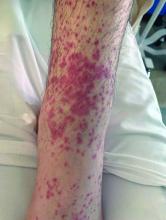
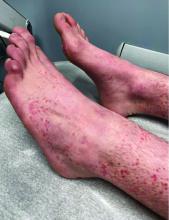
User login
When the evidence suggests that placebo is best
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
Words from the wise
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Britney Spears – Reflections on conservatorship
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
Embezzlement: It can happen to you
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Sea buckthorn: What is it and what is it good for?
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
What is the diagnosis?
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Aaron Beck: An appreciation
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Unmasking Our Grief
Since the start of the pandemic, health care systems have requested many in-services for staff on self-care and stress management to help health care workers (HCWs) cope with the heavy toll of COVID-19. The pandemic has set off a global mental health crisis, with unprecedented numbers of individuals meeting criteria for anxiety, depression, and other mental health disorders in response to the intense stressors of living through a pandemic. These calls to assist staff with self-care and burnout prevention have been especially salient for psychologists working in palliative care and geriatrics, where fears of COVID-19 infection and numbers of patient deaths have been high.
Throughout these painful times, we have been grateful for an online community of palliative care psychologists within the US Department of Veterans Affairs (VA) from across the continuum of care and across the country. This community brought together many of us who were both struggling ourselves and striving to support the teams and HCWs around us. We are psychologists who provide home-care services in North Carolina, inpatient hospice and long-term care services in California, and long-term care and outpatient palliative care services in Massachusetts. Through our shared struggles and challenges navigating the pandemic, we realized that our respective teams requested similar services, all focused on staff support.
The psychological impact of COVID-19 on HCWs was clear from the beginning. Early in the pandemic our respective teams requested us to provide staff support and education about coping to our local HCWs. Soon national groups for long-term care staff requested education programs. Through this work, we realized that the emotional needs of HCWs ran much deeper than simple self-care. At the onset of the pandemic, before realizing its chronicity, the trainings we offered focused on stress and coping strategies. We cited several frameworks for staff support and eagerly shared anything that might help us, and our colleagues, survive the immediate anxiety and tumult surrounding us.1-3 In this paper, we briefly discuss the distress affecting the geriatric care workforce, reflect on our efforts to cope as HCWs, and offer recommendations at individual and organization levels to help address our collective grief.
Impact of COVID-19
As the death toll mounted and hospitals were pushed to the brink, we saw the suffering of our fellow HCWs. The lack of personal protective equipment (PPE) and testing supplies led to evolving and increasing anxiety for HCWs about contracting COVID-19, potentially spreading it to one’s social circle or family, fears of becoming sick and dying, and fears of inadvertently spreading the virus to medically-vulnerable patients. Increasing demands on staff required many to work outside their areas of expertise. Clinical practice guidelines changed frequently as information emerged about the virus. Staff members struggled to keep pace with the increasing number of patients, many of whom died despite heroic efforts to save them.
As the medical crisis grew, so too did social uprisings as the general public gained a strengthened awareness of the legacy and ongoing effects of systemic oppression, racism, and social inequities in the United States. Individuals grappled with their own privileges, which often hid such disparities from view. Many HCWs and clinicians of color had to navigate unsolicited questions and discussions about racial injustices while also trying to survive. As psychologists, we strove to support the HCWs around us while also struggling with our own stressors. As the magnitude of the pandemic and ongoing social injustices came into view, we realized that presentations on self-care and burnout prevention did not suffice. We needed discussions on unmasking our grief, acknowledging our traumas, and working toward collective healing.
Geriatric Care Workers
Experiences of grief and trauma hit the geriatric care workforce and especially long-term care facilities particularly hard given the high morbidity and mortality rates of COVID-19.4 The geriatric care workforce itself suffers from institutional vulnerabilities. Individuals are often underpaid, undertrained, and work within a system that continually experiences staffing shortages, high burnout, and consequently high levels of turnover.5,6 Recent immigrants and racial/ethnic minorities disproportionately make up this workforce, who often live in multigenerational households and work in multiple facilities to get by.7,8 Amid the pandemic these HCWs continued to work despite demoralizing negative media coverage of nursing homes.9 Notably, facilities with unionized staff were less likely to need second or third jobs to survive, thus reducing spread across facilities. This along with better access to PPE may have contributed to their lower COVID-19 infection and mortality rates relative to non-unionized staff.10
Similar to long-term care workers, home-care staff had related fears and anxieties, magnified by the need to enter multiple homes. This often overlooked but growing sector of the geriatric care workforce faced the added anxiety of the unknown as they entered multiple homes to provide care to their patients. These staff have little control over who may be in the home when they arrive, the sanitation/PPE practices of the patient/family, and therefore little control over their potential exposure to COVID-19. This also applies to home health aides who, although not providing medical services, are a critical part of home-care services and allow older adults to remain living independently in their home.
Reflection on Grief
As we witnessed the interactive effects of the pandemic and social inequities in geriatrics and palliative care, we frequently sought solace in online communities of psychologists working in similar settings. Over time, our regular community meetings developed a different tone: discussions about caring for others shifted to caring for ourselves. It seemed that in holding others’ pain, many of us neglected to address our own. We needed emotional support. We needed to acknowledge that we were not all okay; that the masks we wear for protection also reveal our vulnerabilities; and that protective equipment in hospitals do not protect us from the hate and bias targeting many of us face everywhere we go.
As we let ourselves be vulnerable with each other, we saw the true face of our pain: it was not stress, it was grief. We were sad, broken, mourning innumerable losses, and grieving, mostly alone. It felt overwhelming. Our minds and hearts often grew numb to find respite from pain. At times we found ourselves seeking haven in our offices, convincing ourselves that paperwork needed to be done when in reality we had no space to hold anyone else’s pain; we could barely contain our own. We could only take so much.
Without space to process, grief festers and eats away at our remaining compassion. How do we hold grace for ourselves, dare to be vulnerable, and allow ourselves to feel, when doing so opens the door to our own grief? How do we allow room for emotional processing when we learned to numb-out in order to function? And as women with diverse intersectional identities, how do we honor our humanity when we live in a society that reflects its indifference? We needed to process our pain in order to heal in the slow and uneven way that grief heals.
Caring During Tough Times
The pain we feel is real and it tears at us over time. Pushing it away disenfranchises ourselves of the opportunity to heal and grow. Our collective grief and trauma demand collective healing and acknowledgment of our individual suffering. We must honor our shared humanity and find commonality amid our differences. Typical self-care (healthy eating, sleep, basic hygiene) may not be enough to mitigate the enormity of these stressors. A glass of wine or a virtual dinner with friends may distract but does not heal our wounds.
Self-care, by definition, centers the self and ignores the larger systemic factors that maintain our struggles. It keeps the focus on the individual and in so doing, risks inducing self-blame should we continue feeling burnout. We must do more. We can advocate that systems acknowledge our grief and suffering as well as our strengths and resiliencies. We can demand that organizations recognize human limits and provide support, rather than promote environments that encourage silent perseverance. And we can deconstruct the cultural narrative that vulnerability is weakness or that we are the “heroes.” Heroism suggests superhuman qualities or extreme courage and often negates the fear and trepidation in its midst.11,12 We can also recognize how intersectional aspects of our identities make navigating the pandemic and systemic racism harder and more dangerous for some than for others.
As noted by President Biden in a speech honoring those lost to COVID-19, “We have to resist becoming numb to the sorrow.”13 The nature of our work (and that of most clinicians) is that it is expected and sometimes necessary to compartmentalize and turn off the emotions so that we can function in a professional manner. But this way of being also serves to hold us back. It does not make space for the very real emotions of trauma and grief that have pervaded HCWs during this pandemic. We must learn a different way of functioning—one where grief is acknowledged and even actively processed while still going about our work. Grief therapist Megan Devine proposes to “tend to pain and grief by bearing witness” and notes that “when we allow the reality of grief to exist, we can focus on helping ourselves—and one another—survive inside pain.”14 She advocates for self-compassion and directs us to “find ways to show our grief to others, in ways that honor the truth of our experience” saying, “we have to be willing to stop diminishing our own pain so that others can be comfortable around us.” But what does this look like among health care teams who are traumatized and grieving?
In our experience, caring for ourselves and our teams in times of prolonged stress, trauma, and grief is essential to maintain functioning over time. We strongly believe that it must occur at both the organizational and individual levels. In the throes of a crisis, teams need support immediately. To offer a timely response, we gathered knowledge of team-based care and collaboration to develop practical strategies that can be implemented swiftly to provide support across the team.15-19
The strategies we developed offer steps for creating and maintaining a supportive, compassionate, and psychologically safe work environment. First, the CARES Strategies for Practical Team Intervention highlights the importance of clear communication, assessing team needs regularly, recognizing the stress that is occurring, engaging staff in discussions, and ensuring psychological safety and comfort (Figure 1). Next, the SHARE approach is laid out to allow for interpersonal support among team members (Figure 2). Showing each other empathy, hoping for better days, acknowledging each other’s pain, reaching out for assistance, and expressing our needs allow HCWs to open up about their grief, stress, and trauma. Of note, we found these sets of strategies interdependent: a team that does not believe the leader/organization CARES is not likely to SHARE. Therefore, we also feel that it is especially important that team leaders work to create or enhance the sense of psychological safety for the team. If team members do not feel safe, they will not disclose their grief and remain stuck in the old mode of suffering in silence.
Conclusions
This pandemic and the collective efforts toward social justice advocacy have revealed our vulnerabilities as well as our strengths. These experiences have forced us to reckon with our past and consider possible futures. It has revealed the inequities in our health care system, including our failure to protect those on the ground who keep our systems running, and prompted us to consider new ways of operating in low-resourced and high-demand environments. These experiences also present us with opportunities to be better and do better as both professionals and people; to reflect on our past and consider what we want different in our lives. As we yearn for better days and brace ourselves for what is to come, we hope that teams and organizations will take advantage of these opportunities for self-reflection and continue unmasking our grief, healing our wounds, and honoring our shared humanity.
1. Blake H, Bermingham F. Psychological wellbeing for health care workers: mitigating the impact of covid-19. Version 2.0. Updated June 18, 2020. Accessed October 12, 2021. https://www.nottingham.ac.uk/toolkits/play_22794
2. Harris R. FACE COVID: how to respond effectively to the corona crisis. Published 2020. Accessed October 12, 2021. http://louisville.edu/counseling/coping-with-covid-19/face-covid-by-dr-russ-harris/view
3. Norcross JC, Phillips CM. Psychologist self-care during the pandemic: now more than ever [published online ahead of print, 2020 May 2]. J Health Serv Psychol. 2020;1-5. doi:10.1007/s42843-020-00010-5
4. Kaiser Family Foundation. State reports of long-term care facility cases and deaths related to COVID-19. 2020. Published April 23, 2020. Accessed October 12, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/state-reporting-of-cases-and-deaths-due-to-covid-19-in-long-term-care-facilities
5. Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Intern Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
6. Stone R, Wilhelm J, Bishop CE, Bryant NS, Hermer L, Squillace MR. Predictors of intent to leave the job among home health workers: analysis of the National Home Health Aide Survey. Gerontologist. 2017;57(5):890-899. doi:10.1093/geront/gnw075
7. Scales K. It’s time to care: a detailed profile of America’s direct care workforce. PHI. 2020. Published January 21, 2020. Accessed October 12, 2021. https://phinational.org/wp-content/uploads/2020/01/Its-Time-to-Care-2020-PHI.pdf
8. Wolfe R, Harknett K, Schneider D. Inequities at work and the toll of COVID-19. Health Aff Health Policy Brief. Published June 4, 2021. doi: 10.1377/hpb20210428.863621
9. White EM, Wetle TF, Reddy A, Baier RR. Front-line nursing home staff experiences during the COVID-19 pandemic [published correction appears in J Am Med Dir Assoc. 2021 May;22(5):1123]. J Am Med Dir Assoc. 2021;22(1):199-203. doi:10.1016/j.jamda.2020.11.022
10. Dean A, Venkataramani A, Kimmel S. Mortality rates from COVID-19 are lower In unionized nursing homes. Health Aff (Millwood). 2020;39(11):1993-2001.doi:10.1377/hlthaff.2020.01011
11. Cox CL. ‘Healthcare Heroes’: problems with media focus on heroism from healthcare workers during the COVID-19 pandemic. J Med Ethics. 2020;46(8):510-513. doi:10.1136/medethics-2020-106398
12. Stokes-Parish J, Elliott R, Rolls K, Massey D. Angels and heroes: the unintended consequence of the hero narrative. J Nurs Scholarsh. 2020;52(5):462-466. doi:10.1111/jnu.12591
13. Biden J. Remarks by President Biden on the more than 500,000 American lives lost to COVID-19. Published February 22, 2021. Accessed October 12, 2021. https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/02/22/remarks-by-president-biden-on-the-more-than-500000-american-lives-lost-to-covid-19
14. Devine M. It’s Okay That You’re Not Okay: Meeting Grief and Loss in a Culture That Doesn’t Understand. Sounds True; 2017.
15. Center for the Study of Traumatic Stress. Grief leadership during COVID-19. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Grief_Leadership_During_COVID19.pdf
16. Center for the Study of Traumatic Stress. Sustaining the well-being of healthcare personnel during coronavirus and other infectious disease outbreaks. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Sustaining_Well_Being_Health care_Personnel_during.pdf
17. Fessell D, Cherniss C. Coronavirus disease 2019 (COVID-19) and beyond: micropractices for burnout prevention and emotional wellness. J Am Coll Radiol. 2020;17(6):746-748. doi:10.1016/j.jacr.2020.03.013
18. US Department of Veterans Affairs, National Center for PTSD. Managing healthcare workers’ stress associated with the COVID-19 virus outbreak. Updated March 25, 2020, Accessed October 12, 2021. https://www.ptsd.va.gov/covid/COVID_healthcare_workers.asp
19. US Department of Veterans Affairs, Veterans Health Administration, National Center for Organization Development (NCOD). Team Development Guide. 2017. https://vaww.va.gov/NCOD/docs/Team_Development_Guide.docx [Nonpublic source, not verified.]
Since the start of the pandemic, health care systems have requested many in-services for staff on self-care and stress management to help health care workers (HCWs) cope with the heavy toll of COVID-19. The pandemic has set off a global mental health crisis, with unprecedented numbers of individuals meeting criteria for anxiety, depression, and other mental health disorders in response to the intense stressors of living through a pandemic. These calls to assist staff with self-care and burnout prevention have been especially salient for psychologists working in palliative care and geriatrics, where fears of COVID-19 infection and numbers of patient deaths have been high.
Throughout these painful times, we have been grateful for an online community of palliative care psychologists within the US Department of Veterans Affairs (VA) from across the continuum of care and across the country. This community brought together many of us who were both struggling ourselves and striving to support the teams and HCWs around us. We are psychologists who provide home-care services in North Carolina, inpatient hospice and long-term care services in California, and long-term care and outpatient palliative care services in Massachusetts. Through our shared struggles and challenges navigating the pandemic, we realized that our respective teams requested similar services, all focused on staff support.
The psychological impact of COVID-19 on HCWs was clear from the beginning. Early in the pandemic our respective teams requested us to provide staff support and education about coping to our local HCWs. Soon national groups for long-term care staff requested education programs. Through this work, we realized that the emotional needs of HCWs ran much deeper than simple self-care. At the onset of the pandemic, before realizing its chronicity, the trainings we offered focused on stress and coping strategies. We cited several frameworks for staff support and eagerly shared anything that might help us, and our colleagues, survive the immediate anxiety and tumult surrounding us.1-3 In this paper, we briefly discuss the distress affecting the geriatric care workforce, reflect on our efforts to cope as HCWs, and offer recommendations at individual and organization levels to help address our collective grief.
Impact of COVID-19
As the death toll mounted and hospitals were pushed to the brink, we saw the suffering of our fellow HCWs. The lack of personal protective equipment (PPE) and testing supplies led to evolving and increasing anxiety for HCWs about contracting COVID-19, potentially spreading it to one’s social circle or family, fears of becoming sick and dying, and fears of inadvertently spreading the virus to medically-vulnerable patients. Increasing demands on staff required many to work outside their areas of expertise. Clinical practice guidelines changed frequently as information emerged about the virus. Staff members struggled to keep pace with the increasing number of patients, many of whom died despite heroic efforts to save them.
As the medical crisis grew, so too did social uprisings as the general public gained a strengthened awareness of the legacy and ongoing effects of systemic oppression, racism, and social inequities in the United States. Individuals grappled with their own privileges, which often hid such disparities from view. Many HCWs and clinicians of color had to navigate unsolicited questions and discussions about racial injustices while also trying to survive. As psychologists, we strove to support the HCWs around us while also struggling with our own stressors. As the magnitude of the pandemic and ongoing social injustices came into view, we realized that presentations on self-care and burnout prevention did not suffice. We needed discussions on unmasking our grief, acknowledging our traumas, and working toward collective healing.
Geriatric Care Workers
Experiences of grief and trauma hit the geriatric care workforce and especially long-term care facilities particularly hard given the high morbidity and mortality rates of COVID-19.4 The geriatric care workforce itself suffers from institutional vulnerabilities. Individuals are often underpaid, undertrained, and work within a system that continually experiences staffing shortages, high burnout, and consequently high levels of turnover.5,6 Recent immigrants and racial/ethnic minorities disproportionately make up this workforce, who often live in multigenerational households and work in multiple facilities to get by.7,8 Amid the pandemic these HCWs continued to work despite demoralizing negative media coverage of nursing homes.9 Notably, facilities with unionized staff were less likely to need second or third jobs to survive, thus reducing spread across facilities. This along with better access to PPE may have contributed to their lower COVID-19 infection and mortality rates relative to non-unionized staff.10
Similar to long-term care workers, home-care staff had related fears and anxieties, magnified by the need to enter multiple homes. This often overlooked but growing sector of the geriatric care workforce faced the added anxiety of the unknown as they entered multiple homes to provide care to their patients. These staff have little control over who may be in the home when they arrive, the sanitation/PPE practices of the patient/family, and therefore little control over their potential exposure to COVID-19. This also applies to home health aides who, although not providing medical services, are a critical part of home-care services and allow older adults to remain living independently in their home.
Reflection on Grief
As we witnessed the interactive effects of the pandemic and social inequities in geriatrics and palliative care, we frequently sought solace in online communities of psychologists working in similar settings. Over time, our regular community meetings developed a different tone: discussions about caring for others shifted to caring for ourselves. It seemed that in holding others’ pain, many of us neglected to address our own. We needed emotional support. We needed to acknowledge that we were not all okay; that the masks we wear for protection also reveal our vulnerabilities; and that protective equipment in hospitals do not protect us from the hate and bias targeting many of us face everywhere we go.
As we let ourselves be vulnerable with each other, we saw the true face of our pain: it was not stress, it was grief. We were sad, broken, mourning innumerable losses, and grieving, mostly alone. It felt overwhelming. Our minds and hearts often grew numb to find respite from pain. At times we found ourselves seeking haven in our offices, convincing ourselves that paperwork needed to be done when in reality we had no space to hold anyone else’s pain; we could barely contain our own. We could only take so much.
Without space to process, grief festers and eats away at our remaining compassion. How do we hold grace for ourselves, dare to be vulnerable, and allow ourselves to feel, when doing so opens the door to our own grief? How do we allow room for emotional processing when we learned to numb-out in order to function? And as women with diverse intersectional identities, how do we honor our humanity when we live in a society that reflects its indifference? We needed to process our pain in order to heal in the slow and uneven way that grief heals.
Caring During Tough Times
The pain we feel is real and it tears at us over time. Pushing it away disenfranchises ourselves of the opportunity to heal and grow. Our collective grief and trauma demand collective healing and acknowledgment of our individual suffering. We must honor our shared humanity and find commonality amid our differences. Typical self-care (healthy eating, sleep, basic hygiene) may not be enough to mitigate the enormity of these stressors. A glass of wine or a virtual dinner with friends may distract but does not heal our wounds.
Self-care, by definition, centers the self and ignores the larger systemic factors that maintain our struggles. It keeps the focus on the individual and in so doing, risks inducing self-blame should we continue feeling burnout. We must do more. We can advocate that systems acknowledge our grief and suffering as well as our strengths and resiliencies. We can demand that organizations recognize human limits and provide support, rather than promote environments that encourage silent perseverance. And we can deconstruct the cultural narrative that vulnerability is weakness or that we are the “heroes.” Heroism suggests superhuman qualities or extreme courage and often negates the fear and trepidation in its midst.11,12 We can also recognize how intersectional aspects of our identities make navigating the pandemic and systemic racism harder and more dangerous for some than for others.
As noted by President Biden in a speech honoring those lost to COVID-19, “We have to resist becoming numb to the sorrow.”13 The nature of our work (and that of most clinicians) is that it is expected and sometimes necessary to compartmentalize and turn off the emotions so that we can function in a professional manner. But this way of being also serves to hold us back. It does not make space for the very real emotions of trauma and grief that have pervaded HCWs during this pandemic. We must learn a different way of functioning—one where grief is acknowledged and even actively processed while still going about our work. Grief therapist Megan Devine proposes to “tend to pain and grief by bearing witness” and notes that “when we allow the reality of grief to exist, we can focus on helping ourselves—and one another—survive inside pain.”14 She advocates for self-compassion and directs us to “find ways to show our grief to others, in ways that honor the truth of our experience” saying, “we have to be willing to stop diminishing our own pain so that others can be comfortable around us.” But what does this look like among health care teams who are traumatized and grieving?
In our experience, caring for ourselves and our teams in times of prolonged stress, trauma, and grief is essential to maintain functioning over time. We strongly believe that it must occur at both the organizational and individual levels. In the throes of a crisis, teams need support immediately. To offer a timely response, we gathered knowledge of team-based care and collaboration to develop practical strategies that can be implemented swiftly to provide support across the team.15-19
The strategies we developed offer steps for creating and maintaining a supportive, compassionate, and psychologically safe work environment. First, the CARES Strategies for Practical Team Intervention highlights the importance of clear communication, assessing team needs regularly, recognizing the stress that is occurring, engaging staff in discussions, and ensuring psychological safety and comfort (Figure 1). Next, the SHARE approach is laid out to allow for interpersonal support among team members (Figure 2). Showing each other empathy, hoping for better days, acknowledging each other’s pain, reaching out for assistance, and expressing our needs allow HCWs to open up about their grief, stress, and trauma. Of note, we found these sets of strategies interdependent: a team that does not believe the leader/organization CARES is not likely to SHARE. Therefore, we also feel that it is especially important that team leaders work to create or enhance the sense of psychological safety for the team. If team members do not feel safe, they will not disclose their grief and remain stuck in the old mode of suffering in silence.
Conclusions
This pandemic and the collective efforts toward social justice advocacy have revealed our vulnerabilities as well as our strengths. These experiences have forced us to reckon with our past and consider possible futures. It has revealed the inequities in our health care system, including our failure to protect those on the ground who keep our systems running, and prompted us to consider new ways of operating in low-resourced and high-demand environments. These experiences also present us with opportunities to be better and do better as both professionals and people; to reflect on our past and consider what we want different in our lives. As we yearn for better days and brace ourselves for what is to come, we hope that teams and organizations will take advantage of these opportunities for self-reflection and continue unmasking our grief, healing our wounds, and honoring our shared humanity.
Since the start of the pandemic, health care systems have requested many in-services for staff on self-care and stress management to help health care workers (HCWs) cope with the heavy toll of COVID-19. The pandemic has set off a global mental health crisis, with unprecedented numbers of individuals meeting criteria for anxiety, depression, and other mental health disorders in response to the intense stressors of living through a pandemic. These calls to assist staff with self-care and burnout prevention have been especially salient for psychologists working in palliative care and geriatrics, where fears of COVID-19 infection and numbers of patient deaths have been high.
Throughout these painful times, we have been grateful for an online community of palliative care psychologists within the US Department of Veterans Affairs (VA) from across the continuum of care and across the country. This community brought together many of us who were both struggling ourselves and striving to support the teams and HCWs around us. We are psychologists who provide home-care services in North Carolina, inpatient hospice and long-term care services in California, and long-term care and outpatient palliative care services in Massachusetts. Through our shared struggles and challenges navigating the pandemic, we realized that our respective teams requested similar services, all focused on staff support.
The psychological impact of COVID-19 on HCWs was clear from the beginning. Early in the pandemic our respective teams requested us to provide staff support and education about coping to our local HCWs. Soon national groups for long-term care staff requested education programs. Through this work, we realized that the emotional needs of HCWs ran much deeper than simple self-care. At the onset of the pandemic, before realizing its chronicity, the trainings we offered focused on stress and coping strategies. We cited several frameworks for staff support and eagerly shared anything that might help us, and our colleagues, survive the immediate anxiety and tumult surrounding us.1-3 In this paper, we briefly discuss the distress affecting the geriatric care workforce, reflect on our efforts to cope as HCWs, and offer recommendations at individual and organization levels to help address our collective grief.
Impact of COVID-19
As the death toll mounted and hospitals were pushed to the brink, we saw the suffering of our fellow HCWs. The lack of personal protective equipment (PPE) and testing supplies led to evolving and increasing anxiety for HCWs about contracting COVID-19, potentially spreading it to one’s social circle or family, fears of becoming sick and dying, and fears of inadvertently spreading the virus to medically-vulnerable patients. Increasing demands on staff required many to work outside their areas of expertise. Clinical practice guidelines changed frequently as information emerged about the virus. Staff members struggled to keep pace with the increasing number of patients, many of whom died despite heroic efforts to save them.
As the medical crisis grew, so too did social uprisings as the general public gained a strengthened awareness of the legacy and ongoing effects of systemic oppression, racism, and social inequities in the United States. Individuals grappled with their own privileges, which often hid such disparities from view. Many HCWs and clinicians of color had to navigate unsolicited questions and discussions about racial injustices while also trying to survive. As psychologists, we strove to support the HCWs around us while also struggling with our own stressors. As the magnitude of the pandemic and ongoing social injustices came into view, we realized that presentations on self-care and burnout prevention did not suffice. We needed discussions on unmasking our grief, acknowledging our traumas, and working toward collective healing.
Geriatric Care Workers
Experiences of grief and trauma hit the geriatric care workforce and especially long-term care facilities particularly hard given the high morbidity and mortality rates of COVID-19.4 The geriatric care workforce itself suffers from institutional vulnerabilities. Individuals are often underpaid, undertrained, and work within a system that continually experiences staffing shortages, high burnout, and consequently high levels of turnover.5,6 Recent immigrants and racial/ethnic minorities disproportionately make up this workforce, who often live in multigenerational households and work in multiple facilities to get by.7,8 Amid the pandemic these HCWs continued to work despite demoralizing negative media coverage of nursing homes.9 Notably, facilities with unionized staff were less likely to need second or third jobs to survive, thus reducing spread across facilities. This along with better access to PPE may have contributed to their lower COVID-19 infection and mortality rates relative to non-unionized staff.10
Similar to long-term care workers, home-care staff had related fears and anxieties, magnified by the need to enter multiple homes. This often overlooked but growing sector of the geriatric care workforce faced the added anxiety of the unknown as they entered multiple homes to provide care to their patients. These staff have little control over who may be in the home when they arrive, the sanitation/PPE practices of the patient/family, and therefore little control over their potential exposure to COVID-19. This also applies to home health aides who, although not providing medical services, are a critical part of home-care services and allow older adults to remain living independently in their home.
Reflection on Grief
As we witnessed the interactive effects of the pandemic and social inequities in geriatrics and palliative care, we frequently sought solace in online communities of psychologists working in similar settings. Over time, our regular community meetings developed a different tone: discussions about caring for others shifted to caring for ourselves. It seemed that in holding others’ pain, many of us neglected to address our own. We needed emotional support. We needed to acknowledge that we were not all okay; that the masks we wear for protection also reveal our vulnerabilities; and that protective equipment in hospitals do not protect us from the hate and bias targeting many of us face everywhere we go.
As we let ourselves be vulnerable with each other, we saw the true face of our pain: it was not stress, it was grief. We were sad, broken, mourning innumerable losses, and grieving, mostly alone. It felt overwhelming. Our minds and hearts often grew numb to find respite from pain. At times we found ourselves seeking haven in our offices, convincing ourselves that paperwork needed to be done when in reality we had no space to hold anyone else’s pain; we could barely contain our own. We could only take so much.
Without space to process, grief festers and eats away at our remaining compassion. How do we hold grace for ourselves, dare to be vulnerable, and allow ourselves to feel, when doing so opens the door to our own grief? How do we allow room for emotional processing when we learned to numb-out in order to function? And as women with diverse intersectional identities, how do we honor our humanity when we live in a society that reflects its indifference? We needed to process our pain in order to heal in the slow and uneven way that grief heals.
Caring During Tough Times
The pain we feel is real and it tears at us over time. Pushing it away disenfranchises ourselves of the opportunity to heal and grow. Our collective grief and trauma demand collective healing and acknowledgment of our individual suffering. We must honor our shared humanity and find commonality amid our differences. Typical self-care (healthy eating, sleep, basic hygiene) may not be enough to mitigate the enormity of these stressors. A glass of wine or a virtual dinner with friends may distract but does not heal our wounds.
Self-care, by definition, centers the self and ignores the larger systemic factors that maintain our struggles. It keeps the focus on the individual and in so doing, risks inducing self-blame should we continue feeling burnout. We must do more. We can advocate that systems acknowledge our grief and suffering as well as our strengths and resiliencies. We can demand that organizations recognize human limits and provide support, rather than promote environments that encourage silent perseverance. And we can deconstruct the cultural narrative that vulnerability is weakness or that we are the “heroes.” Heroism suggests superhuman qualities or extreme courage and often negates the fear and trepidation in its midst.11,12 We can also recognize how intersectional aspects of our identities make navigating the pandemic and systemic racism harder and more dangerous for some than for others.
As noted by President Biden in a speech honoring those lost to COVID-19, “We have to resist becoming numb to the sorrow.”13 The nature of our work (and that of most clinicians) is that it is expected and sometimes necessary to compartmentalize and turn off the emotions so that we can function in a professional manner. But this way of being also serves to hold us back. It does not make space for the very real emotions of trauma and grief that have pervaded HCWs during this pandemic. We must learn a different way of functioning—one where grief is acknowledged and even actively processed while still going about our work. Grief therapist Megan Devine proposes to “tend to pain and grief by bearing witness” and notes that “when we allow the reality of grief to exist, we can focus on helping ourselves—and one another—survive inside pain.”14 She advocates for self-compassion and directs us to “find ways to show our grief to others, in ways that honor the truth of our experience” saying, “we have to be willing to stop diminishing our own pain so that others can be comfortable around us.” But what does this look like among health care teams who are traumatized and grieving?
In our experience, caring for ourselves and our teams in times of prolonged stress, trauma, and grief is essential to maintain functioning over time. We strongly believe that it must occur at both the organizational and individual levels. In the throes of a crisis, teams need support immediately. To offer a timely response, we gathered knowledge of team-based care and collaboration to develop practical strategies that can be implemented swiftly to provide support across the team.15-19
The strategies we developed offer steps for creating and maintaining a supportive, compassionate, and psychologically safe work environment. First, the CARES Strategies for Practical Team Intervention highlights the importance of clear communication, assessing team needs regularly, recognizing the stress that is occurring, engaging staff in discussions, and ensuring psychological safety and comfort (Figure 1). Next, the SHARE approach is laid out to allow for interpersonal support among team members (Figure 2). Showing each other empathy, hoping for better days, acknowledging each other’s pain, reaching out for assistance, and expressing our needs allow HCWs to open up about their grief, stress, and trauma. Of note, we found these sets of strategies interdependent: a team that does not believe the leader/organization CARES is not likely to SHARE. Therefore, we also feel that it is especially important that team leaders work to create or enhance the sense of psychological safety for the team. If team members do not feel safe, they will not disclose their grief and remain stuck in the old mode of suffering in silence.
Conclusions
This pandemic and the collective efforts toward social justice advocacy have revealed our vulnerabilities as well as our strengths. These experiences have forced us to reckon with our past and consider possible futures. It has revealed the inequities in our health care system, including our failure to protect those on the ground who keep our systems running, and prompted us to consider new ways of operating in low-resourced and high-demand environments. These experiences also present us with opportunities to be better and do better as both professionals and people; to reflect on our past and consider what we want different in our lives. As we yearn for better days and brace ourselves for what is to come, we hope that teams and organizations will take advantage of these opportunities for self-reflection and continue unmasking our grief, healing our wounds, and honoring our shared humanity.
1. Blake H, Bermingham F. Psychological wellbeing for health care workers: mitigating the impact of covid-19. Version 2.0. Updated June 18, 2020. Accessed October 12, 2021. https://www.nottingham.ac.uk/toolkits/play_22794
2. Harris R. FACE COVID: how to respond effectively to the corona crisis. Published 2020. Accessed October 12, 2021. http://louisville.edu/counseling/coping-with-covid-19/face-covid-by-dr-russ-harris/view
3. Norcross JC, Phillips CM. Psychologist self-care during the pandemic: now more than ever [published online ahead of print, 2020 May 2]. J Health Serv Psychol. 2020;1-5. doi:10.1007/s42843-020-00010-5
4. Kaiser Family Foundation. State reports of long-term care facility cases and deaths related to COVID-19. 2020. Published April 23, 2020. Accessed October 12, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/state-reporting-of-cases-and-deaths-due-to-covid-19-in-long-term-care-facilities
5. Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Intern Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
6. Stone R, Wilhelm J, Bishop CE, Bryant NS, Hermer L, Squillace MR. Predictors of intent to leave the job among home health workers: analysis of the National Home Health Aide Survey. Gerontologist. 2017;57(5):890-899. doi:10.1093/geront/gnw075
7. Scales K. It’s time to care: a detailed profile of America’s direct care workforce. PHI. 2020. Published January 21, 2020. Accessed October 12, 2021. https://phinational.org/wp-content/uploads/2020/01/Its-Time-to-Care-2020-PHI.pdf
8. Wolfe R, Harknett K, Schneider D. Inequities at work and the toll of COVID-19. Health Aff Health Policy Brief. Published June 4, 2021. doi: 10.1377/hpb20210428.863621
9. White EM, Wetle TF, Reddy A, Baier RR. Front-line nursing home staff experiences during the COVID-19 pandemic [published correction appears in J Am Med Dir Assoc. 2021 May;22(5):1123]. J Am Med Dir Assoc. 2021;22(1):199-203. doi:10.1016/j.jamda.2020.11.022
10. Dean A, Venkataramani A, Kimmel S. Mortality rates from COVID-19 are lower In unionized nursing homes. Health Aff (Millwood). 2020;39(11):1993-2001.doi:10.1377/hlthaff.2020.01011
11. Cox CL. ‘Healthcare Heroes’: problems with media focus on heroism from healthcare workers during the COVID-19 pandemic. J Med Ethics. 2020;46(8):510-513. doi:10.1136/medethics-2020-106398
12. Stokes-Parish J, Elliott R, Rolls K, Massey D. Angels and heroes: the unintended consequence of the hero narrative. J Nurs Scholarsh. 2020;52(5):462-466. doi:10.1111/jnu.12591
13. Biden J. Remarks by President Biden on the more than 500,000 American lives lost to COVID-19. Published February 22, 2021. Accessed October 12, 2021. https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/02/22/remarks-by-president-biden-on-the-more-than-500000-american-lives-lost-to-covid-19
14. Devine M. It’s Okay That You’re Not Okay: Meeting Grief and Loss in a Culture That Doesn’t Understand. Sounds True; 2017.
15. Center for the Study of Traumatic Stress. Grief leadership during COVID-19. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Grief_Leadership_During_COVID19.pdf
16. Center for the Study of Traumatic Stress. Sustaining the well-being of healthcare personnel during coronavirus and other infectious disease outbreaks. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Sustaining_Well_Being_Health care_Personnel_during.pdf
17. Fessell D, Cherniss C. Coronavirus disease 2019 (COVID-19) and beyond: micropractices for burnout prevention and emotional wellness. J Am Coll Radiol. 2020;17(6):746-748. doi:10.1016/j.jacr.2020.03.013
18. US Department of Veterans Affairs, National Center for PTSD. Managing healthcare workers’ stress associated with the COVID-19 virus outbreak. Updated March 25, 2020, Accessed October 12, 2021. https://www.ptsd.va.gov/covid/COVID_healthcare_workers.asp
19. US Department of Veterans Affairs, Veterans Health Administration, National Center for Organization Development (NCOD). Team Development Guide. 2017. https://vaww.va.gov/NCOD/docs/Team_Development_Guide.docx [Nonpublic source, not verified.]
1. Blake H, Bermingham F. Psychological wellbeing for health care workers: mitigating the impact of covid-19. Version 2.0. Updated June 18, 2020. Accessed October 12, 2021. https://www.nottingham.ac.uk/toolkits/play_22794
2. Harris R. FACE COVID: how to respond effectively to the corona crisis. Published 2020. Accessed October 12, 2021. http://louisville.edu/counseling/coping-with-covid-19/face-covid-by-dr-russ-harris/view
3. Norcross JC, Phillips CM. Psychologist self-care during the pandemic: now more than ever [published online ahead of print, 2020 May 2]. J Health Serv Psychol. 2020;1-5. doi:10.1007/s42843-020-00010-5
4. Kaiser Family Foundation. State reports of long-term care facility cases and deaths related to COVID-19. 2020. Published April 23, 2020. Accessed October 12, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/state-reporting-of-cases-and-deaths-due-to-covid-19-in-long-term-care-facilities
5. Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Intern Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
6. Stone R, Wilhelm J, Bishop CE, Bryant NS, Hermer L, Squillace MR. Predictors of intent to leave the job among home health workers: analysis of the National Home Health Aide Survey. Gerontologist. 2017;57(5):890-899. doi:10.1093/geront/gnw075
7. Scales K. It’s time to care: a detailed profile of America’s direct care workforce. PHI. 2020. Published January 21, 2020. Accessed October 12, 2021. https://phinational.org/wp-content/uploads/2020/01/Its-Time-to-Care-2020-PHI.pdf
8. Wolfe R, Harknett K, Schneider D. Inequities at work and the toll of COVID-19. Health Aff Health Policy Brief. Published June 4, 2021. doi: 10.1377/hpb20210428.863621
9. White EM, Wetle TF, Reddy A, Baier RR. Front-line nursing home staff experiences during the COVID-19 pandemic [published correction appears in J Am Med Dir Assoc. 2021 May;22(5):1123]. J Am Med Dir Assoc. 2021;22(1):199-203. doi:10.1016/j.jamda.2020.11.022
10. Dean A, Venkataramani A, Kimmel S. Mortality rates from COVID-19 are lower In unionized nursing homes. Health Aff (Millwood). 2020;39(11):1993-2001.doi:10.1377/hlthaff.2020.01011
11. Cox CL. ‘Healthcare Heroes’: problems with media focus on heroism from healthcare workers during the COVID-19 pandemic. J Med Ethics. 2020;46(8):510-513. doi:10.1136/medethics-2020-106398
12. Stokes-Parish J, Elliott R, Rolls K, Massey D. Angels and heroes: the unintended consequence of the hero narrative. J Nurs Scholarsh. 2020;52(5):462-466. doi:10.1111/jnu.12591
13. Biden J. Remarks by President Biden on the more than 500,000 American lives lost to COVID-19. Published February 22, 2021. Accessed October 12, 2021. https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/02/22/remarks-by-president-biden-on-the-more-than-500000-american-lives-lost-to-covid-19
14. Devine M. It’s Okay That You’re Not Okay: Meeting Grief and Loss in a Culture That Doesn’t Understand. Sounds True; 2017.
15. Center for the Study of Traumatic Stress. Grief leadership during COVID-19. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Grief_Leadership_During_COVID19.pdf
16. Center for the Study of Traumatic Stress. Sustaining the well-being of healthcare personnel during coronavirus and other infectious disease outbreaks. Accessed October 12, 2021. https://www.cstsonline.org/assets/media/documents/CSTS_FS_Sustaining_Well_Being_Health care_Personnel_during.pdf
17. Fessell D, Cherniss C. Coronavirus disease 2019 (COVID-19) and beyond: micropractices for burnout prevention and emotional wellness. J Am Coll Radiol. 2020;17(6):746-748. doi:10.1016/j.jacr.2020.03.013
18. US Department of Veterans Affairs, National Center for PTSD. Managing healthcare workers’ stress associated with the COVID-19 virus outbreak. Updated March 25, 2020, Accessed October 12, 2021. https://www.ptsd.va.gov/covid/COVID_healthcare_workers.asp
19. US Department of Veterans Affairs, Veterans Health Administration, National Center for Organization Development (NCOD). Team Development Guide. 2017. https://vaww.va.gov/NCOD/docs/Team_Development_Guide.docx [Nonpublic source, not verified.]