User login
New test edges closer to rapid, accurate ID of active TB
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection.
Major finding: The assay had a sensitivity of 86%.
Study details: A machine learning and validation study involving patients with chronic cough from multiple countries.
Disclosures: The Bill and Melinda Gates Foundation funded the study. The investigators reported relationships with Quanterix Corporation and FIND.
Source: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
Vitamin C–based regimens in sepsis plausible, need more data, expert says
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
EXPERT ANALYSIS FROM CHEST 2019
CDC: Don’t vape, especially THC
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
FROM MMWR
In-hospital flu shot reduced readmissions in pneumonia patients
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
REPORTING FROM CHEST 2019
How should anticoagulation be managed in a patient with cirrhosis?
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).
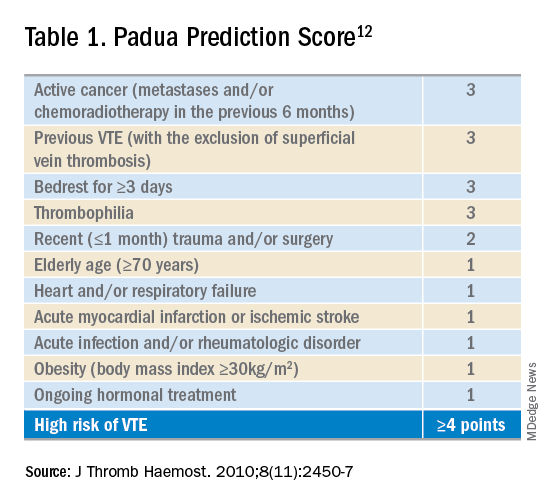
In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment
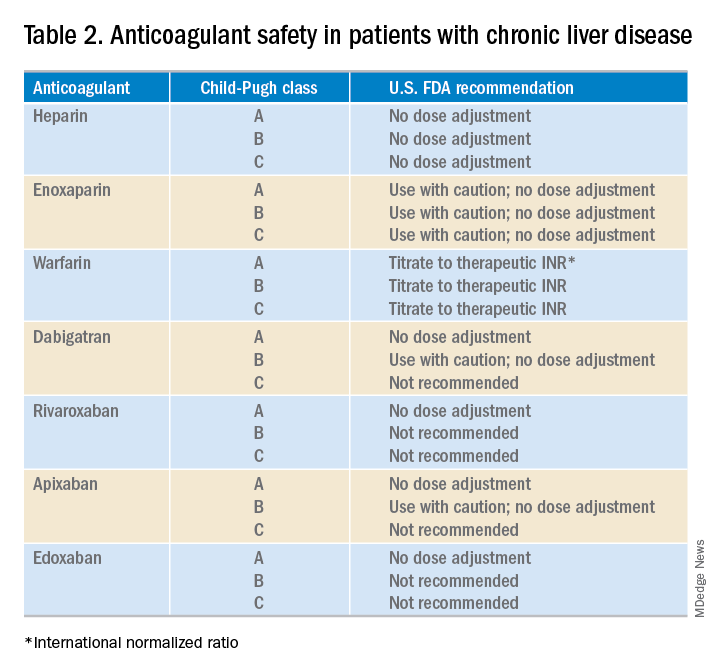
What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.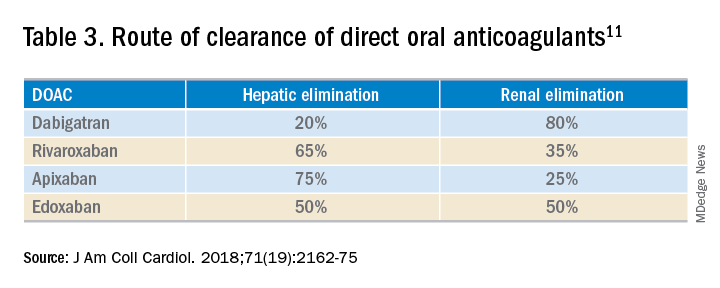
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
DOACs may be a practical option for some CLD patients
DOACs may be a practical option for some CLD patients
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Case
A 60-year-old man with cirrhosis is admitted to the hospital with concern for spontaneous bacterial peritonitis. His body mass index is 35 kg/m2. He is severely deconditioned and largely bed bound. His admission labs show thrombocytopenia (platelets 65,000/mcL) and an elevated international normalized ratio (INR) of 1.6. Should this patient be placed on venous thromboembolism (VTE) prophylaxis on admission?
Brief overview
Patients with chronic liver disease (CLD) have previously been considered “auto-anticoagulated” because of markers of increased bleeding risk, including a decreased platelet count and elevated INR, prothrombin time, and activated partial thromboplastin time. It is being increasingly recognized, however, that CLD often represents a hypercoagulable state despite these abnormalities.1
While cirrhotic patients produce less of several procoagulant substances (such as factors II, V, VII, X, XI, XII, XIII, and fibrinogen), they are also deficient in multiple anticoagulant factors (such as proteins C and S and antithrombin) and fibrinolytics (plasminogen). While the prothrombin time and activated partial thromboplastin time are sensitive to levels of procoagulant proteins in plasma, they do not measure response to the natural anticoagulants and therefore do not reflect an accurate picture of a cirrhotic patient’s risk of developing thrombosis. In addition, cirrhotic patients have many other risk factors for thrombosis, including poor functional status, frequent hospitalization, and elevated estrogen levels.
Overview of the data
VTE incidence among patients with CLD has varied across studies, ranging from 0.5% to 6.3%.2 A systemic review of VTE risk in cirrhotic patients concluded that they “have a significant risk of VTE, if not higher than noncirrhotic patients and this risk cannot be trivialized or ignored.”2
In a nationwide Danish case-control study, patients with cirrhosis had a 1.7 times increased risk of VTE, compared with the general population.3 Hypoalbuminemia appears to be one of the strongest associated risk factors for VTE in these patients, likely as a reflection of the degree of liver synthetic dysfunction (and therefore decreased synthesis of anticoagulant factors). One study showed that patients with an albumin of less than 1.9 g/dL had a VTE risk five times higher than patients with an albumin of 2.8 g/dL or higher.4
Prophylaxis
Given the increased risks of bleeding and thrombosis in patients with cirrhosis, how should VTE prophylaxis be managed in hospitalized patients? While current guidelines do not specifically address the use of pharmacologic prophylaxis in cirrhotic patients, the Padua Predictor Score, which is used to assess VTE risk in the general hospital population, has also been shown to be helpful in the subpopulation of patients with CLD (Table 1).

In one study, cirrhotic patients who were “high risk” by Padua Predictor score were over 12 times more likely to develop VTE than those who were “low risk.”5 Bleeding risk appears to be fairly low, and similar to those patients not receiving prophylactic anticoagulation. One retrospective case series of hospitalized cirrhotic patients receiving thromboprophylaxis showed a rate of GI bleeding of 2.5% (9 of 355 patients); the rate of major bleeding was less than 1%.6
Selection of anticoagulant for VTE prophylaxis should be similar to non-CLD patients. The choice of agent (low-molecular-weight heparin (LMWH) or unfractionated heparin) and dosing depends on factors including renal function and bodyweight. If anticoagulation is contraindicated (because of thrombocytopenia, for example), then mechanical prophylaxis should be considered.7
Treatment

What about anticoagulation in patients with a known VTE? Food and Drug Administration safety recommendations are based on Child-Pugh class, although the current data on the safety and efficacy of full dose anticoagulation therapy for VTE in patients with cirrhosis are limited (Table 2). At this point, LMWH is often the preferred choice for anticoagulation in CLD patients. However, some limitations exist including the need for frequent subcutaneous injections and limited reliability of anti–factor Xa levels.
Cirrhotic patients often fail to achieve target anti–factor Xa levels on standard prophylactic and therapeutic doses of enoxaparin. This, however, is likely a lab anomaly as in vitro studies have shown that cirrhotic patients may show an increased response to LMWH despite reduced anti–factor Xa levels.8 Thus, LMWH remains the standard of care for many CLD patients.
The use of vitamin K antagonists (VKAs) such as warfarin for VTE treatment can be difficult to manage. Traditionally CLD patients have been started on lower doses of warfarin but given their already elevated INR, this may lead to a subtherapeutic dose of VKAs. A recent study of 23 patients with cirrhosis demonstrated that a target INR of 2-3 can be reached with VKA doses similar to those in noncirrhotic patients.9 These data support the practice of using the same VKA dosing strategies for CLD patients, and selecting a starting dose based on patient parameters such as age and weight.
While the use of direct oral anticoagulants (DOACs) for this patient population is still not a common practice, they may be a practical option for some CLD patients. A meta-analysis found that the currently used DOACs have no significant risk of drug-induced hepatic injury.10 Currently, only observational data are available to assess the benefits and risks of bleeding with DOACs in this patient population, as patients with significant liver disease were excluded from the major randomized trials.11 DOACs may also represent a complicated choice for some patients given the effect of liver injury on their metabolism (Table 3).
Application of data to the original case
This patient should be assessed for both risk of VTE and risk of bleeding during the hospital admission. CLD patients likely have a risk of VTE similar to (or even greater than) that of general medical patients. The Padua score for this patient is 4 (bed rest, body mass index) indicating that he is at high risk of VTE. While he is thrombocytopenic, he is not below the threshold for receiving anticoagulation. His INR is elevated but this does not confer any reduced risk of VTE.
Bottom line
This patient should receive VTE prophylaxis with either subcutaneous heparin or LMWH during his hospital admission.
References
1. Khoury T et al. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016 Mar;93(2):149-59.
2. Aggarwal A. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J Gastroenterol. 2014 May 21;20(19):5737-45.
3. Søgaard KK et al. Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol. 2009 Jan;104(1):96-101.
4. Walsh KA et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333-9.
5. Bogari H et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb Res. 2014 Dec;134(6):1220-3.
6. Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014 Jan;34(1):26-32.
7. Pincus KJ et al. Risk of venous thromboembolism in patients with chronic liver disease and the utility of venous thromboembolism prophylaxis. Ann Pharmacother. 2012 Jun;46(6):873-8.
8. Lishman T et al. Established and new-generation antithrombotic drugs in patients with cirrhosis – possibilities and caveats. J Hepatol. 2013 Aug;59(2):358-66.
9. Tripodi A et al. Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists. Dig Liver Dis. 2016 Oct;48(10):1208-13.
10. Caldeira D et al. Risk of drug-induced liver injury with the new oral anticoagulants: Systematic review and meta-analysis. Heart. 2014 Apr;100(7):550-6.
11. Qamar A et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018 May 15;71(19):2162-75.
12. Barbar S et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010 Nov;8(11):2450-7.
Early palliative care consult decreases in-hospital mortality
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
REPORTING FROM CHEST 2019
Updated international consensus recommendations on management of acute upper GI bleeding
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
FROM ANNALS OF INTERNAL MEDICINE
Dapagliflozin approved for reducing HF hospitalization in diabetes
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
Certain diabetes drugs may thwart dementia
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
REPORTING FROM ECNP 2019
Guidelines updated for treating community-acquired pneumonia
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE