User login
69%: hospitals with perfect hand-hygiene compliance
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
69%: the percentage of hospitals that had perfect compliance with the Leapfrog Group employer coalition’s safe practices for hand hygiene in its 2013 annual quality survey of 1,437 U.S. hospitals.
The CDC estimates 2 million patients annually acquire hospital-acquired infections (HAIs), often spread by contaminated hands of healthcare workers.
Urban hospitals performed better than rural hospitals in compliance with Leapfrog’s standard.
CDC names 35 hospitals capable of treating Ebola patients
The Centers for Disease Control and Prevention says that 35 U.S. hospitals have been designated as Ebola treatment centers.
The hospitals, of which CDC published a list on Dec. 2, are in 12 states and the District of Columbia. The hospitals received their designations from state health departments and after on-site reviews by CDC teams.
“Ebola treatment centers are staffed, equipped, and have been assessed to have current capabilities, training, and resources to provide the complex treatment necessary to care for a person with Ebola while minimizing risk to health care workers,” the CDC said in a news release about the new centers.
More hospitals are expected to be named Ebola treatment centers in the coming weeks, the agency said. The CDC has published guidelines to hospitals and state and local health departments on the preparation of these centers, with standards for patient transportation, laboratories, personal protective equipment, and waste management, among other key areas of capability.
The CDC said that the new centers supplement the existing biocontainment facilities at Emory University Hospital in Atlanta, Nebraska Medical Center in Omaha, and the National Institutes of Health in Bethesda, Md., which will continue to play an important role in Ebola treatment – particularly in cases of medical evacuation from overseas.
Some 80% of travelers returning from Ebola-affected countries live within 200 miles of a designated Ebola treatment center, the agency affirmed, and the addition of new facilities in the weeks to come will “further broaden geographic reach.”While Texas is home to two of the designated centers, Texas Health Presbyterian, the Dallas hospital that treated the first Ebola case presenting in the United States, is not currently on the list.
The Centers for Disease Control and Prevention says that 35 U.S. hospitals have been designated as Ebola treatment centers.
The hospitals, of which CDC published a list on Dec. 2, are in 12 states and the District of Columbia. The hospitals received their designations from state health departments and after on-site reviews by CDC teams.
“Ebola treatment centers are staffed, equipped, and have been assessed to have current capabilities, training, and resources to provide the complex treatment necessary to care for a person with Ebola while minimizing risk to health care workers,” the CDC said in a news release about the new centers.
More hospitals are expected to be named Ebola treatment centers in the coming weeks, the agency said. The CDC has published guidelines to hospitals and state and local health departments on the preparation of these centers, with standards for patient transportation, laboratories, personal protective equipment, and waste management, among other key areas of capability.
The CDC said that the new centers supplement the existing biocontainment facilities at Emory University Hospital in Atlanta, Nebraska Medical Center in Omaha, and the National Institutes of Health in Bethesda, Md., which will continue to play an important role in Ebola treatment – particularly in cases of medical evacuation from overseas.
Some 80% of travelers returning from Ebola-affected countries live within 200 miles of a designated Ebola treatment center, the agency affirmed, and the addition of new facilities in the weeks to come will “further broaden geographic reach.”While Texas is home to two of the designated centers, Texas Health Presbyterian, the Dallas hospital that treated the first Ebola case presenting in the United States, is not currently on the list.
The Centers for Disease Control and Prevention says that 35 U.S. hospitals have been designated as Ebola treatment centers.
The hospitals, of which CDC published a list on Dec. 2, are in 12 states and the District of Columbia. The hospitals received their designations from state health departments and after on-site reviews by CDC teams.
“Ebola treatment centers are staffed, equipped, and have been assessed to have current capabilities, training, and resources to provide the complex treatment necessary to care for a person with Ebola while minimizing risk to health care workers,” the CDC said in a news release about the new centers.
More hospitals are expected to be named Ebola treatment centers in the coming weeks, the agency said. The CDC has published guidelines to hospitals and state and local health departments on the preparation of these centers, with standards for patient transportation, laboratories, personal protective equipment, and waste management, among other key areas of capability.
The CDC said that the new centers supplement the existing biocontainment facilities at Emory University Hospital in Atlanta, Nebraska Medical Center in Omaha, and the National Institutes of Health in Bethesda, Md., which will continue to play an important role in Ebola treatment – particularly in cases of medical evacuation from overseas.
Some 80% of travelers returning from Ebola-affected countries live within 200 miles of a designated Ebola treatment center, the agency affirmed, and the addition of new facilities in the weeks to come will “further broaden geographic reach.”While Texas is home to two of the designated centers, Texas Health Presbyterian, the Dallas hospital that treated the first Ebola case presenting in the United States, is not currently on the list.
Medicare Readmissions Penalties Expected to Reach $428 Million
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.
Ebola, Smoking, and Defense Health Agency Top AMSUS Agenda
Reacting quickly, the AMSUS Continuing Education Meeting will hold multiple sessions with experts from the CDC, DoD, and PHS on the evolving federal response to Ebola. The meeting will be held December 2-5, 2014, in Washington, DC. Details, registration, and an updated agenda can be found at http://amsusmeetings.org.
“This is one of the few opportunities for federal health leaders as well as practitioners to meet and know and learn from their peers,” VADM Michael Cowan, MD, executive director of AMSUS, told Federal Practitioner.
Another major development at the conference will be the “coming-out party” for the Defense Health Agency. “This is the biggest reorganization of military medicine, in living memory,” VADM Cowan explained. “They are a year into it, and this is the opportunity for all of us to hear straight from the horse’s mouth what they are doing.”
In one of the conference highlights, RADM Boris Lushniak, Acting Surgeon General (see "Acting Surgeon General Confident in the Battle Against Tobacco, Ebola, and Preventable Diseases"), will also be delivering a plenary session at the conference, marking the 50th anniversary of the original Surgeon General’s report on the public health impact of tobacco smoke. The session will focus on the progress in the past 50 years as well as the future directions of tobacco cessation and communities still at risk.
“This is a unique chance to meet one’s peers and colleagues; not only across [agencies], but also across international boundaries,” VADM Cowan said.
Reacting quickly, the AMSUS Continuing Education Meeting will hold multiple sessions with experts from the CDC, DoD, and PHS on the evolving federal response to Ebola. The meeting will be held December 2-5, 2014, in Washington, DC. Details, registration, and an updated agenda can be found at http://amsusmeetings.org.
“This is one of the few opportunities for federal health leaders as well as practitioners to meet and know and learn from their peers,” VADM Michael Cowan, MD, executive director of AMSUS, told Federal Practitioner.
Another major development at the conference will be the “coming-out party” for the Defense Health Agency. “This is the biggest reorganization of military medicine, in living memory,” VADM Cowan explained. “They are a year into it, and this is the opportunity for all of us to hear straight from the horse’s mouth what they are doing.”
In one of the conference highlights, RADM Boris Lushniak, Acting Surgeon General (see "Acting Surgeon General Confident in the Battle Against Tobacco, Ebola, and Preventable Diseases"), will also be delivering a plenary session at the conference, marking the 50th anniversary of the original Surgeon General’s report on the public health impact of tobacco smoke. The session will focus on the progress in the past 50 years as well as the future directions of tobacco cessation and communities still at risk.
“This is a unique chance to meet one’s peers and colleagues; not only across [agencies], but also across international boundaries,” VADM Cowan said.
Reacting quickly, the AMSUS Continuing Education Meeting will hold multiple sessions with experts from the CDC, DoD, and PHS on the evolving federal response to Ebola. The meeting will be held December 2-5, 2014, in Washington, DC. Details, registration, and an updated agenda can be found at http://amsusmeetings.org.
“This is one of the few opportunities for federal health leaders as well as practitioners to meet and know and learn from their peers,” VADM Michael Cowan, MD, executive director of AMSUS, told Federal Practitioner.
Another major development at the conference will be the “coming-out party” for the Defense Health Agency. “This is the biggest reorganization of military medicine, in living memory,” VADM Cowan explained. “They are a year into it, and this is the opportunity for all of us to hear straight from the horse’s mouth what they are doing.”
In one of the conference highlights, RADM Boris Lushniak, Acting Surgeon General (see "Acting Surgeon General Confident in the Battle Against Tobacco, Ebola, and Preventable Diseases"), will also be delivering a plenary session at the conference, marking the 50th anniversary of the original Surgeon General’s report on the public health impact of tobacco smoke. The session will focus on the progress in the past 50 years as well as the future directions of tobacco cessation and communities still at risk.
“This is a unique chance to meet one’s peers and colleagues; not only across [agencies], but also across international boundaries,” VADM Cowan said.
FDA approves extended-release hydrocodone with abuse-deterrent features
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
FROM THE FDA
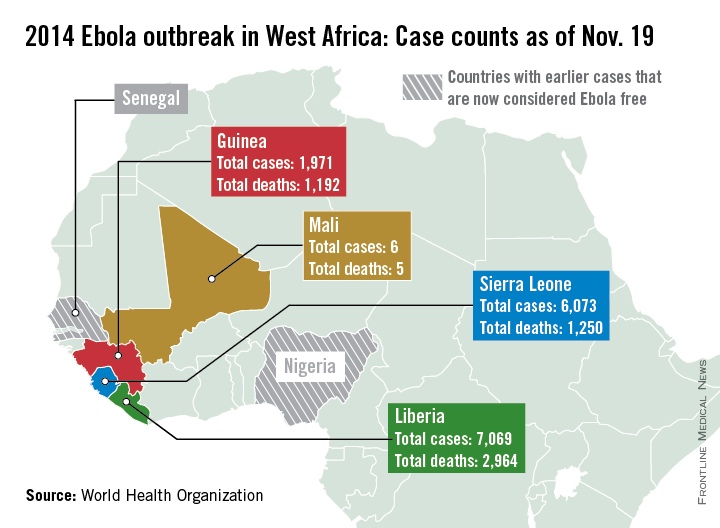
Ebola outbreak spreads further in Mali
Additional cases of Ebola have been reported in the capital of Mali this week, according to a report from the World Health Organization.
There have been six cases and five deaths from Ebola in Mali, with the initial case of a young child unrelated to the five cases reported in the capital, Bamako. The second outbreak began with an imam who crossed the border from Guinea and died in Mali from an undiagnosed condition. Although the man died in Mali, his case has been classified as a case in Guinea, as he developed symptoms there. Of the nearly 400 contacts in Mali, about a quarter are health care workers, the WHO said.
There were more than 1,000 new cases in the past week throughout Liberia, Sierra Leone, and Guinea, bringing the total to over 15,100 cases and 5,400 deaths. Currently, Liberia has the most reported cases of Ebola, with nearly 7,100 cases and nearly 3,000 reported deaths. With just over 700 cases in the past week, Sierra Leone has now reported over 6,000 cases and 1,250 deaths. Guinea has reported nearly 2,000 cases and just under 1,200 deaths, according to the WHO.
There have been no new localized cases of Ebola in Spain or in the United States. As of today, 42 days have passed since the last Ebola test came back negative in the unrelated outbreak in the Democratic Republic of the Congo, and the country should soon be declared Ebola free, the WHO reported.
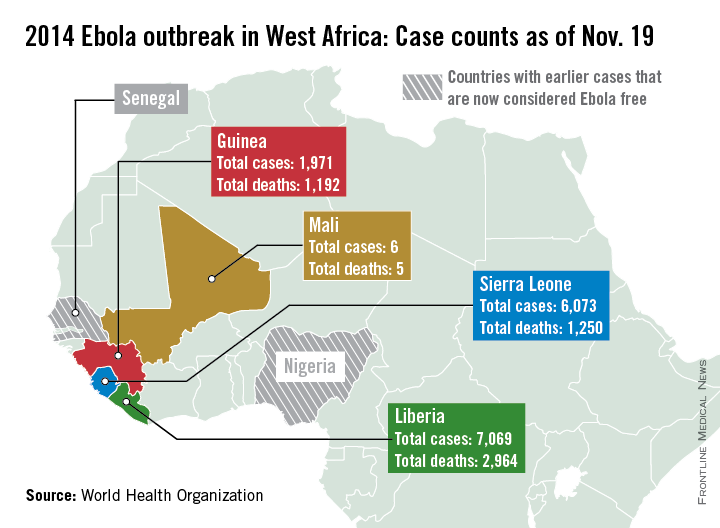
Additional cases of Ebola have been reported in the capital of Mali this week, according to a report from the World Health Organization.
There have been six cases and five deaths from Ebola in Mali, with the initial case of a young child unrelated to the five cases reported in the capital, Bamako. The second outbreak began with an imam who crossed the border from Guinea and died in Mali from an undiagnosed condition. Although the man died in Mali, his case has been classified as a case in Guinea, as he developed symptoms there. Of the nearly 400 contacts in Mali, about a quarter are health care workers, the WHO said.
There were more than 1,000 new cases in the past week throughout Liberia, Sierra Leone, and Guinea, bringing the total to over 15,100 cases and 5,400 deaths. Currently, Liberia has the most reported cases of Ebola, with nearly 7,100 cases and nearly 3,000 reported deaths. With just over 700 cases in the past week, Sierra Leone has now reported over 6,000 cases and 1,250 deaths. Guinea has reported nearly 2,000 cases and just under 1,200 deaths, according to the WHO.
There have been no new localized cases of Ebola in Spain or in the United States. As of today, 42 days have passed since the last Ebola test came back negative in the unrelated outbreak in the Democratic Republic of the Congo, and the country should soon be declared Ebola free, the WHO reported.

Additional cases of Ebola have been reported in the capital of Mali this week, according to a report from the World Health Organization.
There have been six cases and five deaths from Ebola in Mali, with the initial case of a young child unrelated to the five cases reported in the capital, Bamako. The second outbreak began with an imam who crossed the border from Guinea and died in Mali from an undiagnosed condition. Although the man died in Mali, his case has been classified as a case in Guinea, as he developed symptoms there. Of the nearly 400 contacts in Mali, about a quarter are health care workers, the WHO said.
There were more than 1,000 new cases in the past week throughout Liberia, Sierra Leone, and Guinea, bringing the total to over 15,100 cases and 5,400 deaths. Currently, Liberia has the most reported cases of Ebola, with nearly 7,100 cases and nearly 3,000 reported deaths. With just over 700 cases in the past week, Sierra Leone has now reported over 6,000 cases and 1,250 deaths. Guinea has reported nearly 2,000 cases and just under 1,200 deaths, according to the WHO.
There have been no new localized cases of Ebola in Spain or in the United States. As of today, 42 days have passed since the last Ebola test came back negative in the unrelated outbreak in the Democratic Republic of the Congo, and the country should soon be declared Ebola free, the WHO reported.

Feds try to clarify meaningful use attestation, hardship rules
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
AT THE AMIA 2014 ANNUAL SYMPOSIUM
Mali added to list of countries for enhanced Ebola screening
Travelers arriving in the United States from the West African nation of Mali will be subject to the same enhanced entry screening as are those coming from the Ebola-stricken countries of Liberia, Sierra Leone, and Guinea, because of a rise in the number of confirmed Ebola cases within Mali.
The measure, announced Nov. 16 by the Centers for Disease Control and Prevention and the Department of Homeland Security, take effect Monday, Nov. 17. In a written statement, both agencies noted that while there are no direct flights from Mali to the United States, an average of 15-20 passengers per day – most of whom are U.S. citizens or lawful permanent residents – begin their flight itineraries in Mali with the United States as their eventual destination.
Enhanced entry screenings began in October in an effort to track potential Ebola cases within the United States before they spread. Once passengers land, health officials will collect contact information for all passengers and their friends or relatives in the United States for monitoring purposes. Travelers will then be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the country. If travelers are free of symptoms for 21 days, they are no longer at risk of having or spreading the Ebola virus.
“For ease of administration, we will work with the airlines to ensure rerouting for the few travelers from Mali not already scheduled to land at one of the five airports in the United States (New York JFK, Newark, Washington-Dulles, Chicago-O’Hare, and Atlanta Hartsfield- Jackson) already performing screening on passengers from the other affected West African nations,” the agencies said in the statement.
The first confirmed case of Ebola in the United States was Thomas Eric Duncan, who was diagnosed in Dallas on Sept. 30 and became the country’s first Ebola casualty on Oct. 8. On Monday, Nov. 17, U.S. permanent resident Dr. Martin Salia died at Nebraska Medical Center after working with Ebola patients in Sierra Leone.
Travelers arriving in the United States from the West African nation of Mali will be subject to the same enhanced entry screening as are those coming from the Ebola-stricken countries of Liberia, Sierra Leone, and Guinea, because of a rise in the number of confirmed Ebola cases within Mali.
The measure, announced Nov. 16 by the Centers for Disease Control and Prevention and the Department of Homeland Security, take effect Monday, Nov. 17. In a written statement, both agencies noted that while there are no direct flights from Mali to the United States, an average of 15-20 passengers per day – most of whom are U.S. citizens or lawful permanent residents – begin their flight itineraries in Mali with the United States as their eventual destination.
Enhanced entry screenings began in October in an effort to track potential Ebola cases within the United States before they spread. Once passengers land, health officials will collect contact information for all passengers and their friends or relatives in the United States for monitoring purposes. Travelers will then be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the country. If travelers are free of symptoms for 21 days, they are no longer at risk of having or spreading the Ebola virus.
“For ease of administration, we will work with the airlines to ensure rerouting for the few travelers from Mali not already scheduled to land at one of the five airports in the United States (New York JFK, Newark, Washington-Dulles, Chicago-O’Hare, and Atlanta Hartsfield- Jackson) already performing screening on passengers from the other affected West African nations,” the agencies said in the statement.
The first confirmed case of Ebola in the United States was Thomas Eric Duncan, who was diagnosed in Dallas on Sept. 30 and became the country’s first Ebola casualty on Oct. 8. On Monday, Nov. 17, U.S. permanent resident Dr. Martin Salia died at Nebraska Medical Center after working with Ebola patients in Sierra Leone.
Travelers arriving in the United States from the West African nation of Mali will be subject to the same enhanced entry screening as are those coming from the Ebola-stricken countries of Liberia, Sierra Leone, and Guinea, because of a rise in the number of confirmed Ebola cases within Mali.
The measure, announced Nov. 16 by the Centers for Disease Control and Prevention and the Department of Homeland Security, take effect Monday, Nov. 17. In a written statement, both agencies noted that while there are no direct flights from Mali to the United States, an average of 15-20 passengers per day – most of whom are U.S. citizens or lawful permanent residents – begin their flight itineraries in Mali with the United States as their eventual destination.
Enhanced entry screenings began in October in an effort to track potential Ebola cases within the United States before they spread. Once passengers land, health officials will collect contact information for all passengers and their friends or relatives in the United States for monitoring purposes. Travelers will then be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the country. If travelers are free of symptoms for 21 days, they are no longer at risk of having or spreading the Ebola virus.
“For ease of administration, we will work with the airlines to ensure rerouting for the few travelers from Mali not already scheduled to land at one of the five airports in the United States (New York JFK, Newark, Washington-Dulles, Chicago-O’Hare, and Atlanta Hartsfield- Jackson) already performing screening on passengers from the other affected West African nations,” the agencies said in the statement.
The first confirmed case of Ebola in the United States was Thomas Eric Duncan, who was diagnosed in Dallas on Sept. 30 and became the country’s first Ebola casualty on Oct. 8. On Monday, Nov. 17, U.S. permanent resident Dr. Martin Salia died at Nebraska Medical Center after working with Ebola patients in Sierra Leone.
Court: Patients can sue over HIPAA breaches
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
VIDEO: Hepatitis C screening rises, but where are the positive cases?
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON– The number of hepatitis C virus antibody tests increased by 15.4% after the 2012 Centers for Disease Control and Prevention task force recommendation calling for one-time HCV testing in baby boomers, according to preliminary results from an analysis of 4.5 million tests.
Surprisingly, that increase in testing did not lead to an increase in the number of positive tests, which actually declined by 4.1%, R. Monina Klevens, D.D.S., MPH, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is a huge question that we need to look at for implementation,” said Dr. Klevens, a medical epidemiologist with the CDC.
For a deep dive into the data and to hear what’s next, click here to see an interview with Dr Klevens.
Dr. Klevens reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE LIVER MEETING 2014