User login
Adherence to Evidence-Based Outpatient Antimicrobial Prescribing Guidelines at a Tribal Health System
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
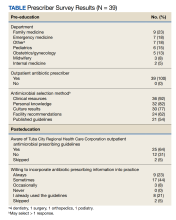
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
Augmented Reality Demonstration Survey Results From a Veteran Affairs Medical Center
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
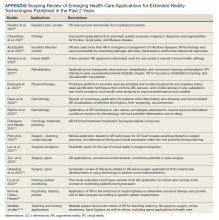
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
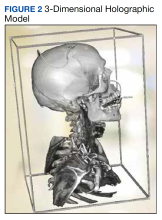
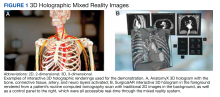
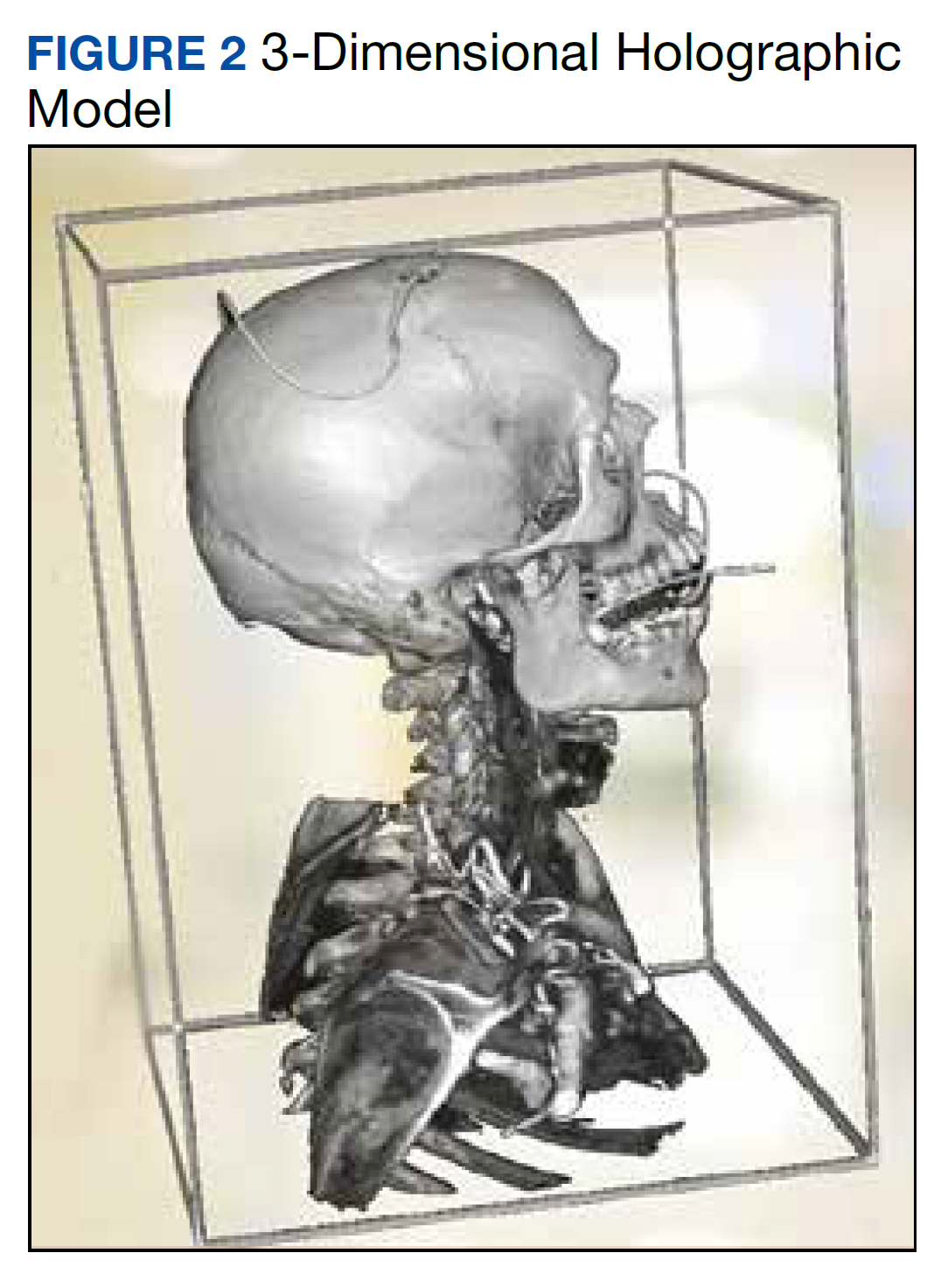
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
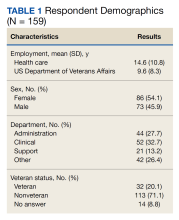
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
Characterization of Blood-borne Pathogen Exposures During Dermatologic Procedures: The Mayo Clinic Experience
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).
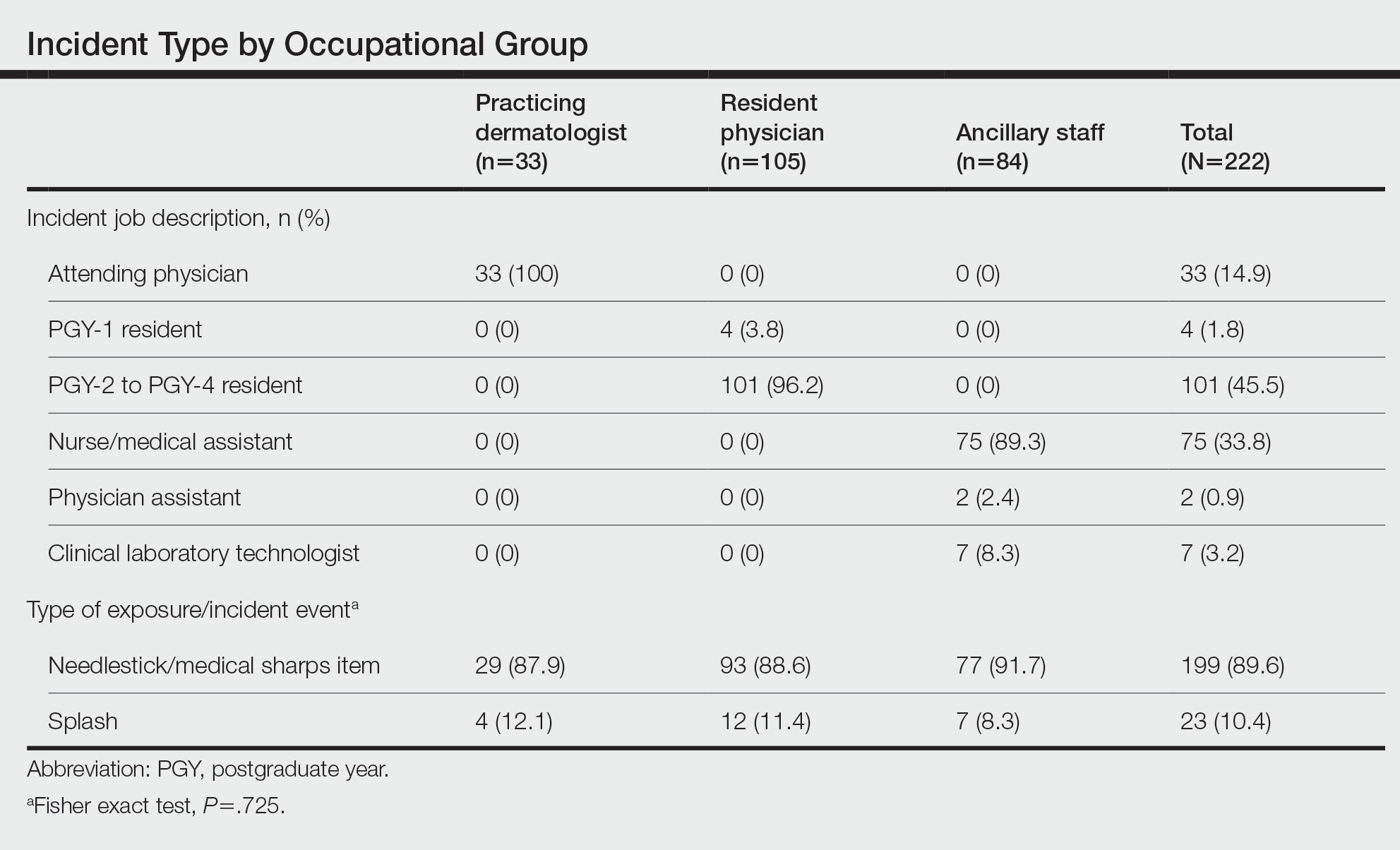
Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.
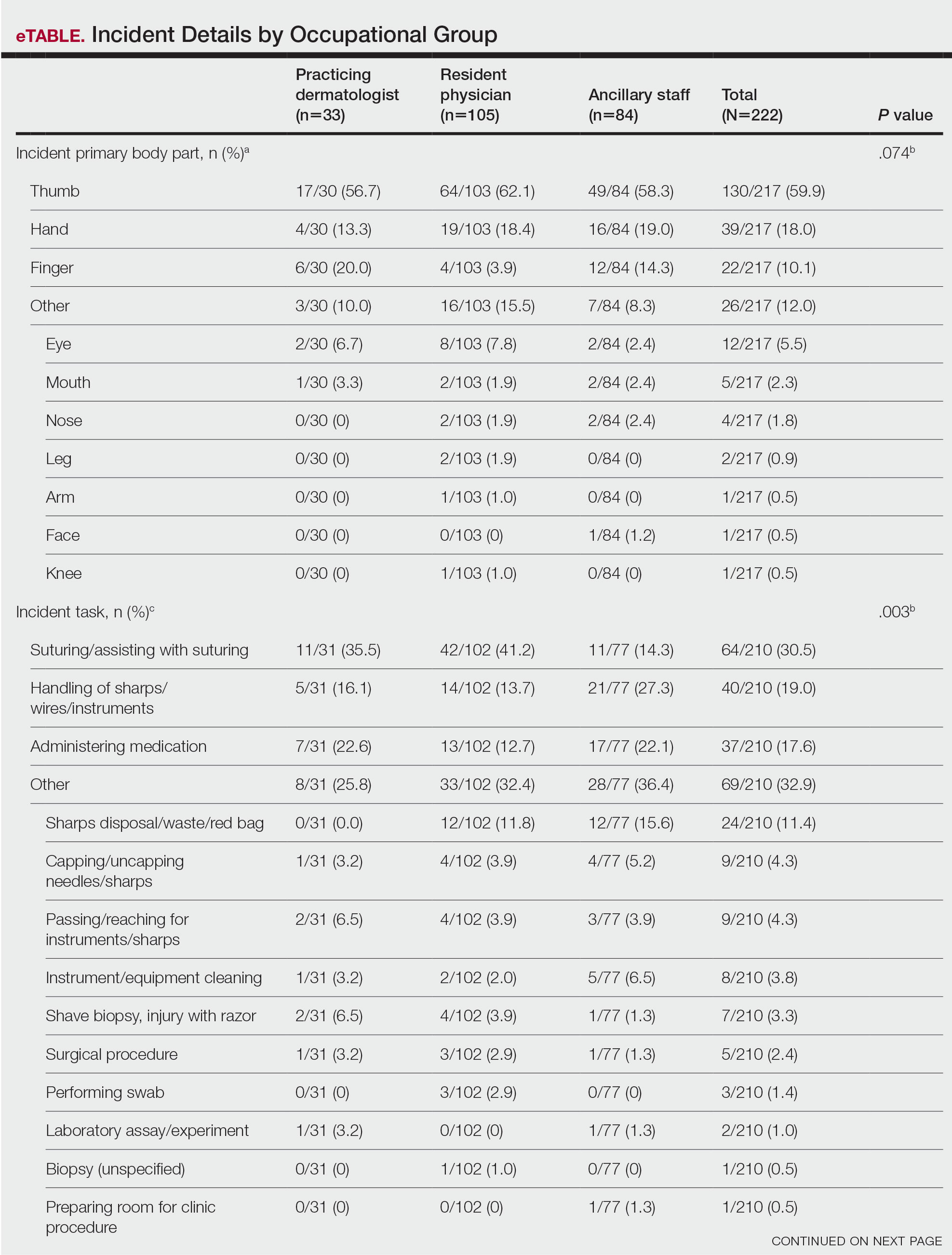
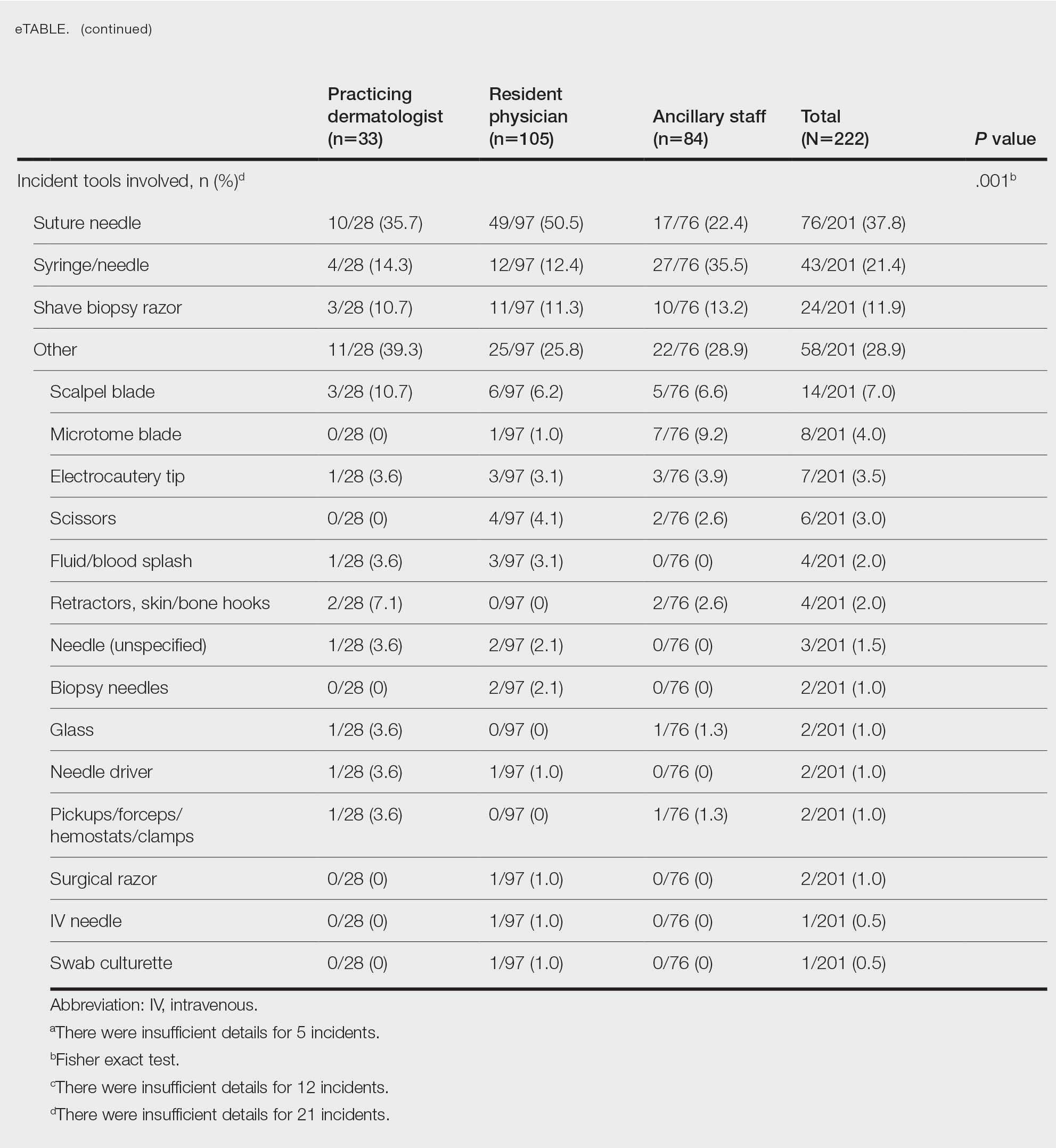
Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Practice Points
- Most blood-borne pathogen (BBP) exposures in dermatologic staff occur due to medical sharps as opposed to splash incidents.
- The most common implicated task in resident physicians and practicing dermatologists is suturing or assisting with suturing, and the most commonly associated instrument is the suture needle. In contrast, ancillary staff experience most BBP exposures during handling of sharps, wires, or instruments, and the injection syringe/needle is the most common instrument of injury.
- Quality improvement measures are needed in prevention of BBP exposures and should focus on identified risk factors among occupational groups in the workplace.
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
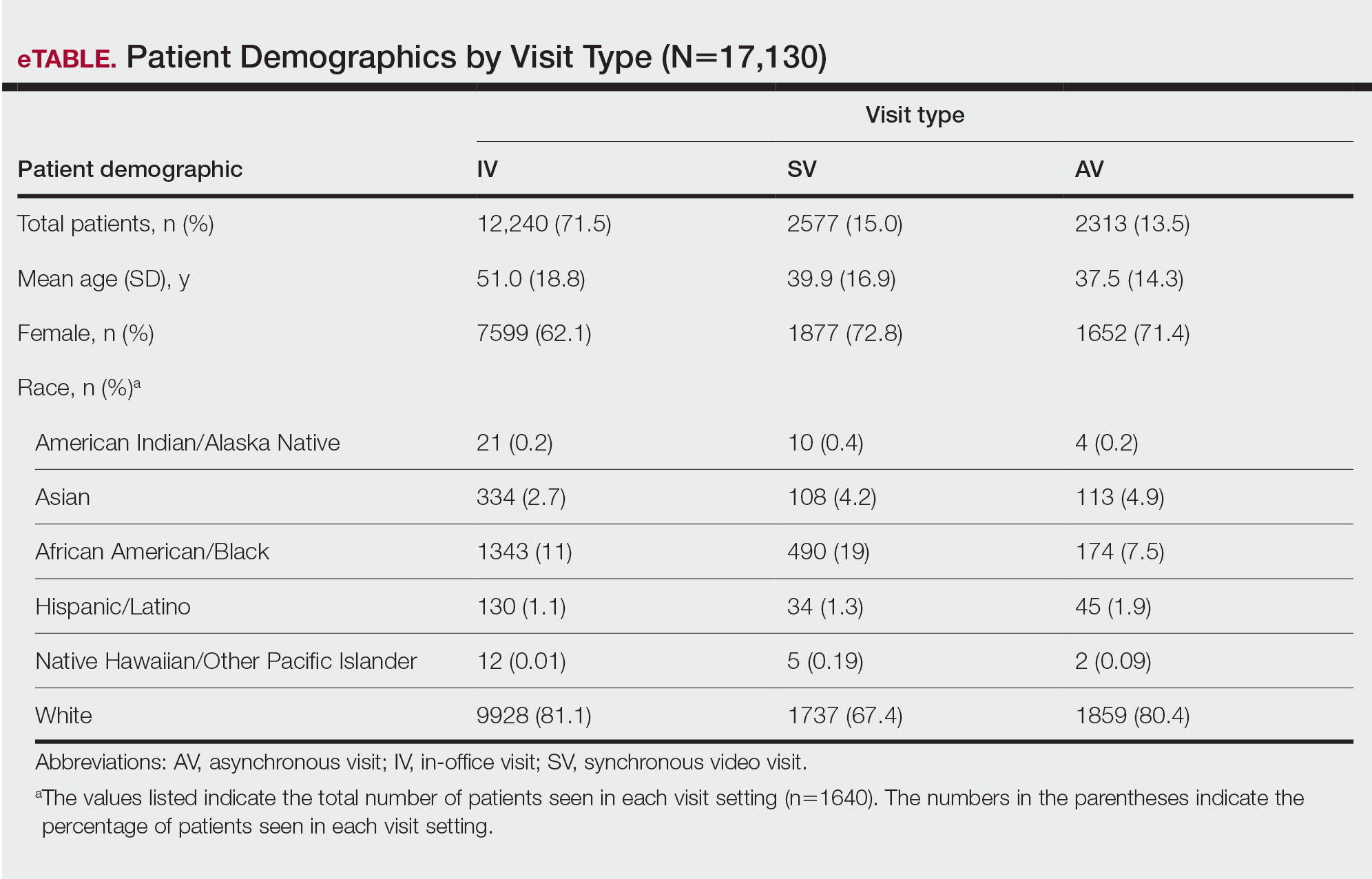
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
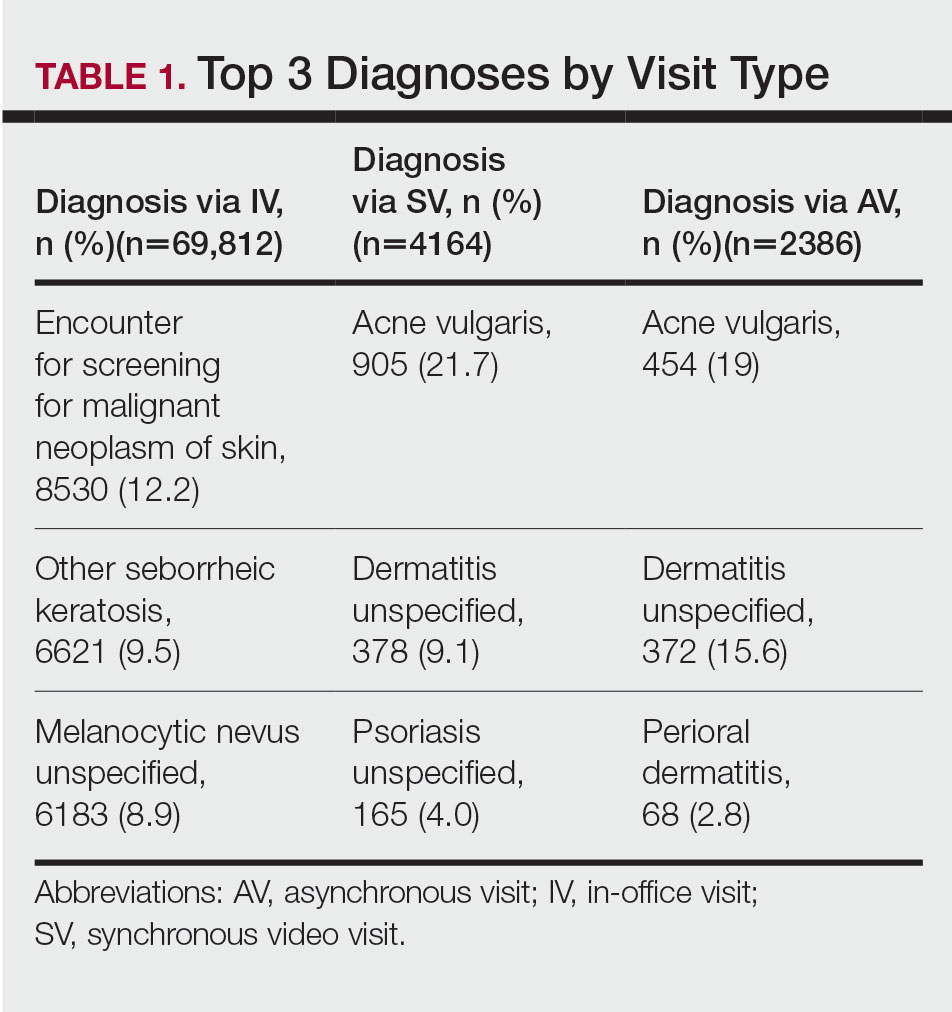
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
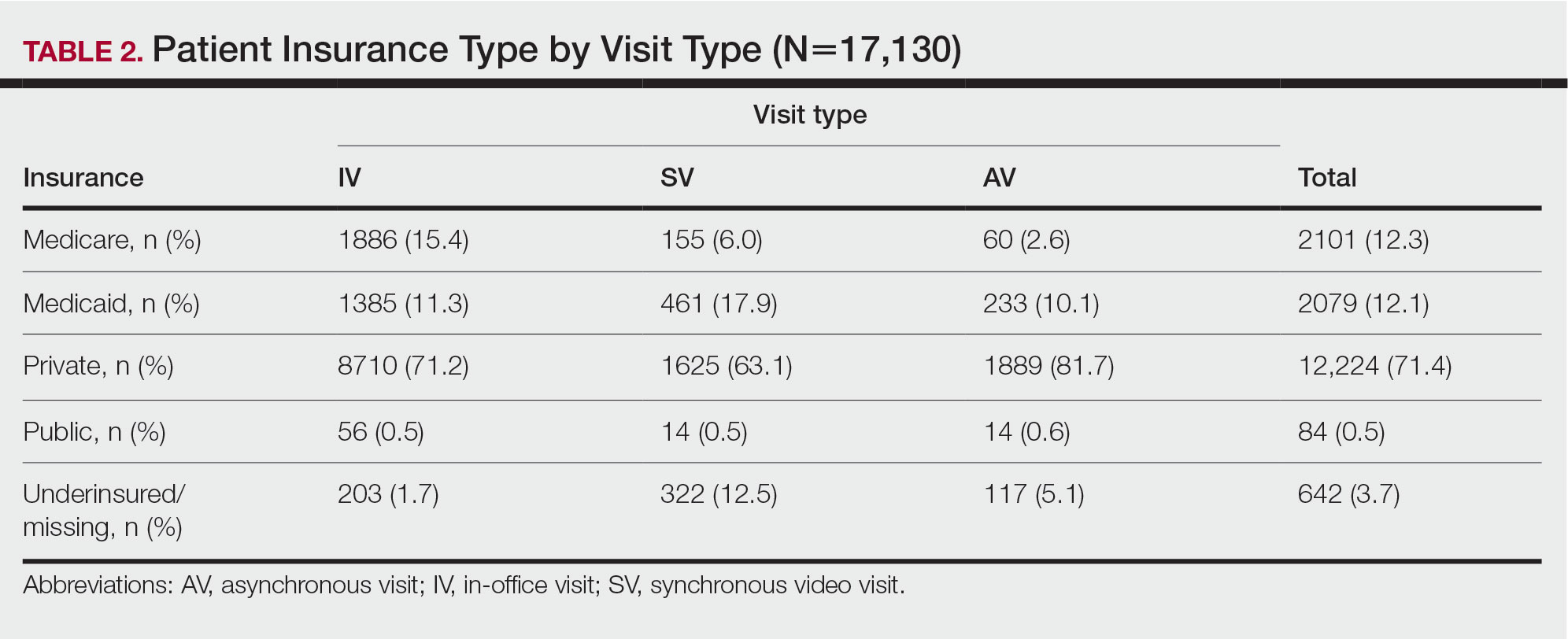
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
Muscle-Related Adverse Events Associated With PCSK9 Inhibitors in a Veteran Population
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
Evaluation of the Appropriateness of Aspirin Therapy in a Veteran Population
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
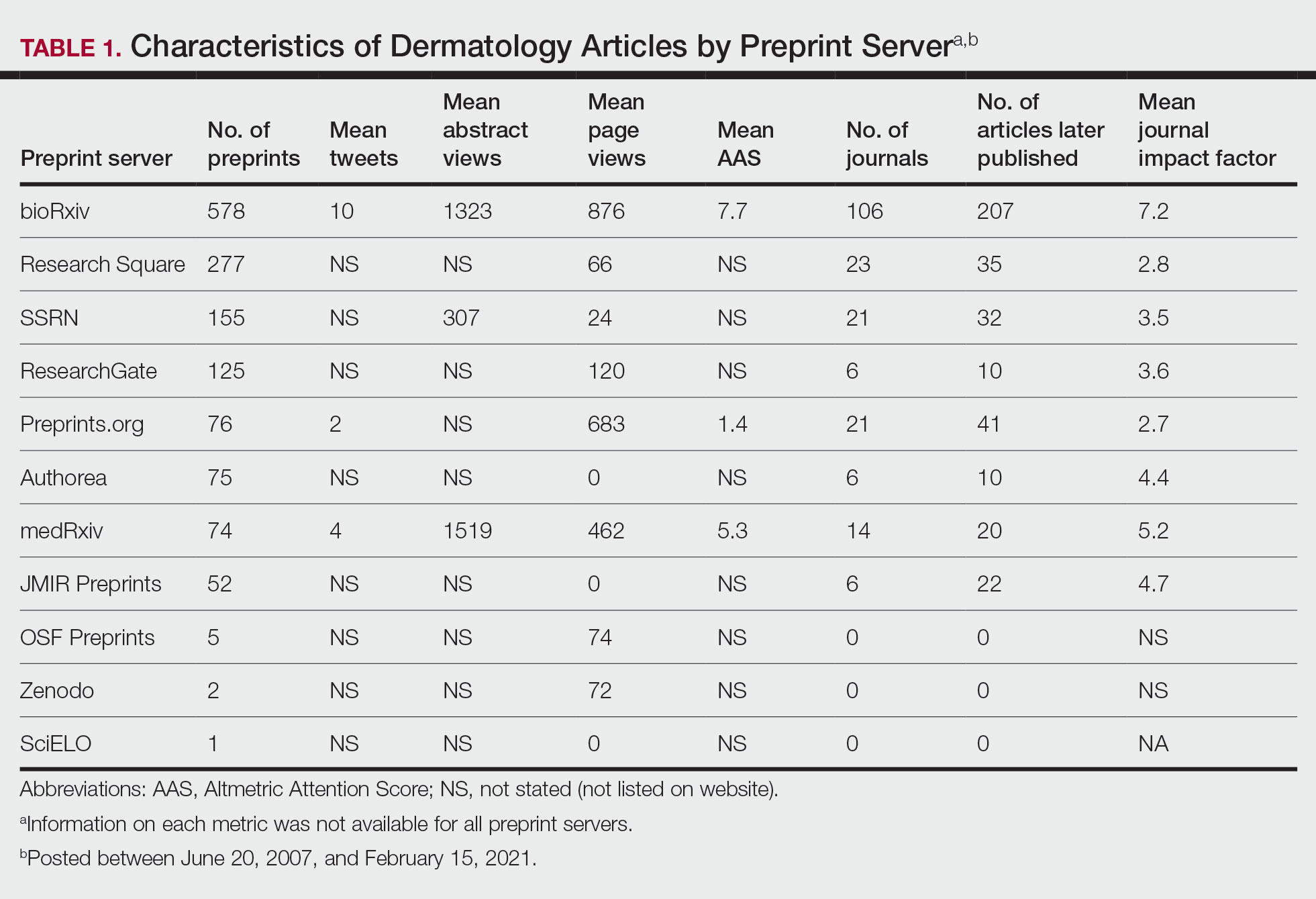
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
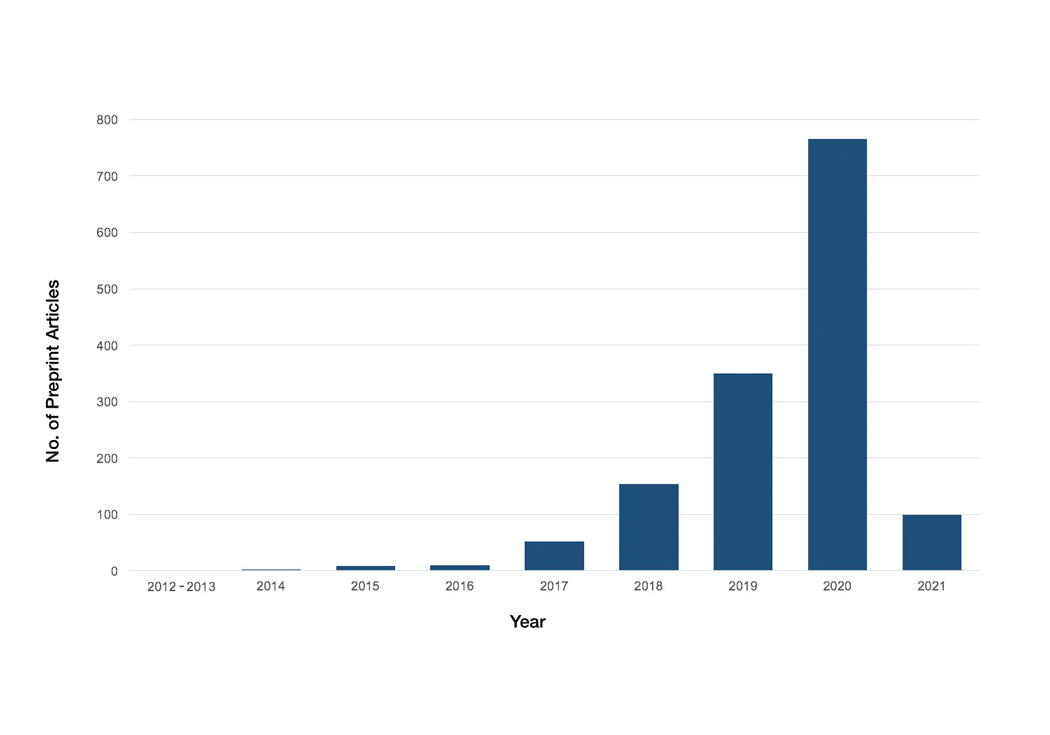
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
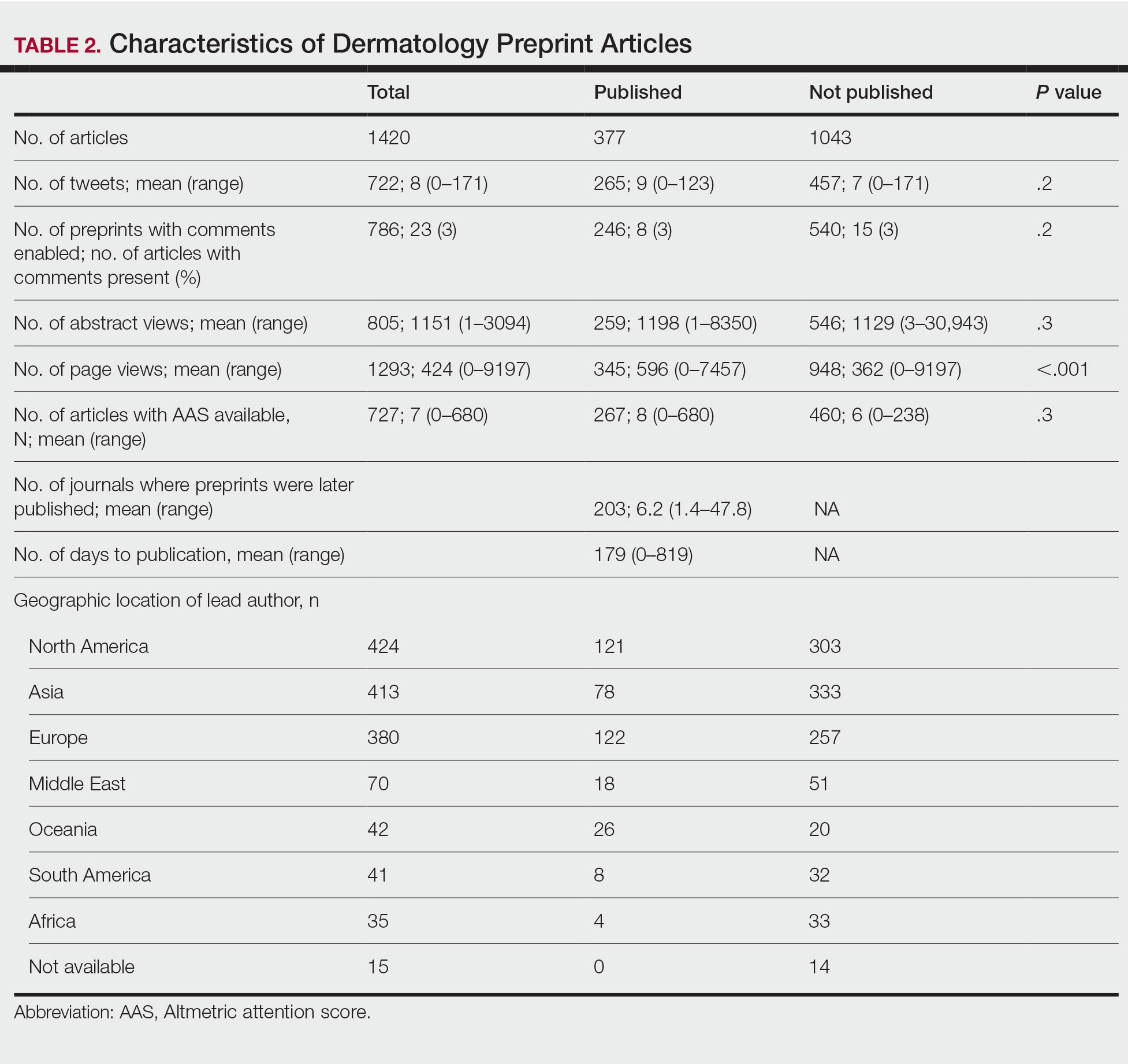
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
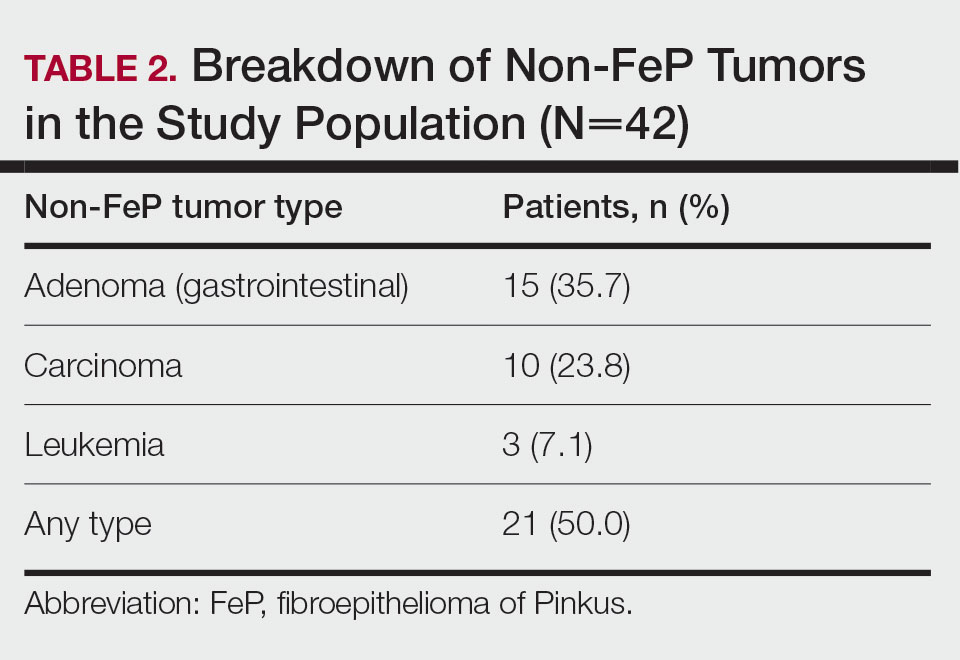
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
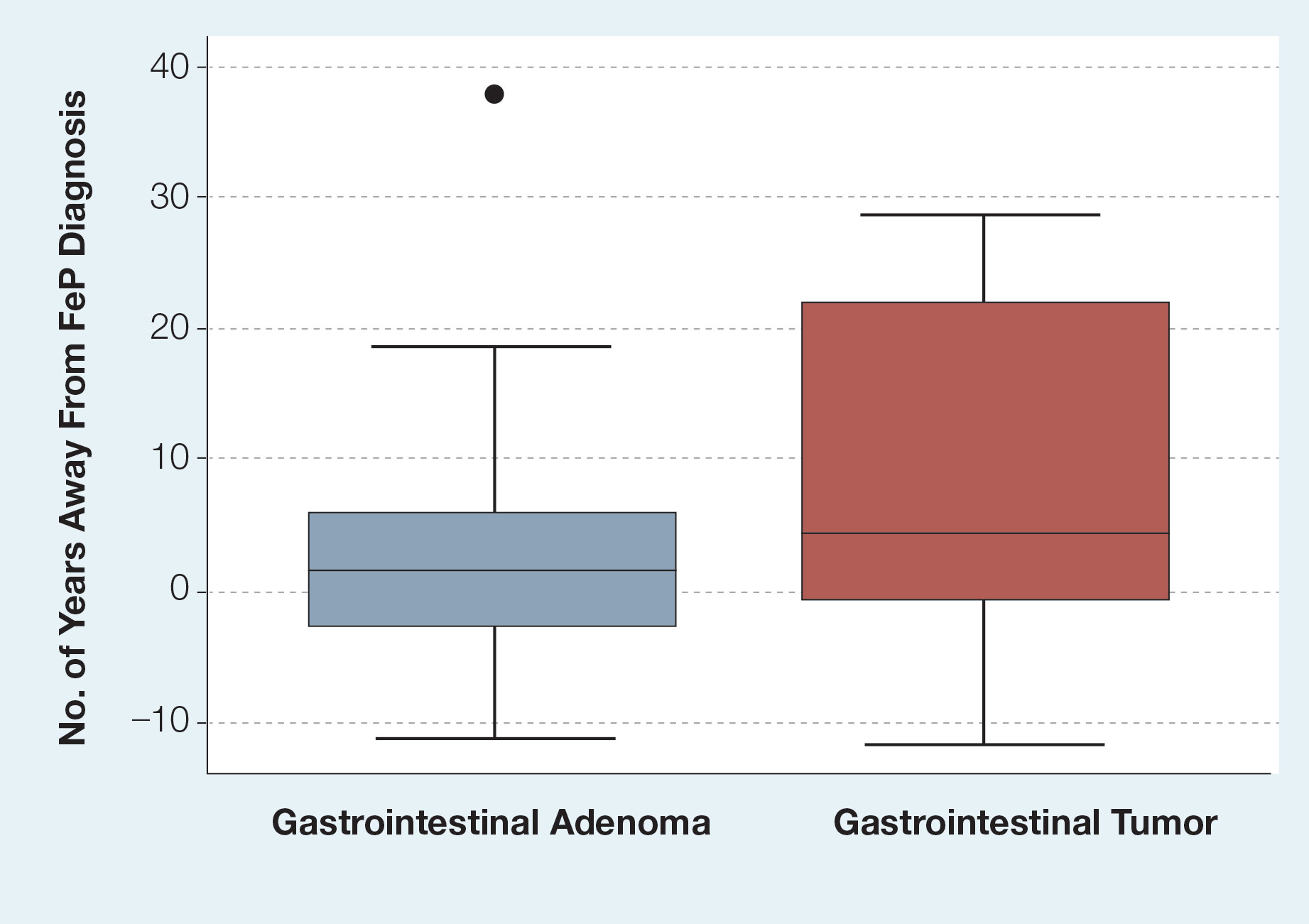
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
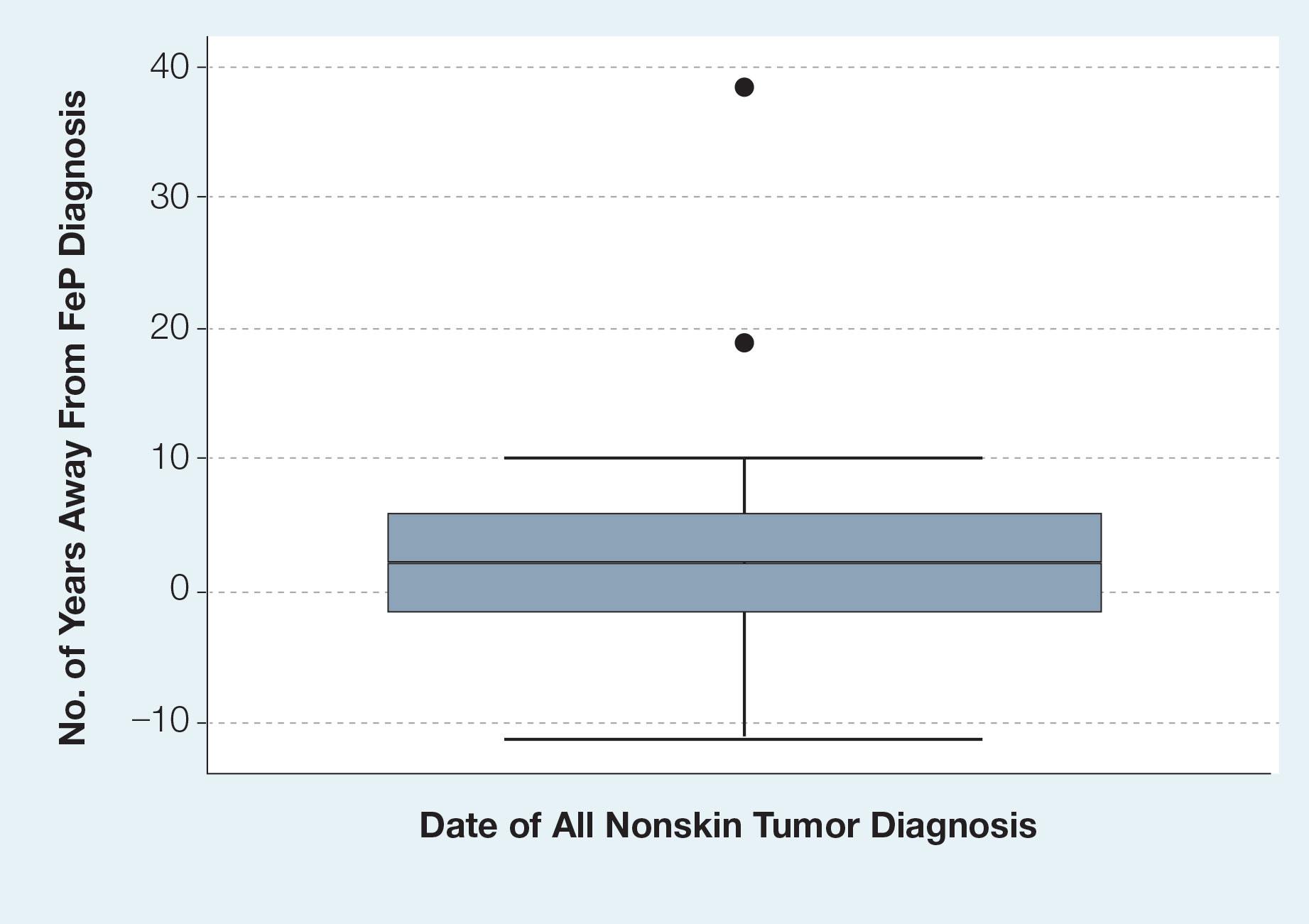
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
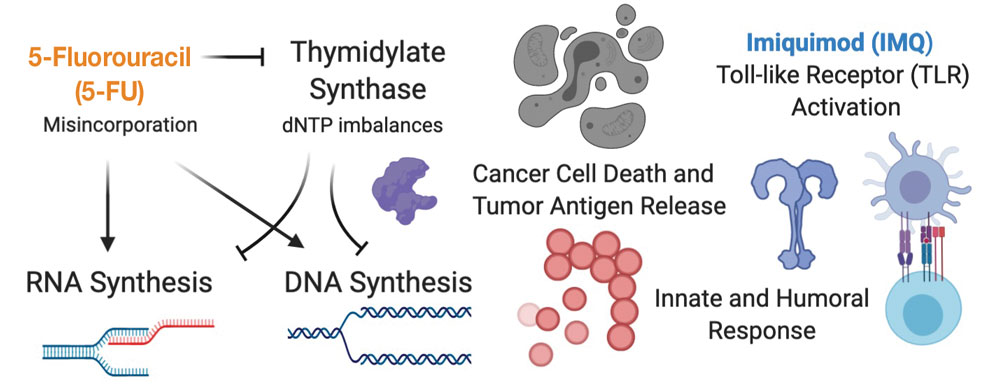
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea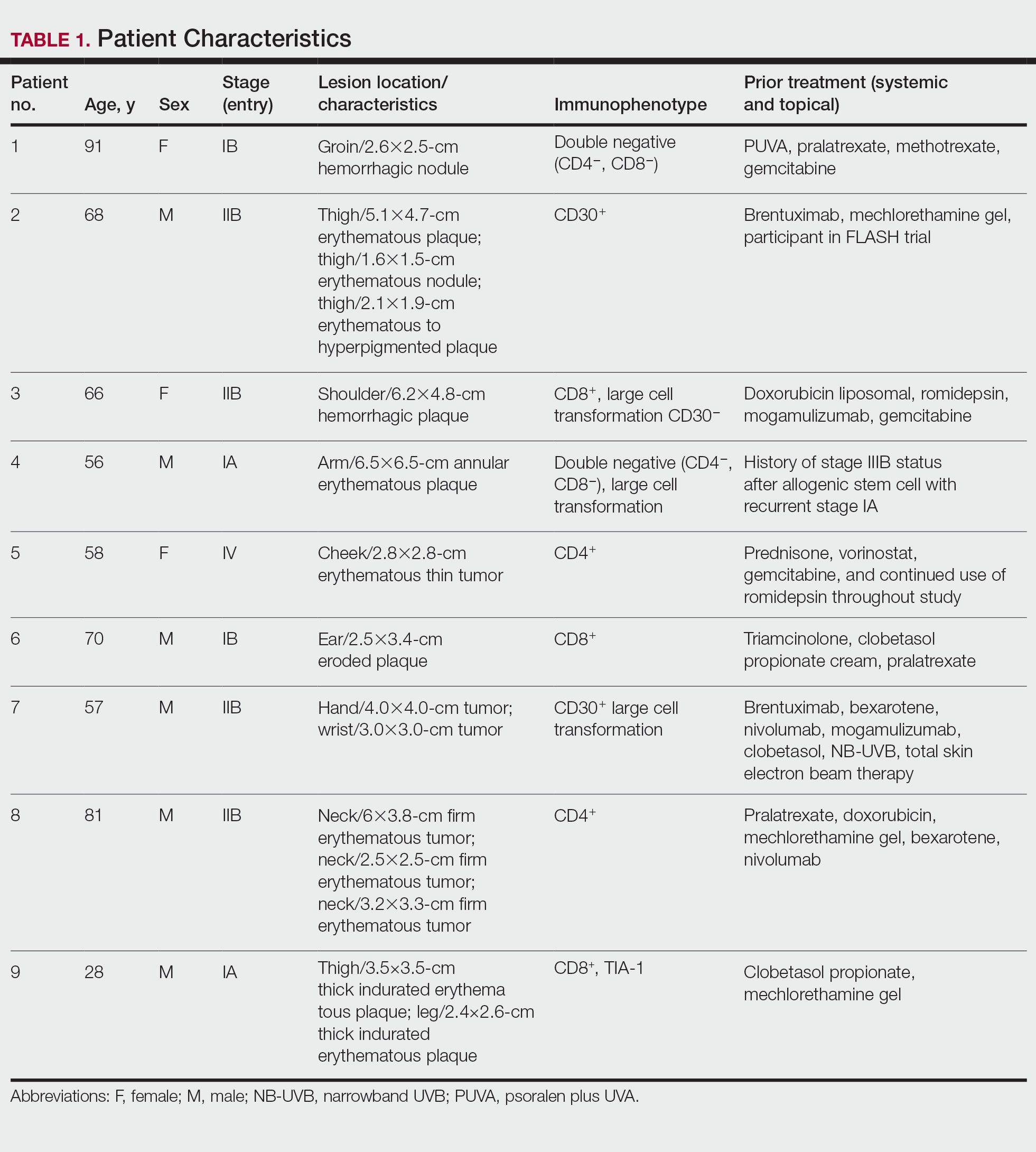
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
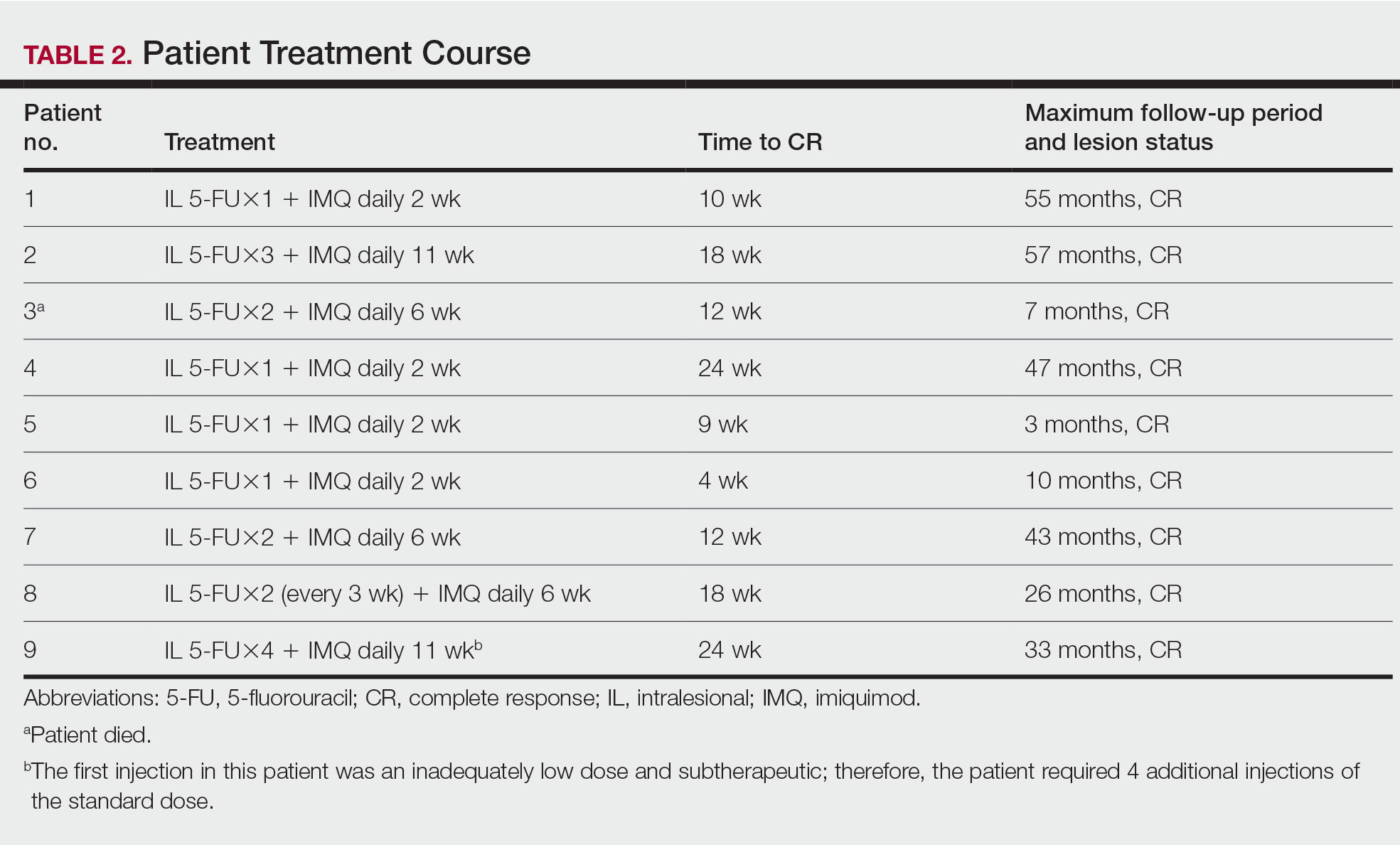
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
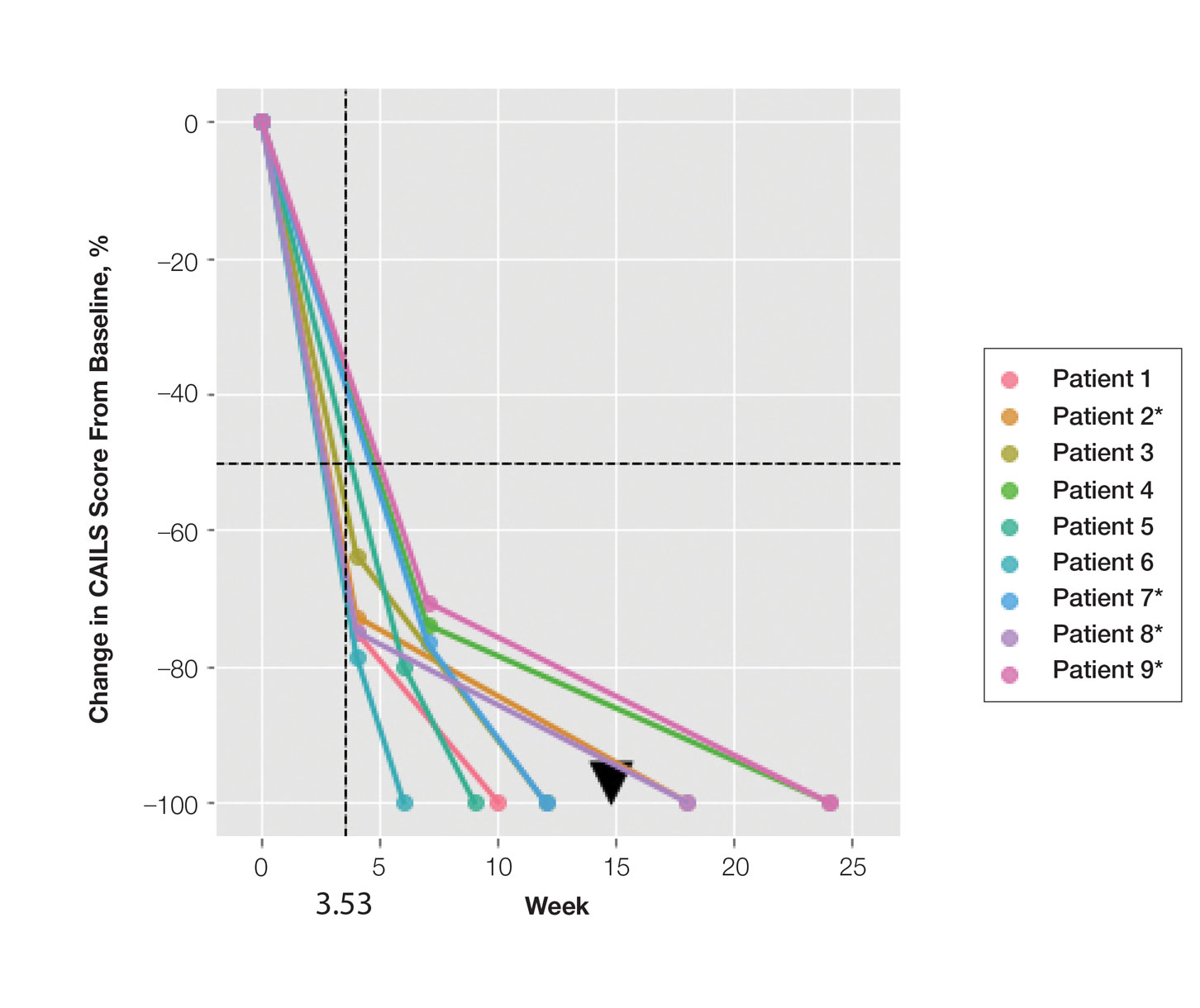
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
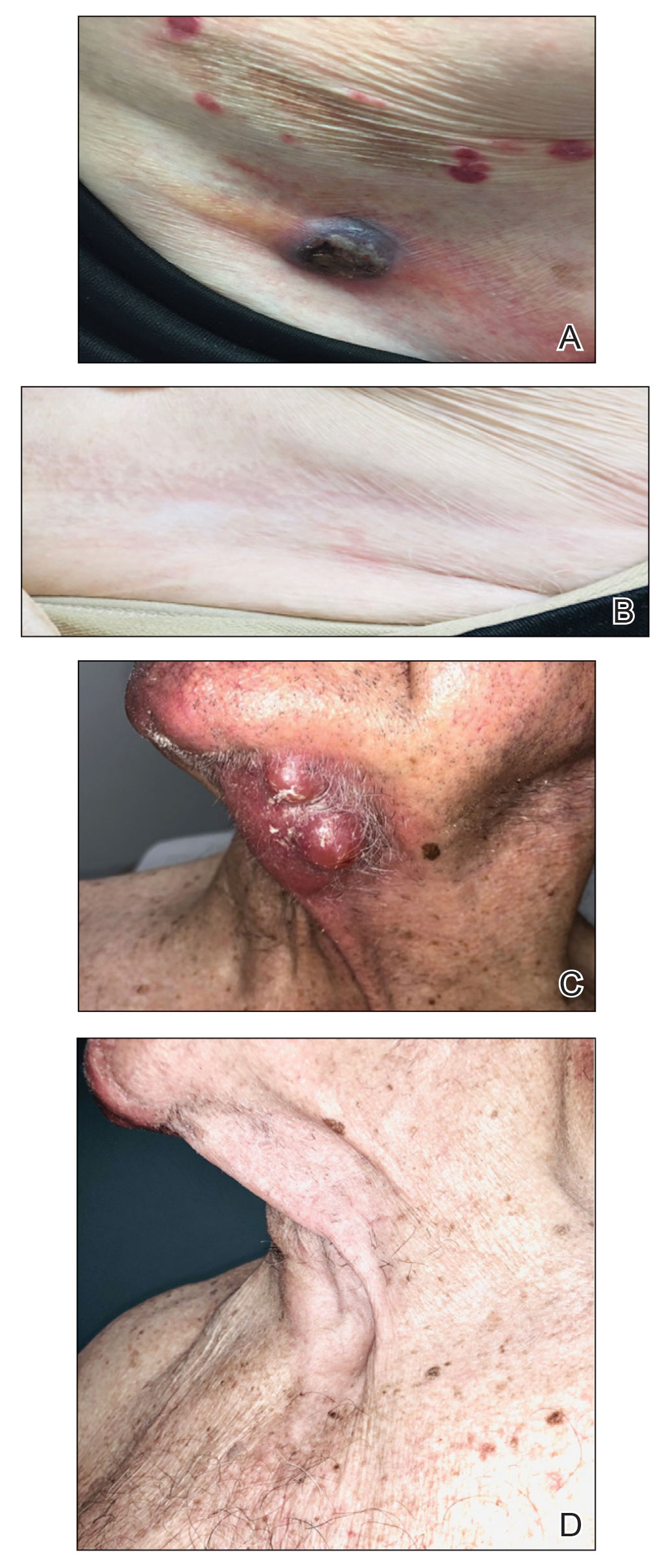
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.