User login
Perfect storm of SARS-CoV-2 during flu season
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.
Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
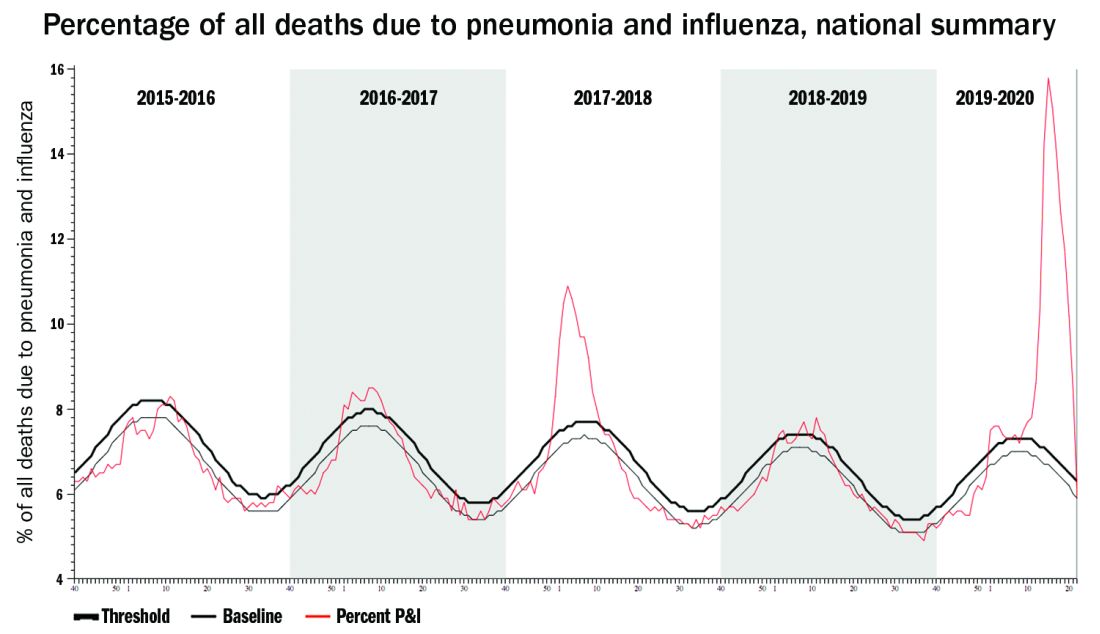
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.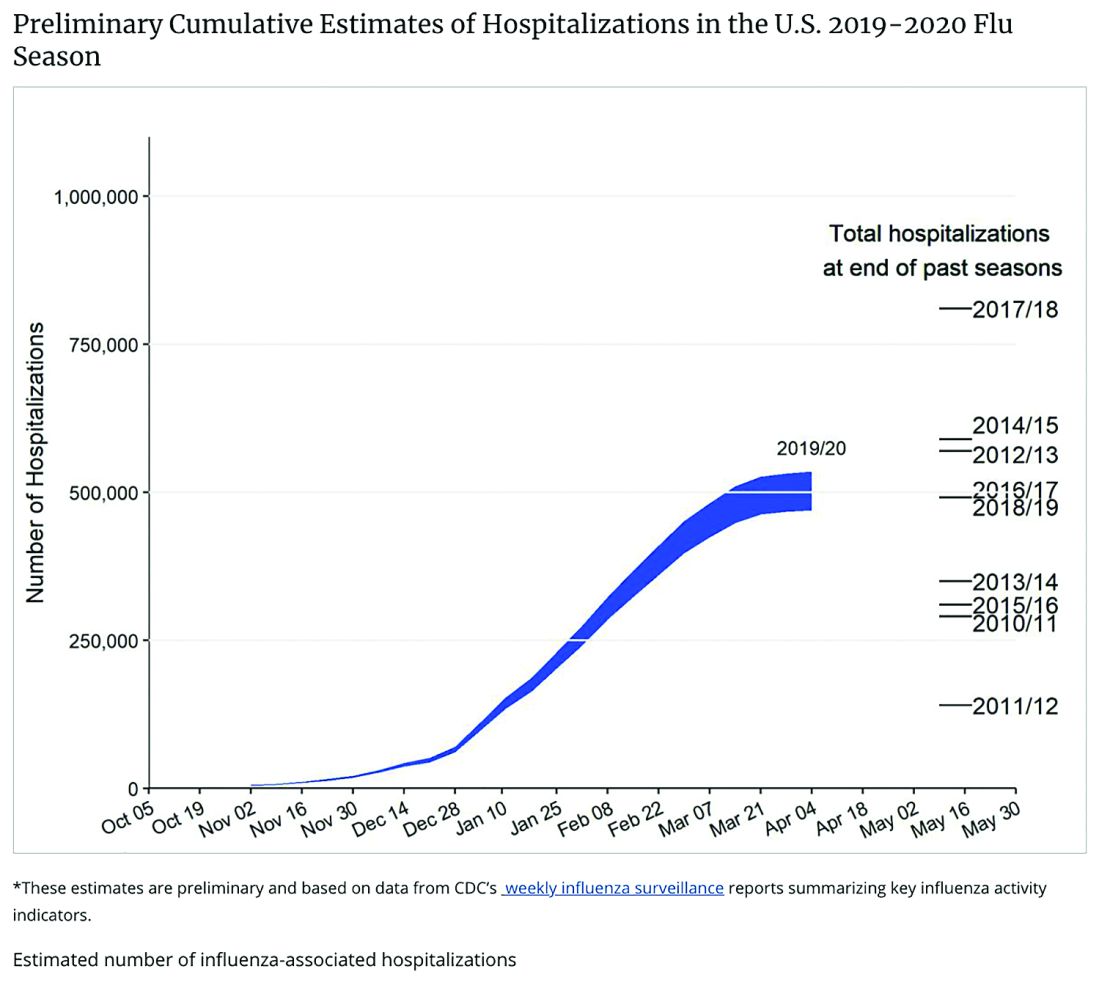
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
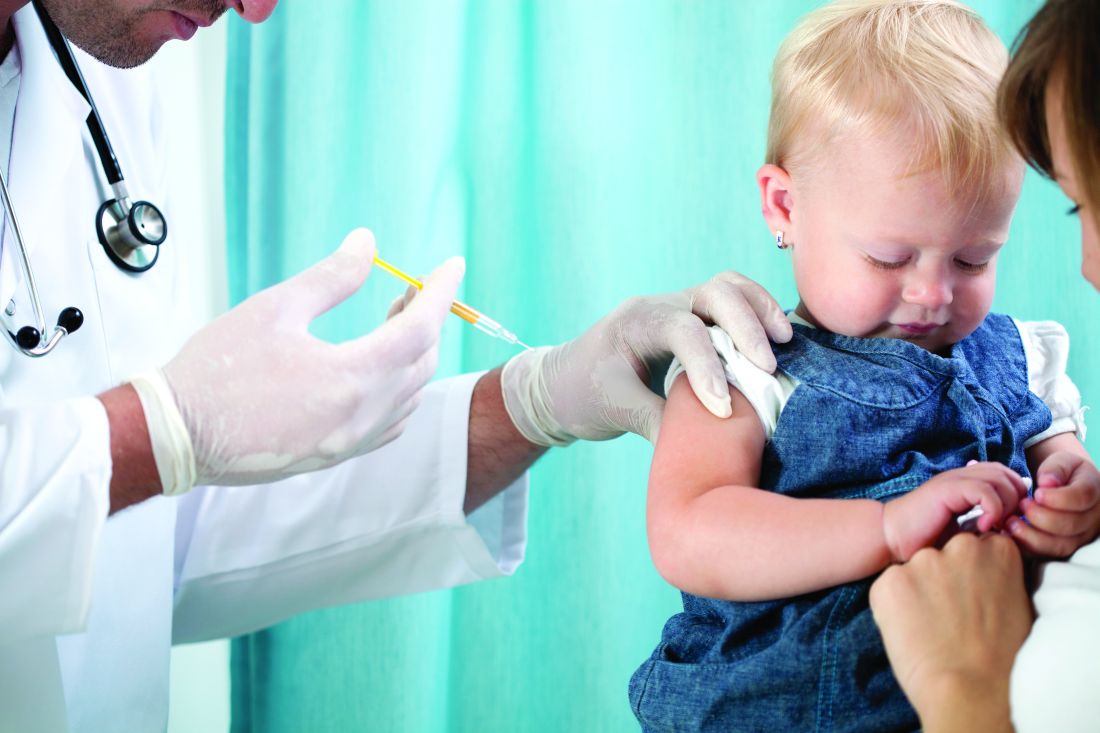
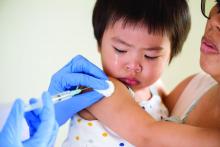
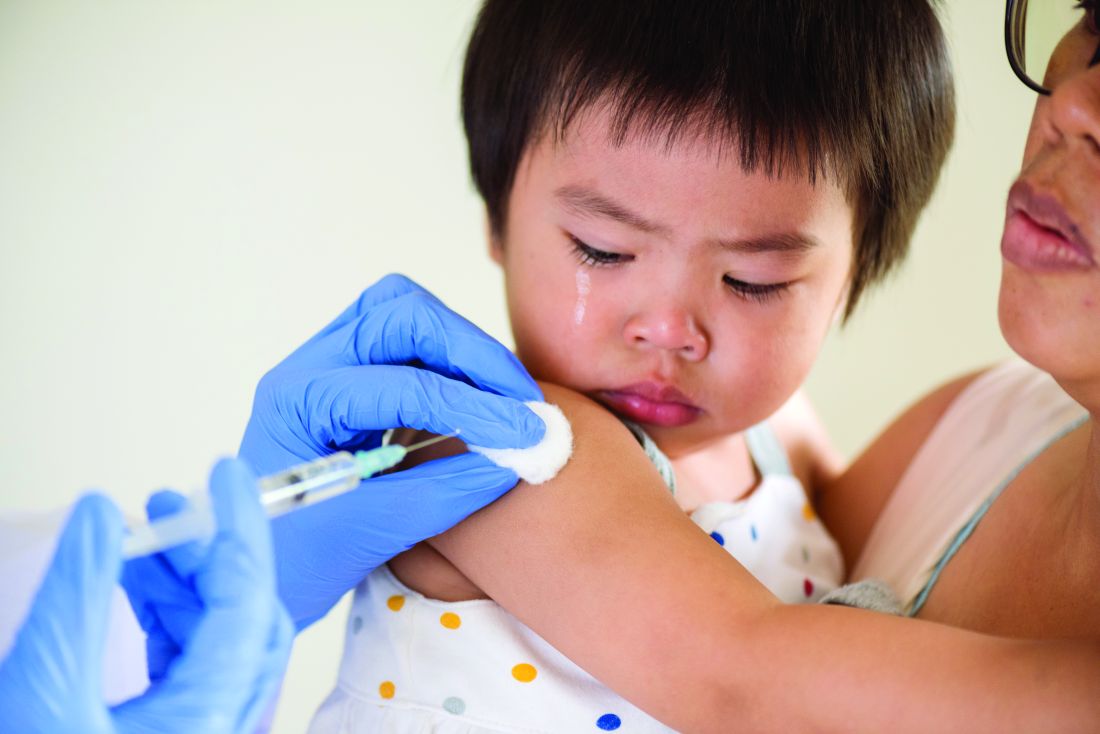
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
Consider COVID-19–associated multisystem hyperinflammatory syndrome
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
2019-nCoV outbreak: A few lessons learned for pediatric practices
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
ID Consult: It’s not necessarily over when measles infection clears
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
Don’t let a foodborne illness dampen the holiday season
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.
Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Is your office ready for a case of measles?
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.