User login
Aspirin plus a DOAC may do more harm than good in some
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
REPORTING FROM ASH 2019
Influenza already in midseason form
It’s been a decade since flu activity levels were this high this early in the season.
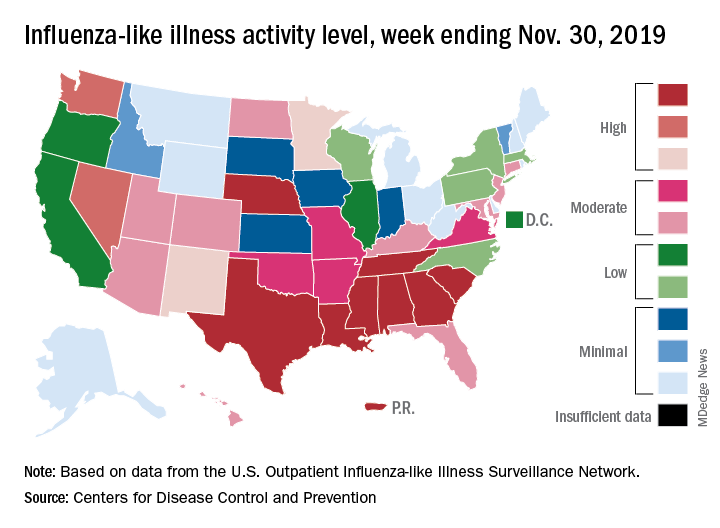
For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
Experts bring clarity to end of life difficulties
Understanding family perspective is an important factor
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
Understanding family perspective is an important factor
Understanding family perspective is an important factor
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
Gunshot wound victims are at high risk for readmission
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
REPORTING FROM RSNA 2019
2019-2020 flu season starts off full throttle
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
Anticipating the A.I. revolution
Goal is to augment human performance
Artificial intelligence (A.I.) is likely to change almost everything in medical practice, according to a new book called “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again,” by Eric Topol, MD.
Dr. Topol told The Hospitalist that his book’s subtitle “is the paradox: the unexpected, far-reaching goal of A.I. that can, if used properly, restore the most important part of medicine – a deep patient-doctor relationship.”
That’s because A.I. can do more than enhance diagnoses; it can also help with tasks such as note-taking and reading scans, making it possible for hospitalists to spend more time connecting with their patients. “Hospitalists could have a much better handle on a patient’s dataset via algorithmic processing, providing alerts and augmented performance of hospitalists (when validated),” Dr. Topol said. “They can also expect far less keyboard use with the help of speech recognition, natural language processing, and deep learning.”In an interview with the New York Times, Dr. Topol said that by augmenting human performance, A.I. has the potential to markedly improve productivity, efficiency, work flow, accuracy and speed, both for doctors and for patients, giving more charge and control to consumers through algorithmic support of their data.
“We can’t, and will never, rely on only algorithms for interpretation of life and death matters,” he said. “That requires human expert contextualization, something machines can’t do.”Of course, there could be pitfalls. “The liabilities include breaches of privacy and security, hacking, the lack of explainability of most A.I. algorithms, the potential to worsen inequities, the embedded bias, and ethical quandaries,” he said.
Reference
1. O’Connor A. How Artificial Intelligence Could Transform Medicine. New York Times. March 11, 2019. https://www.nytimes.com/2019/03/11/well/live/how-artificial-intelligence-could-transform-medicine.html.
Goal is to augment human performance
Goal is to augment human performance
Artificial intelligence (A.I.) is likely to change almost everything in medical practice, according to a new book called “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again,” by Eric Topol, MD.
Dr. Topol told The Hospitalist that his book’s subtitle “is the paradox: the unexpected, far-reaching goal of A.I. that can, if used properly, restore the most important part of medicine – a deep patient-doctor relationship.”
That’s because A.I. can do more than enhance diagnoses; it can also help with tasks such as note-taking and reading scans, making it possible for hospitalists to spend more time connecting with their patients. “Hospitalists could have a much better handle on a patient’s dataset via algorithmic processing, providing alerts and augmented performance of hospitalists (when validated),” Dr. Topol said. “They can also expect far less keyboard use with the help of speech recognition, natural language processing, and deep learning.”In an interview with the New York Times, Dr. Topol said that by augmenting human performance, A.I. has the potential to markedly improve productivity, efficiency, work flow, accuracy and speed, both for doctors and for patients, giving more charge and control to consumers through algorithmic support of their data.
“We can’t, and will never, rely on only algorithms for interpretation of life and death matters,” he said. “That requires human expert contextualization, something machines can’t do.”Of course, there could be pitfalls. “The liabilities include breaches of privacy and security, hacking, the lack of explainability of most A.I. algorithms, the potential to worsen inequities, the embedded bias, and ethical quandaries,” he said.
Reference
1. O’Connor A. How Artificial Intelligence Could Transform Medicine. New York Times. March 11, 2019. https://www.nytimes.com/2019/03/11/well/live/how-artificial-intelligence-could-transform-medicine.html.
Artificial intelligence (A.I.) is likely to change almost everything in medical practice, according to a new book called “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again,” by Eric Topol, MD.
Dr. Topol told The Hospitalist that his book’s subtitle “is the paradox: the unexpected, far-reaching goal of A.I. that can, if used properly, restore the most important part of medicine – a deep patient-doctor relationship.”
That’s because A.I. can do more than enhance diagnoses; it can also help with tasks such as note-taking and reading scans, making it possible for hospitalists to spend more time connecting with their patients. “Hospitalists could have a much better handle on a patient’s dataset via algorithmic processing, providing alerts and augmented performance of hospitalists (when validated),” Dr. Topol said. “They can also expect far less keyboard use with the help of speech recognition, natural language processing, and deep learning.”In an interview with the New York Times, Dr. Topol said that by augmenting human performance, A.I. has the potential to markedly improve productivity, efficiency, work flow, accuracy and speed, both for doctors and for patients, giving more charge and control to consumers through algorithmic support of their data.
“We can’t, and will never, rely on only algorithms for interpretation of life and death matters,” he said. “That requires human expert contextualization, something machines can’t do.”Of course, there could be pitfalls. “The liabilities include breaches of privacy and security, hacking, the lack of explainability of most A.I. algorithms, the potential to worsen inequities, the embedded bias, and ethical quandaries,” he said.
Reference
1. O’Connor A. How Artificial Intelligence Could Transform Medicine. New York Times. March 11, 2019. https://www.nytimes.com/2019/03/11/well/live/how-artificial-intelligence-could-transform-medicine.html.
DAPA-HF: Dapagliflozin benefits regardless of age, HF severity
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Large population-based study underscores link between gout, CVD event risk
ATLANTA – Gout is associated with an increased risk of both fatal and nonfatal cardiovascular disease events, according to a large population-based health data linkage study in New Zealand.
“Overall, the survival was quite good within both cohorts, but ... there is a clear and statistically significant difference in the survival between the people with gout and those without gout,” Ken Cai, MBBS, reported at the annual meeting of the American College of Rheumatology, noting that a similarly “significant and clear” difference was seen in nonfatal CVD events between the groups.
Of 968,387 individuals included in the analysis, 34,056 had gout, said Dr. Cai, a rheumatology clinical fellow at the University of Auckland (New Zealand). After adjusting for population-level estimated 5-year CVD risk for cardiovascular death, nonfatal myocardial infarction, stroke, or other vascular event, the adjusted hazard ratios were 1.20 for fatal and 1.32 for nonfatal first CVD events in patients with gout. The CVD risk score used in the analysis accounted for age, gender, ethnicity, level of social deprivation, diabetes status, previous hospitalization for atrial fibrillation, and baseline dispensing of blood pressure–lowering, lipid-lowering, and antiplatelet/anticoagulant medications.
“To allow for any other differences between the gout and nongout cohorts with respect to gender, age, ethnicity, and social deprivation, we further adjusted for these factors again, even though they had been accounted for within our CVD risk score,” he said, noting that “gout continued to demonstrate an increased adjusted hazard ratio” for fatal and nonfatal events after that adjustment (HRs, 1.40 and 1.35, respectively)
Additional analysis in the gout patients showed that CVD risk was similarly increased both in those who had been dispensed allopurinol at least once in the prior 5 years and those who had not (adjusted HRs for fatal events, 1.41 and 1.33; and for nonfatal, first CVD events, 1.34 and 1.38, respectively), and “there was no significant difference between these two groups, compared to people without gout,” he said.
Adjustment for serum urate levels in gout patients also showed similarly increased risk for fatal and nonfatal events for those with levels less than 6 mg/dL and those with levels of 6 mg/dL or greater (adjusted HRs of 1.32 and 1.42 for fatal events, and 1.27 and 1.43 for nonfatal first CVD events, respectively).
Again, no significant difference was seen in the risk of events between these two groups and those without gout, Dr. Cai said, noting that patients with no serum urate monitoring also had an increased risk of events (adjusted HR of 1.41 for fatal events and 1.29 for nonfatal, first CVD events).
Gout and hyperuricemia have previously been reported to be independent risk factors for CVD and CVD events, and urate-lowering therapy such as allopurinol have been thought to potentially be associated with reduced risk of CVD, he said, noting that the relationships are of particular concern in New Zealand, where gout affects more than 4% of the adult population.
“Maori, who are the indigenous people of New Zealand, and Pasifika people are disproportionately affected by gout; 8.5% of Maori, and 13.9% of Pasifika adults have gout,” he said, adding that an estimated one-third of Maori and Pasifika adults over age 65 years have gout.
To further assess the relationships between gout and CVD risk, he and his colleagues used validated population-level risk-prediction equations and linked National Health Identifier (NHI) data, he said.
National registries of medicines dispensing data, hospitalization, and death were linked to the Auckland/Northland regional repository of laboratory results from Jan. 1, 2012 to Dec. 31, 2016.
“We included all New Zealand residents aged 20 years or older who were in contact with publicly funded services in 2011 and were alive at the end of December, 2011,” he said, adding that those with a previous hospitalization for CVD or heart failure prior to the end of December 2011 were excluded, as were those with primary residence outside of the region for the prior 3 years and those missing predictor variable data.
Although the findings are limited by an inability to adjust for smoking status, body mass index, and blood pressure – as such data are not collected at the national level, and by the population-based nature of the study, which does not allow determination about causation, they nevertheless reinforce the association between gout and an increased estimated risk of CVD events, Dr. Cai said.
“Even after adjustment for estimated 5-year CVD risk and the additional weighting of risk factors within it, gout independently increased the hazard ratio for fatal and nonfatal events,” he said. “In our study, this effect was not ameliorated by allopurinol use or serum urate lowering to treatment target.”
Similar studies are needed in other populations, he said.
Dr. Cai reported grant support from Arthritis Australia.
SOURCE: Cai K et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2732.
ATLANTA – Gout is associated with an increased risk of both fatal and nonfatal cardiovascular disease events, according to a large population-based health data linkage study in New Zealand.
“Overall, the survival was quite good within both cohorts, but ... there is a clear and statistically significant difference in the survival between the people with gout and those without gout,” Ken Cai, MBBS, reported at the annual meeting of the American College of Rheumatology, noting that a similarly “significant and clear” difference was seen in nonfatal CVD events between the groups.
Of 968,387 individuals included in the analysis, 34,056 had gout, said Dr. Cai, a rheumatology clinical fellow at the University of Auckland (New Zealand). After adjusting for population-level estimated 5-year CVD risk for cardiovascular death, nonfatal myocardial infarction, stroke, or other vascular event, the adjusted hazard ratios were 1.20 for fatal and 1.32 for nonfatal first CVD events in patients with gout. The CVD risk score used in the analysis accounted for age, gender, ethnicity, level of social deprivation, diabetes status, previous hospitalization for atrial fibrillation, and baseline dispensing of blood pressure–lowering, lipid-lowering, and antiplatelet/anticoagulant medications.
“To allow for any other differences between the gout and nongout cohorts with respect to gender, age, ethnicity, and social deprivation, we further adjusted for these factors again, even though they had been accounted for within our CVD risk score,” he said, noting that “gout continued to demonstrate an increased adjusted hazard ratio” for fatal and nonfatal events after that adjustment (HRs, 1.40 and 1.35, respectively)
Additional analysis in the gout patients showed that CVD risk was similarly increased both in those who had been dispensed allopurinol at least once in the prior 5 years and those who had not (adjusted HRs for fatal events, 1.41 and 1.33; and for nonfatal, first CVD events, 1.34 and 1.38, respectively), and “there was no significant difference between these two groups, compared to people without gout,” he said.
Adjustment for serum urate levels in gout patients also showed similarly increased risk for fatal and nonfatal events for those with levels less than 6 mg/dL and those with levels of 6 mg/dL or greater (adjusted HRs of 1.32 and 1.42 for fatal events, and 1.27 and 1.43 for nonfatal first CVD events, respectively).
Again, no significant difference was seen in the risk of events between these two groups and those without gout, Dr. Cai said, noting that patients with no serum urate monitoring also had an increased risk of events (adjusted HR of 1.41 for fatal events and 1.29 for nonfatal, first CVD events).
Gout and hyperuricemia have previously been reported to be independent risk factors for CVD and CVD events, and urate-lowering therapy such as allopurinol have been thought to potentially be associated with reduced risk of CVD, he said, noting that the relationships are of particular concern in New Zealand, where gout affects more than 4% of the adult population.
“Maori, who are the indigenous people of New Zealand, and Pasifika people are disproportionately affected by gout; 8.5% of Maori, and 13.9% of Pasifika adults have gout,” he said, adding that an estimated one-third of Maori and Pasifika adults over age 65 years have gout.
To further assess the relationships between gout and CVD risk, he and his colleagues used validated population-level risk-prediction equations and linked National Health Identifier (NHI) data, he said.
National registries of medicines dispensing data, hospitalization, and death were linked to the Auckland/Northland regional repository of laboratory results from Jan. 1, 2012 to Dec. 31, 2016.
“We included all New Zealand residents aged 20 years or older who were in contact with publicly funded services in 2011 and were alive at the end of December, 2011,” he said, adding that those with a previous hospitalization for CVD or heart failure prior to the end of December 2011 were excluded, as were those with primary residence outside of the region for the prior 3 years and those missing predictor variable data.
Although the findings are limited by an inability to adjust for smoking status, body mass index, and blood pressure – as such data are not collected at the national level, and by the population-based nature of the study, which does not allow determination about causation, they nevertheless reinforce the association between gout and an increased estimated risk of CVD events, Dr. Cai said.
“Even after adjustment for estimated 5-year CVD risk and the additional weighting of risk factors within it, gout independently increased the hazard ratio for fatal and nonfatal events,” he said. “In our study, this effect was not ameliorated by allopurinol use or serum urate lowering to treatment target.”
Similar studies are needed in other populations, he said.
Dr. Cai reported grant support from Arthritis Australia.
SOURCE: Cai K et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2732.
ATLANTA – Gout is associated with an increased risk of both fatal and nonfatal cardiovascular disease events, according to a large population-based health data linkage study in New Zealand.
“Overall, the survival was quite good within both cohorts, but ... there is a clear and statistically significant difference in the survival between the people with gout and those without gout,” Ken Cai, MBBS, reported at the annual meeting of the American College of Rheumatology, noting that a similarly “significant and clear” difference was seen in nonfatal CVD events between the groups.
Of 968,387 individuals included in the analysis, 34,056 had gout, said Dr. Cai, a rheumatology clinical fellow at the University of Auckland (New Zealand). After adjusting for population-level estimated 5-year CVD risk for cardiovascular death, nonfatal myocardial infarction, stroke, or other vascular event, the adjusted hazard ratios were 1.20 for fatal and 1.32 for nonfatal first CVD events in patients with gout. The CVD risk score used in the analysis accounted for age, gender, ethnicity, level of social deprivation, diabetes status, previous hospitalization for atrial fibrillation, and baseline dispensing of blood pressure–lowering, lipid-lowering, and antiplatelet/anticoagulant medications.
“To allow for any other differences between the gout and nongout cohorts with respect to gender, age, ethnicity, and social deprivation, we further adjusted for these factors again, even though they had been accounted for within our CVD risk score,” he said, noting that “gout continued to demonstrate an increased adjusted hazard ratio” for fatal and nonfatal events after that adjustment (HRs, 1.40 and 1.35, respectively)
Additional analysis in the gout patients showed that CVD risk was similarly increased both in those who had been dispensed allopurinol at least once in the prior 5 years and those who had not (adjusted HRs for fatal events, 1.41 and 1.33; and for nonfatal, first CVD events, 1.34 and 1.38, respectively), and “there was no significant difference between these two groups, compared to people without gout,” he said.
Adjustment for serum urate levels in gout patients also showed similarly increased risk for fatal and nonfatal events for those with levels less than 6 mg/dL and those with levels of 6 mg/dL or greater (adjusted HRs of 1.32 and 1.42 for fatal events, and 1.27 and 1.43 for nonfatal first CVD events, respectively).
Again, no significant difference was seen in the risk of events between these two groups and those without gout, Dr. Cai said, noting that patients with no serum urate monitoring also had an increased risk of events (adjusted HR of 1.41 for fatal events and 1.29 for nonfatal, first CVD events).
Gout and hyperuricemia have previously been reported to be independent risk factors for CVD and CVD events, and urate-lowering therapy such as allopurinol have been thought to potentially be associated with reduced risk of CVD, he said, noting that the relationships are of particular concern in New Zealand, where gout affects more than 4% of the adult population.
“Maori, who are the indigenous people of New Zealand, and Pasifika people are disproportionately affected by gout; 8.5% of Maori, and 13.9% of Pasifika adults have gout,” he said, adding that an estimated one-third of Maori and Pasifika adults over age 65 years have gout.
To further assess the relationships between gout and CVD risk, he and his colleagues used validated population-level risk-prediction equations and linked National Health Identifier (NHI) data, he said.
National registries of medicines dispensing data, hospitalization, and death were linked to the Auckland/Northland regional repository of laboratory results from Jan. 1, 2012 to Dec. 31, 2016.
“We included all New Zealand residents aged 20 years or older who were in contact with publicly funded services in 2011 and were alive at the end of December, 2011,” he said, adding that those with a previous hospitalization for CVD or heart failure prior to the end of December 2011 were excluded, as were those with primary residence outside of the region for the prior 3 years and those missing predictor variable data.
Although the findings are limited by an inability to adjust for smoking status, body mass index, and blood pressure – as such data are not collected at the national level, and by the population-based nature of the study, which does not allow determination about causation, they nevertheless reinforce the association between gout and an increased estimated risk of CVD events, Dr. Cai said.
“Even after adjustment for estimated 5-year CVD risk and the additional weighting of risk factors within it, gout independently increased the hazard ratio for fatal and nonfatal events,” he said. “In our study, this effect was not ameliorated by allopurinol use or serum urate lowering to treatment target.”
Similar studies are needed in other populations, he said.
Dr. Cai reported grant support from Arthritis Australia.
SOURCE: Cai K et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2732.
REPORTING FROM ACR 2019
What’s new in hepatitis C: Four themes that dominated at the Liver Meeting
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
REPORTING FROM THE LIVER MEETING 2019
Guideline: Diagnosis and treatment of adults with community-acquired pneumonia
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.