User login
Update on the Pediatric Dermatology Workforce Shortage
Pediatric dermatology is a relatively young subspecialty. The Society for Pediatric Dermatology (SPD) was established in 1975, followed by the creation of the journal Pediatric Dermatology in 1982 and the American Academy of Pediatrics Section on Dermatology in 1986.1 In 2000, the Accreditation Council for Graduate Medical Education (ACGME) officially recognized pediatric dermatology as a unique subspecialty of the American Board of Dermatology (ABD). During that time, informal fellowship experiences emerged, and formal 1-year training programs approved by the ABD evolved by 2006. A subspecialty certification examination was created and has been administered every other year since 2004.1 Data provided by the SPD indicate that approximately 431 US dermatologists have passed the ABD’s pediatric dermatology board certification examination thus far (unpublished data, September 2021).
In 1986, the first systematic evaluation of the US pediatric dermatology workforce revealed a total of 57 practicing pediatric dermatologists and concluded that job opportunities appeared to be limited at that time.2 Since then, the demand for pediatric dermatology services has continued to grow steadily, and the number of board-certified pediatric dermatologists practicing in the United States has increased to at least 317 per data from a 2020 survey.3 However, given that there are more than 11,000 board-certified dermatologists in the United States, there continues to be a severe shortage of pediatric dermatologists.1
Increased Demand for Pediatric Dermatologists
Approximately 10% to 30% of almost 200 million annual outpatient pediatric primary care visits involve a skin concern. Although many of these problems can be handled by primary care physicians, more than 80% of pediatricians report having difficulty accessing dermatology services for their patients.4 In surveys of pediatricians, pediatric dermatology has the third highest referral rate but has consistently ranked third among the specialties deemed most difficult to access.5-7 In addition, it is not uncommon for the wait time to see a pediatric dermatologist to be 6 weeks or longer.5,8
Recent population data estimate that there are 73 million children living in the United States.9 If there are roughly 317 practicing board-certified pediatric dermatologists, that translates into approximately 4.3 pediatric dermatologists per million children. This number is far smaller than the 4 general dermatologists per 100,000 individuals recommended by Glazer et al10 in 2017. To meet this suggested ratio goal, the workforce of pediatric dermatologists would have to increase to 2920. In addition to this severe workforce shortage, there is an additional problem with geographic maldistribution of pediatric dermatologists. More than 98% of pediatric dermatologists practice in metropolitan areas. At least 8 states and 95% of counties have no pediatric dermatologist, and there are no pediatric dermatologists practicing in rural counties.9 This disparity has considerable implications for barriers to care and lack of access for children living in underserved areas. Suggestions for attracting pediatric dermatologists to practice in these areas have included loan forgiveness programs as well as remote mentorship programs to provide professional support.8,9
Training in Pediatrics
There currently are 38 ABD-approved pediatric dermatology fellowship training programs in the United States. Beginning in 2009, pediatric dermatology fellowship programs have participated in the SF Match program. Data provided by the SPD show that, since 2012, up to 27 programs have participated in the annual Match, offering a total number of positions ranging from 27 to 38; however, only 11 to 21 positions have been filled each year, leaving a large number of post-Match vacancies (unpublished data, September 2021).
Surveys have explored the reasons behind this lack of interest in pediatric dermatology training among dermatology residents. Factors that have been mentioned include lack of exposure and mentorship in medical school and residency, the financial hardship of an additional year of fellowship training, and historically lower salaries for pediatric dermatologists compared to general dermatologists.3,6
A 2004 survey revealed that more than 75% of dermatology department chairs believed it was important to have a pediatric dermatologist on the faculty; however, at that time only 48% of dermatology programs reported having at least 1 full-time pediatric dermatology faculty member.11 By 2008, a follow-up survey showed an increase to 70% of dermatology training programs reporting at least 1 full-time pediatric dermatologist; however, 43% of departments still had at least 1 open position, and 76% of those programs shared that they had been searching for more than 1 year.2 Currently, the Accreditation Data System of the ACGME shows a total of 144 accredited US dermatology training programs. Of those, 117 programs have 1 or more board-certified pediatric dermatology faculty member, and 27 programs still have none (unpublished data, September 2021).
A shortage of pediatric dermatologists in training programs contributes to the lack of exposure and mentorship for medical students and residents during a critical time in professional development. Studies show that up to 91% of pediatric dermatologists decided to pursue training in pediatric dermatology during medical school, pediatrics residency, or dermatology residency. In one survey, 84% of respondents (N=109) cited early mentorship as the most important factor in their decision to pursue pediatric dermatology.6
A lack of pediatric dermatologists also results in suboptimal dermatology training for residents who care for children in primary care specialties, including pediatrics, combined internal medicine and pediatrics, and family practice. Multiple surveys have shown that many pediatricians feel they received inadequate training in dermatology during residency. Up to 38% have cited a need for more pediatric dermatology education (N=755).5,6 In addition, studies show a wide disparity in diagnostic accuracy between dermatologists and pediatricians, with one concluding that more than one-third of referrals to pediatric dermatologists were initially misdiagnosed and/or incorrectly treated.5,7
Recruitment Efforts for Pediatric Dermatologists
There are multiple strategies for recruiting trainees into the pediatric dermatology workforce. First, given the importance of early exposure to the field and role models/mentors, pediatric dermatologists must take advantage of every opportunity to interact with medical students and residents. They can share their genuine enthusiasm and love for the specialty while encouraging and supporting those who show interest. They also should seek opportunities for teaching, lecturing, and advising at every level of training. In addition, they can enhance visibility of the specialty by participating in career forums and/or assuming leadership roles within their departments or institutions.12 Another suggestion is for dermatology training programs to consider giving priority to qualified applicants who express sincere interest in pursuing pediatric dermatology training (including those who have already completed pediatrics residency). Although a 2008 survey revealed that 39% of dermatology residency programs (N=80) favored giving priority to applicants demonstrating interest in pediatric dermatology, others were against it, citing issues such as lack of funding for additional residency training, lack of pediatric dermatology mentors within the program, and an overall mistrust of applicants’ sincerity.2
Final Thoughts
The subspecialty of pediatric dermatology has experienced remarkable growth over the last 40 years; however, demand for pediatric dermatology services has continued to outpace supply, resulting in a persistent and notable workforce shortage. Overall, the current supply of pediatric dermatologists can neither meet the clinical demands of the pediatric population nor fulfill academic needs of existing training programs. We must continue to develop novel strategies for increasing the pool of students and residents who are interested in pursuing careers in pediatric dermatology. Ultimately, we also must create incentives and develop tactics to address the geographic maldistribution that exists within the specialty.
- Prindaville B, Antaya R, Siegfried E. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12.
- Craiglow BG, Resneck JS, Lucky AW, et al. Pediatric dermatology workforce shortage: perspectives from academia. J Am Acad Dermatol. 2008;59:986-989.
- Ashrafzadeh S, Peters G, Brandling-Bennett H, et al. The geographic distribution of the US pediatric dermatologist workforce: a national cross-sectional study. Pediatr Dermatol. 2020;37:1098-1105.
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. Pediatr Dermatol. 2019;36:893-897.
- Prindaville B, Simon S, Horii K. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Admani S, Caufield M, Kim S, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Prindaville B, Horii K, Siegfried E, et al. Pediatric dermatology workforce in the United States. Pediatr Dermatol. 2019;36:166-168.
- Ugwu-Dike P, Nambudiri V. Access as equity: addressing the distribution of the pediatric dermatology workforce [published online August 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14665
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Wright TS, Huang JT. Comment on “pediatric dermatology workforce in the United States”. Pediatr Dermatol. 2019;36:177-178.
Pediatric dermatology is a relatively young subspecialty. The Society for Pediatric Dermatology (SPD) was established in 1975, followed by the creation of the journal Pediatric Dermatology in 1982 and the American Academy of Pediatrics Section on Dermatology in 1986.1 In 2000, the Accreditation Council for Graduate Medical Education (ACGME) officially recognized pediatric dermatology as a unique subspecialty of the American Board of Dermatology (ABD). During that time, informal fellowship experiences emerged, and formal 1-year training programs approved by the ABD evolved by 2006. A subspecialty certification examination was created and has been administered every other year since 2004.1 Data provided by the SPD indicate that approximately 431 US dermatologists have passed the ABD’s pediatric dermatology board certification examination thus far (unpublished data, September 2021).
In 1986, the first systematic evaluation of the US pediatric dermatology workforce revealed a total of 57 practicing pediatric dermatologists and concluded that job opportunities appeared to be limited at that time.2 Since then, the demand for pediatric dermatology services has continued to grow steadily, and the number of board-certified pediatric dermatologists practicing in the United States has increased to at least 317 per data from a 2020 survey.3 However, given that there are more than 11,000 board-certified dermatologists in the United States, there continues to be a severe shortage of pediatric dermatologists.1
Increased Demand for Pediatric Dermatologists
Approximately 10% to 30% of almost 200 million annual outpatient pediatric primary care visits involve a skin concern. Although many of these problems can be handled by primary care physicians, more than 80% of pediatricians report having difficulty accessing dermatology services for their patients.4 In surveys of pediatricians, pediatric dermatology has the third highest referral rate but has consistently ranked third among the specialties deemed most difficult to access.5-7 In addition, it is not uncommon for the wait time to see a pediatric dermatologist to be 6 weeks or longer.5,8
Recent population data estimate that there are 73 million children living in the United States.9 If there are roughly 317 practicing board-certified pediatric dermatologists, that translates into approximately 4.3 pediatric dermatologists per million children. This number is far smaller than the 4 general dermatologists per 100,000 individuals recommended by Glazer et al10 in 2017. To meet this suggested ratio goal, the workforce of pediatric dermatologists would have to increase to 2920. In addition to this severe workforce shortage, there is an additional problem with geographic maldistribution of pediatric dermatologists. More than 98% of pediatric dermatologists practice in metropolitan areas. At least 8 states and 95% of counties have no pediatric dermatologist, and there are no pediatric dermatologists practicing in rural counties.9 This disparity has considerable implications for barriers to care and lack of access for children living in underserved areas. Suggestions for attracting pediatric dermatologists to practice in these areas have included loan forgiveness programs as well as remote mentorship programs to provide professional support.8,9
Training in Pediatrics
There currently are 38 ABD-approved pediatric dermatology fellowship training programs in the United States. Beginning in 2009, pediatric dermatology fellowship programs have participated in the SF Match program. Data provided by the SPD show that, since 2012, up to 27 programs have participated in the annual Match, offering a total number of positions ranging from 27 to 38; however, only 11 to 21 positions have been filled each year, leaving a large number of post-Match vacancies (unpublished data, September 2021).
Surveys have explored the reasons behind this lack of interest in pediatric dermatology training among dermatology residents. Factors that have been mentioned include lack of exposure and mentorship in medical school and residency, the financial hardship of an additional year of fellowship training, and historically lower salaries for pediatric dermatologists compared to general dermatologists.3,6
A 2004 survey revealed that more than 75% of dermatology department chairs believed it was important to have a pediatric dermatologist on the faculty; however, at that time only 48% of dermatology programs reported having at least 1 full-time pediatric dermatology faculty member.11 By 2008, a follow-up survey showed an increase to 70% of dermatology training programs reporting at least 1 full-time pediatric dermatologist; however, 43% of departments still had at least 1 open position, and 76% of those programs shared that they had been searching for more than 1 year.2 Currently, the Accreditation Data System of the ACGME shows a total of 144 accredited US dermatology training programs. Of those, 117 programs have 1 or more board-certified pediatric dermatology faculty member, and 27 programs still have none (unpublished data, September 2021).
A shortage of pediatric dermatologists in training programs contributes to the lack of exposure and mentorship for medical students and residents during a critical time in professional development. Studies show that up to 91% of pediatric dermatologists decided to pursue training in pediatric dermatology during medical school, pediatrics residency, or dermatology residency. In one survey, 84% of respondents (N=109) cited early mentorship as the most important factor in their decision to pursue pediatric dermatology.6
A lack of pediatric dermatologists also results in suboptimal dermatology training for residents who care for children in primary care specialties, including pediatrics, combined internal medicine and pediatrics, and family practice. Multiple surveys have shown that many pediatricians feel they received inadequate training in dermatology during residency. Up to 38% have cited a need for more pediatric dermatology education (N=755).5,6 In addition, studies show a wide disparity in diagnostic accuracy between dermatologists and pediatricians, with one concluding that more than one-third of referrals to pediatric dermatologists were initially misdiagnosed and/or incorrectly treated.5,7
Recruitment Efforts for Pediatric Dermatologists
There are multiple strategies for recruiting trainees into the pediatric dermatology workforce. First, given the importance of early exposure to the field and role models/mentors, pediatric dermatologists must take advantage of every opportunity to interact with medical students and residents. They can share their genuine enthusiasm and love for the specialty while encouraging and supporting those who show interest. They also should seek opportunities for teaching, lecturing, and advising at every level of training. In addition, they can enhance visibility of the specialty by participating in career forums and/or assuming leadership roles within their departments or institutions.12 Another suggestion is for dermatology training programs to consider giving priority to qualified applicants who express sincere interest in pursuing pediatric dermatology training (including those who have already completed pediatrics residency). Although a 2008 survey revealed that 39% of dermatology residency programs (N=80) favored giving priority to applicants demonstrating interest in pediatric dermatology, others were against it, citing issues such as lack of funding for additional residency training, lack of pediatric dermatology mentors within the program, and an overall mistrust of applicants’ sincerity.2
Final Thoughts
The subspecialty of pediatric dermatology has experienced remarkable growth over the last 40 years; however, demand for pediatric dermatology services has continued to outpace supply, resulting in a persistent and notable workforce shortage. Overall, the current supply of pediatric dermatologists can neither meet the clinical demands of the pediatric population nor fulfill academic needs of existing training programs. We must continue to develop novel strategies for increasing the pool of students and residents who are interested in pursuing careers in pediatric dermatology. Ultimately, we also must create incentives and develop tactics to address the geographic maldistribution that exists within the specialty.
Pediatric dermatology is a relatively young subspecialty. The Society for Pediatric Dermatology (SPD) was established in 1975, followed by the creation of the journal Pediatric Dermatology in 1982 and the American Academy of Pediatrics Section on Dermatology in 1986.1 In 2000, the Accreditation Council for Graduate Medical Education (ACGME) officially recognized pediatric dermatology as a unique subspecialty of the American Board of Dermatology (ABD). During that time, informal fellowship experiences emerged, and formal 1-year training programs approved by the ABD evolved by 2006. A subspecialty certification examination was created and has been administered every other year since 2004.1 Data provided by the SPD indicate that approximately 431 US dermatologists have passed the ABD’s pediatric dermatology board certification examination thus far (unpublished data, September 2021).
In 1986, the first systematic evaluation of the US pediatric dermatology workforce revealed a total of 57 practicing pediatric dermatologists and concluded that job opportunities appeared to be limited at that time.2 Since then, the demand for pediatric dermatology services has continued to grow steadily, and the number of board-certified pediatric dermatologists practicing in the United States has increased to at least 317 per data from a 2020 survey.3 However, given that there are more than 11,000 board-certified dermatologists in the United States, there continues to be a severe shortage of pediatric dermatologists.1
Increased Demand for Pediatric Dermatologists
Approximately 10% to 30% of almost 200 million annual outpatient pediatric primary care visits involve a skin concern. Although many of these problems can be handled by primary care physicians, more than 80% of pediatricians report having difficulty accessing dermatology services for their patients.4 In surveys of pediatricians, pediatric dermatology has the third highest referral rate but has consistently ranked third among the specialties deemed most difficult to access.5-7 In addition, it is not uncommon for the wait time to see a pediatric dermatologist to be 6 weeks or longer.5,8
Recent population data estimate that there are 73 million children living in the United States.9 If there are roughly 317 practicing board-certified pediatric dermatologists, that translates into approximately 4.3 pediatric dermatologists per million children. This number is far smaller than the 4 general dermatologists per 100,000 individuals recommended by Glazer et al10 in 2017. To meet this suggested ratio goal, the workforce of pediatric dermatologists would have to increase to 2920. In addition to this severe workforce shortage, there is an additional problem with geographic maldistribution of pediatric dermatologists. More than 98% of pediatric dermatologists practice in metropolitan areas. At least 8 states and 95% of counties have no pediatric dermatologist, and there are no pediatric dermatologists practicing in rural counties.9 This disparity has considerable implications for barriers to care and lack of access for children living in underserved areas. Suggestions for attracting pediatric dermatologists to practice in these areas have included loan forgiveness programs as well as remote mentorship programs to provide professional support.8,9
Training in Pediatrics
There currently are 38 ABD-approved pediatric dermatology fellowship training programs in the United States. Beginning in 2009, pediatric dermatology fellowship programs have participated in the SF Match program. Data provided by the SPD show that, since 2012, up to 27 programs have participated in the annual Match, offering a total number of positions ranging from 27 to 38; however, only 11 to 21 positions have been filled each year, leaving a large number of post-Match vacancies (unpublished data, September 2021).
Surveys have explored the reasons behind this lack of interest in pediatric dermatology training among dermatology residents. Factors that have been mentioned include lack of exposure and mentorship in medical school and residency, the financial hardship of an additional year of fellowship training, and historically lower salaries for pediatric dermatologists compared to general dermatologists.3,6
A 2004 survey revealed that more than 75% of dermatology department chairs believed it was important to have a pediatric dermatologist on the faculty; however, at that time only 48% of dermatology programs reported having at least 1 full-time pediatric dermatology faculty member.11 By 2008, a follow-up survey showed an increase to 70% of dermatology training programs reporting at least 1 full-time pediatric dermatologist; however, 43% of departments still had at least 1 open position, and 76% of those programs shared that they had been searching for more than 1 year.2 Currently, the Accreditation Data System of the ACGME shows a total of 144 accredited US dermatology training programs. Of those, 117 programs have 1 or more board-certified pediatric dermatology faculty member, and 27 programs still have none (unpublished data, September 2021).
A shortage of pediatric dermatologists in training programs contributes to the lack of exposure and mentorship for medical students and residents during a critical time in professional development. Studies show that up to 91% of pediatric dermatologists decided to pursue training in pediatric dermatology during medical school, pediatrics residency, or dermatology residency. In one survey, 84% of respondents (N=109) cited early mentorship as the most important factor in their decision to pursue pediatric dermatology.6
A lack of pediatric dermatologists also results in suboptimal dermatology training for residents who care for children in primary care specialties, including pediatrics, combined internal medicine and pediatrics, and family practice. Multiple surveys have shown that many pediatricians feel they received inadequate training in dermatology during residency. Up to 38% have cited a need for more pediatric dermatology education (N=755).5,6 In addition, studies show a wide disparity in diagnostic accuracy between dermatologists and pediatricians, with one concluding that more than one-third of referrals to pediatric dermatologists were initially misdiagnosed and/or incorrectly treated.5,7
Recruitment Efforts for Pediatric Dermatologists
There are multiple strategies for recruiting trainees into the pediatric dermatology workforce. First, given the importance of early exposure to the field and role models/mentors, pediatric dermatologists must take advantage of every opportunity to interact with medical students and residents. They can share their genuine enthusiasm and love for the specialty while encouraging and supporting those who show interest. They also should seek opportunities for teaching, lecturing, and advising at every level of training. In addition, they can enhance visibility of the specialty by participating in career forums and/or assuming leadership roles within their departments or institutions.12 Another suggestion is for dermatology training programs to consider giving priority to qualified applicants who express sincere interest in pursuing pediatric dermatology training (including those who have already completed pediatrics residency). Although a 2008 survey revealed that 39% of dermatology residency programs (N=80) favored giving priority to applicants demonstrating interest in pediatric dermatology, others were against it, citing issues such as lack of funding for additional residency training, lack of pediatric dermatology mentors within the program, and an overall mistrust of applicants’ sincerity.2
Final Thoughts
The subspecialty of pediatric dermatology has experienced remarkable growth over the last 40 years; however, demand for pediatric dermatology services has continued to outpace supply, resulting in a persistent and notable workforce shortage. Overall, the current supply of pediatric dermatologists can neither meet the clinical demands of the pediatric population nor fulfill academic needs of existing training programs. We must continue to develop novel strategies for increasing the pool of students and residents who are interested in pursuing careers in pediatric dermatology. Ultimately, we also must create incentives and develop tactics to address the geographic maldistribution that exists within the specialty.
- Prindaville B, Antaya R, Siegfried E. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12.
- Craiglow BG, Resneck JS, Lucky AW, et al. Pediatric dermatology workforce shortage: perspectives from academia. J Am Acad Dermatol. 2008;59:986-989.
- Ashrafzadeh S, Peters G, Brandling-Bennett H, et al. The geographic distribution of the US pediatric dermatologist workforce: a national cross-sectional study. Pediatr Dermatol. 2020;37:1098-1105.
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. Pediatr Dermatol. 2019;36:893-897.
- Prindaville B, Simon S, Horii K. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Admani S, Caufield M, Kim S, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Prindaville B, Horii K, Siegfried E, et al. Pediatric dermatology workforce in the United States. Pediatr Dermatol. 2019;36:166-168.
- Ugwu-Dike P, Nambudiri V. Access as equity: addressing the distribution of the pediatric dermatology workforce [published online August 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14665
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Wright TS, Huang JT. Comment on “pediatric dermatology workforce in the United States”. Pediatr Dermatol. 2019;36:177-178.
- Prindaville B, Antaya R, Siegfried E. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12.
- Craiglow BG, Resneck JS, Lucky AW, et al. Pediatric dermatology workforce shortage: perspectives from academia. J Am Acad Dermatol. 2008;59:986-989.
- Ashrafzadeh S, Peters G, Brandling-Bennett H, et al. The geographic distribution of the US pediatric dermatologist workforce: a national cross-sectional study. Pediatr Dermatol. 2020;37:1098-1105.
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. Pediatr Dermatol. 2019;36:893-897.
- Prindaville B, Simon S, Horii K. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Admani S, Caufield M, Kim S, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Prindaville B, Horii K, Siegfried E, et al. Pediatric dermatology workforce in the United States. Pediatr Dermatol. 2019;36:166-168.
- Ugwu-Dike P, Nambudiri V. Access as equity: addressing the distribution of the pediatric dermatology workforce [published online August 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14665
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Wright TS, Huang JT. Comment on “pediatric dermatology workforce in the United States”. Pediatr Dermatol. 2019;36:177-178.
Time to retire race- and ethnicity-based carrier screening
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
TANS Syndrome: Tanorexia, Anorexia, and Nonmelanoma Skin Cancer
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
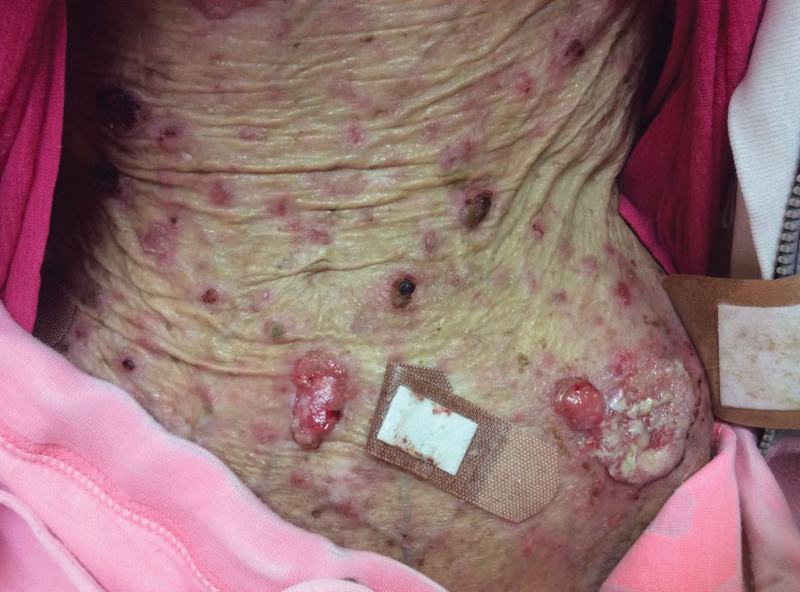
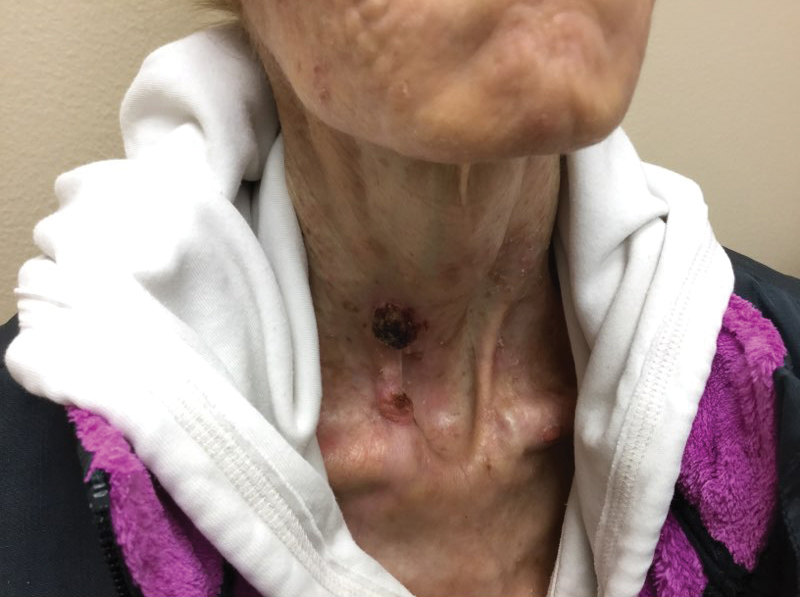
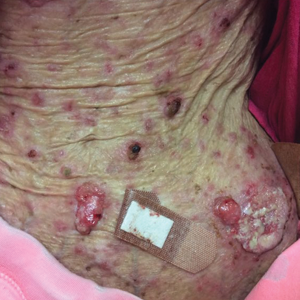
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
Practice Points
- Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.
- Comorbidities related to TANS syndrome make it challenging to effectively treat cutaneous squamous cell carcinoma.
Q&A: Meeting the challenge of giving COVID vaccines to younger kids
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
Time to attack hypoactivity in our children
My 50th medical school reunion has come and gone. This milestone offered me another opportunity to look back over the last 5 decades of pediatrics that I have watched pass under the bridge. Triggered by the discovery of two recently published studies, this particular view back over my shoulder induced a wave of sadness, anger, and frustration that I have had trouble shaking.
The first study demonstrated a strong positive effect of exercise on academic achievement, the other found that children who were more physically active have weathered the pandemic with fewer mental health problems.
These studies are just two pieces of a growing body of evidence that our sedentary lifestyles are shortening our lives and launching our children into adulthood burdened with a raft of health risks they could possibly have avoided by being more physically active. Encountering these two papers just as the alumni office was inviting me to engage in an orgy of retrospection and introspection made me consider how little I and others in my profession have done to substantially address this scourge on our young people.
Yes, I have tried to encourage my patients to be less sedentary and more active. Yes, I have tried to set a very visible example by bicycling and walking around town. Yes, I have coached youth sports teams. All of my children and grandchildren are leading active lives and appear to be reaping the benefits. But in the grander scheme of things I feel that neither I nor the American Academy of Pediatrics has made a difference.
In March of 2020 the AAP published a clinical report that lists the numerous positive associations between activity and health that includes a comprehensive collection of suggestions for providers on how we might assess the problem of inactivity and then play a role in addressing it with our patients and our communities. Unfortunately, the message’s importance was lost in the glut of pandemic news.
While the AAP’s report should have been published many decades ago, I doubt the delay lessened its impact significantly because the report is primarily a compendium of recommendations that in the long run will be seen as just another example of us believers preaching to the choir.
Making lifestyle changes on the order of magnitude necessary to convert an increasingly sedentary population into one that unconsciously becomes physically active requires more than recommendations. It is only natural that folks have trouble saying “No.”
No to the entertainment of electronic devices. No to the comforts of all-weather enclosed transportation. No to hours on the couch. Overcoming the inertia built into our society is going to require more than encouragement, recommendations, and professional sports–sponsored presidential initiatives.
Mandate has become a politically charged dirty word. But our current experience with the COVID-19 vaccines should help us realize that there is a significant segment of the population that doesn’t like being told what to do even if the outcome is in their best interest. Education and rewards have fallen short, but the evidence is mounting that mandates can work.
There was a time when physical activity was built into every child’s school day. For a variety of bad reasons, vigorous physical education classes and once- or twice-daily outdoor recesses have disappeared from the educational landscape. It is time to return to them in a robust form. Unfortunately, because activity isn’t happening at home it will take a government mandate.
There will be pushback. Even from some educators whose observations should have shown them the critical role of physical activity in health and academic success. We must move the distraction of the phenomenon once known simply as hyperactivity to the back burner and tackle the real epidemic of hypoactivity that is destroying our children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
My 50th medical school reunion has come and gone. This milestone offered me another opportunity to look back over the last 5 decades of pediatrics that I have watched pass under the bridge. Triggered by the discovery of two recently published studies, this particular view back over my shoulder induced a wave of sadness, anger, and frustration that I have had trouble shaking.
The first study demonstrated a strong positive effect of exercise on academic achievement, the other found that children who were more physically active have weathered the pandemic with fewer mental health problems.
These studies are just two pieces of a growing body of evidence that our sedentary lifestyles are shortening our lives and launching our children into adulthood burdened with a raft of health risks they could possibly have avoided by being more physically active. Encountering these two papers just as the alumni office was inviting me to engage in an orgy of retrospection and introspection made me consider how little I and others in my profession have done to substantially address this scourge on our young people.
Yes, I have tried to encourage my patients to be less sedentary and more active. Yes, I have tried to set a very visible example by bicycling and walking around town. Yes, I have coached youth sports teams. All of my children and grandchildren are leading active lives and appear to be reaping the benefits. But in the grander scheme of things I feel that neither I nor the American Academy of Pediatrics has made a difference.
In March of 2020 the AAP published a clinical report that lists the numerous positive associations between activity and health that includes a comprehensive collection of suggestions for providers on how we might assess the problem of inactivity and then play a role in addressing it with our patients and our communities. Unfortunately, the message’s importance was lost in the glut of pandemic news.
While the AAP’s report should have been published many decades ago, I doubt the delay lessened its impact significantly because the report is primarily a compendium of recommendations that in the long run will be seen as just another example of us believers preaching to the choir.
Making lifestyle changes on the order of magnitude necessary to convert an increasingly sedentary population into one that unconsciously becomes physically active requires more than recommendations. It is only natural that folks have trouble saying “No.”
No to the entertainment of electronic devices. No to the comforts of all-weather enclosed transportation. No to hours on the couch. Overcoming the inertia built into our society is going to require more than encouragement, recommendations, and professional sports–sponsored presidential initiatives.
Mandate has become a politically charged dirty word. But our current experience with the COVID-19 vaccines should help us realize that there is a significant segment of the population that doesn’t like being told what to do even if the outcome is in their best interest. Education and rewards have fallen short, but the evidence is mounting that mandates can work.
There was a time when physical activity was built into every child’s school day. For a variety of bad reasons, vigorous physical education classes and once- or twice-daily outdoor recesses have disappeared from the educational landscape. It is time to return to them in a robust form. Unfortunately, because activity isn’t happening at home it will take a government mandate.
There will be pushback. Even from some educators whose observations should have shown them the critical role of physical activity in health and academic success. We must move the distraction of the phenomenon once known simply as hyperactivity to the back burner and tackle the real epidemic of hypoactivity that is destroying our children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
My 50th medical school reunion has come and gone. This milestone offered me another opportunity to look back over the last 5 decades of pediatrics that I have watched pass under the bridge. Triggered by the discovery of two recently published studies, this particular view back over my shoulder induced a wave of sadness, anger, and frustration that I have had trouble shaking.
The first study demonstrated a strong positive effect of exercise on academic achievement, the other found that children who were more physically active have weathered the pandemic with fewer mental health problems.
These studies are just two pieces of a growing body of evidence that our sedentary lifestyles are shortening our lives and launching our children into adulthood burdened with a raft of health risks they could possibly have avoided by being more physically active. Encountering these two papers just as the alumni office was inviting me to engage in an orgy of retrospection and introspection made me consider how little I and others in my profession have done to substantially address this scourge on our young people.
Yes, I have tried to encourage my patients to be less sedentary and more active. Yes, I have tried to set a very visible example by bicycling and walking around town. Yes, I have coached youth sports teams. All of my children and grandchildren are leading active lives and appear to be reaping the benefits. But in the grander scheme of things I feel that neither I nor the American Academy of Pediatrics has made a difference.
In March of 2020 the AAP published a clinical report that lists the numerous positive associations between activity and health that includes a comprehensive collection of suggestions for providers on how we might assess the problem of inactivity and then play a role in addressing it with our patients and our communities. Unfortunately, the message’s importance was lost in the glut of pandemic news.
While the AAP’s report should have been published many decades ago, I doubt the delay lessened its impact significantly because the report is primarily a compendium of recommendations that in the long run will be seen as just another example of us believers preaching to the choir.
Making lifestyle changes on the order of magnitude necessary to convert an increasingly sedentary population into one that unconsciously becomes physically active requires more than recommendations. It is only natural that folks have trouble saying “No.”
No to the entertainment of electronic devices. No to the comforts of all-weather enclosed transportation. No to hours on the couch. Overcoming the inertia built into our society is going to require more than encouragement, recommendations, and professional sports–sponsored presidential initiatives.
Mandate has become a politically charged dirty word. But our current experience with the COVID-19 vaccines should help us realize that there is a significant segment of the population that doesn’t like being told what to do even if the outcome is in their best interest. Education and rewards have fallen short, but the evidence is mounting that mandates can work.
There was a time when physical activity was built into every child’s school day. For a variety of bad reasons, vigorous physical education classes and once- or twice-daily outdoor recesses have disappeared from the educational landscape. It is time to return to them in a robust form. Unfortunately, because activity isn’t happening at home it will take a government mandate.
There will be pushback. Even from some educators whose observations should have shown them the critical role of physical activity in health and academic success. We must move the distraction of the phenomenon once known simply as hyperactivity to the back burner and tackle the real epidemic of hypoactivity that is destroying our children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Boxed warnings: Legal risks that many physicians never see coming
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Giving thanks
Thanksgiving has long been my favorite holiday: a chance to reconnect with family and friends as well as time for reflection, gratitude, and hope. While Thanksgiving 2020 (sadly) was spent eating takeout turkey on the couch due to the pandemic, I am hopeful that Thanksgiving 2021 will for most of us bring a return to the holiday traditions that sustain us.
In this month’s issue of GIHN, we highlight several important studies impacting frontline clinical practice. Relevant to patients with liver disease, we highlight work evaluating the potential supra-additive effects of alcohol intake and obesity in impacting cirrhosis incidence and assessing the comparative performance of non-invasive screening tests in detecting NASH-related fibrosis. Another study of note, relevant to clinical management of GERD, suggests that combinations of abnormal pH-impedance monitoring metrics may predict PPI nonresponders better than individual metrics and could be used to identify patients more likely to respond to invasive GERD management.
We also wish to acknowledge in this issue the outstanding work that AGA and its fellow societies do on behalf of the gastroenterology community in developing and harmonizing ACGME Reporting Milestones for GI and Transplant Hepatology fellowship programs to assist with trainee assessment. Our fellowship trainees represent the future of our profession, and it is of critical importance that we train competent, compassionate professionals who will provide outstanding clinical care to our patients. Kudos to the team, including Dr. Brijen Shah, GI & Hepatology News associate editor Dr. Janice Jou, and others, for their hard work on Milestones 2.0!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Thanksgiving has long been my favorite holiday: a chance to reconnect with family and friends as well as time for reflection, gratitude, and hope. While Thanksgiving 2020 (sadly) was spent eating takeout turkey on the couch due to the pandemic, I am hopeful that Thanksgiving 2021 will for most of us bring a return to the holiday traditions that sustain us.
In this month’s issue of GIHN, we highlight several important studies impacting frontline clinical practice. Relevant to patients with liver disease, we highlight work evaluating the potential supra-additive effects of alcohol intake and obesity in impacting cirrhosis incidence and assessing the comparative performance of non-invasive screening tests in detecting NASH-related fibrosis. Another study of note, relevant to clinical management of GERD, suggests that combinations of abnormal pH-impedance monitoring metrics may predict PPI nonresponders better than individual metrics and could be used to identify patients more likely to respond to invasive GERD management.
We also wish to acknowledge in this issue the outstanding work that AGA and its fellow societies do on behalf of the gastroenterology community in developing and harmonizing ACGME Reporting Milestones for GI and Transplant Hepatology fellowship programs to assist with trainee assessment. Our fellowship trainees represent the future of our profession, and it is of critical importance that we train competent, compassionate professionals who will provide outstanding clinical care to our patients. Kudos to the team, including Dr. Brijen Shah, GI & Hepatology News associate editor Dr. Janice Jou, and others, for their hard work on Milestones 2.0!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Thanksgiving has long been my favorite holiday: a chance to reconnect with family and friends as well as time for reflection, gratitude, and hope. While Thanksgiving 2020 (sadly) was spent eating takeout turkey on the couch due to the pandemic, I am hopeful that Thanksgiving 2021 will for most of us bring a return to the holiday traditions that sustain us.
In this month’s issue of GIHN, we highlight several important studies impacting frontline clinical practice. Relevant to patients with liver disease, we highlight work evaluating the potential supra-additive effects of alcohol intake and obesity in impacting cirrhosis incidence and assessing the comparative performance of non-invasive screening tests in detecting NASH-related fibrosis. Another study of note, relevant to clinical management of GERD, suggests that combinations of abnormal pH-impedance monitoring metrics may predict PPI nonresponders better than individual metrics and could be used to identify patients more likely to respond to invasive GERD management.
We also wish to acknowledge in this issue the outstanding work that AGA and its fellow societies do on behalf of the gastroenterology community in developing and harmonizing ACGME Reporting Milestones for GI and Transplant Hepatology fellowship programs to assist with trainee assessment. Our fellowship trainees represent the future of our profession, and it is of critical importance that we train competent, compassionate professionals who will provide outstanding clinical care to our patients. Kudos to the team, including Dr. Brijen Shah, GI & Hepatology News associate editor Dr. Janice Jou, and others, for their hard work on Milestones 2.0!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Medical transition in transgender patients
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Iatrogenic hyponatremia in a patient with bipolar disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.
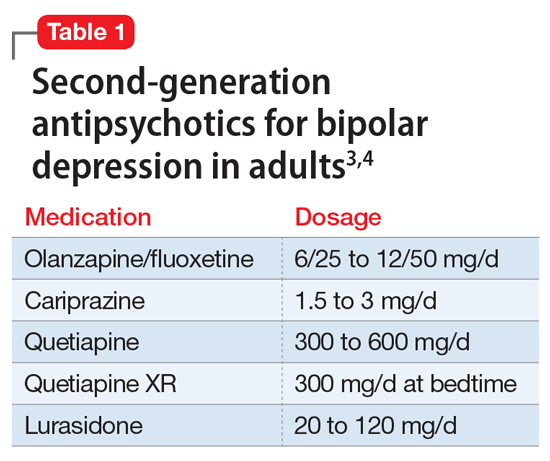
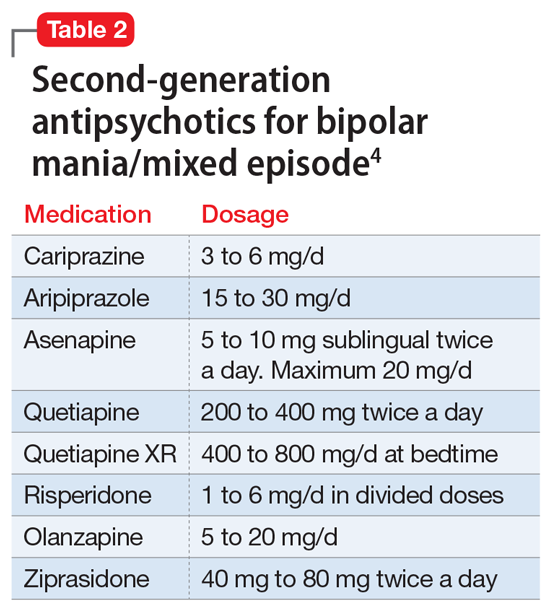
The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.
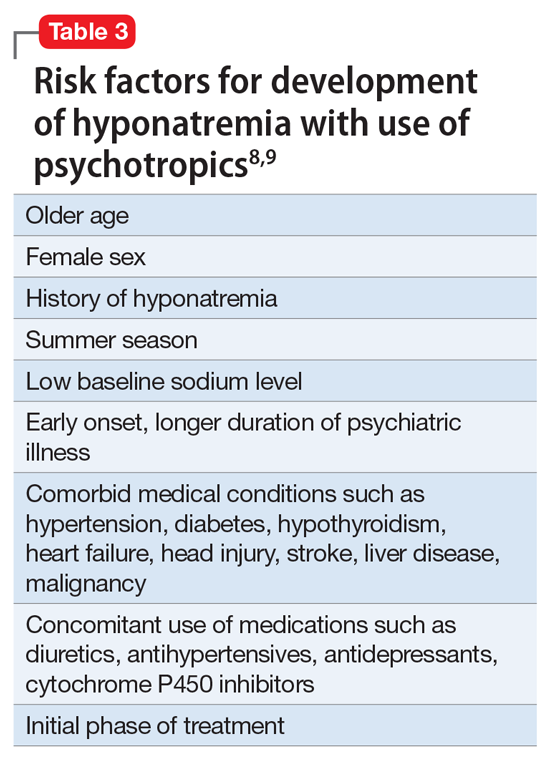
Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.