User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Dietary changes to microbiome may improve lung function
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT CHEST 2023
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.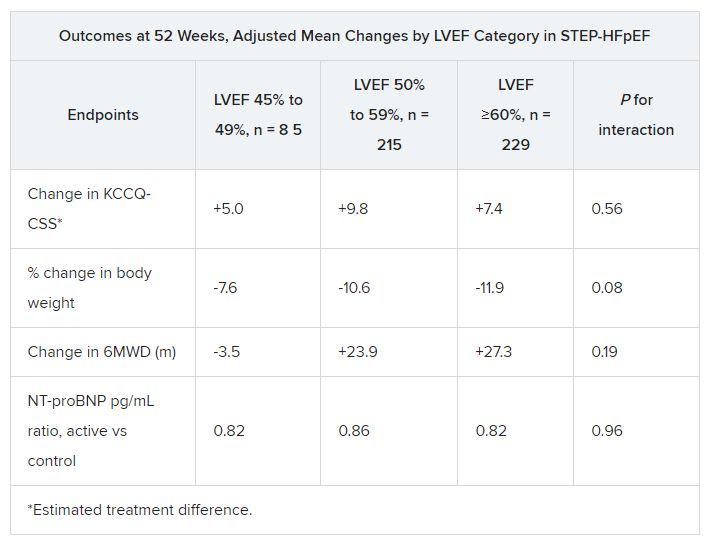
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
Short, long-lasting bronchodilators similar for exacerbated COPD
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
AT CHEST 2023
Head, neck cancer radiotherapy regimen saves time when resources limited
SAN DIEGO – In low- and middle-income countries with high incidence and mortality from head and neck cancer, resources remain limited. Patients often can’t travel far for treatment or afford to stay near a treatment center for the length of time required for conventionally fractionated radiotherapy.
in these settings.
The phase 3 randomized HYPNO trial, conducted in 10 low- and middle-income countries, revealed that the hypofractionated regimen shortened total treatment time by a median of 11.5 days and was noninferior to conventional fractionation for tumor control and safety.
The primary trial results were presented by Søren Bentzen, PhD, DMSc, at the annual meeting of the American Society for Radiation Oncology.
“It was Usain Bolt who said, ‘I train for 4 years to run 9 seconds,’ and that was the feeling that I had when we did the noninferiority test,” said Dr. Bentzen, from the University of Maryland School of Medicine in Baltimore. “We had not looked at the data while the data were being accumulated, and guess what? It actually turned out that we had noninferiority with respect to both locoregional control and the late effects.”
In the HYPNO trial, Dr. Bentzen and colleagues wanted to determine whether a streamlined approach to the treatment of patients in low- and middle-income countries could improve access to care and still achieve strong outcomes.
The investigators used mathematical modeling to devise a strategy to reduce the number of fractions and put this hypothesis to the test in a pragmatic trial.
Patients from Uruguay, Brazil, Argentina, Cuba, South Africa, India, Pakistan, Thailand, Indonesia, and the Philippines were enrolled. After stratification by performance status, tumor subsite, institution, and previous treatment with chemotherapy, the 792 patients in the trial were randomly assigned in a 1:1 ratio to receive either 66 Gy in 33 fractions 6 days each week over 5.5 weeks, or 55 Gy in 20 fractions 5 days per week over 4 weeks. In both groups, weekly cisplatin was optional.
Compliance with the regimens was high in both arms, with 95% of patients assigned to conventional fractionation and 99% assigned to hypofractionation receiving the total planned doses.
At 3 years’ follow-up, the rates of locoregional control were 50.7% in the hypofractionation arm and 51.2% in the conventional fractionation arm (P = .40). No significant differences between the groups have emerged over 5 years, Dr. Bentzen said.
Rates of late toxicities of grade 3 or greater at 3 years’ follow-up were similar between the groups, at 18.8% in the hypofractionation arm and 20.2% in the conventional fractionation arm (P = .68).
Three-year overall survival rates also did not differ between the groups – 54.1% in the hypofractionation arm vs. 55.5% in the conventional arm (P = .62) – nor did rates of progression-free survival – 44.0% vs. 45.3%.
“Head and neck cancer caused by factors other than the human papillomavirus (HPV) remains a significant burden especially in lower- and middle-income countries,” Dr. Bentzen said in a press release. “This is a trial that directly informs how you can effectively deliver radiation therapy to patients in a resource-scarce environment.”
Beth Beadle, MD, PhD, the invited discussant at a media briefing where Dr. Bentzen summarized the findings, said, “I think this trial is going to change practice in low- and middle-income countries and will improve access to care.”
Although the approach used in the HYPNO trial will likely allow more patients to receive treatment and will save lives in countries with limited resources, the strategy likely won’t apply to U.S. practice, noted Dr. Beadle, a professor of radiation oncology at Stanford University, California.
“The one thing I do caution, and that Dr. Bentzen brought up, is that this is a very different population than the one that we see in the United States now,” Dr. Beadle said. “In fact, it’s very challenging to find a similar patient population to even serve as a comparison in the modern era and modern techniques.”
The HYPNO trial was sponsored by the International Atomic Energy Agency. Dr. Bentzen and Dr. Beadle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – In low- and middle-income countries with high incidence and mortality from head and neck cancer, resources remain limited. Patients often can’t travel far for treatment or afford to stay near a treatment center for the length of time required for conventionally fractionated radiotherapy.
in these settings.
The phase 3 randomized HYPNO trial, conducted in 10 low- and middle-income countries, revealed that the hypofractionated regimen shortened total treatment time by a median of 11.5 days and was noninferior to conventional fractionation for tumor control and safety.
The primary trial results were presented by Søren Bentzen, PhD, DMSc, at the annual meeting of the American Society for Radiation Oncology.
“It was Usain Bolt who said, ‘I train for 4 years to run 9 seconds,’ and that was the feeling that I had when we did the noninferiority test,” said Dr. Bentzen, from the University of Maryland School of Medicine in Baltimore. “We had not looked at the data while the data were being accumulated, and guess what? It actually turned out that we had noninferiority with respect to both locoregional control and the late effects.”
In the HYPNO trial, Dr. Bentzen and colleagues wanted to determine whether a streamlined approach to the treatment of patients in low- and middle-income countries could improve access to care and still achieve strong outcomes.
The investigators used mathematical modeling to devise a strategy to reduce the number of fractions and put this hypothesis to the test in a pragmatic trial.
Patients from Uruguay, Brazil, Argentina, Cuba, South Africa, India, Pakistan, Thailand, Indonesia, and the Philippines were enrolled. After stratification by performance status, tumor subsite, institution, and previous treatment with chemotherapy, the 792 patients in the trial were randomly assigned in a 1:1 ratio to receive either 66 Gy in 33 fractions 6 days each week over 5.5 weeks, or 55 Gy in 20 fractions 5 days per week over 4 weeks. In both groups, weekly cisplatin was optional.
Compliance with the regimens was high in both arms, with 95% of patients assigned to conventional fractionation and 99% assigned to hypofractionation receiving the total planned doses.
At 3 years’ follow-up, the rates of locoregional control were 50.7% in the hypofractionation arm and 51.2% in the conventional fractionation arm (P = .40). No significant differences between the groups have emerged over 5 years, Dr. Bentzen said.
Rates of late toxicities of grade 3 or greater at 3 years’ follow-up were similar between the groups, at 18.8% in the hypofractionation arm and 20.2% in the conventional fractionation arm (P = .68).
Three-year overall survival rates also did not differ between the groups – 54.1% in the hypofractionation arm vs. 55.5% in the conventional arm (P = .62) – nor did rates of progression-free survival – 44.0% vs. 45.3%.
“Head and neck cancer caused by factors other than the human papillomavirus (HPV) remains a significant burden especially in lower- and middle-income countries,” Dr. Bentzen said in a press release. “This is a trial that directly informs how you can effectively deliver radiation therapy to patients in a resource-scarce environment.”
Beth Beadle, MD, PhD, the invited discussant at a media briefing where Dr. Bentzen summarized the findings, said, “I think this trial is going to change practice in low- and middle-income countries and will improve access to care.”
Although the approach used in the HYPNO trial will likely allow more patients to receive treatment and will save lives in countries with limited resources, the strategy likely won’t apply to U.S. practice, noted Dr. Beadle, a professor of radiation oncology at Stanford University, California.
“The one thing I do caution, and that Dr. Bentzen brought up, is that this is a very different population than the one that we see in the United States now,” Dr. Beadle said. “In fact, it’s very challenging to find a similar patient population to even serve as a comparison in the modern era and modern techniques.”
The HYPNO trial was sponsored by the International Atomic Energy Agency. Dr. Bentzen and Dr. Beadle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – In low- and middle-income countries with high incidence and mortality from head and neck cancer, resources remain limited. Patients often can’t travel far for treatment or afford to stay near a treatment center for the length of time required for conventionally fractionated radiotherapy.
in these settings.
The phase 3 randomized HYPNO trial, conducted in 10 low- and middle-income countries, revealed that the hypofractionated regimen shortened total treatment time by a median of 11.5 days and was noninferior to conventional fractionation for tumor control and safety.
The primary trial results were presented by Søren Bentzen, PhD, DMSc, at the annual meeting of the American Society for Radiation Oncology.
“It was Usain Bolt who said, ‘I train for 4 years to run 9 seconds,’ and that was the feeling that I had when we did the noninferiority test,” said Dr. Bentzen, from the University of Maryland School of Medicine in Baltimore. “We had not looked at the data while the data were being accumulated, and guess what? It actually turned out that we had noninferiority with respect to both locoregional control and the late effects.”
In the HYPNO trial, Dr. Bentzen and colleagues wanted to determine whether a streamlined approach to the treatment of patients in low- and middle-income countries could improve access to care and still achieve strong outcomes.
The investigators used mathematical modeling to devise a strategy to reduce the number of fractions and put this hypothesis to the test in a pragmatic trial.
Patients from Uruguay, Brazil, Argentina, Cuba, South Africa, India, Pakistan, Thailand, Indonesia, and the Philippines were enrolled. After stratification by performance status, tumor subsite, institution, and previous treatment with chemotherapy, the 792 patients in the trial were randomly assigned in a 1:1 ratio to receive either 66 Gy in 33 fractions 6 days each week over 5.5 weeks, or 55 Gy in 20 fractions 5 days per week over 4 weeks. In both groups, weekly cisplatin was optional.
Compliance with the regimens was high in both arms, with 95% of patients assigned to conventional fractionation and 99% assigned to hypofractionation receiving the total planned doses.
At 3 years’ follow-up, the rates of locoregional control were 50.7% in the hypofractionation arm and 51.2% in the conventional fractionation arm (P = .40). No significant differences between the groups have emerged over 5 years, Dr. Bentzen said.
Rates of late toxicities of grade 3 or greater at 3 years’ follow-up were similar between the groups, at 18.8% in the hypofractionation arm and 20.2% in the conventional fractionation arm (P = .68).
Three-year overall survival rates also did not differ between the groups – 54.1% in the hypofractionation arm vs. 55.5% in the conventional arm (P = .62) – nor did rates of progression-free survival – 44.0% vs. 45.3%.
“Head and neck cancer caused by factors other than the human papillomavirus (HPV) remains a significant burden especially in lower- and middle-income countries,” Dr. Bentzen said in a press release. “This is a trial that directly informs how you can effectively deliver radiation therapy to patients in a resource-scarce environment.”
Beth Beadle, MD, PhD, the invited discussant at a media briefing where Dr. Bentzen summarized the findings, said, “I think this trial is going to change practice in low- and middle-income countries and will improve access to care.”
Although the approach used in the HYPNO trial will likely allow more patients to receive treatment and will save lives in countries with limited resources, the strategy likely won’t apply to U.S. practice, noted Dr. Beadle, a professor of radiation oncology at Stanford University, California.
“The one thing I do caution, and that Dr. Bentzen brought up, is that this is a very different population than the one that we see in the United States now,” Dr. Beadle said. “In fact, it’s very challenging to find a similar patient population to even serve as a comparison in the modern era and modern techniques.”
The HYPNO trial was sponsored by the International Atomic Energy Agency. Dr. Bentzen and Dr. Beadle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASTRO 2023
‘Reassuring’ follow-up validates radiation strategy for early breast cancer
SAN DIEGO –
The follow-up analysis of the 2,016-subject IMPORT LOW study found that 10-year overall survival rates were 87.8% (95% confidence interval, 84.9-90.1) for a full-breast radiation group, 87.2% (95% CI, 84.3-89.6) for a reduced-dose group, and 90.3% (95% CI, 87.7-92.4) in a partial-breast group. Breast cancer radiotherapy specialist Anna Kirby, MB, MD (Res), MA, of the Royal Marsden and Institute of Cancer Research, London, reported the results at the annual meeting of the American Society for Radiation Oncology.
Ipsilateral breast tumor relapse was also similar in the three groups at the 10-year mark at 2.8% (95% CI, 1.8-4.5), 1.9% (95% CI, 1.1-3.4), and 2.8% (95% CI, 1.7-4.5), respectively. Moderate/marked adverse effects were deemed to be low.
Dr. Kirby said the new findings are not “practice-changing.” However, “this complementary data supporting the change in practice that happened in the UK and elsewhere following the publication back in 2017.”
The findings are “reassuring,” breast cancer radiology specialist Robert W. Mutter, MD, of the Mayo Clinic, Rochester, Minn., said in an interview after reviewing the study findings. While the outcomes and adverse events are similar between the groups, “partial-breast irradiation is attractive because it exposes less normal tissue such as the heart and lungs than whole-breast irradiation. This could lead to fewer side effects for patients later in life at a population level. Partial-breast irradiation should be considered a standard of care in selected patients.”
In a presentation at ASTRO, Dr. Kirby provided background about the motivation for the study. It was clear that radiotherapy reduces local recurrence by up to two-thirds in early breast cancer, she said. “But in a population with excellent prognosis, this translates into relatively small absolute benefits from radiotherapy for many of our lower-risk patients,” she said. “All patients treated are at risk of radiotherapy side effects, and these become the main hazard for many women. Radiotherapy that’s focused on the part of the breast that contained the tumor – the so-called tumor bed – may reduce the long-term complications from radiotherapy, particularly in the breast, and potentially in the heart and lung, whilst hopefully maintaining low local recurrence rates.”
The initial 5-year study was a randomized, multicenter, phase III trial of patients ≥ age 50 in the United Kingdom who were tracked since recruitment in 2007-2010 (median age, 63). All had undergone breast conservation surgery. The patients were assigned to the control group (n = 674, 40 Gy), reduced-dose (n = 673, 40 Gy) and partial-breast (n = 669, 40 Gy to partial breast only) in 15 daily treatment fractions. The initial results, published in The Lancet, reported noninferiority for both reduced-dose and partial-breast radiotherapy. Adverse effects were similar in the three groups except for change in breast appearance, which was better in partial-breast therapy vs. whole-breast, and breast harder or firmer, which was better in both partial- and reduced-dose groups vs. whole-breast.
Dr. Mutter described the IMPORT LOW trial as “a practice-changing study.”
The trial was unique since both the whole-breast and partial-breast arms received the same dosing schedule, he said, which “enables an unbiased assessment of the impact of target volume on treatment outcomes.” This contrasts “with other partial-breast irradiation studies where a different dosing schedule was employed for whole-breast and partial-breast irradiation.”
The new analysis tracked patients for a median of 121 months. “There is no difference in local recurrence rate across the three arms,” Dr. Kirby said. There were no differences in overall survival, breast cancer or cardiac deaths, she added, and “neither was there any difference in the time to any moderate or marked clinician-assessed breast normal tissue endpoint.”
Heart and lung outcomes may improve over time in the lower-dose groups because of less radiation exposure, “but we haven’t shown that yet with this data set.”
Dr. Mutter cautioned that “the results of this trial may not necessarily be extrapolated to other partial-breast irradiation techniques that treat a much smaller volume of breast tissue such as intracavitary brachytherapy and intraoperative radiotherapy. Whether these same excellent outcomes can be achieved with smaller treatment volumes is an area for further investigation.”
Funding information was not provided; the initial study was funded by Cancer Research UK. Dr. Kirby discloses travel costs paid by European Society of Radiotherapy and Oncology, and other authors have various disclosures including relationships with companies such as Pfizer, Seagen, AstraZeneca, Eli Lilly, Bayer, and Janssen. Dr. Mutter has no disclosures.
SAN DIEGO –
The follow-up analysis of the 2,016-subject IMPORT LOW study found that 10-year overall survival rates were 87.8% (95% confidence interval, 84.9-90.1) for a full-breast radiation group, 87.2% (95% CI, 84.3-89.6) for a reduced-dose group, and 90.3% (95% CI, 87.7-92.4) in a partial-breast group. Breast cancer radiotherapy specialist Anna Kirby, MB, MD (Res), MA, of the Royal Marsden and Institute of Cancer Research, London, reported the results at the annual meeting of the American Society for Radiation Oncology.
Ipsilateral breast tumor relapse was also similar in the three groups at the 10-year mark at 2.8% (95% CI, 1.8-4.5), 1.9% (95% CI, 1.1-3.4), and 2.8% (95% CI, 1.7-4.5), respectively. Moderate/marked adverse effects were deemed to be low.
Dr. Kirby said the new findings are not “practice-changing.” However, “this complementary data supporting the change in practice that happened in the UK and elsewhere following the publication back in 2017.”
The findings are “reassuring,” breast cancer radiology specialist Robert W. Mutter, MD, of the Mayo Clinic, Rochester, Minn., said in an interview after reviewing the study findings. While the outcomes and adverse events are similar between the groups, “partial-breast irradiation is attractive because it exposes less normal tissue such as the heart and lungs than whole-breast irradiation. This could lead to fewer side effects for patients later in life at a population level. Partial-breast irradiation should be considered a standard of care in selected patients.”
In a presentation at ASTRO, Dr. Kirby provided background about the motivation for the study. It was clear that radiotherapy reduces local recurrence by up to two-thirds in early breast cancer, she said. “But in a population with excellent prognosis, this translates into relatively small absolute benefits from radiotherapy for many of our lower-risk patients,” she said. “All patients treated are at risk of radiotherapy side effects, and these become the main hazard for many women. Radiotherapy that’s focused on the part of the breast that contained the tumor – the so-called tumor bed – may reduce the long-term complications from radiotherapy, particularly in the breast, and potentially in the heart and lung, whilst hopefully maintaining low local recurrence rates.”
The initial 5-year study was a randomized, multicenter, phase III trial of patients ≥ age 50 in the United Kingdom who were tracked since recruitment in 2007-2010 (median age, 63). All had undergone breast conservation surgery. The patients were assigned to the control group (n = 674, 40 Gy), reduced-dose (n = 673, 40 Gy) and partial-breast (n = 669, 40 Gy to partial breast only) in 15 daily treatment fractions. The initial results, published in The Lancet, reported noninferiority for both reduced-dose and partial-breast radiotherapy. Adverse effects were similar in the three groups except for change in breast appearance, which was better in partial-breast therapy vs. whole-breast, and breast harder or firmer, which was better in both partial- and reduced-dose groups vs. whole-breast.
Dr. Mutter described the IMPORT LOW trial as “a practice-changing study.”
The trial was unique since both the whole-breast and partial-breast arms received the same dosing schedule, he said, which “enables an unbiased assessment of the impact of target volume on treatment outcomes.” This contrasts “with other partial-breast irradiation studies where a different dosing schedule was employed for whole-breast and partial-breast irradiation.”
The new analysis tracked patients for a median of 121 months. “There is no difference in local recurrence rate across the three arms,” Dr. Kirby said. There were no differences in overall survival, breast cancer or cardiac deaths, she added, and “neither was there any difference in the time to any moderate or marked clinician-assessed breast normal tissue endpoint.”
Heart and lung outcomes may improve over time in the lower-dose groups because of less radiation exposure, “but we haven’t shown that yet with this data set.”
Dr. Mutter cautioned that “the results of this trial may not necessarily be extrapolated to other partial-breast irradiation techniques that treat a much smaller volume of breast tissue such as intracavitary brachytherapy and intraoperative radiotherapy. Whether these same excellent outcomes can be achieved with smaller treatment volumes is an area for further investigation.”
Funding information was not provided; the initial study was funded by Cancer Research UK. Dr. Kirby discloses travel costs paid by European Society of Radiotherapy and Oncology, and other authors have various disclosures including relationships with companies such as Pfizer, Seagen, AstraZeneca, Eli Lilly, Bayer, and Janssen. Dr. Mutter has no disclosures.
SAN DIEGO –
The follow-up analysis of the 2,016-subject IMPORT LOW study found that 10-year overall survival rates were 87.8% (95% confidence interval, 84.9-90.1) for a full-breast radiation group, 87.2% (95% CI, 84.3-89.6) for a reduced-dose group, and 90.3% (95% CI, 87.7-92.4) in a partial-breast group. Breast cancer radiotherapy specialist Anna Kirby, MB, MD (Res), MA, of the Royal Marsden and Institute of Cancer Research, London, reported the results at the annual meeting of the American Society for Radiation Oncology.
Ipsilateral breast tumor relapse was also similar in the three groups at the 10-year mark at 2.8% (95% CI, 1.8-4.5), 1.9% (95% CI, 1.1-3.4), and 2.8% (95% CI, 1.7-4.5), respectively. Moderate/marked adverse effects were deemed to be low.
Dr. Kirby said the new findings are not “practice-changing.” However, “this complementary data supporting the change in practice that happened in the UK and elsewhere following the publication back in 2017.”
The findings are “reassuring,” breast cancer radiology specialist Robert W. Mutter, MD, of the Mayo Clinic, Rochester, Minn., said in an interview after reviewing the study findings. While the outcomes and adverse events are similar between the groups, “partial-breast irradiation is attractive because it exposes less normal tissue such as the heart and lungs than whole-breast irradiation. This could lead to fewer side effects for patients later in life at a population level. Partial-breast irradiation should be considered a standard of care in selected patients.”
In a presentation at ASTRO, Dr. Kirby provided background about the motivation for the study. It was clear that radiotherapy reduces local recurrence by up to two-thirds in early breast cancer, she said. “But in a population with excellent prognosis, this translates into relatively small absolute benefits from radiotherapy for many of our lower-risk patients,” she said. “All patients treated are at risk of radiotherapy side effects, and these become the main hazard for many women. Radiotherapy that’s focused on the part of the breast that contained the tumor – the so-called tumor bed – may reduce the long-term complications from radiotherapy, particularly in the breast, and potentially in the heart and lung, whilst hopefully maintaining low local recurrence rates.”
The initial 5-year study was a randomized, multicenter, phase III trial of patients ≥ age 50 in the United Kingdom who were tracked since recruitment in 2007-2010 (median age, 63). All had undergone breast conservation surgery. The patients were assigned to the control group (n = 674, 40 Gy), reduced-dose (n = 673, 40 Gy) and partial-breast (n = 669, 40 Gy to partial breast only) in 15 daily treatment fractions. The initial results, published in The Lancet, reported noninferiority for both reduced-dose and partial-breast radiotherapy. Adverse effects were similar in the three groups except for change in breast appearance, which was better in partial-breast therapy vs. whole-breast, and breast harder or firmer, which was better in both partial- and reduced-dose groups vs. whole-breast.
Dr. Mutter described the IMPORT LOW trial as “a practice-changing study.”
The trial was unique since both the whole-breast and partial-breast arms received the same dosing schedule, he said, which “enables an unbiased assessment of the impact of target volume on treatment outcomes.” This contrasts “with other partial-breast irradiation studies where a different dosing schedule was employed for whole-breast and partial-breast irradiation.”
The new analysis tracked patients for a median of 121 months. “There is no difference in local recurrence rate across the three arms,” Dr. Kirby said. There were no differences in overall survival, breast cancer or cardiac deaths, she added, and “neither was there any difference in the time to any moderate or marked clinician-assessed breast normal tissue endpoint.”
Heart and lung outcomes may improve over time in the lower-dose groups because of less radiation exposure, “but we haven’t shown that yet with this data set.”
Dr. Mutter cautioned that “the results of this trial may not necessarily be extrapolated to other partial-breast irradiation techniques that treat a much smaller volume of breast tissue such as intracavitary brachytherapy and intraoperative radiotherapy. Whether these same excellent outcomes can be achieved with smaller treatment volumes is an area for further investigation.”
Funding information was not provided; the initial study was funded by Cancer Research UK. Dr. Kirby discloses travel costs paid by European Society of Radiotherapy and Oncology, and other authors have various disclosures including relationships with companies such as Pfizer, Seagen, AstraZeneca, Eli Lilly, Bayer, and Janssen. Dr. Mutter has no disclosures.
AT ASTRO 2023
Optimal antiplatelet regimen in ‘bi-risk’ ACS?
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Does an elevated TSH value always require therapy?
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Choosing which biologic to prescribe for psoriasis
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
AT CALDERM 2023
Pelvic yoga, physical conditioning both improve urinary incontinence
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
AT NAMS 2023
Respiratory infections, asthma rise before type 2 diabetes
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
AT EASD 2023

