain he might be experiencing. She took a deep breath, silenced her phone, and entered the room.
Interview
The physician sat at the bedside and interviewed the patient using a calm and nonjudgmental tone. It was quickly obvious to her that he was experiencing real pain. His cellulitis appeared severe and was tender to even minimal palpation. She learned that the pain in his leg had been worsening over the past week to the point that it was becoming difficult to ambulate, sleep and perform his daily hygiene routine. He was taking 4 mg tablets of hydromorphone every 2 hours, and he had run out a few days ago. He added that his mood was increasingly depressed, and he had even admitted to occasional suicidal thoughts because the pain was so unbearable.
When asked directly, he admitted that he was worried he was addicted to hydromorphone. He had first received it for low back pain after the motor vehicle accident, and it been refilled multiple times for ongoing pain over the course of a year. Importantly, she also learned that he felt he was often treated as an addict by medical professionals and felt that doctors no longer listened to him or believed him.
Empathize
As the conversation went on, the physician offered empathetic statements, recognizing the way it might feel to have your pain ignored or minimized by doctors. She expressed how frustrating it is to not be able to perform basic functions and how difficult it must be to constantly live in pain.
She said, “I don’t want you to suffer in pain. I care about you and my goal is to treat your pain so that you can return to doing the things in life that you find meaningful.” She also recognized the severity of his depression and discussed with him the role and importance of psychiatric consultation.
Wrap Up
The physician wrapped up the encounter by summarizing her plan to treat the infection and work together with him to treat his pain with the goal that he could ambulate and perform activities of daily living.
She reviewed the side effects of both acute and long-term use of opioids and discussed the risks and benefits. Given the fact that patient was on chronic baseline opioids and also had objective signs of acute pain, she started an initial regimen of hydromorphone 6 mg tablets every 4 hours as needed (a 50% increase over his home dose) and added acetaminophen 1000 mg every 6 hours and ibuprofen 600 mg every 8 hours.
She informed him that she would check on him in the afternoon and that the ultimate plan would be to taper down on his hydromorphone dose each day as his cellulitis improved. She also communicated that bidirectional respect between the patient and care team members was critical to a successful pain management.
Finally, she explained that there was going to be a different doctor covering at night and major changes to the prescription regimen would be deferred to daytime hours.
When she left the room, she summarized the plan with the patient’s nurse and shared a few details about the patient’s difficult past. At the end of the shift, the physician signed out to the overnight team that the patient had objective signs of pain and recommended a visit to the bedside if the patient’s symptoms were reported as worsening.
During his hospital stay, she monitored the patient’s nonverbal responses to movement, participation in physical therapy, and ability to sleep. She tapered the hydromorphone down each day as the patient’s cellulitis improved. At discharge, he was prescribed a 3-day supply of his home dose of hydromorphone and the same acetaminophen and ibuprofen regimen he had been on in the hospital with instructions for tapering. Finally, after coming to an agreement with the patient, she arranged for follow-up in the opioid taper clinic and communicated the plan with the patient’s primary care provider.
Dr. Horman is a hospitalist and assistant professor of medicine at UC San Diego Health. Dr. Richards is a hospitalist and assistant professor of medicine at the University of Nebraska Medical Center in Omaha. Dr. Horman and Dr. Richards note that they wrote this article in collaboration with the Society of Hospital Medicine Patient Experience Committee.
Key points
- Spend adequate time to fully visit patients’ history as it relates to their current pain complaints.
- Review notes and prescription data to better understand past and current pain regimen.
- Be vigilant about taking a mindful moment to visit your thoughts and potential biases.
- Interview patients using a calm tone and nonjudgmental, reassuring words.
- Empathize with patients and validate any frustrations and experience of pain.
- Wrap-up by summarizing your recommendations with patients, their families, the care team, and subsequent providers.
References
1. Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: A Systematic Review of Existing Guidelines. J Hosp Med. 2018;13(4):256-62.
2. An PG et al. (MEM Investigators). Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169(4):410-4.
3. Sanyer O, Fortenberry K. Using Mindfulness Techniques to improve difficult clinical encounters. Am Fam Physician. 2013;87(6):402.
4. Beckman HB et al. The impact of a program in mindful communication on primary care physicians. Acad Med. 2012;87(6):815-8.
5. Krasner MS et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12):1284-93.
6. Dean M, Street R. A 3-Stage model of patient centered communication for addressing cancer patients’ emotional distress. Patient Educ Couns. 2014;94(2):143-8.
7. Howick J et al. Effects of empathic and positive communication in healthcare consultations: A systematic review and meta-analysis. J R Soc Med. 2018;111(7):240-52.
8. Mistiaen P et al. The effect of patient-practitioner communication on pain: A systematic review. Eur J Pain. 2016;20:675-88.
9. Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
Case
A 55-year-old male with a history of diabetes mellitus, lumbar degenerative disc disease, and chronic low back pain was admitted overnight with right lower extremity cellulitis. He reported taking oral hydromorphone for chronic pain, but review of the Prescription Drug Monitoring Program (PDMP) revealed multiple short-term prescriptions from various ED providers, as well as monthly prescriptions from a variety of primary care providers.
Throughout the EHR, he is described as manipulative and narcotic-seeking with notation of multiple ED visits for pain. Multiple discharges against medical advice were noted. He was given two doses of IV hydromorphone in the ED and requested that this be continued. He was admitted for IV antibiotics for severe leg pain that he rated 15/10.
Background
The Society of Hospital Medicine published a consensus statement in the Journal of Hospital Medicine in 2018 that included 16 clinical recommendations on the safe use of opioids for the treatment of acute pain in hospitalized adults.1 In regard to communication about pain, clinicians are encouraged to set realistic goals and expectations of opioid therapy, closely monitor response to opioid therapy, and provide education about the side effects and potential risks of opioid therapy for patients and their families.
However, even when these strategies are employed, the social and behavioral complexities of individual patients can contribute to unsatisfactory interactions with health care staff. Because difficult encounters have been linked to provider burnout, enhanced communication strategies can benefit both the patient and physician.2
SHM’s Patient Experience Committee saw an opportunity to provide complementary evidence-based best-practice tips for communication about pain. Specifically, the committee worked collectively to develop a framework that can be applied to more challenging encounters.
The VIEW Framework
VISIT the patient’s chart and your own mental state.
First, visit the patient’s chart to review information relevant to the patient’s pain history. The EHR can be leveraged through filters and search functions to identify encounters, consultations, and notes relevant to pain management.
Look at the prior to admission medication list and active medication list and see if there are discrepancies. The medication administration record (MAR) can help identify adjunctive medications that the patient may be refusing. PDMP data should be screened for signs of aberrant use, including multiple pharmacies, multiple prescribers, short intervals between prescriptions, and serially prescribed, multiple, low-quantity prescriptions.
While documented pain scores can be a marker of patient distress, objective aspects of the patient’s functional status can shed light on how much his/her discomfort impairs day-to-day living. Examples of these measures include nutritional intake, sleep cycle, out of bed activity, and participation with therapy. Lastly, assess for opioid-related side-effects including constipation, decreased respiratory rate, and any notation of over sedation in narrative documentation from ancillary services.
Once this information has been accrued, it is important to take a moment of mindfulness before meeting with the patient. Take steps to minimize interruptions with electronic devices by silencing your pager/cell phone and disengaging from computers/tablets. Some examples of mindfulness-based practices include taking cycles of deep breathing, going for a short walk to appreciate hospital artwork or view points, or focusing on the sensory aspects of washing your hands prior to seeing the patient. Self-reflection on prior meaningful encounters can also help reset your state of mind. These activities can help clear prior subconscious thoughts and frustrations and prepare for the task ahead of you.3
Intense focus and awareness can enhance your recognition of patient distress, increase your ability to engage in active listening, and enable you to be more receptive to verbal and nonverbal cues.2 Additionally, mindful behaviors have been shown to contribute to decreased burnout and improved empathy.4,5
INTERVIEW the patient.
Once you enter the room, introduce yourself to the patient and others who are present. Interview the patient by eliciting subjective information. Use open-ended and nonjudgmental language, and take moments to summarize the patient’s perspective.
Inquire about the patient’s home baseline pain scores and past levels of acceptable function. Further explore the patient’s performance goals related to activities of daily living and quality of life. Ask about any prior history of addiction to any substance, and if needed, discuss your specific concerns related to substance misuse and abuse.
EMPATHIZE with the patient.
Integrate empathy into your interview by validating any frustrations and experience of pain. Identifying with loss of function and quality of life can help you connect with the patient and initiate a therapeutic relationship. Observe both verbal and nonverbal behaviors that reveal signs of emotional discomfort.6 Use open-ended questions to create space and trust for patients to share their feelings.
Pause to summarize the patient’s perspective while acknowledging and validating emotions that he or she may be experiencing such as anxiety, fear, frustration and anger.6 Statements such as “ I know it is frustrating to ... ” or “I can’t imagine what it must feel like to ... ” can help convey empathy. Multiple studies have suggested that enhanced provider empathy and positive messaging can also reduce patient pain and anxiety and increase quality of life.7,8 Empathic responses to negative emotional expressions from patients have also been associated with higher ratings of communication.9
WRAP UP.
Finally, wrap up by aligning expectations with the patient for pain control and summarize your management recommendations. Educate the patient and his/her family on the risks and benefits of recommended therapy as well as the expected course of recovery. Setting shared goals for functionality relevant to the patient’s personal values and quality of life can build connection between you and your patient.
While handing over the patient to the next provider, refrain from using stereotypical language such as “narcotic-seeking patient.” Clearly communicate the management plan and milestones to other team members, such as nurses, physical therapists, and oncoming hospitalists, to maintain consistency. This will help align patients and their care team and may stave off maladaptive patient behaviors such as splitting.
Applying the VIEW framework to the case
Visit
Upon visiting the medical chart, the physician realized that the patient’s opioid use began in his 20s when he injured his back in a traumatic motor vehicle accident. His successful athletic career came to a halt after this injury and opioid dependence ensued.
While reviewing past notes and prescription data via the PDMP, the physician noted that the patient had been visiting many different providers in order to get more pain medications. The most recent prescription was for oral hydromorphone 4 mg every 4 hours as needed, filled 1 week prior to this presentation.
She reviewed his vital signs and found that he had been persistently hypertensive and tachycardic. His nurse mentioned that he appeared to be in severe pain because of facial grimacing with standing and walking.
Prior to entering the patient’s room, the physician took a moment of mindfulness to become aware of her emotional state because she recognized that she was worried this could be a difficult encounter. She considered how hard his life has been and how much emotional and physical pain he might be experiencing. She took a deep breath, silenced her phone, and entered the room.
Interview
The physician sat at the bedside and interviewed the patient using a calm and nonjudgmental tone. It was quickly obvious to her that he was experiencing real pain. His cellulitis appeared severe and was tender to even minimal palpation. She learned that the pain in his leg had been worsening over the past week to the point that it was becoming difficult to ambulate, sleep and perform his daily hygiene routine. He was taking 4 mg tablets of hydromorphone every 2 hours, and he had run out a few days ago. He added that his mood was increasingly depressed, and he had even admitted to occasional suicidal thoughts because the pain was so unbearable.
When asked directly, he admitted that he was worried he was addicted to hydromorphone. He had first received it for low back pain after the motor vehicle accident, and it been refilled multiple times for ongoing pain over the course of a year. Importantly, she also learned that he felt he was often treated as an addict by medical professionals and felt that doctors no longer listened to him or believed him.
Empathize
As the conversation went on, the physician offered empathetic statements, recognizing the way it might feel to have your pain ignored or minimized by doctors. She expressed how frustrating it is to not be able to perform basic functions and how difficult it must be to constantly live in pain.
She said, “I don’t want you to suffer in pain. I care about you and my goal is to treat your pain so that you can return to doing the things in life that you find meaningful.” She also recognized the severity of his depression and discussed with him the role and importance of psychiatric consultation.
Wrap Up
The physician wrapped up the encounter by summarizing her plan to treat the infection and work together with him to treat his pain with the goal that he could ambulate and perform activities of daily living.
She reviewed the side effects of both acute and long-term use of opioids and discussed the risks and benefits. Given the fact that patient was on chronic baseline opioids and also had objective signs of acute pain, she started an initial regimen of hydromorphone 6 mg tablets every 4 hours as needed (a 50% increase over his home dose) and added acetaminophen 1000 mg every 6 hours and ibuprofen 600 mg every 8 hours.
She informed him that she would check on him in the afternoon and that the ultimate plan would be to taper down on his hydromorphone dose each day as his cellulitis improved. She also communicated that bidirectional respect between the patient and care team members was critical to a successful pain management.
Finally, she explained that there was going to be a different doctor covering at night and major changes to the prescription regimen would be deferred to daytime hours.
When she left the room, she summarized the plan with the patient’s nurse and shared a few details about the patient’s difficult past. At the end of the shift, the physician signed out to the overnight team that the patient had objective signs of pain and recommended a visit to the bedside if the patient’s symptoms were reported as worsening.
During his hospital stay, she monitored the patient’s nonverbal responses to movement, participation in physical therapy, and ability to sleep. She tapered the hydromorphone down each day as the patient’s cellulitis improved. At discharge, he was prescribed a 3-day supply of his home dose of hydromorphone and the same acetaminophen and ibuprofen regimen he had been on in the hospital with instructions for tapering. Finally, after coming to an agreement with the patient, she arranged for follow-up in the opioid taper clinic and communicated the plan with the patient’s primary care provider.
Dr. Horman is a hospitalist and assistant professor of medicine at UC San Diego Health. Dr. Richards is a hospitalist and assistant professor of medicine at the University of Nebraska Medical Center in Omaha. Dr. Horman and Dr. Richards note that they wrote this article in collaboration with the Society of Hospital Medicine Patient Experience Committee.
Key points
- Spend adequate time to fully visit patients’ history as it relates to their current pain complaints.
- Review notes and prescription data to better understand past and current pain regimen.
- Be vigilant about taking a mindful moment to visit your thoughts and potential biases.
- Interview patients using a calm tone and nonjudgmental, reassuring words.
- Empathize with patients and validate any frustrations and experience of pain.
- Wrap-up by summarizing your recommendations with patients, their families, the care team, and subsequent providers.
References
1. Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: A Systematic Review of Existing Guidelines. J Hosp Med. 2018;13(4):256-62.
2. An PG et al. (MEM Investigators). Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169(4):410-4.
3. Sanyer O, Fortenberry K. Using Mindfulness Techniques to improve difficult clinical encounters. Am Fam Physician. 2013;87(6):402.
4. Beckman HB et al. The impact of a program in mindful communication on primary care physicians. Acad Med. 2012;87(6):815-8.
5. Krasner MS et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA. 2009;302(12):1284-93.
6. Dean M, Street R. A 3-Stage model of patient centered communication for addressing cancer patients’ emotional distress. Patient Educ Couns. 2014;94(2):143-8.
7. Howick J et al. Effects of empathic and positive communication in healthcare consultations: A systematic review and meta-analysis. J R Soc Med. 2018;111(7):240-52.
8. Mistiaen P et al. The effect of patient-practitioner communication on pain: A systematic review. Eur J Pain. 2016;20:675-88.
9. Weiss R et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-10.
FDA warns gabapentin, pregabalin may cause serious breathing problems
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
Elderly patients who take these drugs also are at increased risk of breathing problems, the announcement said.
Gabapentin (marketed as Neurontin, Gralise, and Horizant) and pregabalin (Lyrica and Lyrica CR) are used to treat seizures, nerve pain, and restless legs syndrome. Physicians increasingly are prescribing these medications, and people are misusing and abusing these drugs more frequently, the agency said. Gabapentin and pregabalin often are combined with central nervous system depressants such as opioids, antianxiety medicines, antidepressants, and antihistamines, which increases the risk of respiratory depression.
Conditions that reduce lung function, including chronic obstructive pulmonary disease (COPD), also increase the likelihood of breathing problems when taking gabapentin and pregabalin.
“There is less evidence supporting the risk of serious breathing difficulties in healthy individuals taking gabapentinoids alone. We will continue to monitor these medicines as part of our routine monitoring of all FDA-approved drugs,” the announcement said.
The FDA is requiring new warnings about the risk of respiratory depression in the prescribing information of gabapentinoids. In addition, drug manufacturers must further assess the abuse potential of these drugs, particularly in combination with opioids.
Patients and caregivers should seek immediate medical attention for respiratory problems, which can be life threatening. Symptoms include confusion or disorientation; unusual dizziness or lightheadedness; extreme sleepiness or lethargy; slowed, shallow, or difficult breathing; unresponsiveness; and bluish-colored or tinted skin, especially on the lips, fingers, and toes.
Physicians should start gabapentinoids at the lowest dose and monitor patients for symptoms of respiratory depression and sedation when coprescribing these drugs with an opioid or other central nervous system depressant such as a benzodiazepine, according to the FDA.
The agency reviewed 49 case reports that were submitted between 2012 and 2017. Among these cases, 12 people died from respiratory depression with gabapentinoids. All of the patients who died had at least one risk factor.
Gabapentin first was approved in 1993, and pregabalin was approved in 2004. Drug adverse events and side effects can be reported online, the agency noted.
ENGAGE AF-TIMI: Insulin linked to greater risk for stroke, CV death, bleeding
LOS ANGELES – Patients with diabetes had significantly higher adjusted risk of bleeding, cardiovascular-related death, and poorer net outcomes, particularly those treated with insulin, a subanalysis of the ENGAGE AF-TIMI 48 trial has shown.
In addition, the pharmacokinetic and pharmacodynamic profile of the study drug, edoxaban – a novel oral anticoagulant drug and a direct factor Xa inhibitor – was generally similar in patients with and without diabetes.
“We know that atrial fibrillation is associated with a fivefold increased risk of stroke,” Anna Plitt, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Type 2 diabetes is associated with a twofold increased risk of stroke, and longer duration of diabetes is associated with even higher ischemic event rates. The coexistence of [atrial fibrillation] and type 2 diabetes further increases thromboembolic risk.”
Dr. Plitt, a cardiology fellow at Mount Sinai Hospital, New York, noted that, although type 2 diabetes is characterized by a prothrombotic and inflammatory state, the mechanism of action by which hyperglycemia and/or insulin resistance leads to the development of atrial fibrillation (AFib) remains unknown. “Given the complex clinical interactions between AFib and type 2 diabetes, care for these patients remains challenging,” she said. “Recommendations for anticoagulation managements vary based on the presence of additional risk factors and which guidelines are followed.”
In the ENGAGE AF-TIMI 48 trial, 21,105 patients with documented AFib within the previous 12 months were randomized to standard-care warfarin or high-dose edoxaban (60 mg daily) or low-dose edoxaban (30 mg daily). The edoxaban dose was reduced by 50% if creatinine clearance reached 30-50 mL/min, patient weight reached 60 kg or less, or there was concomitant use of a P-glycoprotein inhibitor (N Engl J Med. 2013;369:2093-104). The median follow-up was 2.8 years, and the primary efficacy endpoint was stroke or systemic embolic events (SEEs). The primary safety endpoint was major bleeding, as defined by the International Society on Thrombosis and Haemostasis criteria.
The findings showed that edoxaban was noninferior to warfarin in preventing stroke/SEEs. It also significantly reduced major bleeding, cardiovascular death, and net outcomes. “Therefore, the higher dose of edoxaban was approved globally for treating patients with AFib,” Dr. Plitt said. “The lower-dose regimen was not approved because there was less protection from ischemic stroke, compared with warfarin.”
For the current subanalysis, Dr. Plitt and colleagues set out to further evaluate outcomes of patients enrolled in the ENGAGE AF-TIMI 48 trial, excluding those who were in the low-dose edoxaban group. The presence or absence of diabetes was determined by the local investigator at randomization. The investigators further stratified patients into insulin-treated and non–insulin treated groups and used multivariate Cox regression models to adjust for baseline characteristics across the groups stratified by diabetes status. Next, they analyzed edoxaban concentration, anti–factor Xa activity, and international normalized ratio data and compared outcomes of high-dose edoxaban with those of warfarin.
The primary endpoint and the primary safety endpoint of interest were the same as in the main ENGAGE AF-TIMI 48 trial. Key secondary endpoints included in the subanalysis were cardiovascular death, stroke/SEE, major adverse cardiovascular events (MACE, a composite of myocardial infarction, stroke, SEE, or death because of cardiovascular cause or bleeding), and all-cause death.
In all, 7,624 of the 21,105 patients in the ENGAGE AF-TIMI 48 trial had diabetes, for a rate of 36%. Most of the patients with diabetes did not require insulin (30%), while 6% did. There were fewer female patients with diabetes than without (37% vs. 39%, respectively). Of note was that history of prior stroke/transient ischemic attack was higher in the no-diabetes group than in the diabetes group (33% vs. 21%), as was congestive heart failure (63% vs. 48%).
The mean CHA2DS2-VASc score for predicting thromboembolic risk (0, low risk; greater than 1, high risk) was 4.6 in the diabetes group and 4.2 in the no-diabetes group. When diabetes was not included in the score, the mean CHA2DS2-VASc score was 3.6 in the diabetes group. “Because the trial entry criteria required a minimum CHADS2 score of 2, patients without diabetes were enriched with stroke risk factors other than diabetes,” Dr. Plitt said.
Adjusted outcomes from the subanalysis showed that the risk of stroke/SEE was similar between patients with and without diabetes (hazard ratio, 1.08). However, patients with diabetes were at higher adjusted risk for cardiovascular death than patients without diabetes (HR, 1.29), MACE (HR, 1.28), major bleed (HR, 1.28), and the net outcome of stroke, SEE, major bleed, or all-cause death (HR, 1.25).
The researchers also analyzed the pharmacodynamic and pharmacokinetic data of high-dose edoxaban, stratified by diabetes status. They found that the parameters were generally similar between patients with and without diabetes, including trough concentrations of edoxaban (34.3 and 37.2 ng/mL, respectively; P = .04), trough exogenous anti–factor Xa activity (0.59 and 0.68 IU/mL; P = .11), and the percentage change from baseline in the peak endogenous anti–factor Xa activity (P = .66). The percentage changes from baseline of the trough endogenous anti–factor Xa activity was slightly lower in patients with diabetes, compared with patients without diabetes (P less than .001). “However, these modest differences between the two groups are of unclear clinical significance,” Dr. Plitt said.
Results from the main ENGAGE AF-TIMI 48 showed that the rates of stroke/SEE were reduced by 13% on high-dose edoxaban. However, the subanalysis found no significant effect modification in the reduction in stroke/SEE with edoxaban, compared with warfarin, when stratified by diabetes status (reductions of 16% vs. 7% in the no-diabetes and diabetes groups, respectively; P for interaction = .54). The researchers also observed similar reductions with edoxaban in the risks of secondary outcomes when patients were stratified by diabetes status.
In another finding, patients with diabetes who were treated with insulin were at a higher adjusted risk for all outcomes, compared with those with diabetes who were not treated with insulin. This included stroke/SEE (HR, 1.44), cardiovascular-related death (HR, 1.83), MACE (HR, 1.78), major bleed (HR, 1.31), and net outcome (HR, 1.57).
Next, the researchers compared the study endpoints of high-dose edoxaban and warfarin, with and without insulin. “None of the efficacy, safety, or net outcomes demonstrated evidence of treatment effect modification related to the use of insulin among [patients with diabetes],” she said.
Dr. Plitt disclosed having received honoraria for educational activities from Bristol-Myers Squibb.
LOS ANGELES – Patients with diabetes had significantly higher adjusted risk of bleeding, cardiovascular-related death, and poorer net outcomes, particularly those treated with insulin, a subanalysis of the ENGAGE AF-TIMI 48 trial has shown.
In addition, the pharmacokinetic and pharmacodynamic profile of the study drug, edoxaban – a novel oral anticoagulant drug and a direct factor Xa inhibitor – was generally similar in patients with and without diabetes.
“We know that atrial fibrillation is associated with a fivefold increased risk of stroke,” Anna Plitt, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Type 2 diabetes is associated with a twofold increased risk of stroke, and longer duration of diabetes is associated with even higher ischemic event rates. The coexistence of [atrial fibrillation] and type 2 diabetes further increases thromboembolic risk.”
Dr. Plitt, a cardiology fellow at Mount Sinai Hospital, New York, noted that, although type 2 diabetes is characterized by a prothrombotic and inflammatory state, the mechanism of action by which hyperglycemia and/or insulin resistance leads to the development of atrial fibrillation (AFib) remains unknown. “Given the complex clinical interactions between AFib and type 2 diabetes, care for these patients remains challenging,” she said. “Recommendations for anticoagulation managements vary based on the presence of additional risk factors and which guidelines are followed.”
In the ENGAGE AF-TIMI 48 trial, 21,105 patients with documented AFib within the previous 12 months were randomized to standard-care warfarin or high-dose edoxaban (60 mg daily) or low-dose edoxaban (30 mg daily). The edoxaban dose was reduced by 50% if creatinine clearance reached 30-50 mL/min, patient weight reached 60 kg or less, or there was concomitant use of a P-glycoprotein inhibitor (N Engl J Med. 2013;369:2093-104). The median follow-up was 2.8 years, and the primary efficacy endpoint was stroke or systemic embolic events (SEEs). The primary safety endpoint was major bleeding, as defined by the International Society on Thrombosis and Haemostasis criteria.
The findings showed that edoxaban was noninferior to warfarin in preventing stroke/SEEs. It also significantly reduced major bleeding, cardiovascular death, and net outcomes. “Therefore, the higher dose of edoxaban was approved globally for treating patients with AFib,” Dr. Plitt said. “The lower-dose regimen was not approved because there was less protection from ischemic stroke, compared with warfarin.”
For the current subanalysis, Dr. Plitt and colleagues set out to further evaluate outcomes of patients enrolled in the ENGAGE AF-TIMI 48 trial, excluding those who were in the low-dose edoxaban group. The presence or absence of diabetes was determined by the local investigator at randomization. The investigators further stratified patients into insulin-treated and non–insulin treated groups and used multivariate Cox regression models to adjust for baseline characteristics across the groups stratified by diabetes status. Next, they analyzed edoxaban concentration, anti–factor Xa activity, and international normalized ratio data and compared outcomes of high-dose edoxaban with those of warfarin.
The primary endpoint and the primary safety endpoint of interest were the same as in the main ENGAGE AF-TIMI 48 trial. Key secondary endpoints included in the subanalysis were cardiovascular death, stroke/SEE, major adverse cardiovascular events (MACE, a composite of myocardial infarction, stroke, SEE, or death because of cardiovascular cause or bleeding), and all-cause death.
In all, 7,624 of the 21,105 patients in the ENGAGE AF-TIMI 48 trial had diabetes, for a rate of 36%. Most of the patients with diabetes did not require insulin (30%), while 6% did. There were fewer female patients with diabetes than without (37% vs. 39%, respectively). Of note was that history of prior stroke/transient ischemic attack was higher in the no-diabetes group than in the diabetes group (33% vs. 21%), as was congestive heart failure (63% vs. 48%).
The mean CHA2DS2-VASc score for predicting thromboembolic risk (0, low risk; greater than 1, high risk) was 4.6 in the diabetes group and 4.2 in the no-diabetes group. When diabetes was not included in the score, the mean CHA2DS2-VASc score was 3.6 in the diabetes group. “Because the trial entry criteria required a minimum CHADS2 score of 2, patients without diabetes were enriched with stroke risk factors other than diabetes,” Dr. Plitt said.
Adjusted outcomes from the subanalysis showed that the risk of stroke/SEE was similar between patients with and without diabetes (hazard ratio, 1.08). However, patients with diabetes were at higher adjusted risk for cardiovascular death than patients without diabetes (HR, 1.29), MACE (HR, 1.28), major bleed (HR, 1.28), and the net outcome of stroke, SEE, major bleed, or all-cause death (HR, 1.25).
The researchers also analyzed the pharmacodynamic and pharmacokinetic data of high-dose edoxaban, stratified by diabetes status. They found that the parameters were generally similar between patients with and without diabetes, including trough concentrations of edoxaban (34.3 and 37.2 ng/mL, respectively; P = .04), trough exogenous anti–factor Xa activity (0.59 and 0.68 IU/mL; P = .11), and the percentage change from baseline in the peak endogenous anti–factor Xa activity (P = .66). The percentage changes from baseline of the trough endogenous anti–factor Xa activity was slightly lower in patients with diabetes, compared with patients without diabetes (P less than .001). “However, these modest differences between the two groups are of unclear clinical significance,” Dr. Plitt said.
Results from the main ENGAGE AF-TIMI 48 showed that the rates of stroke/SEE were reduced by 13% on high-dose edoxaban. However, the subanalysis found no significant effect modification in the reduction in stroke/SEE with edoxaban, compared with warfarin, when stratified by diabetes status (reductions of 16% vs. 7% in the no-diabetes and diabetes groups, respectively; P for interaction = .54). The researchers also observed similar reductions with edoxaban in the risks of secondary outcomes when patients were stratified by diabetes status.
In another finding, patients with diabetes who were treated with insulin were at a higher adjusted risk for all outcomes, compared with those with diabetes who were not treated with insulin. This included stroke/SEE (HR, 1.44), cardiovascular-related death (HR, 1.83), MACE (HR, 1.78), major bleed (HR, 1.31), and net outcome (HR, 1.57).
Next, the researchers compared the study endpoints of high-dose edoxaban and warfarin, with and without insulin. “None of the efficacy, safety, or net outcomes demonstrated evidence of treatment effect modification related to the use of insulin among [patients with diabetes],” she said.
Dr. Plitt disclosed having received honoraria for educational activities from Bristol-Myers Squibb.
LOS ANGELES – Patients with diabetes had significantly higher adjusted risk of bleeding, cardiovascular-related death, and poorer net outcomes, particularly those treated with insulin, a subanalysis of the ENGAGE AF-TIMI 48 trial has shown.
In addition, the pharmacokinetic and pharmacodynamic profile of the study drug, edoxaban – a novel oral anticoagulant drug and a direct factor Xa inhibitor – was generally similar in patients with and without diabetes.
“We know that atrial fibrillation is associated with a fivefold increased risk of stroke,” Anna Plitt, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “Type 2 diabetes is associated with a twofold increased risk of stroke, and longer duration of diabetes is associated with even higher ischemic event rates. The coexistence of [atrial fibrillation] and type 2 diabetes further increases thromboembolic risk.”
Dr. Plitt, a cardiology fellow at Mount Sinai Hospital, New York, noted that, although type 2 diabetes is characterized by a prothrombotic and inflammatory state, the mechanism of action by which hyperglycemia and/or insulin resistance leads to the development of atrial fibrillation (AFib) remains unknown. “Given the complex clinical interactions between AFib and type 2 diabetes, care for these patients remains challenging,” she said. “Recommendations for anticoagulation managements vary based on the presence of additional risk factors and which guidelines are followed.”
In the ENGAGE AF-TIMI 48 trial, 21,105 patients with documented AFib within the previous 12 months were randomized to standard-care warfarin or high-dose edoxaban (60 mg daily) or low-dose edoxaban (30 mg daily). The edoxaban dose was reduced by 50% if creatinine clearance reached 30-50 mL/min, patient weight reached 60 kg or less, or there was concomitant use of a P-glycoprotein inhibitor (N Engl J Med. 2013;369:2093-104). The median follow-up was 2.8 years, and the primary efficacy endpoint was stroke or systemic embolic events (SEEs). The primary safety endpoint was major bleeding, as defined by the International Society on Thrombosis and Haemostasis criteria.
The findings showed that edoxaban was noninferior to warfarin in preventing stroke/SEEs. It also significantly reduced major bleeding, cardiovascular death, and net outcomes. “Therefore, the higher dose of edoxaban was approved globally for treating patients with AFib,” Dr. Plitt said. “The lower-dose regimen was not approved because there was less protection from ischemic stroke, compared with warfarin.”
For the current subanalysis, Dr. Plitt and colleagues set out to further evaluate outcomes of patients enrolled in the ENGAGE AF-TIMI 48 trial, excluding those who were in the low-dose edoxaban group. The presence or absence of diabetes was determined by the local investigator at randomization. The investigators further stratified patients into insulin-treated and non–insulin treated groups and used multivariate Cox regression models to adjust for baseline characteristics across the groups stratified by diabetes status. Next, they analyzed edoxaban concentration, anti–factor Xa activity, and international normalized ratio data and compared outcomes of high-dose edoxaban with those of warfarin.
The primary endpoint and the primary safety endpoint of interest were the same as in the main ENGAGE AF-TIMI 48 trial. Key secondary endpoints included in the subanalysis were cardiovascular death, stroke/SEE, major adverse cardiovascular events (MACE, a composite of myocardial infarction, stroke, SEE, or death because of cardiovascular cause or bleeding), and all-cause death.
In all, 7,624 of the 21,105 patients in the ENGAGE AF-TIMI 48 trial had diabetes, for a rate of 36%. Most of the patients with diabetes did not require insulin (30%), while 6% did. There were fewer female patients with diabetes than without (37% vs. 39%, respectively). Of note was that history of prior stroke/transient ischemic attack was higher in the no-diabetes group than in the diabetes group (33% vs. 21%), as was congestive heart failure (63% vs. 48%).
The mean CHA2DS2-VASc score for predicting thromboembolic risk (0, low risk; greater than 1, high risk) was 4.6 in the diabetes group and 4.2 in the no-diabetes group. When diabetes was not included in the score, the mean CHA2DS2-VASc score was 3.6 in the diabetes group. “Because the trial entry criteria required a minimum CHADS2 score of 2, patients without diabetes were enriched with stroke risk factors other than diabetes,” Dr. Plitt said.
Adjusted outcomes from the subanalysis showed that the risk of stroke/SEE was similar between patients with and without diabetes (hazard ratio, 1.08). However, patients with diabetes were at higher adjusted risk for cardiovascular death than patients without diabetes (HR, 1.29), MACE (HR, 1.28), major bleed (HR, 1.28), and the net outcome of stroke, SEE, major bleed, or all-cause death (HR, 1.25).
The researchers also analyzed the pharmacodynamic and pharmacokinetic data of high-dose edoxaban, stratified by diabetes status. They found that the parameters were generally similar between patients with and without diabetes, including trough concentrations of edoxaban (34.3 and 37.2 ng/mL, respectively; P = .04), trough exogenous anti–factor Xa activity (0.59 and 0.68 IU/mL; P = .11), and the percentage change from baseline in the peak endogenous anti–factor Xa activity (P = .66). The percentage changes from baseline of the trough endogenous anti–factor Xa activity was slightly lower in patients with diabetes, compared with patients without diabetes (P less than .001). “However, these modest differences between the two groups are of unclear clinical significance,” Dr. Plitt said.
Results from the main ENGAGE AF-TIMI 48 showed that the rates of stroke/SEE were reduced by 13% on high-dose edoxaban. However, the subanalysis found no significant effect modification in the reduction in stroke/SEE with edoxaban, compared with warfarin, when stratified by diabetes status (reductions of 16% vs. 7% in the no-diabetes and diabetes groups, respectively; P for interaction = .54). The researchers also observed similar reductions with edoxaban in the risks of secondary outcomes when patients were stratified by diabetes status.
In another finding, patients with diabetes who were treated with insulin were at a higher adjusted risk for all outcomes, compared with those with diabetes who were not treated with insulin. This included stroke/SEE (HR, 1.44), cardiovascular-related death (HR, 1.83), MACE (HR, 1.78), major bleed (HR, 1.31), and net outcome (HR, 1.57).
Next, the researchers compared the study endpoints of high-dose edoxaban and warfarin, with and without insulin. “None of the efficacy, safety, or net outcomes demonstrated evidence of treatment effect modification related to the use of insulin among [patients with diabetes],” she said.
Dr. Plitt disclosed having received honoraria for educational activities from Bristol-Myers Squibb.
REPORTING FROM THE WCIRDC 2019
Improving sepsis-related outcomes
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Early diagnosis a key goal
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
New ASH guideline: VTE prophylaxis after major surgery
ORLANDO – The latest American Society of Hematology guideline on venous thromboembolism (VTE) tackles 30 key questions regarding prophylaxis in hospitalized patients undergoing surgery, according to the chair of the guideline panel, who highlighted 9 of those questions during a special session at the society’s annual meeting.
The clinical practice guideline, published just about a week before the annual meeting of the American Society of Hematology, focuses mainly on pharmacologic prophylaxis in specific surgical settings, said David R. Anderson, MD, dean of the faculty of medicine of Dalhousie University, Halifax, N.S.
“Our guidelines focused upon clinically important symptomatic outcomes, with less emphasis being placed on asymptomatic deep vein thrombosis detected by screening tests,” Dr. Anderson said.
At the special education session, Dr. Anderson highlighted several specific recommendations on prophylaxis in surgical patients.
Pharmacologic prophylaxis is not recommended for patients experiencing major trauma deemed to be at high risk of bleeding. Its use does reduce risk of symptomatic pulmonary embolism (PE) and deep vein thrombosis (DVT) by about 10 events per 1,000 patients treated; however, Dr. Anderson said, the panel’s opinion was that this benefit was outweighed by increased risk of major bleeding, at 24 events per 1,000 patients treated.
“We do recommend, however that this risk of bleeding must be reevaluated over the course of recovery of patients, and this may change the decision around this intervention over time,” Dr. Anderson told attendees at the special session.
That’s because pharmacologic prophylaxis is recommended in surgical patients at low to moderate risk of bleeding. In this scenario, the incremental risk of major bleeding (14 events per 1,000 patients treated) is outweighed by the benefit of the reduction of symptomatic VTE events, according to Dr. Anderson.
When pharmacologic prophylaxis is used, the panel recommends combined prophylaxis – mechanical prophylaxis in addition to pharmacologic prophylaxis – especially in those patients at high or very high risk of VTE. Evidence shows that the combination approach significantly reduces risk of PE, and strongly suggests it may also reduce risk of symptomatic proximal DVT, Dr. Anderson said.
In surgical patients not receiving pharmacologic prophylaxis, mechanical prophylaxis is recommended over no mechanical prophylaxis, he added. Moreover, in those patients receiving mechanical prophylaxis, the ASH panel recommends use of intermittent compression devices over graduated compression stockings.
The panel comes out against prophylactic inferior vena cava (IVC) filter insertion in the guidelines. Dr. Anderson said that the “small reduction” in PE risk seen in observational studies is outweighed by increased risk of DVT, and a resulting trend for increased mortality, associated with insertion of the devices.
“We did not consider other risks of IVC filters such as filter embolization or perforation, which again would be complications that would support our recommendation against routine use of these devices in patients undergoing major surgery,” he said.
In terms of the type of pharmacologic prophylaxis to use, the panel said low-molecular-weight heparin or unfractionated heparin would be reasonable choices in this setting. Available data do not demonstrate any significant differences between these choices for major clinical outcomes, Dr. Anderson added.
The guideline also addresses duration of pharmacologic prophylaxis, stating that extended prophylaxis – of at least 3 weeks – is favored over short-term prophylaxis, or up to 2 weeks of treatment. The extended approach significantly reduces risk of symptomatic PE and proximal DVT, though most of the supporting data come from studies of major joint arthroplasty and major general surgical procedures for patients with cancer. “We need more studies in other clinical areas to examine this particular question,” Dr. Anderson said.
The guideline on prophylaxis in surgical patients was published in Blood Advances (2019 Dec 3;3[23]:3898-944). Six other ASH VTE guidelines, all published in 2018, covered prophylaxis in medical patients, diagnosis, VTE in pregnancy, optimal anticoagulation, heparin-induced thrombocytopenia, and pediatric considerations. The guidelines are available on the ASH website.
Dr. Anderson reported having no relevant conflicts of interest.
ORLANDO – The latest American Society of Hematology guideline on venous thromboembolism (VTE) tackles 30 key questions regarding prophylaxis in hospitalized patients undergoing surgery, according to the chair of the guideline panel, who highlighted 9 of those questions during a special session at the society’s annual meeting.
The clinical practice guideline, published just about a week before the annual meeting of the American Society of Hematology, focuses mainly on pharmacologic prophylaxis in specific surgical settings, said David R. Anderson, MD, dean of the faculty of medicine of Dalhousie University, Halifax, N.S.
“Our guidelines focused upon clinically important symptomatic outcomes, with less emphasis being placed on asymptomatic deep vein thrombosis detected by screening tests,” Dr. Anderson said.
At the special education session, Dr. Anderson highlighted several specific recommendations on prophylaxis in surgical patients.
Pharmacologic prophylaxis is not recommended for patients experiencing major trauma deemed to be at high risk of bleeding. Its use does reduce risk of symptomatic pulmonary embolism (PE) and deep vein thrombosis (DVT) by about 10 events per 1,000 patients treated; however, Dr. Anderson said, the panel’s opinion was that this benefit was outweighed by increased risk of major bleeding, at 24 events per 1,000 patients treated.
“We do recommend, however that this risk of bleeding must be reevaluated over the course of recovery of patients, and this may change the decision around this intervention over time,” Dr. Anderson told attendees at the special session.
That’s because pharmacologic prophylaxis is recommended in surgical patients at low to moderate risk of bleeding. In this scenario, the incremental risk of major bleeding (14 events per 1,000 patients treated) is outweighed by the benefit of the reduction of symptomatic VTE events, according to Dr. Anderson.
When pharmacologic prophylaxis is used, the panel recommends combined prophylaxis – mechanical prophylaxis in addition to pharmacologic prophylaxis – especially in those patients at high or very high risk of VTE. Evidence shows that the combination approach significantly reduces risk of PE, and strongly suggests it may also reduce risk of symptomatic proximal DVT, Dr. Anderson said.
In surgical patients not receiving pharmacologic prophylaxis, mechanical prophylaxis is recommended over no mechanical prophylaxis, he added. Moreover, in those patients receiving mechanical prophylaxis, the ASH panel recommends use of intermittent compression devices over graduated compression stockings.
The panel comes out against prophylactic inferior vena cava (IVC) filter insertion in the guidelines. Dr. Anderson said that the “small reduction” in PE risk seen in observational studies is outweighed by increased risk of DVT, and a resulting trend for increased mortality, associated with insertion of the devices.
“We did not consider other risks of IVC filters such as filter embolization or perforation, which again would be complications that would support our recommendation against routine use of these devices in patients undergoing major surgery,” he said.
In terms of the type of pharmacologic prophylaxis to use, the panel said low-molecular-weight heparin or unfractionated heparin would be reasonable choices in this setting. Available data do not demonstrate any significant differences between these choices for major clinical outcomes, Dr. Anderson added.
The guideline also addresses duration of pharmacologic prophylaxis, stating that extended prophylaxis – of at least 3 weeks – is favored over short-term prophylaxis, or up to 2 weeks of treatment. The extended approach significantly reduces risk of symptomatic PE and proximal DVT, though most of the supporting data come from studies of major joint arthroplasty and major general surgical procedures for patients with cancer. “We need more studies in other clinical areas to examine this particular question,” Dr. Anderson said.
The guideline on prophylaxis in surgical patients was published in Blood Advances (2019 Dec 3;3[23]:3898-944). Six other ASH VTE guidelines, all published in 2018, covered prophylaxis in medical patients, diagnosis, VTE in pregnancy, optimal anticoagulation, heparin-induced thrombocytopenia, and pediatric considerations. The guidelines are available on the ASH website.
Dr. Anderson reported having no relevant conflicts of interest.
ORLANDO – The latest American Society of Hematology guideline on venous thromboembolism (VTE) tackles 30 key questions regarding prophylaxis in hospitalized patients undergoing surgery, according to the chair of the guideline panel, who highlighted 9 of those questions during a special session at the society’s annual meeting.
The clinical practice guideline, published just about a week before the annual meeting of the American Society of Hematology, focuses mainly on pharmacologic prophylaxis in specific surgical settings, said David R. Anderson, MD, dean of the faculty of medicine of Dalhousie University, Halifax, N.S.
“Our guidelines focused upon clinically important symptomatic outcomes, with less emphasis being placed on asymptomatic deep vein thrombosis detected by screening tests,” Dr. Anderson said.
At the special education session, Dr. Anderson highlighted several specific recommendations on prophylaxis in surgical patients.
Pharmacologic prophylaxis is not recommended for patients experiencing major trauma deemed to be at high risk of bleeding. Its use does reduce risk of symptomatic pulmonary embolism (PE) and deep vein thrombosis (DVT) by about 10 events per 1,000 patients treated; however, Dr. Anderson said, the panel’s opinion was that this benefit was outweighed by increased risk of major bleeding, at 24 events per 1,000 patients treated.
“We do recommend, however that this risk of bleeding must be reevaluated over the course of recovery of patients, and this may change the decision around this intervention over time,” Dr. Anderson told attendees at the special session.
That’s because pharmacologic prophylaxis is recommended in surgical patients at low to moderate risk of bleeding. In this scenario, the incremental risk of major bleeding (14 events per 1,000 patients treated) is outweighed by the benefit of the reduction of symptomatic VTE events, according to Dr. Anderson.
When pharmacologic prophylaxis is used, the panel recommends combined prophylaxis – mechanical prophylaxis in addition to pharmacologic prophylaxis – especially in those patients at high or very high risk of VTE. Evidence shows that the combination approach significantly reduces risk of PE, and strongly suggests it may also reduce risk of symptomatic proximal DVT, Dr. Anderson said.
In surgical patients not receiving pharmacologic prophylaxis, mechanical prophylaxis is recommended over no mechanical prophylaxis, he added. Moreover, in those patients receiving mechanical prophylaxis, the ASH panel recommends use of intermittent compression devices over graduated compression stockings.
The panel comes out against prophylactic inferior vena cava (IVC) filter insertion in the guidelines. Dr. Anderson said that the “small reduction” in PE risk seen in observational studies is outweighed by increased risk of DVT, and a resulting trend for increased mortality, associated with insertion of the devices.
“We did not consider other risks of IVC filters such as filter embolization or perforation, which again would be complications that would support our recommendation against routine use of these devices in patients undergoing major surgery,” he said.
In terms of the type of pharmacologic prophylaxis to use, the panel said low-molecular-weight heparin or unfractionated heparin would be reasonable choices in this setting. Available data do not demonstrate any significant differences between these choices for major clinical outcomes, Dr. Anderson added.
The guideline also addresses duration of pharmacologic prophylaxis, stating that extended prophylaxis – of at least 3 weeks – is favored over short-term prophylaxis, or up to 2 weeks of treatment. The extended approach significantly reduces risk of symptomatic PE and proximal DVT, though most of the supporting data come from studies of major joint arthroplasty and major general surgical procedures for patients with cancer. “We need more studies in other clinical areas to examine this particular question,” Dr. Anderson said.
The guideline on prophylaxis in surgical patients was published in Blood Advances (2019 Dec 3;3[23]:3898-944). Six other ASH VTE guidelines, all published in 2018, covered prophylaxis in medical patients, diagnosis, VTE in pregnancy, optimal anticoagulation, heparin-induced thrombocytopenia, and pediatric considerations. The guidelines are available on the ASH website.
Dr. Anderson reported having no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASH 2019
Cardiac arrhythmia heightens mortality risk during epilepsy hospitalizations
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
REPORTING FROM AES 2019
EVALI outbreak ongoing, but new cases decline
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).
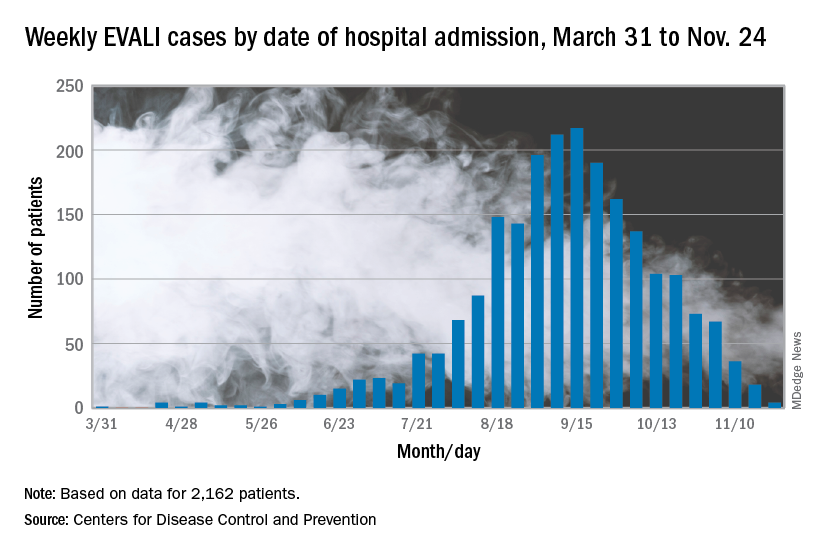
CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
FROM THE MMWR
Genetic test stratified AFib patients with low CHA2DS2-VASc scores
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
REPORTING FROM AHA 2019
New heart failure trial data presage guideline revisions
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
EXPERT ANALYSIS FROM AHA 2019

















