User login
High school students using less tobacco, vape products, CDC report shows
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FDA to health care providers: Double-check COVID vaccine dose for children
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
FDA OKs new treatment for erosive esophagitis
also known as erosive gastroesophageal reflux disease (GERD), as well as relief of associated heartburn, the company has announced.
Vonoprazan, an oral potassium-competitive acid blocker (PCAB), provides more potent inhibition of gastric acid than do proton pump inhibitors (PPIs) and is seen as a potential alternative.
The approval of vonoprazan for erosive GERD was based on results from the phase 3 PHALCON-EE study.
The randomized, double-blind, multicenter study enrolled 1,024 patients with erosive GERD in the United States and Europe and compared vonoprazan with the PPI lansoprazole (Prevacid) in the healing and maintenance of healing of erosive GERD and associated heartburn symptom relief.
Vonoprazan 20 mg was noninferior to lansoprazole 30 mg for complete healing by week 8 in patients with all grades of erosive GERD, with healing rates of 93% vs. 85% for lansoprazole.
In addition, vonoprazan showed superior rates of healing in patients with moderate to severe disease (LA Grade C/D) at week 2 (70% vs. 53% with lansoprazole). Vonoprazan was also noninferior to lansoprazole in terms of heartburn-free days over the healing period.
In the maintenance phase of the trial, vonoprazan 10 mg was superior to lansoprazole 15 mg in maintaining healing at 6 months in all patients who were randomly assigned (79% vs. 72%) and in the subset of patients with moderate to severe erosive GERD (75% vs. 61%).
Adverse event (AE) rates for vonoprazan were comparable to lansoprazole. The most common AEs in the healing phase (≥ 2% with vonoprazan) were gastritis, diarrhea, abdominal distention, abdominal pain, and nausea.
The most common AEs in the maintenance phase (≥ 3% with vonoprazan) were gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection.
“For many GERD patients with erosive esophagitis, the response to current treatment is suboptimal, leaving them with incomplete healing and ongoing symptoms,” Colin W. Howden, MD, professor emeritus, University of Tennessee, Memphis, said in the news release.
Vonoprazan provides clinicians with a “new first-in-class therapeutic option that demonstrated faster healing in the more difficult-to-treat GERD patients with erosive esophagitis,” Dr. Howden added.
Vonoprazan is expected to be available in the United States in December.
The FDA also recently approved reformulated vonoprazan tablets for Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) for the treatment of Helicobacter pylori infection in adults, Phathom Pharmaceuticals announced.
In February, the FDA had put both the vonoprazan new drug application for erosive esophagitis and the postapproval supplement for H. pylori on hold until the company addressed concerns over the presence of nitrosamine impurities.
Dr. Howden is a former editor-in-chief of GI&Hepatology News. A version of this article appeared on Medscape.com.
also known as erosive gastroesophageal reflux disease (GERD), as well as relief of associated heartburn, the company has announced.
Vonoprazan, an oral potassium-competitive acid blocker (PCAB), provides more potent inhibition of gastric acid than do proton pump inhibitors (PPIs) and is seen as a potential alternative.
The approval of vonoprazan for erosive GERD was based on results from the phase 3 PHALCON-EE study.
The randomized, double-blind, multicenter study enrolled 1,024 patients with erosive GERD in the United States and Europe and compared vonoprazan with the PPI lansoprazole (Prevacid) in the healing and maintenance of healing of erosive GERD and associated heartburn symptom relief.
Vonoprazan 20 mg was noninferior to lansoprazole 30 mg for complete healing by week 8 in patients with all grades of erosive GERD, with healing rates of 93% vs. 85% for lansoprazole.
In addition, vonoprazan showed superior rates of healing in patients with moderate to severe disease (LA Grade C/D) at week 2 (70% vs. 53% with lansoprazole). Vonoprazan was also noninferior to lansoprazole in terms of heartburn-free days over the healing period.
In the maintenance phase of the trial, vonoprazan 10 mg was superior to lansoprazole 15 mg in maintaining healing at 6 months in all patients who were randomly assigned (79% vs. 72%) and in the subset of patients with moderate to severe erosive GERD (75% vs. 61%).
Adverse event (AE) rates for vonoprazan were comparable to lansoprazole. The most common AEs in the healing phase (≥ 2% with vonoprazan) were gastritis, diarrhea, abdominal distention, abdominal pain, and nausea.
The most common AEs in the maintenance phase (≥ 3% with vonoprazan) were gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection.
“For many GERD patients with erosive esophagitis, the response to current treatment is suboptimal, leaving them with incomplete healing and ongoing symptoms,” Colin W. Howden, MD, professor emeritus, University of Tennessee, Memphis, said in the news release.
Vonoprazan provides clinicians with a “new first-in-class therapeutic option that demonstrated faster healing in the more difficult-to-treat GERD patients with erosive esophagitis,” Dr. Howden added.
Vonoprazan is expected to be available in the United States in December.
The FDA also recently approved reformulated vonoprazan tablets for Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) for the treatment of Helicobacter pylori infection in adults, Phathom Pharmaceuticals announced.
In February, the FDA had put both the vonoprazan new drug application for erosive esophagitis and the postapproval supplement for H. pylori on hold until the company addressed concerns over the presence of nitrosamine impurities.
Dr. Howden is a former editor-in-chief of GI&Hepatology News. A version of this article appeared on Medscape.com.
also known as erosive gastroesophageal reflux disease (GERD), as well as relief of associated heartburn, the company has announced.
Vonoprazan, an oral potassium-competitive acid blocker (PCAB), provides more potent inhibition of gastric acid than do proton pump inhibitors (PPIs) and is seen as a potential alternative.
The approval of vonoprazan for erosive GERD was based on results from the phase 3 PHALCON-EE study.
The randomized, double-blind, multicenter study enrolled 1,024 patients with erosive GERD in the United States and Europe and compared vonoprazan with the PPI lansoprazole (Prevacid) in the healing and maintenance of healing of erosive GERD and associated heartburn symptom relief.
Vonoprazan 20 mg was noninferior to lansoprazole 30 mg for complete healing by week 8 in patients with all grades of erosive GERD, with healing rates of 93% vs. 85% for lansoprazole.
In addition, vonoprazan showed superior rates of healing in patients with moderate to severe disease (LA Grade C/D) at week 2 (70% vs. 53% with lansoprazole). Vonoprazan was also noninferior to lansoprazole in terms of heartburn-free days over the healing period.
In the maintenance phase of the trial, vonoprazan 10 mg was superior to lansoprazole 15 mg in maintaining healing at 6 months in all patients who were randomly assigned (79% vs. 72%) and in the subset of patients with moderate to severe erosive GERD (75% vs. 61%).
Adverse event (AE) rates for vonoprazan were comparable to lansoprazole. The most common AEs in the healing phase (≥ 2% with vonoprazan) were gastritis, diarrhea, abdominal distention, abdominal pain, and nausea.
The most common AEs in the maintenance phase (≥ 3% with vonoprazan) were gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection.
“For many GERD patients with erosive esophagitis, the response to current treatment is suboptimal, leaving them with incomplete healing and ongoing symptoms,” Colin W. Howden, MD, professor emeritus, University of Tennessee, Memphis, said in the news release.
Vonoprazan provides clinicians with a “new first-in-class therapeutic option that demonstrated faster healing in the more difficult-to-treat GERD patients with erosive esophagitis,” Dr. Howden added.
Vonoprazan is expected to be available in the United States in December.
The FDA also recently approved reformulated vonoprazan tablets for Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) for the treatment of Helicobacter pylori infection in adults, Phathom Pharmaceuticals announced.
In February, the FDA had put both the vonoprazan new drug application for erosive esophagitis and the postapproval supplement for H. pylori on hold until the company addressed concerns over the presence of nitrosamine impurities.
Dr. Howden is a former editor-in-chief of GI&Hepatology News. A version of this article appeared on Medscape.com.
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves abatacept for pediatric patients with psoriatic arthritis
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
FDA warns of hidden ingredients in arthritis, pain products
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Some of these products contain active ingredients found in anti-inflammatory prescription medication.
“These products may cause potentially serious side effects and may interact with medications or dietary supplements a consumer is taking,” the FDA said in a statement. “It is clear from the results of our decade of testing that retailers and distributors, including online marketplaces, do not effectively prevent these types of potentially harmful products from being sold to consumers.”
Unlike prescription medication and over-the-counter drugs such as loratadine (Claritin) or acetaminophen (Tylenol), supplements do not need FDA approval before they can be sold. Only after a complaint is made or FDA testing reveals illegal or unsafe ingredients can the FDA get involved.
From August 2013 to September 2023, the FDA identified 22 arthritis and pain products with active ingredients not disclosed on the product label. The most common hidden ingredients detected in these supplements were prescription-only corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and muscle relaxants, said Candy Tsourounis, PharmD, a professor in the department of clinical pharmacy at the University of California, San Francisco.
Kuka Flex Forte and Reumo Flex, both promoted for joint pain and arthritis, both contain the NSAID diclofenac. Tapee Tea, a product promoted for pain relief, contains dexamethasone and piroxicam. AK Forte, also sold for joint pain and arthritis, contains diclofenac, dexamethasone, and methocarbamol not disclosed on the label.
“It is interesting that these products have hidden ingredients that are used to reduce swelling and inflammation,” Dr. Tsourounis said. “I don’t know if this was intentional, but it seems suspicious that a product marketed to reduce joint pain and inflammation contains prescription-only ingredients that are used for this purpose.”
Certain products also contained antihistamines including cyproheptadine and chlorpheniramine.
These types of products are likely targeted toward underserved and immigrant communities, added Pieter Cohen, MD, a primary care physician and an assistant professor of medicine at Harvard Medical School, Boston, who studies dietary supplements. They might be sold in mom-and-pop shops or gas stations to individuals with limited access to health care or insurance, he noted.
The FDA warned that this list included “only a small fraction of the potentially dangerous products marketed to consumers online and in stores. Even if a product is not included in this list, consumers should exercise caution before using these types of arthritis and pain management products.”
Advising patients
Research suggests that most patients do not tell doctors about the supplements they are taking, and often, clinicians do not ask, said Dr. Cohen. “Most of the time it’s a total black box – we don’t know what’s going on,” he added.
He advised raising the subject of supplements in a very nonjudgmental way, particularly when treating patients in marginalized and immigrant communities. One approach he suggested was first mentioning that other patients in your care dealing with joint pain have bought remedies locally or have tried treatments that friends recommend. You can then ask a patient about their own use, framing it as a way to better help with treatment decisions.
Once a clinician understands what their patient is taking, they can then give advice and discuss if a product is safe to combine with prescription drugs, Dr. Cohen said. “If they come down too hard, I think the patients will just clam up and not talk about it anymore,” he said.
If a patient begins to experience side effects or gets sick, a clinician will already be informed of what their patient is taking and can ask that patient to bring the product or supplement in, so they can look over the product together, Dr. Cohen noted. Any side effects or other adverse events potentially related to the use of these products should then be reported to FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Tips for safe shopping
To make sure supplements and other over-the-counter products are safe to use, Dr. Tsourounis recommends that consumers:
- Buy products from well-known retailers like Target or large pharmacies like CVS or Walgreens.
- Avoid buying products with labels in another language that you cannot read or products with no drug label.
- Be cautious of buying products online or from other countries.
- Look up suspicious products on the FDA’s health fraud database.
- Be wary of any product that offers miracle cures or relies on personal testimonies without evidence.
In general, do not base purchasing decisions on any health claims on a product label because companies selling supplements making these claims “don’t have to have any clinical data to back them up,” Dr. Cohen said.
Dr. Cohen also recommends sticking with individual ingredients. “If you want echinacea, buy echinacea. Don’t buy a complicated mix that is supposed to be good for arthritis with 10 different botanical [ingredients]. That’s more likely to run [you] into trouble,” he said.
Last, Dr. Cohen recommended buying supplements that are certified by NSF International or United States Pharmacopeia, both respected third-party testing organizations. “If it has an NSF International or USP stamp, that gives us more certainty that what’s in the bottle is going to be what’s listed on label,” he said.
Dr. Tsourounis noted that if you are skeptical of a product, you can also try calling the manufacturer number on the product label.
“I always encourage people to call that number to see if somebody answers,” she said. “Sometimes, you can tell a lot about that company just by calling that number.”
Dr. Cohen has received research support from the Consumers Union and PEW Charitable Trusts and royalties from UpToDate. He has collaborated in research with NSF International. Dr. Tsourounis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
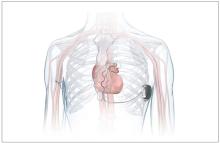
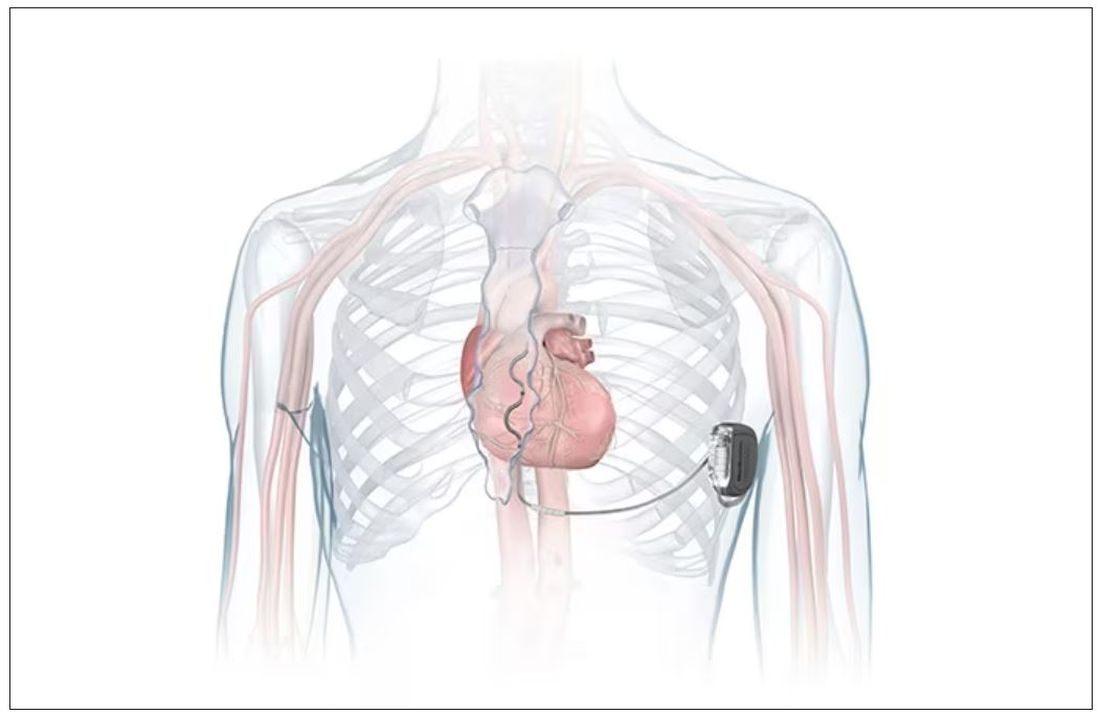
FDA okays first extravascular ICD system
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
FDA approves subcutaneous infliximab for IBD
Infliximab-dyyb is a subcutaneous formulation of Celltrion’s infliximab. The FDA approval provides an alternative administration option for delivering the drug, which blocks the action of tumor necrosis factor alpha.
The new formulation was approved based on phase 3 pivotal trials that evaluated the safety and efficacy of infliximab-dyyb as maintenance therapy in patients with moderately to severely active UC (LIBERTY-UC) and CD (LIBERTY-CD).
In both 54-week trials, infliximab-dyyb demonstrated superiority to placebo in the primary endpoints of clinical remission (UC and CD) and endoscopic response (CD) when given as maintenance therapy after induction therapy with IV infliximab.
The overall safety profile of infliximab-dyyb was similar to that of the placebo during the maintenance period in both studies, with no new safety signals seen.
In the randomized, placebo-controlled, double-blind LIBERTY-UC study, 438 patients with moderately to severely active UC after induction therapy with IV infliximab were randomly assigned at week 10. The rate of clinical remission at week 54 was significantly greater with infliximab-dyyb (43.2%), compared with placebo (20.8%).
The most common adverse events were COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain.
In the similarly designed LIBERTY-CD study, 343 patients with moderately to severely active CD after induction therapy were randomly assigned at week 10. At week 54, the clinical remission rate was greater with infliximab-dyyb (62.3%) than with placebo (32.1%).
In parallel, the endoscopic response rate at week 54 was also greater in the infliximab-dyyb arm than in the placebo arm (51.1% vs. 17.9%, respectively).
The safety profile during the maintenance phase was generally comparable between the two trial arms. The most common adverse events were COVID-19, upper respiratory tract infection, headache, injection site reaction, diarrhea, increased alanine aminotransferase, increased blood creatine phosphokinase, neutropenia, hypertension, urinary tract infection, dizziness, and leukopenia.
Full prescribing information is available online.
“As someone dedicated to improving the lives of patients with IBD, I am excited to see data supporting the efficacy and safety of a new formulation offering convenience and improved access to a well-known and proven drug,” Andres Yarur, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a news release.
The data “validate a convenient treatment option that could allow more patients in the United States to have greater control of their disease management,” added Jean-Frederic Colombel, MD, of Icahn School of Medicine at Mount Sinai, New York.
A version of this article first appeared on Medscape.com.
Infliximab-dyyb is a subcutaneous formulation of Celltrion’s infliximab. The FDA approval provides an alternative administration option for delivering the drug, which blocks the action of tumor necrosis factor alpha.
The new formulation was approved based on phase 3 pivotal trials that evaluated the safety and efficacy of infliximab-dyyb as maintenance therapy in patients with moderately to severely active UC (LIBERTY-UC) and CD (LIBERTY-CD).
In both 54-week trials, infliximab-dyyb demonstrated superiority to placebo in the primary endpoints of clinical remission (UC and CD) and endoscopic response (CD) when given as maintenance therapy after induction therapy with IV infliximab.
The overall safety profile of infliximab-dyyb was similar to that of the placebo during the maintenance period in both studies, with no new safety signals seen.
In the randomized, placebo-controlled, double-blind LIBERTY-UC study, 438 patients with moderately to severely active UC after induction therapy with IV infliximab were randomly assigned at week 10. The rate of clinical remission at week 54 was significantly greater with infliximab-dyyb (43.2%), compared with placebo (20.8%).
The most common adverse events were COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain.
In the similarly designed LIBERTY-CD study, 343 patients with moderately to severely active CD after induction therapy were randomly assigned at week 10. At week 54, the clinical remission rate was greater with infliximab-dyyb (62.3%) than with placebo (32.1%).
In parallel, the endoscopic response rate at week 54 was also greater in the infliximab-dyyb arm than in the placebo arm (51.1% vs. 17.9%, respectively).
The safety profile during the maintenance phase was generally comparable between the two trial arms. The most common adverse events were COVID-19, upper respiratory tract infection, headache, injection site reaction, diarrhea, increased alanine aminotransferase, increased blood creatine phosphokinase, neutropenia, hypertension, urinary tract infection, dizziness, and leukopenia.
Full prescribing information is available online.
“As someone dedicated to improving the lives of patients with IBD, I am excited to see data supporting the efficacy and safety of a new formulation offering convenience and improved access to a well-known and proven drug,” Andres Yarur, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a news release.
The data “validate a convenient treatment option that could allow more patients in the United States to have greater control of their disease management,” added Jean-Frederic Colombel, MD, of Icahn School of Medicine at Mount Sinai, New York.
A version of this article first appeared on Medscape.com.
Infliximab-dyyb is a subcutaneous formulation of Celltrion’s infliximab. The FDA approval provides an alternative administration option for delivering the drug, which blocks the action of tumor necrosis factor alpha.
The new formulation was approved based on phase 3 pivotal trials that evaluated the safety and efficacy of infliximab-dyyb as maintenance therapy in patients with moderately to severely active UC (LIBERTY-UC) and CD (LIBERTY-CD).
In both 54-week trials, infliximab-dyyb demonstrated superiority to placebo in the primary endpoints of clinical remission (UC and CD) and endoscopic response (CD) when given as maintenance therapy after induction therapy with IV infliximab.
The overall safety profile of infliximab-dyyb was similar to that of the placebo during the maintenance period in both studies, with no new safety signals seen.
In the randomized, placebo-controlled, double-blind LIBERTY-UC study, 438 patients with moderately to severely active UC after induction therapy with IV infliximab were randomly assigned at week 10. The rate of clinical remission at week 54 was significantly greater with infliximab-dyyb (43.2%), compared with placebo (20.8%).
The most common adverse events were COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain.
In the similarly designed LIBERTY-CD study, 343 patients with moderately to severely active CD after induction therapy were randomly assigned at week 10. At week 54, the clinical remission rate was greater with infliximab-dyyb (62.3%) than with placebo (32.1%).
In parallel, the endoscopic response rate at week 54 was also greater in the infliximab-dyyb arm than in the placebo arm (51.1% vs. 17.9%, respectively).
The safety profile during the maintenance phase was generally comparable between the two trial arms. The most common adverse events were COVID-19, upper respiratory tract infection, headache, injection site reaction, diarrhea, increased alanine aminotransferase, increased blood creatine phosphokinase, neutropenia, hypertension, urinary tract infection, dizziness, and leukopenia.
Full prescribing information is available online.
“As someone dedicated to improving the lives of patients with IBD, I am excited to see data supporting the efficacy and safety of a new formulation offering convenience and improved access to a well-known and proven drug,” Andres Yarur, MD, of Cedars-Sinai Medical Center, Los Angeles, said in a news release.
The data “validate a convenient treatment option that could allow more patients in the United States to have greater control of their disease management,” added Jean-Frederic Colombel, MD, of Icahn School of Medicine at Mount Sinai, New York.
A version of this article first appeared on Medscape.com.
New meningococcal vaccine wins FDA approval
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
Approximately 20% of U.S. adults are diagnosed with arthritis
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of reported diagnosed arthritis in the United States is highest overall in older adults with comorbid chronic conditions.
METHODOLOGY:
- Researchers reviewed data from the National Health Interview Survey (NHIS) from 2019 to 2021 to update the prevalence of self-reported arthritis in the United States.
- The sample sizes for the 2019, 2020, and 2021 NHIS were 31,997, 21,153, and 29,482, with survey response rates of 59.1%, 48.9%, and 50.9%, respectively.
- The unadjusted and age-standardized prevalence estimates were calculated for adults aged 18 years and older and based on self-reported health and demographic data.
TAKEAWAY:
- Overall, arthritis was diagnosed in 53.2 million adults aged 18 years and older in the United States; of these, 88.3% were aged 45 years and older and 48.3% were 65 years and older.
- Age-standardized prevalence of arthritis was higher in women vs men and among veterans vs nonveterans (20.9% vs 16.3% and 24.2% vs 18.5%, respectively).
- When categorized by race, age-standardized prevalence of arthritis was higher among non-Hispanic White individuals, compared with Hispanic or Latino individuals or non-Hispanic Asian individuals (20.1%, 14.7%, and 10.3%, respectively).
- The prevalence of arthritis also was higher among individuals with self-reported diagnosis of chronic conditions including dementia, chronic obstructive pulmonary disease, stroke, heart disease, diabetes, and cancer than in those without these conditions; approximately half of adults aged 65 years and older with arthritis reported at least one of these conditions.
IN PRACTICE:
“These prevalence estimates can be used to guide public health policies and activities to increase equitable access to physical activity opportunities within the built environment and other community-based, arthritis-appropriate, evidence-based interventions,” the authors write.
SOURCE:
The study was led by Elizabeth A. Fallon, PhD, of the Centers for Disease Control and Prevention, Atlanta, Georgia. The data were published online in the CDC’s Morbidity and Mortality Weekly Report.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality between individual characteristics and arthritis diagnosis; other limitations included the reliance on self-reports, possible response bias, and the inability to calculate prevalence of arthritis subtypes.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.