User login
COVID-19: Delta variant is raising the stakes
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Empathetic conversations with unvaccinated people desperately needed
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Needed: More studies of CSF molecular biomarkers in psychiatric disorders
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
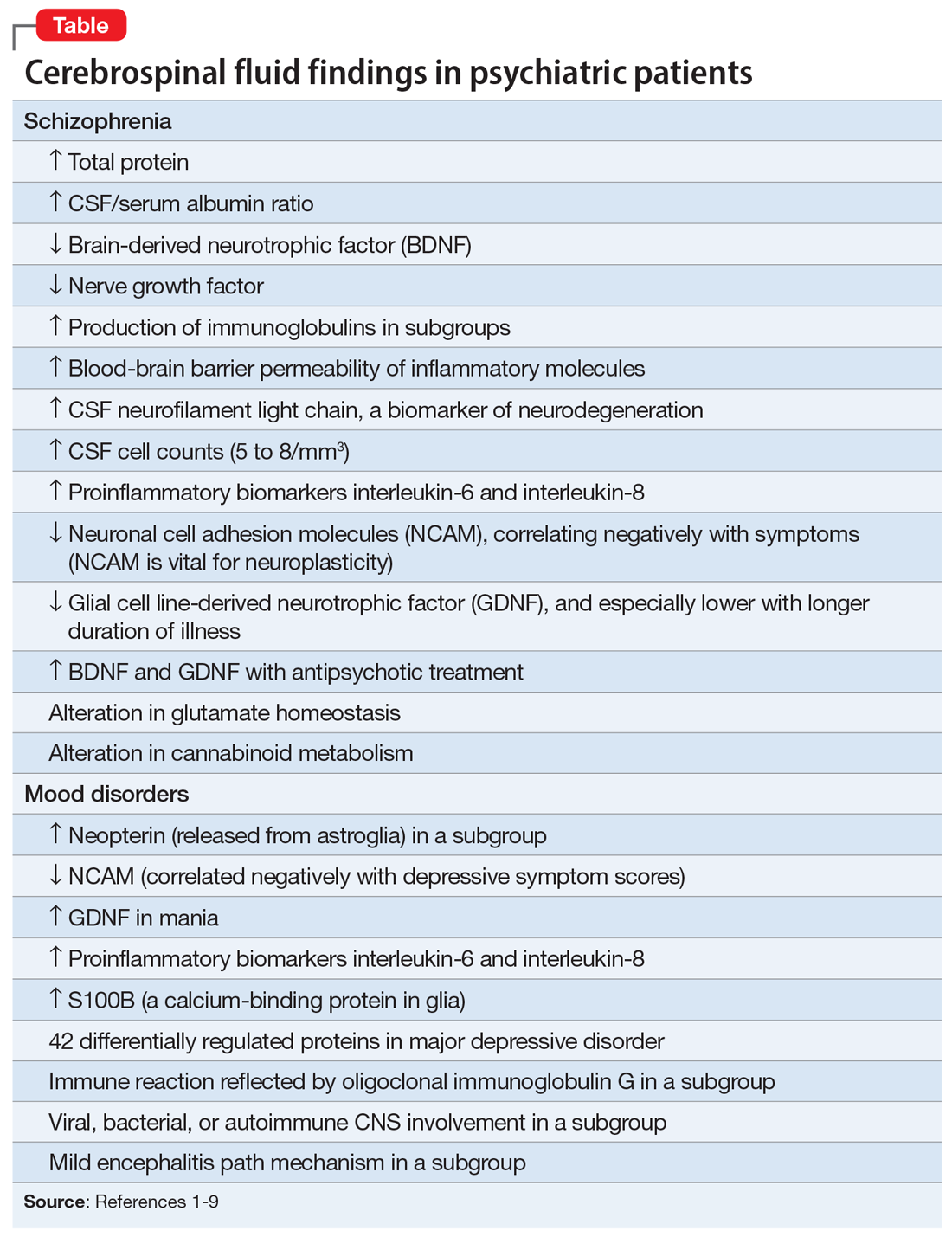
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Medicine’s ‘Big Lie’
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
While today “The Big Lie” mainly refers to the actions of the prior President, an older and bigger lie that has a real effect on every American is one perpetrated by our very own health care conglomerate. Americans pay the highest rates for health care on the planet; health care consumes about 17% of our gross domestic product.1 If we got higher-quality care, faster services, longer lives, or even greater consumer happiness, paying those rates might be worth it. But we don’t.
Worse yet is the idea that “board certification” assures the public that the doctor from whom they receive/purchase care is of a higher quality than one who is not so credentialed. That is our “Big Lie!” For decades, the public has been told that they should seek out board-certified doctors. Doctors in training have been told they must get board-certified. Hospitals brag about employing only board-certified doctors, insurers sometimes mandate board certification for a doctor to get paid, and employers use board certification as a benchmark for hiring and as a factor in compensation.
The sacred secret is that board certification makes no difference. There is no substantial evidence in any branch of medicine that doctors who are board-certified are better. There is no evidence that board-certified doctors get their patients healthier with more frequency, faster, less expensively, or with fewer medical errors than other doctors. The reality is that board certification is a sham. It’s a certificate granted after taking a very expensive test, and it is now part of an industry that is misleading the public and harming the trust the medical profession had once earned. Board certification is the equivalent of a diploma mill or an online certificate in any other field.
Why has this been kept under wraps for so long? Follow the money. The American Board of Medical Specialties (ABMS) oversees 24 specialty boards and reported revenue of $22.2M and expenses of $19.3M on its 2019 IRS Form 990.2 They make profit every year. But, looking further, these “not-for-profit” educational entities are sitting on hundreds of millions of dollars in their “foundations.” Take the American Board of Psychiatry and Neurology, for instance. They had more than $140M in assets in 2019.3 How is this possible? Easy. They have misled the American public and been remarkably successful convincing other organizations, such as the Joint Commission, the Accreditation Council for Graduate Medical Education, and the National Committee for Quality Assurance, that board certification is an assurance of quality. They charge high fees to “candidates” for taking the computer-based test and have developed a system called maintenance of certification (MOC) that is onerous, expensive, and serves as an annuity that forces doctors to pay annually to keep their board certification.
Medicine is a science. In the practice of our discipline, we are expected to follow the science and to adhere to scientific principles. Yet there is neither scientific proof nor good evidence that board certification means anything in terms of competence, safety to the public, or quality of care. Doctors favor life-long learning, and continuing education has long been the standard and should remain so, not board certification or MOC. The mandatory continuing education required in every state to maintain a medical license is sufficient to prove doctors are current in their field of practice and to protect the public.
It is time for the medical community to admit that the emperor wears no clothes, and demand that the money grab of the ABMS and its affiliates be halted. This would result in greater access to care for patients and would reduce the cost of medical care, as the hundreds of millions being “stolen” from doctors today—costs that get passed on to patients—could be recouped and used for treating patients who clearly are in need and are being forgotten as the medical-industrial complex continues to flex its muscles and ensnare more of our national budget in its tentacles.
Neil S. Kaye, MD, DLFAPA
Hockessin, Delaware
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
1. The World Bank. Current health expenditure (% of GDP). Accessed July 12, 2021. https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS
2. American Board of Medical Specialties. 2019 Form 990. Return of Organization Exempt From Income Tax. Accessed July 12, 2021. https://www.abms.org/wp-content/uploads/2021/01/2019-american-board-of-medical-specialties-form-990.pdf
3. ProPublica. American Board of Psychiatry and Neurology. Accessed July 13, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
My palliative care rotation: Lessons of gratitude, mindfulness, and kindness
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
As a psychiatry resident and as a part of consultation-liaison service, I have visited many palliative care patients to assist other physicians in managing psychiatric issues such as depression, anxiety, or delirium. But recently, as the first resident from our Department of Psychiatry who was sent to a palliative care rotation, I followed these patients as a part of a primary palliative care team. Doing so allowed me to see patients from the other side of the bridge.
Palliative care focuses on providing relief from the suffering and stress of a patient’s illness, with the primary goal of improving the quality of life of the patient and their families. The palliative care team works in collaboration with the patient’s other clinicians to provide an extra layer of support. They provide biopsychosociocultural interventions that are in harmony with the needs of the patient rather than the prognosis of the illness. To do so, they first must evaluate the needs of the patient and their family. This is a time-consuming, energy-consuming, emotionally draining job.
During my palliative care rotation, I attended table rounds, bedside rounds, family meetings, long counseling sessions, and disposition planning meetings. This rotation also gave me the opportunity to place my feet in the shoes of a palliative care team and to reflect on how it feels to be the physician of a patient who is dying, which as a psychiatric resident I had seldom experienced. I learned that although working with patients who are dying can cause stress, burnout, and compassion fatigue, it also helps physicians appreciate the little things in life. To appreciate all the blessings we have that we usually take for granted. To practice gratitude. To be kind.
Upon reflection, I learned that the rounds of palliative care are actually mindfulness-based discussions that provide cushions of supportive work, facilitate feelings of being in control, tend to alleviate physical as well as mental suffering, foster clear-sighted hope, and assist in establishing small, subjectively significant, realistic goals for the patient’s immediate future, and to help the patient achieve these goals.
A valuable lesson from a patient
I want to highlight a case of a 65-year-old woman I first visited while I was shadowing my attending, who had been providing palliative care to the patient and her family for several months. The patient was admitted to a tertiary care hospital because cancer had invaded her small bowel and caused mechanical obstruction, resulting in intractable vomiting, abdominal distension, and anorexia. She underwent open laparotomy and ileostomy for symptomatic relief. A nasogastric tube was placed, and she was put on total parenteral nutrition. The day I met her was her third postoperative day. She had been improving significantly, and she wanted to eat. She was missing food. Most of the discussion in the round among my attending, the patient, and her family was centered around how to get to the point where she would be able to eat again and appreciate the taste of biryani.
What my attending did was incredible. After assessing the patient’s needs, he instilled a realistic hope: the hope of tasting food again. The attending, while acknowledging the patient’s apprehensions, respectfully and supportively kept her from wandering into the future, made every possible attempt to bring her attention back to the present moment, and helped her establish goals for the present and her immediate future. My attending was not toxic-positive, forcing his patient to uselessly revisit her current trauma. Instead, he was kind, empathic, and considerate. His primary focus was to understand rather than to be understood, to help her find meaning, and to improve her quality of life—a quality she defined for herself, which was to taste the food of her choice.
That day, when I returned to my working station in the psychiatry ward and had lunch in the break room, I thought, “When I eat, how often do I think about eating?” Mostly I either think about work, tasks, and presentations, or I scroll on social media.
Our taste buds indeed get adapted to repetitive stimulation, but the experience of eating our favorite dish is the naked truth of being alive, and is something that I have been taking for granted for a long time. These are little things in life that I need to appreciate, and learn to cultivate their power.
Introduction: Precision Oncology Changes the Game for VA Health Care (FULL)
For US Army veteran Tam Huynh, the US Department of Veterans Affairs (VA) precision oncology program has been the proverbial game changer. Diagnosed in 2016 with stage IV lung cancer and physically depleted by chemotherapy, Huynh learned that treatment based on the precise molecular makeup of his tumors held the potential for improving quality of life. Through the VA National Precision Oncology Program (NPOP), Huynh was matched to a medication shown to help patients whose tumors had the same genetic mutation as Huynh’s tumors. Today, Huynh is not only free of chemotherapy’s debilitating adverse effects, but able to enjoy time with his family and return to work.
Huynh is one of 400,000 veterans treated for cancer annually at the VA. The life-changing treatment he received is due to the legacy of research, integrated care, and collaboration that is the hallmark of the VA health care system. The NPOP is a natural outgrowth of this legacy, and, as Executive-in-Charge Richard Stone, MD, notes in his Foreword, part of the Veterans Health Administration’s (VHA) evolution as a learning health care system. The articles in this special issue represent a snapshot of the work underway under VHA NPOP as well as the dedication of VHA staff nationwide to provide patient-centric care to every veteran.
Leading off this special issue, NPOP director Michael J. Kelley, MD, provides context for understanding the paradigm shift represented by precision oncology.2 He also discusses how, within 5 years, the program came together from its start as a regional effort to its use today by almost every VA oncology practice. Kelley also explains the complexity behind interpreting next-generation sequencing (NGS) gene panel test results and how VA medical centers can call upon NPOP for assistance with this interpretation. Further, he states the “obligation” for new medical technology to be accessible and notes how NPOP was “intentional” during implementation to ensure rural veterans would be offered testing.2
Following Kelley’s discussion is a series of articles focused on precision oncology for prostate cancer, which affects 15,000 veterans yearly. The first, an overview of the Prostate Cancer Foundation (PCF), provides a short history of the organization and how it came to partner with the VA.3 Written by several PCF staff, including President and CEO Jonathan Simons, MD, the paper notes how the commitment of early leaders like S. Ward Casscells, MD, and Larry Stupski led to PCF’s “no veteran left behind” philosophy; ie, ensuring veteran access to clinical trials and world class care regardless of location. As the first disease-specific national network for oncology trials serving veterans, PCF aims to provide a model for all of US health care in the delivery of precision oncology care.
A critical part of PCF is the Precision Oncology Program for Cancer of the Prostate (POPCaP), which focuses on genetics and genomic testing. Bruce Montgomery, MD, and Matthew Retting, MD—VHA’s leading experts in prostate cancer—shine the spotlight on VA’s research track record, specifically the genomics of metastatic prostate cancer.4 They also note the program’s focus on African American veteran patients who are disproportionately affected by the disease but well represented in the VA. In discussing future directions, the authors explain the importance of expanding genetic testing for those diagnosed with prostate cancer.
Prostate cancer Analysis for Therapy Choice (PATCH) is a clinical trials network that works hand-in-hand with POPCaP to use genetic data collected by POPCaP sites to find patients for trials. In their discussion, authors Julie N. Graff, MD, and Grant D. Huang, MD, who leads VA Research’s Cooperative Studies Program, focus on 3 key areas: (1) the challenges of precision oncology when working with relatively rare mutations; (2) 2 new drug trials at VA that will help clinicians know whether certain targeted therapies work for prostate cancer; and (3) how VA is emerging as a national partner in drug discovery and the approval of precision drugs.5
Turning to lung cancer–the second leading cause of cancer death among veterans–Drew Moghanaki, MD, MPH, and Michael Hagan, MD, discuss 3 multisite initiatives launched in 2016 and 2017.6 The first trial, VA Partnership to Increase Access to Lung Cancer Screening (VA-PALS), is a multisite project sponsored by the VA’s Office of Rural Health and Bristol-Myers Squibb Foundation. The trial’s goal is to reduce lung cancer mortality through a robust early detection program. The second trial, VA Lung Cancer Surgery OR Radiation therapy (VALOR) compares whether radiation or surgery is the best for early-stage lung cancer. Notably, VALOR may be one of the most difficult randomized trial ever attempted in lung cancer research (4 previous phase 3 trials outside the VA closed prematurely). By addressing the previous challenges associated with running such a trial, the VALOR study team already has enrolled more than all of the previous phase 3 efforts combined. The third trial is VA Radiation Oncology Quality Surveillance Program (VA-ROQS), which was created in 2016 to benchmark the treatment of veterans with lung cancer. VA-ROQS aims to create a national network of Lung Cancer Centers of Excellence that work with VISNs to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information.
The final group of authors, led by Maren T. Scheuner, MD, discuss how the advent of germline testing as a standard-of-care practice for certain tumor types presents opportunities and challenges for precision oncology.7 One of the primary challenges they note is the shortage of genetics professionals, both within the VA system and health care generally. To help address this issue, they recommend leveraging VA’s longstanding partnership with its academic affiliates.
Precision oncology clearly demonstrates how applying knowledge regarding one of the smallest of living matter can make a tremendous difference in the matter of living. Tam Huynh’s story is proof positive. Speaking at last year’s AMSUS (Society for Federal Health Professionals) annual meeting about his experience, Huynh said that all veterans should have access to the same life-changing treatment he received. This is exactly where the VA NPOP is heading.
1. How the VA is using AI to target cancer, https://www.theatlantic.com/sponsored/ibm-2018/watson-va-cancer/1925. Accessed August 6, 2020.
2. Kelley MJ. VA National Precision Oncology Program. Fed Pract. 2020;37 (suppl 4):S22-S27. doi:10.12788/fp.0037
3. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi: 10.12788/fp.0035
4. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
5. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi:10.12788/fp.0028
6. Moghanaki D, Hagan M. Strategic initiatives for veterans with lung cancer. Fed Pract. 2020;37(suppl 4):S76-S80. doi:10.12788/fp.0019
7. Scheuner MT, Myrie K, Peredo J, et al. Integrating germline genetics into precision oncology practice in the Veterans Health Administration: challenges and opportunities. Fed Pract. 2020;37(suppl 4):S82-S88. doi:10.12788/fp.0033
For US Army veteran Tam Huynh, the US Department of Veterans Affairs (VA) precision oncology program has been the proverbial game changer. Diagnosed in 2016 with stage IV lung cancer and physically depleted by chemotherapy, Huynh learned that treatment based on the precise molecular makeup of his tumors held the potential for improving quality of life. Through the VA National Precision Oncology Program (NPOP), Huynh was matched to a medication shown to help patients whose tumors had the same genetic mutation as Huynh’s tumors. Today, Huynh is not only free of chemotherapy’s debilitating adverse effects, but able to enjoy time with his family and return to work.
Huynh is one of 400,000 veterans treated for cancer annually at the VA. The life-changing treatment he received is due to the legacy of research, integrated care, and collaboration that is the hallmark of the VA health care system. The NPOP is a natural outgrowth of this legacy, and, as Executive-in-Charge Richard Stone, MD, notes in his Foreword, part of the Veterans Health Administration’s (VHA) evolution as a learning health care system. The articles in this special issue represent a snapshot of the work underway under VHA NPOP as well as the dedication of VHA staff nationwide to provide patient-centric care to every veteran.
Leading off this special issue, NPOP director Michael J. Kelley, MD, provides context for understanding the paradigm shift represented by precision oncology.2 He also discusses how, within 5 years, the program came together from its start as a regional effort to its use today by almost every VA oncology practice. Kelley also explains the complexity behind interpreting next-generation sequencing (NGS) gene panel test results and how VA medical centers can call upon NPOP for assistance with this interpretation. Further, he states the “obligation” for new medical technology to be accessible and notes how NPOP was “intentional” during implementation to ensure rural veterans would be offered testing.2
Following Kelley’s discussion is a series of articles focused on precision oncology for prostate cancer, which affects 15,000 veterans yearly. The first, an overview of the Prostate Cancer Foundation (PCF), provides a short history of the organization and how it came to partner with the VA.3 Written by several PCF staff, including President and CEO Jonathan Simons, MD, the paper notes how the commitment of early leaders like S. Ward Casscells, MD, and Larry Stupski led to PCF’s “no veteran left behind” philosophy; ie, ensuring veteran access to clinical trials and world class care regardless of location. As the first disease-specific national network for oncology trials serving veterans, PCF aims to provide a model for all of US health care in the delivery of precision oncology care.
A critical part of PCF is the Precision Oncology Program for Cancer of the Prostate (POPCaP), which focuses on genetics and genomic testing. Bruce Montgomery, MD, and Matthew Retting, MD—VHA’s leading experts in prostate cancer—shine the spotlight on VA’s research track record, specifically the genomics of metastatic prostate cancer.4 They also note the program’s focus on African American veteran patients who are disproportionately affected by the disease but well represented in the VA. In discussing future directions, the authors explain the importance of expanding genetic testing for those diagnosed with prostate cancer.
Prostate cancer Analysis for Therapy Choice (PATCH) is a clinical trials network that works hand-in-hand with POPCaP to use genetic data collected by POPCaP sites to find patients for trials. In their discussion, authors Julie N. Graff, MD, and Grant D. Huang, MD, who leads VA Research’s Cooperative Studies Program, focus on 3 key areas: (1) the challenges of precision oncology when working with relatively rare mutations; (2) 2 new drug trials at VA that will help clinicians know whether certain targeted therapies work for prostate cancer; and (3) how VA is emerging as a national partner in drug discovery and the approval of precision drugs.5
Turning to lung cancer–the second leading cause of cancer death among veterans–Drew Moghanaki, MD, MPH, and Michael Hagan, MD, discuss 3 multisite initiatives launched in 2016 and 2017.6 The first trial, VA Partnership to Increase Access to Lung Cancer Screening (VA-PALS), is a multisite project sponsored by the VA’s Office of Rural Health and Bristol-Myers Squibb Foundation. The trial’s goal is to reduce lung cancer mortality through a robust early detection program. The second trial, VA Lung Cancer Surgery OR Radiation therapy (VALOR) compares whether radiation or surgery is the best for early-stage lung cancer. Notably, VALOR may be one of the most difficult randomized trial ever attempted in lung cancer research (4 previous phase 3 trials outside the VA closed prematurely). By addressing the previous challenges associated with running such a trial, the VALOR study team already has enrolled more than all of the previous phase 3 efforts combined. The third trial is VA Radiation Oncology Quality Surveillance Program (VA-ROQS), which was created in 2016 to benchmark the treatment of veterans with lung cancer. VA-ROQS aims to create a national network of Lung Cancer Centers of Excellence that work with VISNs to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information.
The final group of authors, led by Maren T. Scheuner, MD, discuss how the advent of germline testing as a standard-of-care practice for certain tumor types presents opportunities and challenges for precision oncology.7 One of the primary challenges they note is the shortage of genetics professionals, both within the VA system and health care generally. To help address this issue, they recommend leveraging VA’s longstanding partnership with its academic affiliates.
Precision oncology clearly demonstrates how applying knowledge regarding one of the smallest of living matter can make a tremendous difference in the matter of living. Tam Huynh’s story is proof positive. Speaking at last year’s AMSUS (Society for Federal Health Professionals) annual meeting about his experience, Huynh said that all veterans should have access to the same life-changing treatment he received. This is exactly where the VA NPOP is heading.
For US Army veteran Tam Huynh, the US Department of Veterans Affairs (VA) precision oncology program has been the proverbial game changer. Diagnosed in 2016 with stage IV lung cancer and physically depleted by chemotherapy, Huynh learned that treatment based on the precise molecular makeup of his tumors held the potential for improving quality of life. Through the VA National Precision Oncology Program (NPOP), Huynh was matched to a medication shown to help patients whose tumors had the same genetic mutation as Huynh’s tumors. Today, Huynh is not only free of chemotherapy’s debilitating adverse effects, but able to enjoy time with his family and return to work.
Huynh is one of 400,000 veterans treated for cancer annually at the VA. The life-changing treatment he received is due to the legacy of research, integrated care, and collaboration that is the hallmark of the VA health care system. The NPOP is a natural outgrowth of this legacy, and, as Executive-in-Charge Richard Stone, MD, notes in his Foreword, part of the Veterans Health Administration’s (VHA) evolution as a learning health care system. The articles in this special issue represent a snapshot of the work underway under VHA NPOP as well as the dedication of VHA staff nationwide to provide patient-centric care to every veteran.
Leading off this special issue, NPOP director Michael J. Kelley, MD, provides context for understanding the paradigm shift represented by precision oncology.2 He also discusses how, within 5 years, the program came together from its start as a regional effort to its use today by almost every VA oncology practice. Kelley also explains the complexity behind interpreting next-generation sequencing (NGS) gene panel test results and how VA medical centers can call upon NPOP for assistance with this interpretation. Further, he states the “obligation” for new medical technology to be accessible and notes how NPOP was “intentional” during implementation to ensure rural veterans would be offered testing.2
Following Kelley’s discussion is a series of articles focused on precision oncology for prostate cancer, which affects 15,000 veterans yearly. The first, an overview of the Prostate Cancer Foundation (PCF), provides a short history of the organization and how it came to partner with the VA.3 Written by several PCF staff, including President and CEO Jonathan Simons, MD, the paper notes how the commitment of early leaders like S. Ward Casscells, MD, and Larry Stupski led to PCF’s “no veteran left behind” philosophy; ie, ensuring veteran access to clinical trials and world class care regardless of location. As the first disease-specific national network for oncology trials serving veterans, PCF aims to provide a model for all of US health care in the delivery of precision oncology care.
A critical part of PCF is the Precision Oncology Program for Cancer of the Prostate (POPCaP), which focuses on genetics and genomic testing. Bruce Montgomery, MD, and Matthew Retting, MD—VHA’s leading experts in prostate cancer—shine the spotlight on VA’s research track record, specifically the genomics of metastatic prostate cancer.4 They also note the program’s focus on African American veteran patients who are disproportionately affected by the disease but well represented in the VA. In discussing future directions, the authors explain the importance of expanding genetic testing for those diagnosed with prostate cancer.
Prostate cancer Analysis for Therapy Choice (PATCH) is a clinical trials network that works hand-in-hand with POPCaP to use genetic data collected by POPCaP sites to find patients for trials. In their discussion, authors Julie N. Graff, MD, and Grant D. Huang, MD, who leads VA Research’s Cooperative Studies Program, focus on 3 key areas: (1) the challenges of precision oncology when working with relatively rare mutations; (2) 2 new drug trials at VA that will help clinicians know whether certain targeted therapies work for prostate cancer; and (3) how VA is emerging as a national partner in drug discovery and the approval of precision drugs.5
Turning to lung cancer–the second leading cause of cancer death among veterans–Drew Moghanaki, MD, MPH, and Michael Hagan, MD, discuss 3 multisite initiatives launched in 2016 and 2017.6 The first trial, VA Partnership to Increase Access to Lung Cancer Screening (VA-PALS), is a multisite project sponsored by the VA’s Office of Rural Health and Bristol-Myers Squibb Foundation. The trial’s goal is to reduce lung cancer mortality through a robust early detection program. The second trial, VA Lung Cancer Surgery OR Radiation therapy (VALOR) compares whether radiation or surgery is the best for early-stage lung cancer. Notably, VALOR may be one of the most difficult randomized trial ever attempted in lung cancer research (4 previous phase 3 trials outside the VA closed prematurely). By addressing the previous challenges associated with running such a trial, the VALOR study team already has enrolled more than all of the previous phase 3 efforts combined. The third trial is VA Radiation Oncology Quality Surveillance Program (VA-ROQS), which was created in 2016 to benchmark the treatment of veterans with lung cancer. VA-ROQS aims to create a national network of Lung Cancer Centers of Excellence that work with VISNs to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information.
The final group of authors, led by Maren T. Scheuner, MD, discuss how the advent of germline testing as a standard-of-care practice for certain tumor types presents opportunities and challenges for precision oncology.7 One of the primary challenges they note is the shortage of genetics professionals, both within the VA system and health care generally. To help address this issue, they recommend leveraging VA’s longstanding partnership with its academic affiliates.
Precision oncology clearly demonstrates how applying knowledge regarding one of the smallest of living matter can make a tremendous difference in the matter of living. Tam Huynh’s story is proof positive. Speaking at last year’s AMSUS (Society for Federal Health Professionals) annual meeting about his experience, Huynh said that all veterans should have access to the same life-changing treatment he received. This is exactly where the VA NPOP is heading.
1. How the VA is using AI to target cancer, https://www.theatlantic.com/sponsored/ibm-2018/watson-va-cancer/1925. Accessed August 6, 2020.
2. Kelley MJ. VA National Precision Oncology Program. Fed Pract. 2020;37 (suppl 4):S22-S27. doi:10.12788/fp.0037
3. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi: 10.12788/fp.0035
4. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
5. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi:10.12788/fp.0028
6. Moghanaki D, Hagan M. Strategic initiatives for veterans with lung cancer. Fed Pract. 2020;37(suppl 4):S76-S80. doi:10.12788/fp.0019
7. Scheuner MT, Myrie K, Peredo J, et al. Integrating germline genetics into precision oncology practice in the Veterans Health Administration: challenges and opportunities. Fed Pract. 2020;37(suppl 4):S82-S88. doi:10.12788/fp.0033
1. How the VA is using AI to target cancer, https://www.theatlantic.com/sponsored/ibm-2018/watson-va-cancer/1925. Accessed August 6, 2020.
2. Kelley MJ. VA National Precision Oncology Program. Fed Pract. 2020;37 (suppl 4):S22-S27. doi:10.12788/fp.0037
3. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi: 10.12788/fp.0035
4. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
5. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi:10.12788/fp.0028
6. Moghanaki D, Hagan M. Strategic initiatives for veterans with lung cancer. Fed Pract. 2020;37(suppl 4):S76-S80. doi:10.12788/fp.0019
7. Scheuner MT, Myrie K, Peredo J, et al. Integrating germline genetics into precision oncology practice in the Veterans Health Administration: challenges and opportunities. Fed Pract. 2020;37(suppl 4):S82-S88. doi:10.12788/fp.0033
Advances in Precision Oncology: Foreword (FULL)
For > 90 years, the US Department of Veterans Affairs (VA) has been in the vanguard of cancer research and treatment—improving the lives of veterans and all Americans. In 1932, recognizing the intrinsic link between research and clinical care, the Edward Hines, Jr. VA Hospital in Chicago, Illinois, established a tumor research laboratory to complement the work of its cancer treatment center. As the first VA laboratory to receive funding specifically for research, the new facility symbolized a paradigm shift in thinking about cancer treatment.
Today, through its National Precision Oncology Program (NPOP), the Veterans Health Administration (VHA) has embarked upon another paradigm shift—one that also puts research front and center by leveraging VHA’s unique assets as a learning health care system. As noted by Montgomery and colleagues, “given its size, integration and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.”1 The articles in this special issue, which focus on the 2 cancers that affects the most veterans—prostate and lung—show the transformative work underway to develop a new model of collaboration in cancer care.
At VHA, research and practice are not just proximal; they are truly integrated in the service of enhancing veterans’ outcomes. For example, > 60% of VA researchers are clinicians who also provide direct patient care. As observed by Levine and colleagues, “meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa.”2
For example, it was physician-scientist Donald Gleason, MD, PhD, who in the 1960s pioneered the standardized system that helps doctors better assess and treat prostate cancer (the Gleason score). More recently, physician-scientists Matthew Rettig, MD, and Bruce Montgomery, MD, both leading experts in prostate cancer research, were instrumental to VA’s partnership with the Prostate Cancer Foundation (PCF) to establish a national network for oncology trials serving veterans.
Having an embedded research program within the nation’s largest integrated health care system also provides the VA with the ability to conduct large-scale, multisite clinical trials. Since the 1940s, the VA Cooperative Studies Program (CSP) has generated key research findings across a range of diseases, including cancer, and provided definitive evidence and learning. In 1994, CSP launched its Prostate Cancer Intervention vs Observation Trial (PIVOT) study to determine whether observation is as effective as surgery for early-stage prostate cancer. Today, through the CSP, VA researchers are conducting a randomized, phase 3 clinical trial called VA Lung cancer surgery Or stereotactic Radiotherapy trial (VALOR) that will assess which of the 2 modalities is better when treating veterans with operable early-stage non-small cell lung cancer.
Additionally, VA is privileged to serve a patient population so dedicated to their country that many volunteer to serve again as participants in VA research clinical trials. In fact, Levine and colleagues credit the patients willing to enter clinical trials for the collective call to action and “critical philanthropic investment” that led to the Precision Oncology Program for Cancer of the Prostate (POPCaP).2
As a learning health care system, we also have been mindful of lessons drawn from the ongoing COVID-19 public health crisis. Almost overnight, VHA shifted from in-person to virtual visits to minimize the risk for veterans and their families. At the same time, we limited in-person clinical research visits to those that were required for the Veterans’ health or well-being and conducted large numbers of virtual research visits. (Notably, the current crisis motivated accelerated study regarding virtual research trials, clarifying which touchpoints must be face-to-face and which have been face-to-face due mainly to convention.) In parallel, we also launched numerous clinical studies focused on the fight against COVID-19. Our capacity to transition both clinical care and research is due in no small part to our preexisting and strong foundation in telehealth.
With one-third of our patient population living in rural areas, these achievements are vital to our commitment of “no veteran left behind.” These efforts were recently boosted by VHA’s newest partnership with the Bristol Myers Squibb Foundation to establish a national teleoncology center that will enable all veterans to benefit from new research advances no matter where they live.
Precision oncology represents a new model of collaboration in cancer care among clinicians, operations leaders, researchers and veterans. By leveraging the many assets that have contributed to VA’s success as a learning health care system, we can fulfill the promise of providing leading edge cancer care to all veterans.
1. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
2. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs Partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi:10.12788/fp.0035
For > 90 years, the US Department of Veterans Affairs (VA) has been in the vanguard of cancer research and treatment—improving the lives of veterans and all Americans. In 1932, recognizing the intrinsic link between research and clinical care, the Edward Hines, Jr. VA Hospital in Chicago, Illinois, established a tumor research laboratory to complement the work of its cancer treatment center. As the first VA laboratory to receive funding specifically for research, the new facility symbolized a paradigm shift in thinking about cancer treatment.
Today, through its National Precision Oncology Program (NPOP), the Veterans Health Administration (VHA) has embarked upon another paradigm shift—one that also puts research front and center by leveraging VHA’s unique assets as a learning health care system. As noted by Montgomery and colleagues, “given its size, integration and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.”1 The articles in this special issue, which focus on the 2 cancers that affects the most veterans—prostate and lung—show the transformative work underway to develop a new model of collaboration in cancer care.
At VHA, research and practice are not just proximal; they are truly integrated in the service of enhancing veterans’ outcomes. For example, > 60% of VA researchers are clinicians who also provide direct patient care. As observed by Levine and colleagues, “meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa.”2
For example, it was physician-scientist Donald Gleason, MD, PhD, who in the 1960s pioneered the standardized system that helps doctors better assess and treat prostate cancer (the Gleason score). More recently, physician-scientists Matthew Rettig, MD, and Bruce Montgomery, MD, both leading experts in prostate cancer research, were instrumental to VA’s partnership with the Prostate Cancer Foundation (PCF) to establish a national network for oncology trials serving veterans.
Having an embedded research program within the nation’s largest integrated health care system also provides the VA with the ability to conduct large-scale, multisite clinical trials. Since the 1940s, the VA Cooperative Studies Program (CSP) has generated key research findings across a range of diseases, including cancer, and provided definitive evidence and learning. In 1994, CSP launched its Prostate Cancer Intervention vs Observation Trial (PIVOT) study to determine whether observation is as effective as surgery for early-stage prostate cancer. Today, through the CSP, VA researchers are conducting a randomized, phase 3 clinical trial called VA Lung cancer surgery Or stereotactic Radiotherapy trial (VALOR) that will assess which of the 2 modalities is better when treating veterans with operable early-stage non-small cell lung cancer.
Additionally, VA is privileged to serve a patient population so dedicated to their country that many volunteer to serve again as participants in VA research clinical trials. In fact, Levine and colleagues credit the patients willing to enter clinical trials for the collective call to action and “critical philanthropic investment” that led to the Precision Oncology Program for Cancer of the Prostate (POPCaP).2
As a learning health care system, we also have been mindful of lessons drawn from the ongoing COVID-19 public health crisis. Almost overnight, VHA shifted from in-person to virtual visits to minimize the risk for veterans and their families. At the same time, we limited in-person clinical research visits to those that were required for the Veterans’ health or well-being and conducted large numbers of virtual research visits. (Notably, the current crisis motivated accelerated study regarding virtual research trials, clarifying which touchpoints must be face-to-face and which have been face-to-face due mainly to convention.) In parallel, we also launched numerous clinical studies focused on the fight against COVID-19. Our capacity to transition both clinical care and research is due in no small part to our preexisting and strong foundation in telehealth.
With one-third of our patient population living in rural areas, these achievements are vital to our commitment of “no veteran left behind.” These efforts were recently boosted by VHA’s newest partnership with the Bristol Myers Squibb Foundation to establish a national teleoncology center that will enable all veterans to benefit from new research advances no matter where they live.
Precision oncology represents a new model of collaboration in cancer care among clinicians, operations leaders, researchers and veterans. By leveraging the many assets that have contributed to VA’s success as a learning health care system, we can fulfill the promise of providing leading edge cancer care to all veterans.
For > 90 years, the US Department of Veterans Affairs (VA) has been in the vanguard of cancer research and treatment—improving the lives of veterans and all Americans. In 1932, recognizing the intrinsic link between research and clinical care, the Edward Hines, Jr. VA Hospital in Chicago, Illinois, established a tumor research laboratory to complement the work of its cancer treatment center. As the first VA laboratory to receive funding specifically for research, the new facility symbolized a paradigm shift in thinking about cancer treatment.
Today, through its National Precision Oncology Program (NPOP), the Veterans Health Administration (VHA) has embarked upon another paradigm shift—one that also puts research front and center by leveraging VHA’s unique assets as a learning health care system. As noted by Montgomery and colleagues, “given its size, integration and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.”1 The articles in this special issue, which focus on the 2 cancers that affects the most veterans—prostate and lung—show the transformative work underway to develop a new model of collaboration in cancer care.
At VHA, research and practice are not just proximal; they are truly integrated in the service of enhancing veterans’ outcomes. For example, > 60% of VA researchers are clinicians who also provide direct patient care. As observed by Levine and colleagues, “meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa.”2
For example, it was physician-scientist Donald Gleason, MD, PhD, who in the 1960s pioneered the standardized system that helps doctors better assess and treat prostate cancer (the Gleason score). More recently, physician-scientists Matthew Rettig, MD, and Bruce Montgomery, MD, both leading experts in prostate cancer research, were instrumental to VA’s partnership with the Prostate Cancer Foundation (PCF) to establish a national network for oncology trials serving veterans.
Having an embedded research program within the nation’s largest integrated health care system also provides the VA with the ability to conduct large-scale, multisite clinical trials. Since the 1940s, the VA Cooperative Studies Program (CSP) has generated key research findings across a range of diseases, including cancer, and provided definitive evidence and learning. In 1994, CSP launched its Prostate Cancer Intervention vs Observation Trial (PIVOT) study to determine whether observation is as effective as surgery for early-stage prostate cancer. Today, through the CSP, VA researchers are conducting a randomized, phase 3 clinical trial called VA Lung cancer surgery Or stereotactic Radiotherapy trial (VALOR) that will assess which of the 2 modalities is better when treating veterans with operable early-stage non-small cell lung cancer.
Additionally, VA is privileged to serve a patient population so dedicated to their country that many volunteer to serve again as participants in VA research clinical trials. In fact, Levine and colleagues credit the patients willing to enter clinical trials for the collective call to action and “critical philanthropic investment” that led to the Precision Oncology Program for Cancer of the Prostate (POPCaP).2
As a learning health care system, we also have been mindful of lessons drawn from the ongoing COVID-19 public health crisis. Almost overnight, VHA shifted from in-person to virtual visits to minimize the risk for veterans and their families. At the same time, we limited in-person clinical research visits to those that were required for the Veterans’ health or well-being and conducted large numbers of virtual research visits. (Notably, the current crisis motivated accelerated study regarding virtual research trials, clarifying which touchpoints must be face-to-face and which have been face-to-face due mainly to convention.) In parallel, we also launched numerous clinical studies focused on the fight against COVID-19. Our capacity to transition both clinical care and research is due in no small part to our preexisting and strong foundation in telehealth.
With one-third of our patient population living in rural areas, these achievements are vital to our commitment of “no veteran left behind.” These efforts were recently boosted by VHA’s newest partnership with the Bristol Myers Squibb Foundation to establish a national teleoncology center that will enable all veterans to benefit from new research advances no matter where they live.
Precision oncology represents a new model of collaboration in cancer care among clinicians, operations leaders, researchers and veterans. By leveraging the many assets that have contributed to VA’s success as a learning health care system, we can fulfill the promise of providing leading edge cancer care to all veterans.
1. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
2. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs Partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi:10.12788/fp.0035
1. Montgomery B, Rettig M, Kasten J, Muralidhar S, Myrie K, Ramoni R. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
2. Levine RD, Ekanayake RN, Martin AC, et al. Prostate Cancer Foundation-Department of Veterans Affairs Partnership: a model of public-private collaboration to advance treatment and care of invasive cancers. Fed Pract. 2020;37(suppl 4):S32-S37. doi:10.12788/fp.0035
Catching up with ourselves
August is a month that we traditionally reserved for rest and recovery. But unfortunately, there seems to be little of either as we recover from COVID-19, deal with the care that has been delayed, try to understand issues of health inequity, and manage our hybrid reimbursement landscape. So let’s set those issues aside for a bit and get back to science.
In this month’s cover stories, we can read about some astounding accomplishments. A fantastic study comes from Dana-Farber Cancer Institute, Boston, where researchers found 900 colorectal cancers from nurses who had participated in the long-running Nurse’s Health Studies. The researchers completed a whole-exome sequence on both normal and tumor tissue and then linked findings to the nutritional information contained in the Health Studies. With this information, they connected a tumor-associated mutation to the ingestion of red meat, which may suggest a causal link for the known association between red meat and CRC.
AGA has published a detailed clinical practice update about endoscopic management of postsurgical complications after bariatric/metabolic surgery. Bariatric therapy is an area in which gastroenterologists should play an increasingly prominent role, in conjunction with our surgical and metabolic colleagues.
Finally, read about a novel oral therapy that may provide substantial relief for celiac patients. This randomized trial of a transglutaminase inhibitor was published in the New England Journal of Medicine and may provide new hope for this difficult condition.
October marks the end of my term as Editor-in-Chief. Megan Adams, MD, JD, MSc, will take over and provide insights and opinions beyond my past missives. I thank Christopher Palmer and the excellent Frontline staff who find topics and compose articles for us. Finally, the publication department at the American Gastroenterological Association is unparalleled, led by Erin Landis with Jillian Schweitzer managing the GI & Hepatology News area. I am fortunate to return to the AGA Governing Board as Secretary/Treasurer and work with our new president, John Inadomi, as well as Tom Serena, a great friend and AGA CEO.
John I Allen, MD, MBA, AGAF
Editor in Chief
August is a month that we traditionally reserved for rest and recovery. But unfortunately, there seems to be little of either as we recover from COVID-19, deal with the care that has been delayed, try to understand issues of health inequity, and manage our hybrid reimbursement landscape. So let’s set those issues aside for a bit and get back to science.
In this month’s cover stories, we can read about some astounding accomplishments. A fantastic study comes from Dana-Farber Cancer Institute, Boston, where researchers found 900 colorectal cancers from nurses who had participated in the long-running Nurse’s Health Studies. The researchers completed a whole-exome sequence on both normal and tumor tissue and then linked findings to the nutritional information contained in the Health Studies. With this information, they connected a tumor-associated mutation to the ingestion of red meat, which may suggest a causal link for the known association between red meat and CRC.
AGA has published a detailed clinical practice update about endoscopic management of postsurgical complications after bariatric/metabolic surgery. Bariatric therapy is an area in which gastroenterologists should play an increasingly prominent role, in conjunction with our surgical and metabolic colleagues.
Finally, read about a novel oral therapy that may provide substantial relief for celiac patients. This randomized trial of a transglutaminase inhibitor was published in the New England Journal of Medicine and may provide new hope for this difficult condition.
October marks the end of my term as Editor-in-Chief. Megan Adams, MD, JD, MSc, will take over and provide insights and opinions beyond my past missives. I thank Christopher Palmer and the excellent Frontline staff who find topics and compose articles for us. Finally, the publication department at the American Gastroenterological Association is unparalleled, led by Erin Landis with Jillian Schweitzer managing the GI & Hepatology News area. I am fortunate to return to the AGA Governing Board as Secretary/Treasurer and work with our new president, John Inadomi, as well as Tom Serena, a great friend and AGA CEO.
John I Allen, MD, MBA, AGAF
Editor in Chief
August is a month that we traditionally reserved for rest and recovery. But unfortunately, there seems to be little of either as we recover from COVID-19, deal with the care that has been delayed, try to understand issues of health inequity, and manage our hybrid reimbursement landscape. So let’s set those issues aside for a bit and get back to science.
In this month’s cover stories, we can read about some astounding accomplishments. A fantastic study comes from Dana-Farber Cancer Institute, Boston, where researchers found 900 colorectal cancers from nurses who had participated in the long-running Nurse’s Health Studies. The researchers completed a whole-exome sequence on both normal and tumor tissue and then linked findings to the nutritional information contained in the Health Studies. With this information, they connected a tumor-associated mutation to the ingestion of red meat, which may suggest a causal link for the known association between red meat and CRC.
AGA has published a detailed clinical practice update about endoscopic management of postsurgical complications after bariatric/metabolic surgery. Bariatric therapy is an area in which gastroenterologists should play an increasingly prominent role, in conjunction with our surgical and metabolic colleagues.
Finally, read about a novel oral therapy that may provide substantial relief for celiac patients. This randomized trial of a transglutaminase inhibitor was published in the New England Journal of Medicine and may provide new hope for this difficult condition.
October marks the end of my term as Editor-in-Chief. Megan Adams, MD, JD, MSc, will take over and provide insights and opinions beyond my past missives. I thank Christopher Palmer and the excellent Frontline staff who find topics and compose articles for us. Finally, the publication department at the American Gastroenterological Association is unparalleled, led by Erin Landis with Jillian Schweitzer managing the GI & Hepatology News area. I am fortunate to return to the AGA Governing Board as Secretary/Treasurer and work with our new president, John Inadomi, as well as Tom Serena, a great friend and AGA CEO.
John I Allen, MD, MBA, AGAF
Editor in Chief
COVID-19, hearings on Jan. 6 attack reignite interest in PTSD
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
Immune reconstitution inflammatory syndrome: ‘Why is my patient getting worse?’
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.