User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
FDA Antidepressant Warnings Tied to Increase in Suicidality
, a new analysis suggests.
Investigators said the totality of evidence supports “reevaluation and possible replacement” of the US Food and Drug Administration (FDA) black box warning with routine warnings in product labeling.
“The sudden, simultaneous, and sweeping effects of these warnings — the reduction in depression treatment and increase in suicide — are documented across 14 years of strong research. The consistency in observed harms and absence of observed benefits after the black box warnings indicate this is not a coincidence,” lead author Stephen Soumerai, ScD, professor of population medicine, Harvard Medical School at Harvard Pilgrim Health Care Institute, Boston, said in a news release.
The study was published online in Health Affairs.
How Did We Get Here?
In October 2003, the FDA warned that antidepressants may be associated with suicidality among people younger than age 18 years soon after starting treatment. In January 2005, the FDA required a permanent black box warning of this risk on product labels and in television and print advertising for all antidepressant drugs.
In May 2007, the FDA expanded the 2005 black box warning to include young adults through age 24, and this broader warning remains in effect today.
Dr. Soumerai and colleagues evaluated the intended and unintended outcomes of the youth antidepressant warnings through a systematic review of “the most credible evidence in the field,” Dr. Soumerai said.
Through an exhaustive literature search, the researchers identified 34 studies of depression and suicide-related outcomes published in peer-reviewed journals after the warnings were issued.
Eleven of these studies measured abrupt changes in outcome trends following the warnings and were included in their analyses. These outcomes included monitoring for suicidality, physician visits for depression, depression diagnoses, psychotherapy visits, antidepressant treatment and use and psychotropic drug poisonings (a proxy for suicide attempts), and suicide deaths.
More Harms Than Benefits
Four studies, with more than 12 million patients, found “consistent evidence of sudden and substantial” long-term declines in doctor visits for depression and depression diagnoses after the FDA warnings, the study team noted.
These studies showed increases in physician visits for depression and depression diagnoses in the years before the warnings and abrupt, sustained declines, ranging from 20% to 45%, in visits and diagnoses after the warnings. “Some spillover occurred in comparison groups of adults, who were not targeted by the FDA warnings,” the study team said.
Seven studies revealed evidence that the FDA warnings were followed by abrupt reductions in antidepressant treatment and use, ranging from 20% to 50%. Most of these studies showed increasing use of antidepressants in the years before the FDA warnings, followed by abrupt and sustained reductions in use afterward.
Three studies found evidence of declining or flat trends in psychotropic drug poisonings and suicide deaths among pediatric patients before the warnings, followed by abrupt increases in these trends after the warnings were issued.
The intent of the warnings was to increase physician monitoring of suicidality of patients treated with antidepressants, but the data suggest that this did not occur.
Less than 5% of pediatric patients were monitored in accordance with FDA’s recommended contact schedule recommendations after the warnings were issued. This low rate was unchanged from the rate before the warnings.
No study documented improvements in mental health care or declines in suicide attempts or suicides after the warnings went into effect.
“The overwhelming evidence suggests that the ongoing use of these warnings may result in more harms than benefits,” the authors wrote.
Concerning Data
The results are “very concerning and provide reason to pause, rethink, and possibly recalibrate boxed warning recommendations as it relates to antidepressants in younger populations,” said Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Canada, and head of the Mood Disorders Psychopharmacology Unit.
Dr. McIntyre, who wasn’t involved in the study, said the data “unfortunately” provide evidence suggesting that the boxed warning had the “unintended consequence of increasing the likelihood that persons would not receive adequate healthcare for their mental disorder, consequently resulting in unfavorable outcomes, including suicidality.”
He added, “Two decades have now passed with additional information available, which not only appears to recalibrate the initial risk assessment but provides an opportunity for us to reduce the externality of decreasing access to healthcare for people living with mental illness during their youth years.”
A spokesperson for the FDA said that “generally, the FDA does not comment on specific studies, but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
The study had no commercial funding. Disclosures for the authors are listed with the original article. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, and Neurocrine.
A version of this article appeared on Medscape.com.
, a new analysis suggests.
Investigators said the totality of evidence supports “reevaluation and possible replacement” of the US Food and Drug Administration (FDA) black box warning with routine warnings in product labeling.
“The sudden, simultaneous, and sweeping effects of these warnings — the reduction in depression treatment and increase in suicide — are documented across 14 years of strong research. The consistency in observed harms and absence of observed benefits after the black box warnings indicate this is not a coincidence,” lead author Stephen Soumerai, ScD, professor of population medicine, Harvard Medical School at Harvard Pilgrim Health Care Institute, Boston, said in a news release.
The study was published online in Health Affairs.
How Did We Get Here?
In October 2003, the FDA warned that antidepressants may be associated with suicidality among people younger than age 18 years soon after starting treatment. In January 2005, the FDA required a permanent black box warning of this risk on product labels and in television and print advertising for all antidepressant drugs.
In May 2007, the FDA expanded the 2005 black box warning to include young adults through age 24, and this broader warning remains in effect today.
Dr. Soumerai and colleagues evaluated the intended and unintended outcomes of the youth antidepressant warnings through a systematic review of “the most credible evidence in the field,” Dr. Soumerai said.
Through an exhaustive literature search, the researchers identified 34 studies of depression and suicide-related outcomes published in peer-reviewed journals after the warnings were issued.
Eleven of these studies measured abrupt changes in outcome trends following the warnings and were included in their analyses. These outcomes included monitoring for suicidality, physician visits for depression, depression diagnoses, psychotherapy visits, antidepressant treatment and use and psychotropic drug poisonings (a proxy for suicide attempts), and suicide deaths.
More Harms Than Benefits
Four studies, with more than 12 million patients, found “consistent evidence of sudden and substantial” long-term declines in doctor visits for depression and depression diagnoses after the FDA warnings, the study team noted.
These studies showed increases in physician visits for depression and depression diagnoses in the years before the warnings and abrupt, sustained declines, ranging from 20% to 45%, in visits and diagnoses after the warnings. “Some spillover occurred in comparison groups of adults, who were not targeted by the FDA warnings,” the study team said.
Seven studies revealed evidence that the FDA warnings were followed by abrupt reductions in antidepressant treatment and use, ranging from 20% to 50%. Most of these studies showed increasing use of antidepressants in the years before the FDA warnings, followed by abrupt and sustained reductions in use afterward.
Three studies found evidence of declining or flat trends in psychotropic drug poisonings and suicide deaths among pediatric patients before the warnings, followed by abrupt increases in these trends after the warnings were issued.
The intent of the warnings was to increase physician monitoring of suicidality of patients treated with antidepressants, but the data suggest that this did not occur.
Less than 5% of pediatric patients were monitored in accordance with FDA’s recommended contact schedule recommendations after the warnings were issued. This low rate was unchanged from the rate before the warnings.
No study documented improvements in mental health care or declines in suicide attempts or suicides after the warnings went into effect.
“The overwhelming evidence suggests that the ongoing use of these warnings may result in more harms than benefits,” the authors wrote.
Concerning Data
The results are “very concerning and provide reason to pause, rethink, and possibly recalibrate boxed warning recommendations as it relates to antidepressants in younger populations,” said Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Canada, and head of the Mood Disorders Psychopharmacology Unit.
Dr. McIntyre, who wasn’t involved in the study, said the data “unfortunately” provide evidence suggesting that the boxed warning had the “unintended consequence of increasing the likelihood that persons would not receive adequate healthcare for their mental disorder, consequently resulting in unfavorable outcomes, including suicidality.”
He added, “Two decades have now passed with additional information available, which not only appears to recalibrate the initial risk assessment but provides an opportunity for us to reduce the externality of decreasing access to healthcare for people living with mental illness during their youth years.”
A spokesperson for the FDA said that “generally, the FDA does not comment on specific studies, but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
The study had no commercial funding. Disclosures for the authors are listed with the original article. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, and Neurocrine.
A version of this article appeared on Medscape.com.
, a new analysis suggests.
Investigators said the totality of evidence supports “reevaluation and possible replacement” of the US Food and Drug Administration (FDA) black box warning with routine warnings in product labeling.
“The sudden, simultaneous, and sweeping effects of these warnings — the reduction in depression treatment and increase in suicide — are documented across 14 years of strong research. The consistency in observed harms and absence of observed benefits after the black box warnings indicate this is not a coincidence,” lead author Stephen Soumerai, ScD, professor of population medicine, Harvard Medical School at Harvard Pilgrim Health Care Institute, Boston, said in a news release.
The study was published online in Health Affairs.
How Did We Get Here?
In October 2003, the FDA warned that antidepressants may be associated with suicidality among people younger than age 18 years soon after starting treatment. In January 2005, the FDA required a permanent black box warning of this risk on product labels and in television and print advertising for all antidepressant drugs.
In May 2007, the FDA expanded the 2005 black box warning to include young adults through age 24, and this broader warning remains in effect today.
Dr. Soumerai and colleagues evaluated the intended and unintended outcomes of the youth antidepressant warnings through a systematic review of “the most credible evidence in the field,” Dr. Soumerai said.
Through an exhaustive literature search, the researchers identified 34 studies of depression and suicide-related outcomes published in peer-reviewed journals after the warnings were issued.
Eleven of these studies measured abrupt changes in outcome trends following the warnings and were included in their analyses. These outcomes included monitoring for suicidality, physician visits for depression, depression diagnoses, psychotherapy visits, antidepressant treatment and use and psychotropic drug poisonings (a proxy for suicide attempts), and suicide deaths.
More Harms Than Benefits
Four studies, with more than 12 million patients, found “consistent evidence of sudden and substantial” long-term declines in doctor visits for depression and depression diagnoses after the FDA warnings, the study team noted.
These studies showed increases in physician visits for depression and depression diagnoses in the years before the warnings and abrupt, sustained declines, ranging from 20% to 45%, in visits and diagnoses after the warnings. “Some spillover occurred in comparison groups of adults, who were not targeted by the FDA warnings,” the study team said.
Seven studies revealed evidence that the FDA warnings were followed by abrupt reductions in antidepressant treatment and use, ranging from 20% to 50%. Most of these studies showed increasing use of antidepressants in the years before the FDA warnings, followed by abrupt and sustained reductions in use afterward.
Three studies found evidence of declining or flat trends in psychotropic drug poisonings and suicide deaths among pediatric patients before the warnings, followed by abrupt increases in these trends after the warnings were issued.
The intent of the warnings was to increase physician monitoring of suicidality of patients treated with antidepressants, but the data suggest that this did not occur.
Less than 5% of pediatric patients were monitored in accordance with FDA’s recommended contact schedule recommendations after the warnings were issued. This low rate was unchanged from the rate before the warnings.
No study documented improvements in mental health care or declines in suicide attempts or suicides after the warnings went into effect.
“The overwhelming evidence suggests that the ongoing use of these warnings may result in more harms than benefits,” the authors wrote.
Concerning Data
The results are “very concerning and provide reason to pause, rethink, and possibly recalibrate boxed warning recommendations as it relates to antidepressants in younger populations,” said Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Canada, and head of the Mood Disorders Psychopharmacology Unit.
Dr. McIntyre, who wasn’t involved in the study, said the data “unfortunately” provide evidence suggesting that the boxed warning had the “unintended consequence of increasing the likelihood that persons would not receive adequate healthcare for their mental disorder, consequently resulting in unfavorable outcomes, including suicidality.”
He added, “Two decades have now passed with additional information available, which not only appears to recalibrate the initial risk assessment but provides an opportunity for us to reduce the externality of decreasing access to healthcare for people living with mental illness during their youth years.”
A spokesperson for the FDA said that “generally, the FDA does not comment on specific studies, but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
The study had no commercial funding. Disclosures for the authors are listed with the original article. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, and Neurocrine.
A version of this article appeared on Medscape.com.
From Health Affairs
Mycosis Fungoides: Measured Approach Key to Treatment
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Ghost Fat: The Unseen Consequences of Weight Loss
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Why Residents Are Joining Unions in Droves
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Crisugabalin Alleviates Postherpetic Neuralgia Symptoms in Phase 3 Study
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nonalcoholic Beer and Underage Drinking
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Isatuximab Quadruplet Approval Could Change the Landscape for Treating Myeloma
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
The findings, presented on September 26 at the annual meeting of the International Myeloma Society, support the four-drug combination known as Isa-VRd as a potential new standard of care (SOC) supplanting VRd alone as the SOC in this setting, according to Meletios Dimopoulos, MD, of the University of Athens, Greece.
The IMROZ findings — the first from a phase 3 study of an anti-CD38 monoclonal antibody given in combination with VRd — were also reported in May at the annual meeting of the American Society of Clinical Oncology (ASCO) and published simultaneously in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” first author Thierry Facon, MD, told this news organization at ASCO.
Dr. Thierry, of the University of Lille, and the French Academy of Medicine in Paris, France, added that Isa-VRd has the potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients.”
Isatuximab in combination with VRd was subsequently approved by the US Food and Drug Administration (FDA) for this indication, as reported on September 23 by this news organization.
So, what will this quadruplet mean for the treatment of multiple myeloma? IMROZ study coauthors Meral Beksac, MD, of Istinye University, Istanbul, and Liv Hospital Ankara, Turkey, and Mohamad Mohty, MD, of Sorbonne University, Saint-Antoine Hospital, Paris, France, provided some insights in a recent interview, telling the European Medical Journal (EMJ) Hematology that Isa-VRd is a “welcome addition” to the multiple myeloma armamentarium.
Should Isa-VRd Be Considered the New First-Choice Frontline Treatment for Transplant-Ineligible Patients?
“The short answer is yes,” Dr. Mohty told EMJ. “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.”
Dr. Beksac agreed that Isa-VRd will play a role in frontline management for transplant-ineligible patients.
However, both noted that despite having a favorable safety profile similar to VRd, Isa-VRd may not be well tolerated in elderly and frail patients. Demonstrably frail patients were excluded from IMROZ, and this is a factor that should be considered in the practice setting, they agreed.
Will Isa-VRd Change How Patients Are Evaluated for Transplant Eligibility?
“The cutoff for transplant eligibility differs from one country to another, and today, we do not have consensus around an agreed-upon age limit,” Dr. Beksac said. “We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well.”
She also noted that “[t]he introduction of very effective systemic regimens with similar efficacy to [hematopoietic stem cell transplant (HSCT)] has seen a shift towards non-transplant regimens, particularly in the USA.”
“In many centers in Europe, these patients [in IMROZ] would be considered transplant eligible. Hence, for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” Dr. Mohty added.
Patient preference and access are also important considerations, as is cost, he noted.
Younger transplant-eligible patients may prefer transplant over continuous treatment for life, whereas some might prefer long-term treatment over a stem cell protocol that will require months off of work, he and Dr. Beksac explained.
“Based on this trial, we will likely see a decline in the number of transplants,” Dr. Mohty predicted. “With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.”
How Will This Combination Be Integrated Into Daily Clinical Practice?
“My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” Dr. Beksac said.
Dr. Mohty added that the multiple myeloma story is changing and evolving.
“It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet, who is fit and who is unfit,” he explained, adding that physicians will likely adapt the Isa-VRd regimen for real-world use based on clinical judgment.
For example, the quadruplet may be combined “in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient,” Dr. Beksac added.
“Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant,” Dr. Mohty said. “So, it’s a most welcome addition to what we do.”
Both the IMROZ study and the EMJ article were funded by Sanofi.
Dr. Dimopoulos reported ties with Amgen, BeiGene, BMS, Janssen, Sanofi, and Takeda. Dr. Beksac disclosed relationships with Amgen, BMS, GSK, Janssen, Sanofi, and Takeda. Dr. Mohty reported ties with Adaptive Biotechnologies, Amgen, Astellas Pharma, BMS, GSK, Janssen-Cilag, Jazz Pharmaceuticals, and others.
A version of this article appeared on Medscape.com.
FROM IMS 2024
Clozapine and Respiratory Infection Risk: What to Know
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
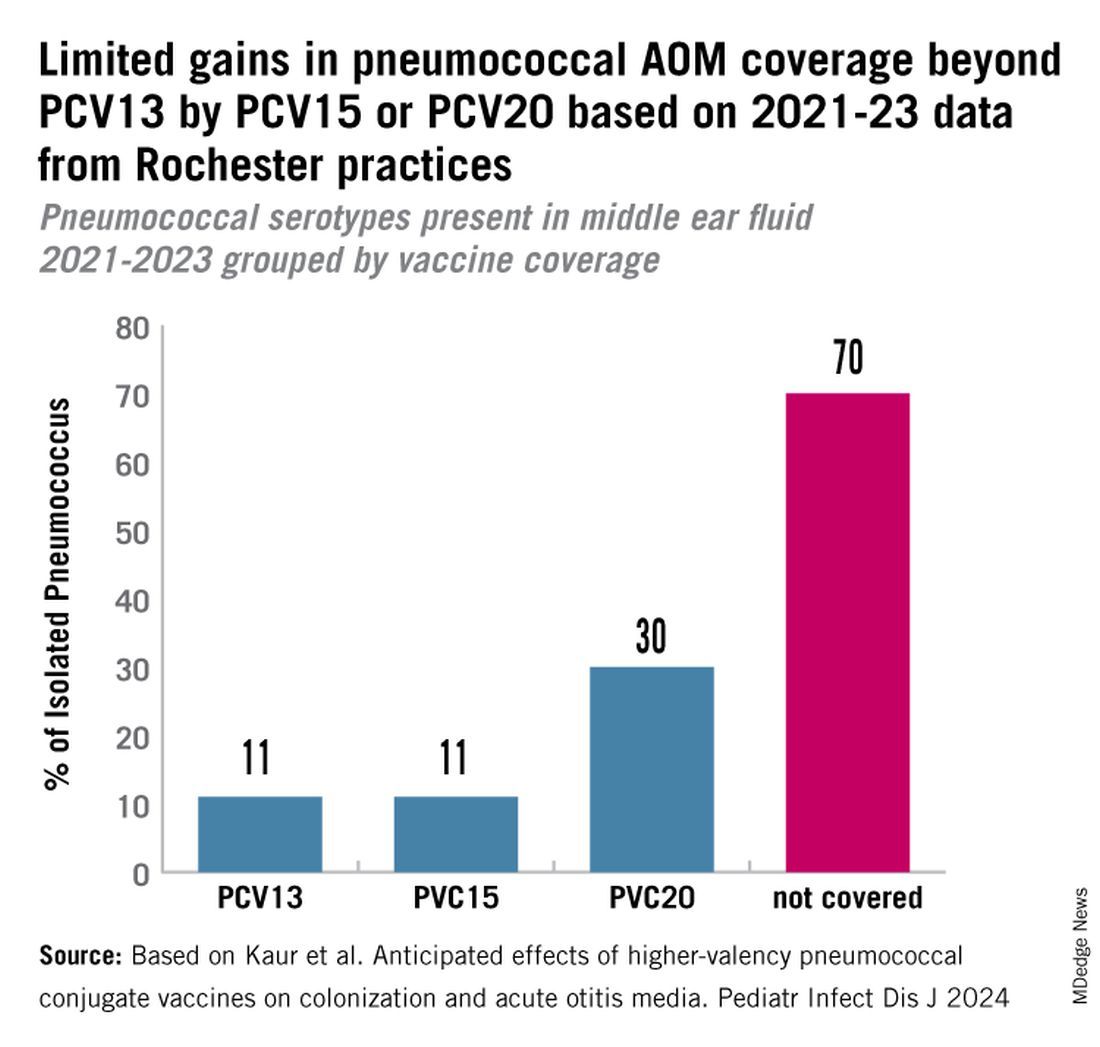
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Tirzepatide Shortage Resolved? FDA Says Yes, Compounders No
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.
The agency wrote in a clarification aimed at compounders that Lilly said it can meet the “present and projected national demand” and that compounders are restricted from making the products.
Nevertheless, patients and prescribers may still see “intermittent localized supply disruptions as the products move through the supply chain,” the FDA noted.
The Alliance for Pharmacy Compounding (APC) responded swiftly, alerting its members and the public to the resolved shortage and stating that compounders “must immediately cease preparing and dispensing compounded copies” of the two drugs.
However, APC CEO Scott Brunner added it often takes a long time for FDA-approved versions of the drug to become widely available to wholesalers, hospitals, and clinics. Even after Lilly announced greater availability for the drugs, including in a new vial format for low doses, “for most pharmacies, they’re lucky to get two or three boxes of Zepbound a day from their wholesaler — for a patient waiting list that can number in the hundreds.”
“We have already heard this morning from APC members that they are unable to fill orders for their patients,” he said.
Furthermore, he contended, “I suspect plenty of patients taking compounded tirzepatide are going to be caught flat-footed by this. They are being cut off cold turkey, their prescription no longer fillable. They’ll need to get in to see their provider to get a new prescription, and that will take some time. It’s possible that so many patients presently taking compounded GLP-1s [glucagon-like peptide 1] will be eventually switched to the FDA-approved versions — if they can afford them, of course — that it will push tirzepatide injection back into shortage.”
Commenting on the shortage resolution, endocrinologist Beverly Tchang, MD, DABOM, an assistant professor of clinical medicine at Weill Cornell Medicine in New York City told this news organization, “we are not yet experiencing relief from the shortages, but I hope this resolves at least one barrier to access for our patients.”
“I don’t think it will create confusion,” she said. “Fortunately or unfortunately, patients and clinicians are adept by now with therapeutic transitions because we’ve been forced to do so whenever insurance withdraws coverage or a shortage recurs or a coupon expires. It’s obviously not ideal but patients are motivated and clinicians don’t give up.”
This news organization has previously reported on the impact of the shortages and how endocrinologists and obesity medicine specialists were handling them, in light of concerns about compounding pharmacies that may or may not be well founded.
Dr. Tchang declared that she is an adviser to Novo Nordisk.
A version of this article appeared on Medscape.com.






