User login
How P-wave indices can improve AFib-related ischemic stroke prediction
Background: Current AFib management guidelines recommend ischemic stroke risk stratification with CHA2DS2-VASc score; however, emerging studies have highlighted limitations of this score.
Study design: Retrospective review of previously obtained prospective cohort study data.
Setting: Fourteen U.S. communities.
Synopsis: For the 2,929 individuals with new incident AFib without anticoagulant use in the prior year, study authors computed P-wave indices (including P-wave axis, P-wave duration, advanced interatrial block, and P-wave terminal force in lead V1) from the most recent sinus rhythm EKG prior to the diagnosis of AFib. Cox proportional hazard models estimated the hazard ratio between PWIs and ischemic stroke. Of the PWIs tested above, abnormal P-wave axis (hazard ratio, 1.88; 95% confidence interval, 1.36-2.61) and advanced interatrial block (HR, 2.93; 95% CI 1.78-4.81) were associated with increased risk of stroke after adjustment for individual CHA2DS2-VASc variables. A P2-CHA2DS2-VASc score that incorporated abnormal P-wave axis measurements demonstrated superior discrimination, compared with the CHA2DS2-VASc score alone, and resulted in improvement in ischemic stroke risk classification.
Bottom line: Abnormal P-wave axis and advanced interatrial block measured during periods of sinus rhythm may be associated with increased risk of ischemic stroke in patients with atrial fibrillation; the P2-CHA2DS2-VASc score incorporating abnormal P-wave axis may be superior to CHA2DS2-VASc in ischemic stroke risk classification.
Citation: Maheshwari A et al. Refining prediction of atrial fibrillation–related stroke using the P2-CHA2DS2-VASc score. Circulation. 2019 Jan 8;139:180-91.
Dr. Cooke is a hospitalist at Beth Israel Deaconess Medical Center.
Background: Current AFib management guidelines recommend ischemic stroke risk stratification with CHA2DS2-VASc score; however, emerging studies have highlighted limitations of this score.
Study design: Retrospective review of previously obtained prospective cohort study data.
Setting: Fourteen U.S. communities.
Synopsis: For the 2,929 individuals with new incident AFib without anticoagulant use in the prior year, study authors computed P-wave indices (including P-wave axis, P-wave duration, advanced interatrial block, and P-wave terminal force in lead V1) from the most recent sinus rhythm EKG prior to the diagnosis of AFib. Cox proportional hazard models estimated the hazard ratio between PWIs and ischemic stroke. Of the PWIs tested above, abnormal P-wave axis (hazard ratio, 1.88; 95% confidence interval, 1.36-2.61) and advanced interatrial block (HR, 2.93; 95% CI 1.78-4.81) were associated with increased risk of stroke after adjustment for individual CHA2DS2-VASc variables. A P2-CHA2DS2-VASc score that incorporated abnormal P-wave axis measurements demonstrated superior discrimination, compared with the CHA2DS2-VASc score alone, and resulted in improvement in ischemic stroke risk classification.
Bottom line: Abnormal P-wave axis and advanced interatrial block measured during periods of sinus rhythm may be associated with increased risk of ischemic stroke in patients with atrial fibrillation; the P2-CHA2DS2-VASc score incorporating abnormal P-wave axis may be superior to CHA2DS2-VASc in ischemic stroke risk classification.
Citation: Maheshwari A et al. Refining prediction of atrial fibrillation–related stroke using the P2-CHA2DS2-VASc score. Circulation. 2019 Jan 8;139:180-91.
Dr. Cooke is a hospitalist at Beth Israel Deaconess Medical Center.
Background: Current AFib management guidelines recommend ischemic stroke risk stratification with CHA2DS2-VASc score; however, emerging studies have highlighted limitations of this score.
Study design: Retrospective review of previously obtained prospective cohort study data.
Setting: Fourteen U.S. communities.
Synopsis: For the 2,929 individuals with new incident AFib without anticoagulant use in the prior year, study authors computed P-wave indices (including P-wave axis, P-wave duration, advanced interatrial block, and P-wave terminal force in lead V1) from the most recent sinus rhythm EKG prior to the diagnosis of AFib. Cox proportional hazard models estimated the hazard ratio between PWIs and ischemic stroke. Of the PWIs tested above, abnormal P-wave axis (hazard ratio, 1.88; 95% confidence interval, 1.36-2.61) and advanced interatrial block (HR, 2.93; 95% CI 1.78-4.81) were associated with increased risk of stroke after adjustment for individual CHA2DS2-VASc variables. A P2-CHA2DS2-VASc score that incorporated abnormal P-wave axis measurements demonstrated superior discrimination, compared with the CHA2DS2-VASc score alone, and resulted in improvement in ischemic stroke risk classification.
Bottom line: Abnormal P-wave axis and advanced interatrial block measured during periods of sinus rhythm may be associated with increased risk of ischemic stroke in patients with atrial fibrillation; the P2-CHA2DS2-VASc score incorporating abnormal P-wave axis may be superior to CHA2DS2-VASc in ischemic stroke risk classification.
Citation: Maheshwari A et al. Refining prediction of atrial fibrillation–related stroke using the P2-CHA2DS2-VASc score. Circulation. 2019 Jan 8;139:180-91.
Dr. Cooke is a hospitalist at Beth Israel Deaconess Medical Center.
Vaping-linked lung injury cases near 1,900
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.
Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
Dose-reduced NOACs may be safer than warfarin in some AFib patients
Background: Prior studies have suggested that NOACs have a favorable risk-benefit profile when compared with warfarin, but it is unclear if this advantage also is present for those high-risk patients for whom NOAC dose reduction is recommended.
Study design: A meta-analysis.
Setting: Three phase 3 randomized, control trials.
Synopsis: From the three randomized, control trials, the authors identified 7,351 of the 46,426 patients as being eligible for dose-reduced NOACs. Of these patients, 3,702 were randomized to take a NOAC and 3,649 were randomized to take warfarin. For the primary outcomes of stroke or systemic embolism, there was no significant difference between patients randomized to receive dose-reduced NOAC versus warfarin. For outcomes of major bleeding, hemorrhagic stroke, intracranial hemorrhage, and fatal bleeding, dose-reduced NOACs had a significantly lower risk, compared with warfarin.
Bottom line: In patients eligible for dose-reduced NOACs, the use of dose-reduced NOACs may have a better safety profile without significant difference in the rate of ischemic stroke or systemic embolism.
Citation: Wang KL et al. Efficacy and safety of reduced-dose non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur Heart J. 2018 Dec 22. doi: 10.1093/eurheartj/ehy802.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
Background: Prior studies have suggested that NOACs have a favorable risk-benefit profile when compared with warfarin, but it is unclear if this advantage also is present for those high-risk patients for whom NOAC dose reduction is recommended.
Study design: A meta-analysis.
Setting: Three phase 3 randomized, control trials.
Synopsis: From the three randomized, control trials, the authors identified 7,351 of the 46,426 patients as being eligible for dose-reduced NOACs. Of these patients, 3,702 were randomized to take a NOAC and 3,649 were randomized to take warfarin. For the primary outcomes of stroke or systemic embolism, there was no significant difference between patients randomized to receive dose-reduced NOAC versus warfarin. For outcomes of major bleeding, hemorrhagic stroke, intracranial hemorrhage, and fatal bleeding, dose-reduced NOACs had a significantly lower risk, compared with warfarin.
Bottom line: In patients eligible for dose-reduced NOACs, the use of dose-reduced NOACs may have a better safety profile without significant difference in the rate of ischemic stroke or systemic embolism.
Citation: Wang KL et al. Efficacy and safety of reduced-dose non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur Heart J. 2018 Dec 22. doi: 10.1093/eurheartj/ehy802.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
Background: Prior studies have suggested that NOACs have a favorable risk-benefit profile when compared with warfarin, but it is unclear if this advantage also is present for those high-risk patients for whom NOAC dose reduction is recommended.
Study design: A meta-analysis.
Setting: Three phase 3 randomized, control trials.
Synopsis: From the three randomized, control trials, the authors identified 7,351 of the 46,426 patients as being eligible for dose-reduced NOACs. Of these patients, 3,702 were randomized to take a NOAC and 3,649 were randomized to take warfarin. For the primary outcomes of stroke or systemic embolism, there was no significant difference between patients randomized to receive dose-reduced NOAC versus warfarin. For outcomes of major bleeding, hemorrhagic stroke, intracranial hemorrhage, and fatal bleeding, dose-reduced NOACs had a significantly lower risk, compared with warfarin.
Bottom line: In patients eligible for dose-reduced NOACs, the use of dose-reduced NOACs may have a better safety profile without significant difference in the rate of ischemic stroke or systemic embolism.
Citation: Wang KL et al. Efficacy and safety of reduced-dose non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur Heart J. 2018 Dec 22. doi: 10.1093/eurheartj/ehy802.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
A sepsis death linked to fecal microbiota transplantation
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two cases of bacteremia – one fatal – have been linked to a fecal microbiota transplant.
Major finding: Two patients developed bacteremia after receiving a fecal microbiota transplant from the same donor.
Study details: Case studies.
Disclosures: The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two authors were attached to a diagnostics company involved in the study.
Source: DeFillip Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
EHR prompt significantly reduced telemetry monitoring during inpatient stays
Background: Prior studies have shown multifaceted interventions that include EHR prompts can reduce the utilization of telemetry monitoring, but it is unclear if EHR prompts alone can reduce utilization.
Study design: Cluster-randomized, control trial.
Setting: November 2016 and May 2017 at a tertiary care medical center on the general medicine service.
Synopsis: The authors designed an EHR prompt for patients ordered for telemetry. The prompt would request the team to either discontinue or continue telemetry. Half of the general medicine teams (representing 499 hospitalizations) were randomized to receive the intervention, and the other half of the general medicine teams (representing 567 hospitalizations) did not receive the intervention. In the intervention group, 62% of prompts were followed by a discontinuation of telemetry. This led to a 17% reduction in the mean hours of telemetry monitoring (50 hours in the control group and 41.3 hours in the intervention group; P = .001). There was no significant difference in the rate of rapid responses or medical emergencies between the two groups.
Bottom line: A targeted EHR prompt alone may lead to a reduction in the utilization of telemetry monitoring.
Citation: Najafi N et al. Assessment of a targeted electronic health record intervention to reduce telemetry duration: A cluster-randomized clinical trial. JAMA Intern Med. 2019 Dec 10;179(1):11-5.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
Background: Prior studies have shown multifaceted interventions that include EHR prompts can reduce the utilization of telemetry monitoring, but it is unclear if EHR prompts alone can reduce utilization.
Study design: Cluster-randomized, control trial.
Setting: November 2016 and May 2017 at a tertiary care medical center on the general medicine service.
Synopsis: The authors designed an EHR prompt for patients ordered for telemetry. The prompt would request the team to either discontinue or continue telemetry. Half of the general medicine teams (representing 499 hospitalizations) were randomized to receive the intervention, and the other half of the general medicine teams (representing 567 hospitalizations) did not receive the intervention. In the intervention group, 62% of prompts were followed by a discontinuation of telemetry. This led to a 17% reduction in the mean hours of telemetry monitoring (50 hours in the control group and 41.3 hours in the intervention group; P = .001). There was no significant difference in the rate of rapid responses or medical emergencies between the two groups.
Bottom line: A targeted EHR prompt alone may lead to a reduction in the utilization of telemetry monitoring.
Citation: Najafi N et al. Assessment of a targeted electronic health record intervention to reduce telemetry duration: A cluster-randomized clinical trial. JAMA Intern Med. 2019 Dec 10;179(1):11-5.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
Background: Prior studies have shown multifaceted interventions that include EHR prompts can reduce the utilization of telemetry monitoring, but it is unclear if EHR prompts alone can reduce utilization.
Study design: Cluster-randomized, control trial.
Setting: November 2016 and May 2017 at a tertiary care medical center on the general medicine service.
Synopsis: The authors designed an EHR prompt for patients ordered for telemetry. The prompt would request the team to either discontinue or continue telemetry. Half of the general medicine teams (representing 499 hospitalizations) were randomized to receive the intervention, and the other half of the general medicine teams (representing 567 hospitalizations) did not receive the intervention. In the intervention group, 62% of prompts were followed by a discontinuation of telemetry. This led to a 17% reduction in the mean hours of telemetry monitoring (50 hours in the control group and 41.3 hours in the intervention group; P = .001). There was no significant difference in the rate of rapid responses or medical emergencies between the two groups.
Bottom line: A targeted EHR prompt alone may lead to a reduction in the utilization of telemetry monitoring.
Citation: Najafi N et al. Assessment of a targeted electronic health record intervention to reduce telemetry duration: A cluster-randomized clinical trial. JAMA Intern Med. 2019 Dec 10;179(1):11-5.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine Harvard Medical School.
Quick Byte: DeepMind emerges
The science of prediction
Hundreds of scientists around the world enter a competition every 2 years called the Critical Assessment of Structure Prediction.
“Tackling a biological puzzle they call ‘the protein folding problem,’ they try to predict the three-dimensional shape of proteins in the human body. No one knows how to solve the problem. Even the winners only chip away at it. But a solution could streamline the way scientists create new medicines and fight disease,” according to a report in the New York Times.
In 2019, those scientists did not win the contest. “It was won by DeepMind, the artificial intelligence lab owned by Google’s parent company. DeepMind specializes in ‘deep learning,’ a type of artificial intelligence that is rapidly changing drug discovery science.”
Reference
1. Metz C. “Making New Drugs With a Dose of Artificial Intelligence,” New York Times. Feb. 5, 2019. https://www.nytimes.com/2019/02/05/technology/artificial-intelligence-drug-research-deepmind.html. Accessed Feb 7, 2019.
The science of prediction
The science of prediction
Hundreds of scientists around the world enter a competition every 2 years called the Critical Assessment of Structure Prediction.
“Tackling a biological puzzle they call ‘the protein folding problem,’ they try to predict the three-dimensional shape of proteins in the human body. No one knows how to solve the problem. Even the winners only chip away at it. But a solution could streamline the way scientists create new medicines and fight disease,” according to a report in the New York Times.
In 2019, those scientists did not win the contest. “It was won by DeepMind, the artificial intelligence lab owned by Google’s parent company. DeepMind specializes in ‘deep learning,’ a type of artificial intelligence that is rapidly changing drug discovery science.”
Reference
1. Metz C. “Making New Drugs With a Dose of Artificial Intelligence,” New York Times. Feb. 5, 2019. https://www.nytimes.com/2019/02/05/technology/artificial-intelligence-drug-research-deepmind.html. Accessed Feb 7, 2019.
Hundreds of scientists around the world enter a competition every 2 years called the Critical Assessment of Structure Prediction.
“Tackling a biological puzzle they call ‘the protein folding problem,’ they try to predict the three-dimensional shape of proteins in the human body. No one knows how to solve the problem. Even the winners only chip away at it. But a solution could streamline the way scientists create new medicines and fight disease,” according to a report in the New York Times.
In 2019, those scientists did not win the contest. “It was won by DeepMind, the artificial intelligence lab owned by Google’s parent company. DeepMind specializes in ‘deep learning,’ a type of artificial intelligence that is rapidly changing drug discovery science.”
Reference
1. Metz C. “Making New Drugs With a Dose of Artificial Intelligence,” New York Times. Feb. 5, 2019. https://www.nytimes.com/2019/02/05/technology/artificial-intelligence-drug-research-deepmind.html. Accessed Feb 7, 2019.
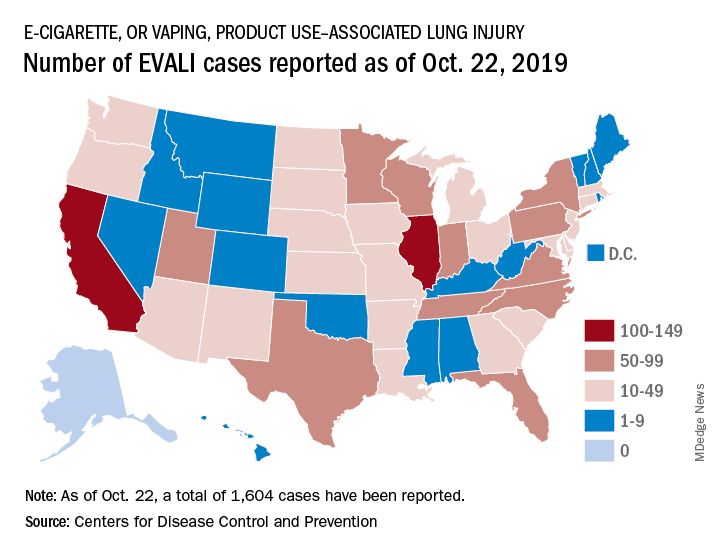
CDC, FDA in hot pursuit of source of vaping lung injuries
The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.
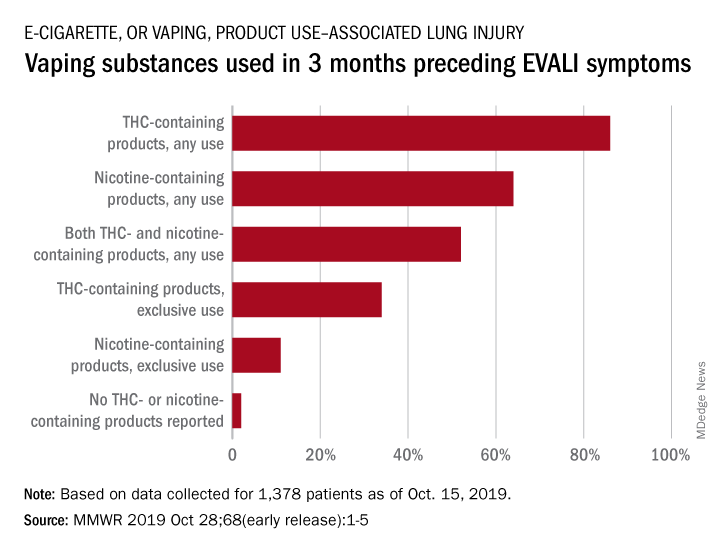
THC use reported in majority of vaping-related illnesses
(EVALI), according to the Centers for Disease Control and Prevention.

In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
(EVALI), according to the Centers for Disease Control and Prevention.

In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
(EVALI), according to the Centers for Disease Control and Prevention.

In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
FROM MMWR
Readmission for COPD exacerbation upped in-hospital mortality risk
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
REPORTING FROM CHEST 2019
Opioids, benzodiazepines carry greater risk of COPD-related hospitalization
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
according to recent research from Annals of the American Thoracic Society.
In addition, the risk of hospitalization because of respiratory events for patients with chronic obstructive pulmonary disease (COPD) was greater when opioid and benzodiazepine medications were combined, compared with patients who did not take either medication, Jacques G. Baillargeon, PhD, of the department of preventive medicine and community health at the University of Texas, Galveston, and colleagues wrote.
“Patients with COPD and their physicians should judiciously assess the risks and benefits of opioids and benzodiazepines, alone and in combination, and preferentially recommend nonopioid and nonbenzodiazepine approaches for pain, sleep, and anxiety management in patients with COPD,” the investigators wrote.
The researchers performed a case-control study of 3,232 Medicare beneficiary cases of COPD patients who were aged at least 66 years. Patients were included if they experienced a hospitalization related to a COPD-related adverse event with a respiratory diagnosis in 2014 and then matched to one or two control patients (total, 6,247 patients) based on age at hospitalization, gender, COPD medication, COPD complexity, obstructive sleep apnea, and socioeconomic status. COPD complexity was assigned to three levels (low, moderate, high) and calculated using the patient’s comorbid respiratory conditions and associated medical procedures in the 12 months prior to their hospitalization.
They found that, in the 30 days before COPD-related hospitalization, use of opioids was associated with greater likelihood of hospitalization (adjusted odds ratio, 1.73; 95% confidence interval, 1.52-1.97), as was use of benzodiazepines (aOR, 1.42; 95% CI, 1.21-1.66). When patients used both opioids and benzodiazepines, they had a significantly higher risk of hospitalization, compared with patients who did not use opioids or benzodiazepines (aOR, 2.32; 95% CI, 1.94-2.77).
In the 60 days prior to hospitalization, there was also a greater likelihood of hospitalization among COPD patients who used opioids (aOR, 1.66; 95% CI, 1.47-1.88), benzodiazepines (aOR, 1.44; 95% CI, 1.24-1.67), and both opioids and benzodiazepines (aOR, 2.27; 95% CI, 1.93-2.67); at 90 days, this higher risk of hospitalization persisted among COPD patients taking opioids (aOR, 1.58; 95% CI, 1.40-1.78), benzodiazepines (aOR, 1.40; 95% CI, 1.20-1.63), and both opioids and benzodiazepines (aOR, 2.21; 95% CI, 1.88-2.59).
The researchers acknowledged that one potential limitation in the study was how COPD diagnoses were obtained through coding performed by clinicians instead of from laboratory testing. Confounding by COPD indication and severity; use of over-the-counter medication or opioids and benzodiazepines received illegally; and lack of analyses of potential confounders such as diet, alcohol use, smoking status and herbal supplement use were other limitations.
This study was supported by an award from the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Baillargeon had no disclosures.
SOURCE: Baillargeon JG et al. Ann Am Thorac Soc. 2019 Oct 1. doi: 10.1513/AnnalsATS.201901-024OC.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY