User login
PACT-HF: Transitional care derives no overall benefit
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
Women respond more to intervention
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
REPORTING FROM AHA 2019
Treating pain with virtual reality
Pilot studies are underway
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
Pilot studies are underway
Pilot studies are underway
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
New hypertension performance measures boost 130/80 mm Hg target
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
REPORTING FROM AHA 2019
Farxiga granted Priority Review for treatment of adults with HFrEF
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
Flu records most active December since 2003
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.
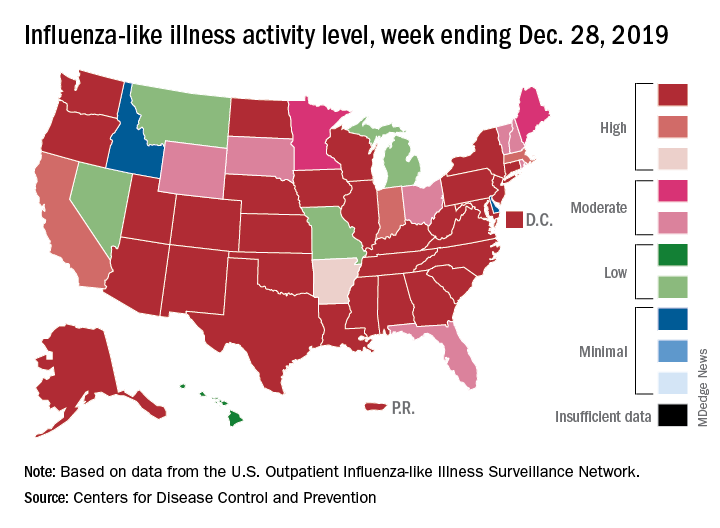
For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
Cultivating patient activation through technology
Tech alone is not enough
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
Tech alone is not enough
Tech alone is not enough
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
Experts call to revise the Uniform Determination of Death Act
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
FROM ANNALS OF INTERNAL MEDICINE
Early increase in flu activity shows no signs of slowing
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
The measles comeback of 2019
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
EVALI readmissions and deaths prompt guideline change

Those who required rehospitalization for e-cigarette or vaping product use–associated lung injury (EVALI) and those who died after discharge were more likely to have one or more chronic conditions than were other EVALI patients, and those “who died also were more likely to have been admitted to an intensive care unit, experienced respiratory failure necessitating intubation and mechanical ventilation, and were significantly older,” Christina A. Mikosz, MD, and associates wrote in the Morbidity and Mortality Weekly Report.
Their analysis included the 1,139 EVALI patients who were discharged on or after Oct. 31, 2019. Of that group, 31 (2.7%) patients were rehospitalized and subsequently discharged and another 7 died after the initial discharge. The median age was 54 years for those who died, 27 years for those who were rehospitalized, and 23 for those who survived without rehospitalization, said Dr. Mikosz of the CDC National Center for Injury Prevention and Control, Atlanta, and associates.
Those findings, along with the rates of one or more comorbidities – 83% for those who died, 71% for those who were rehospitalized, and 26% for those who did not die or get readmitted – prompted the CDC to update its guidance for postdischarge follow-up of EVALI patients.
That update involves six specific recommendations to determine readiness for discharge, which include “confirming no clinically significant fluctuations in vital signs for at least 24-48 hours before discharge [and] preparation for hospital discharge and postdischarge care coordination to reduce risk of rehospitalization and death,” Mary E. Evans, MD, and associates said in a separate CDC communication (MMWR. 2019 Dec. 20. 68[early release]:1-6).
As of Dec. 17, the CDC reports that 2,506 patients have been hospitalized with EVALI since March 31, 2019, and 54 deaths have been confirmed in 27 states and the District of Columbia. The outbreak appears to have peaked in September, but cases are still being reported: 13 during the week of Dec. 1-7 and one case for the week of Dec. 8-14.
SOURCE: Mikosz CA et al. MMWR. 2019 Dec. 20. 68[early release]:1-7.

Those who required rehospitalization for e-cigarette or vaping product use–associated lung injury (EVALI) and those who died after discharge were more likely to have one or more chronic conditions than were other EVALI patients, and those “who died also were more likely to have been admitted to an intensive care unit, experienced respiratory failure necessitating intubation and mechanical ventilation, and were significantly older,” Christina A. Mikosz, MD, and associates wrote in the Morbidity and Mortality Weekly Report.
Their analysis included the 1,139 EVALI patients who were discharged on or after Oct. 31, 2019. Of that group, 31 (2.7%) patients were rehospitalized and subsequently discharged and another 7 died after the initial discharge. The median age was 54 years for those who died, 27 years for those who were rehospitalized, and 23 for those who survived without rehospitalization, said Dr. Mikosz of the CDC National Center for Injury Prevention and Control, Atlanta, and associates.
Those findings, along with the rates of one or more comorbidities – 83% for those who died, 71% for those who were rehospitalized, and 26% for those who did not die or get readmitted – prompted the CDC to update its guidance for postdischarge follow-up of EVALI patients.
That update involves six specific recommendations to determine readiness for discharge, which include “confirming no clinically significant fluctuations in vital signs for at least 24-48 hours before discharge [and] preparation for hospital discharge and postdischarge care coordination to reduce risk of rehospitalization and death,” Mary E. Evans, MD, and associates said in a separate CDC communication (MMWR. 2019 Dec. 20. 68[early release]:1-6).
As of Dec. 17, the CDC reports that 2,506 patients have been hospitalized with EVALI since March 31, 2019, and 54 deaths have been confirmed in 27 states and the District of Columbia. The outbreak appears to have peaked in September, but cases are still being reported: 13 during the week of Dec. 1-7 and one case for the week of Dec. 8-14.
SOURCE: Mikosz CA et al. MMWR. 2019 Dec. 20. 68[early release]:1-7.

Those who required rehospitalization for e-cigarette or vaping product use–associated lung injury (EVALI) and those who died after discharge were more likely to have one or more chronic conditions than were other EVALI patients, and those “who died also were more likely to have been admitted to an intensive care unit, experienced respiratory failure necessitating intubation and mechanical ventilation, and were significantly older,” Christina A. Mikosz, MD, and associates wrote in the Morbidity and Mortality Weekly Report.
Their analysis included the 1,139 EVALI patients who were discharged on or after Oct. 31, 2019. Of that group, 31 (2.7%) patients were rehospitalized and subsequently discharged and another 7 died after the initial discharge. The median age was 54 years for those who died, 27 years for those who were rehospitalized, and 23 for those who survived without rehospitalization, said Dr. Mikosz of the CDC National Center for Injury Prevention and Control, Atlanta, and associates.
Those findings, along with the rates of one or more comorbidities – 83% for those who died, 71% for those who were rehospitalized, and 26% for those who did not die or get readmitted – prompted the CDC to update its guidance for postdischarge follow-up of EVALI patients.
That update involves six specific recommendations to determine readiness for discharge, which include “confirming no clinically significant fluctuations in vital signs for at least 24-48 hours before discharge [and] preparation for hospital discharge and postdischarge care coordination to reduce risk of rehospitalization and death,” Mary E. Evans, MD, and associates said in a separate CDC communication (MMWR. 2019 Dec. 20. 68[early release]:1-6).
As of Dec. 17, the CDC reports that 2,506 patients have been hospitalized with EVALI since March 31, 2019, and 54 deaths have been confirmed in 27 states and the District of Columbia. The outbreak appears to have peaked in September, but cases are still being reported: 13 during the week of Dec. 1-7 and one case for the week of Dec. 8-14.
SOURCE: Mikosz CA et al. MMWR. 2019 Dec. 20. 68[early release]:1-7.
FROM MMWR