User login
Clinical Impact of Initiation of U-500 Insulin vs Continuation of U-100 Insulin in Subjects With Diabetes
More than 70% of Americans are overweight or obese and 1 in 10 has type 2 diabetes mellitus (T2DM). In the last 20 years, the prevalence of obesity and DM has each increased drastically according to the Centers for Disease Control and Prevention.1,2 Thus, an increase in severe insulin-resistant DM is predicted. Severe insulin resistance occurs when insulin doses exceed 200 units per day or 2 units/kg per day.3-5 Treating this condition demands large volumes of U-100 insulin and a high frequency of injections (usually 4-7 per day), which can lead to reduced patient adherence.8-10 Likewise, large injected volumes are more painful and can lead to altered absorption.3,9-11
U-500 insulin (500 units/mL) is 5 times more concentrated than U-100 insulin and has advantages in the management of severe insulin-resistant DM.11-13 Its pharmacokinetic profile is unique, for the clinical effect can last for up to 24 hours.4-6 U-500 can replace basal-bolus and other complex insulin regimens, offering convenient, effective glycemic control with 2 or 3 injections per day.11,14-20 U-500 can also improve the quality of life and adherence compared with formulations that require more frequent injections.7,14,21 Historically, only exceptional or “special” cases were treated with U-500, but demand for concentrated insulins has increased in the last decade as clinicians adjust their care for this growing patient population.17
The purpose of this study was to determine whether a population of subjects with severe insulin-resistant T2DM would benefit from the use of U-500 vs U-100 insulin regimens. The hypothesis was that this population would obtain equal or better glycemic control while achieving improved adherence. Other studies have demonstrated that U-500 yields improvements in glycemic control but also potentially increases hypoglycemic episodes.15-18,22-24 To our knowledge, this study is the first to evaluate the clinical outcomes of subjects with severe insulin-resistant T2DM who changed from U-100 to U-500 vs subjects who remained on high-dose U-100 insulin.
Methods
This was a single-site, retrospective chart review of subjects with T2DM who attended the endocrinology specialty clinic at the James A. Haley Veterans’ Hospital (JAHVA) in Tampa, Florida, between July 2002 and June 2011. The study included a group of subjects using U-500 insulin and a comparison group using U-100 insulin. The study was approved by the JAHVA Research & Development Committee and by the University of South Florida Institutional Review Board.
Inclusion criteria included diagnosis of T2DM, body mass index (BMI) of more than 30, use of U-500 insulin, or > 200 units daily of U-100 insulin. Exclusion criteria included hypoglycemia unawareness, type 1 DM, and use of an insulin pump. A total of 142 subjects met the inclusion criteria (68 in the U-500 group and 74 in the U-100 group).
All study subjects had at least 1 DM education session. U-500 subjects used insulin vials and 1-mL volumetric hypodermal syringes. All U-500 prescriptions were issued electronically in units and volume (U-500 insulin was available exclusively in vials during the time frame from which data were collected). Subjects in the U-100 group used insulin vials or pen devices. Laboratory studies were processed in house by the institution using high-pressure liquid chromatography to determine hemoglobin A1C (Hb A1C) levels. All study subjects required at least 2 Hb A1C measurements over the observed 12 months for inclusion.
Transition to U-500 Insulin
U-500 transition was considered routinely and presented as an option for patients requiring > 200 units of insulin daily. The transition criteria included adherence to medications, follow-up appointments, and glucose monitoring recommendations, and ability to learn and apply insulin self-adjustment instructions. All subjects were given an additional U-500 insulin education session before transition. The endocrinologist calculated all starting doses by reducing the total daily dose by 20%.
Data Collection
Data were collected using the automatic data mining tools within the JAHVA Computerized Patient Record System and confirmed individually by clinical staff. Demographic data included age, race, and sex. Other parameters were weight; BMI; Hb A1C; estimated glomerular filtration rate (eGFR); duration of DM; use of metformin and other oral agents; total daily insulin dose; number of daily injections; prior history of atherosclerotic cardiovascular disease (ASCVD), including coronary artery disease (CAD), cerebrovascular accident (CVA), or peripheral vascular disease (PVD); occurrence of severe hypoglycemia (symptomatic hypoglycemia requiring treatment assistance from another individual) and of new cardiovascular events, classified as CAD, CVA, or PVD.
For the U-500 group, data were collected and analyzed for the 3 months before (baseline) and the 12 months after the initiation of concentrated insulin. For the U-100 group, data were collected and analyzed for the comparable 3 months before (baseline) and the 12 months after the first clinic visit in which the subject started using more than 200 units per day of U-100. Frequency of follow-up visits was individualized according to clinical needs.
Clinical Endpoints
Primary outcomes included changes in Hb A1C from baseline to the following 12 months, and the occurrence of severe hypoglycemia. Secondary outcomes included the occurrence of new ASCVD events during the study, and changes in weight, BMI, and number of injections.
Statistical Analysis
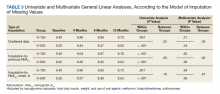
The primary and secondary outcomes were assessed through univariate and multivariate general linear models. Multivariate models were used to compare differences in the variation of Hb A1C over time. Data were incomplete for the Hb A1C in 27 subjects, 6% of the dataset (Each subject had more than one variable or observation). Therefore, a multiple imputation was used to account for the incompleteness on Hb A1C (value substitutions by the mean and by the prior Hb A1C and models were balanced against the unaltered data). A P value of ≤ .05 was used to determine statistical significance. The statistical analyses were performed using IBM SPSS Statistics 21.
Results
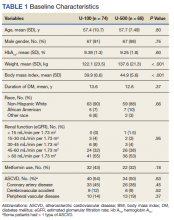
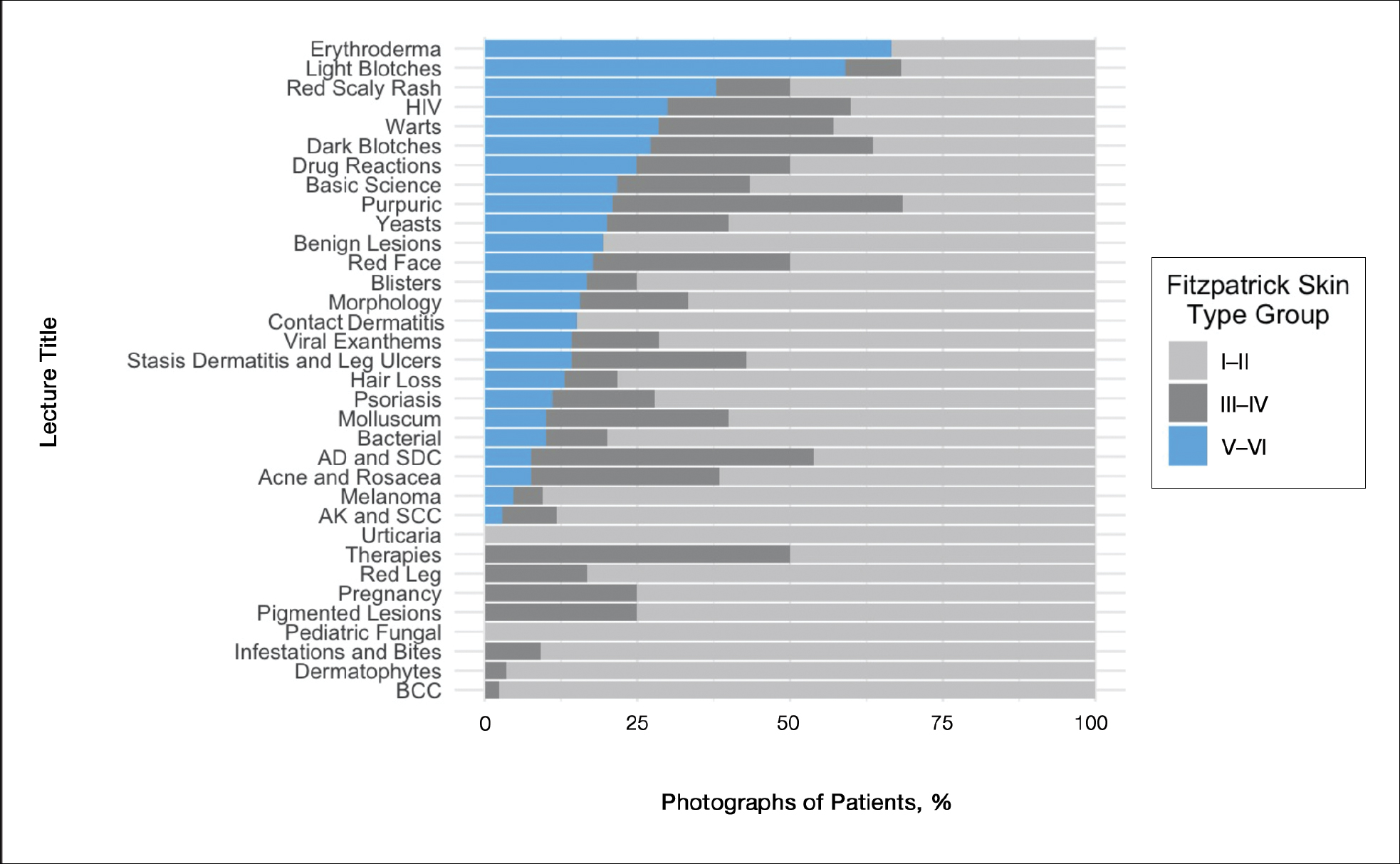
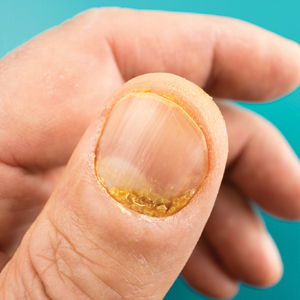
Most patients were male (94%) of white race (86%), with a mean age of 57 years and comparable duration of DM (Table 1). Demographics were balanced between the groups, except for weight and BMI, both higher in the U-500 group (P < .001). Use of oral antidiabetic agents was not significantly different between groups, nor were comorbid conditions, with nearly 50% of subjects in each group affected by CKD and ASCVD, of which CAD was the most common (approximately 40% of both groups). Only about one-third of subjects used metformin and/or other oral agents, likely due to the high prevalence of CKD (contraindicating metformin) and high insulin requirements (due to correlation with β cell failure). A subgroup analysis of subjects on metformin did not demonstrate significant differences in risk of severe hypoglycemia or in Hb A1C levels (data not shown).
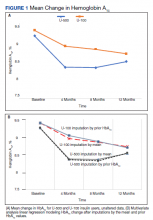
Both groups had similar initial Hb A1C baselines (> 9%) and both improved glycemic control during the study period. However, the Hb A1C reduction was greater in the U-500 group (P= .034), 0.84% vs 0.56% for U-100 and the between-groups difference was 0.4%. (Figure 1, Tables 2 and 3).
The univariate general linear model shows a statistically significant difference in the levels of Hb A1C within each treatment group, regardless of the imputation strategy. However, the differences were not significant when comparing postintervention Hb A1C means between groups with unaltered data (P = .23), because the U-500 group Hb A1C improvement gap narrowed at the end of study. In the multivariate analysis, irrespective of imputation method, the differences in Hb A1C between group treated with U-100 and U-500 were statistically significant (Table 3).
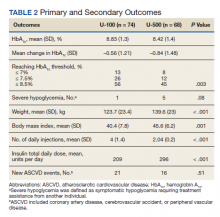
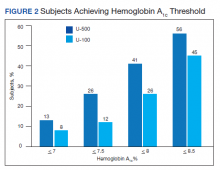
Overall, more subjects in the U-500 group than in the U-100 group achieved Hb A1C levels < 8.5% (56% vs 46%, respectively, P = .003) and the proportion of subjects achieving Hb A1C levels < 7.5% doubled that of the U-100 group (26% vs 12%; Figure 2). Five subjects in the U-500 group experienced severe hypoglycemia, compared with 1 in the U-100 group (P = .08). The total daily insulin dose was significantly higher in the U-500 group (296 units daily) than in the U-100 group (209 units daily) (P < .001) (Table 2). Baseline weight and BMI differences were also significant for the U-500 and U-100 groups (P < .001). Weight gain of approximately 2 kg occurred in both groups, a change that was not statistically significant (P = .79)
There were 21 new ASCVD events in the U-100 and 16 in the U-500 group (P = .51) and there were no statistically significant differences in the incidence of new CAD, PVD or CVA events. The U-500 group required significantly fewer injections than U-100 insulin users (2 vs 4; P < .001).
Discussion
The purpose of the study was to compare subjects with obesity and T2DM using U-500 concentrated insulin with similarly matched subjects using U-100 insulin. Available studies using U-500 insulin, including prospective trials, have reported the experience after transitioning patients who “failed” U-100 regimens.13-16,18,21-24 This failure is a relative and transient condition that, in theory, could be improved with medical intervention and lifestyle changes. Such changes cannot be easily quantified in a clinical trial or retrospective study without a control group. This study was an attempt to fill this knowledge gap.
The U-500 intervention resulted in a 0.8% overall reduction in Hb A1C and a significant 0.4% reduction compared to subjects using U-100. While both groups had improvement in Hb A1C , U-500 was associated with superior reductions in Hb A1C . This finding confirms prior assertions that U-500, compared with U-100, is associated with larger Hb A1C improvement.14-16
The preintervention and postintervention Hb A1C means were > 8% in both groups. This finding suggests that lowering Hb A1C is challenging, similar to published results demonstrating that Hb A1C levels < 7% are achieved by fewer than one-third of U-500 users.16-18 The explanation for this finding remains elusive, due to the methodologic limitations of a retrospective analysis. A possible explanation is the high prevalence of CKD and ASCVD among the study population, conditions which, according to guidelines justify less aggressive glycemic control efforts.25 Multiple prior studies using retrospective data8,13-16 and 2 prospective trials18,22 demonstrated similar Hb A1C reductions after failure of U-100 regimens.
In this study, U-500 resulted in a nominal increase in the risk of severe hypoglycemic episodes. A detailed review of the events found that most of these patients had preestablished CKD and ASCVD, and half of the subjects with sever hypoglycemic episodes had new vascular events during the study (Appendix). These findings suggest a possible correlation between CKD and ASCVD complications and the risk of severe hypoglycemic events. Pharmacokinetic profiles for U-500 have not been studied in subjects with CKD, but the clinical effect of CKD is likely prolonged by the expected reduction in insulin clearance. Similarly, the frailty associated with preexisting ASCVD, or the related polypharmacy, could be factors increasing the risk of hypoglycemia and deserve further study.
Most of the U-500 subjects used it twice daily in this study, which could have contributed to the higher hypoglycemia rate. In a prospective randomized trial Hood and colleagues reported a rate of symptomatic hypoglycemia exceeding 90% in the 2 study groups, and 8 subjects (of 325 total) had severe hypoglycemia during the 6-month observation. The group assigned to 2 daily injections had a significantly higher rate of hypoglycemic events compared with a group that had 3 injections per day.18 Additional studies are required to ascertain whether U-500, compared with specific U-100 regimens (basal-bolus vs premixed; human vs insulin analogs), results in a higher risk of severe hypoglycemia.
This study also investigated the incidence of new cardiovascular events, and no difference was found between the 2 groups. A longer observation would be required to better assess whether U-500 therapy can reduce the incidence of microvascular and macrovascular complications. The similar incidence of complications is further evidence of the similarity between the 2 studied groups. It was also reassuring to find that weight gains were small and nearly identical in both insulin groups.
Strengths and Limitations
This study has several limitations. Data about hospitalizations for congestive heart failure, amputations, progression of diabetic retinopathy, neuropathy, and nephropathy were not collected for this analysis. As both groups of subjects were relatively small, statistical power to assess outcomes is a concern. Retrospective chart reviews may also be affected by incomplete data collections and multiple biases. No data were available for other hypoglycemic episodes, especially to calculate the rate of the more common forms of hypoglycemia. The period of data analyzed spanned only about 15 months. A longer, longitudinal assessment of the differences between these 2 groups may yield more differences, and clearer results and conclusions. Moreover, the data set had aged before publication of this report; however, the authors think that the analysis and information remain highly clinically relevant. The uncommon use of U-500, and prominence as a “special case” insulin may also lead to a detection bias for severe hypoglycemia in the U-500 group. In contrast, lapses in documentation of hypoglycemia in subjects using U-100 could have occurred. Finally, the differences in total daily dose and body weight among groups were significant and may reflect on important physiologic differences between the 2 groups that may affect the reproducibility of our results.
Nevertheless, this study had notable strengths. Comparing U-500 insulin users with similar subjects using U-100 over a period of time provides head-to-head data with potentially important clinical utility. Also, we collected and analyzed a sizable number of clinically important variables, including cardiovascular risk factors, the occurrence of new cardiovascular events, and prevalence of renal disease. The use of linear regression and multivariate analysis using multiple models also strengthened the results. Previous studies compared the outcomes in subjects using U-500 insulin with only their historical selves.8,13-16,18,19,22-25 Therefore, these studies analyzed the data for preconversion and postconversion of U-500 only and consistently favored U-500. This design in a retrospective study cannot eliminate the selection and/or intervention biases, as the subjects of study had inevitably “failed” prior therapies. Similarly, there is no prospective clinical trial data comparing patients on U-500 with patients on high doses of U-100 insulin. Finally, the patients in our study had high rates of comorbidities, which may have increased the applicability of our results to those of “real-life” patients in the community. To our knowledge, no other study has attempted a similar study design approach either prospectively or retrospectively.
Conclusions
In this population of elderly veterans with severely insulin-resistant T2DM, with a high incidence of CKD and ASCVD, U-500 insulin was associated with significantly greater reductions in Hb A1C than U-100 insulin-based regimens, while requiring fewer injections. No difference was noted in the incidence of new ASCVD events. More studies are needed to assess whether U-500 may increase the risk of severe hypoglycemic episodes.
Acknowledgments
The authors recognize the invaluable help provided by the editorial staff of University of South Florida IMpact, the Intramural Review to Support Research and Scientific Publication, and especially to Richard F. Lockey, MD, who has mentored us in this beautiful journey of scientific writing and for his editorial assistance. A portion of this study preliminary data was presented as an abstract at ENDO 2013, The Endocrine Society Annual meeting in San Francisco, CA, June 15-18, 2013.
Appendix. Severe Hypoglycemic Events
Subject 1: U-500 user, 61-year-old African American male. Hypoglycemia occurred during fasting and was associated with a seizure-like event 9 months after transition to concentrated insulin. He was taken by ambulance to a local hospital. No additional data were obtained. Hb A1C was 8.2% in the month before the episode (lowest of the studied period) and increased to 9.1% in the last segment of the study.
Subject 2: U-500 user, 57-year-old white male. The severe hypoglycemic episode occurred approximately 8 months after transition. His Hb A1C was 5.6% around the time of the event, the lowest of the studied period, and increased to 6.8% over the next 4 months. No other data were available.
Subject 3: U-500 user, 67-year-old white male. The event occurred at home in the morning while fasting, 3 months after transition. He was assisted by his family. Hb A1C was 7.1% 10 weeks after the event and was 7% at the end of the studied period. He had a history of CKD and PVD.
Subject 4: U-500 user, 68-year-old white male. He presented with altered consciousness, hypoglycemia, and elevated troponin levels, which was later confirmed as a non-ST elevation myocardial infarction (NSTEMI), 7 months after transition. Hb A1C during the events was 7.1% and was followed by a 9.3% level 9 weeks later. He had history of CKD and PVD.
Subject 5: U-500 user, 67-year-old white man. Hypoglycemia occurred 6 months after transition to U-500. Hb A1C was 8.4% 2 months prior, and was followed by a 7% during the admission for severe hypoglycemia. 3 months later, his HbA1c rose to 8.2%. He had an extensive history of CAD and had a NSTEMI during the study period.
Subject 6: U-100 user, 65-year-old white man. He was found unconscious in the morning while fasting, 6 months after his first clinic visit. He had CKD and advanced ASCVD with prior CAD, PVD, and CVA. He had also had a recent CVA that had affected his movement and cognition.
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief no. 288. Published October 2017. Accessed January 29, 2021. https://www.cdc.gov/nchs/products/databriefs/db288.htm
2. Centers for Disease Control and Prevention. Diabetes and prediabetes: CDC works to prevent type 2 diabetes and improve the health of all people with diabetes. Updated November 30, 2020. Accessed February 17, 2021. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm
3. Cochran E, Gorden P. Use of U-500 insulin in the treatment of severe insulin resistance. Insulin. 2008;3(4):211-218 [Published correction appears in Insulin. 2009;4(1):81]. doi:10.1016/S1557-0843(08)80049-8
4. Shrestha RT, Kumar AF, Taddese A, et al. Duration and onset of action of high dose U-500 regular insulin in severely insulin resistant subjects with type 2 diabetes. Endocrinol Diabetes Metab. 2018;1(4):e00041. Published 2018 Sep 10. doi:10.1002/edm2.41
5. Dailey AM, Tannock LR. Extreme insulin resistance: indications and approaches to the use of U-500 insulin in type 2 diabetes mellitus. Curr Diab Rep. 2011;11(2):77-82. doi:10.1007/s11892-010-0167-6
6. de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects [published correction appears in Diabetes Care. 2014 Aug;37(8):2414]. Diabetes Care. 2011;34(12):2496-2501. doi:10.2337/dc11-0721
7. Brusko C, Jackson JA, de la Peña A. Comparative properties of U-500 and U-100 regular human insulin. Am J Health Syst Pharm. 2013;70(15):1283-1284. doi:10.2146/130117
8. Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88(3):259-264. doi:10.1016/j.diabres.2010.02.012
9. Wysham C, Hood RC, Warren ML, Wang T, Morwick TM, Jackson JA. Effect of total daily dose on efficacy, dosing, and safety of 2 dose titration regimens of human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes. Endocr Pract. 2010;22(6):653-665. doi:10.4158/EP15959.OR
10. Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care. 2010;33(12):2502-2507. doi:10.2337/dc10-1126
11. Schloot NC, Hood RC, Corrigan SM, Panek RL, Heise T. Concentrated insulins in current clinical practice. Diabetes Res Clin Pract. 2019;148:93-101. doi:10.1016/j.diabres.2018.12.007
12. Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin?. Endocr Pract. 2009;15(1):71-79. doi:10.4158/EP.15.1.71
13. Boldo A, Comi RJ. Clinical experience with U500 insulin: risks and benefits. Endocr Pract. 2012;18(1):56-61. doi:10.4158/EP11163.OR
14. Granata JA, Nawarskas AD, Resch ND, Vigil JM. Evaluating the effect of u-500 insulin therapy on glycemic control in veterans with type 2 diabetes. Clin Diabetes. 2015;33(1):14-19. doi:10.2337/diaclin.33.1.14
15. Eby EL, Zagar AJ, Wang P, et al. Healthcare costs and adherence associated with human regular U-500 versus high-dose U-100 insulin in patients with diabetes. Endocr Pract. 2014;20(7):663-670. doi:10.4158/EP13407.OR
16. Eby EL, Curtis BH, Gelwicks SC, et al. Initiation of human regular U-500 insulin use is associated with improved glycemic control: a real-world US cohort study. BMJ Open Diabetes Res Care. 2015;3(1):e000074. Published 2015 Apr 30. doi:10.1136/bmjdrc-2014-000074
17. Jones P, Idris I. The use of U-500 regular insulin in the management of patients with obesity and insulin resistance. Diabetes Obes Metab. 2013;15(10):882-887. doi:10.1111/dom.12094
18. Hood RC, Arakaki RF, Wysham C, Li YG, Settles JA, Jackson JA. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: a randomized, titration-to-target clinical trial. Endocr Pract. 2015;21(7):782-793. doi: 10.4158/EP15612.OR
19. Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients [published correction appears in Diabetes Care. 2007 Feb;30(2):455]. Diabetes Care. 2006;29(11):2504-2505. doi:10.2337/dc06-1478
20. Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2010;33(2):281-283. doi:10.2337/dc09-1490
21. Bulchandani DG, Konrady T, Hamburg MS. Clinical efficacy and patient satisfaction with U-500 insulin pump therapy in patients with type 2 diabetes. Endocr Pract. 2007;13(7):721-725. doi:10.4158/EP.13.7.721
22. Lane WS, Weinrib SL, Rappaport JM, Przestrzelski T. A prospective trial of U500 insulin delivered by Omnipod in patients with type 2 diabetes mellitus and severe insulin resistance [published correction appears in Endocr Pract. 2010 Nov-Dec;16(6):1082]. Endocr Pract. 2010;16(5):778-784. doi:10.4158/EP10014.OR
23. Martin C, Perez-Molinar D, Shah M, Billington C. U500 Disposable Patch Insulin Pump: Results and Discussion of a Veterans Affairs Pilot Study. J Endocr Soc. 2018;2(11):1275-1283. Published 2018 Sep 17. doi:10.1210/js.2018-00198
24. Ziesmer AE, Kelly KC, Guerra PA, George KG, Dunn FL. U500 regular insulin use in insulin-resistant type 2 diabetic veteran patients. Endocr Pract. 2012;18(1):34-38. doi:10.4158/EP11043.OR
25. American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61-S70. doi:10.2337/dc19-S006
More than 70% of Americans are overweight or obese and 1 in 10 has type 2 diabetes mellitus (T2DM). In the last 20 years, the prevalence of obesity and DM has each increased drastically according to the Centers for Disease Control and Prevention.1,2 Thus, an increase in severe insulin-resistant DM is predicted. Severe insulin resistance occurs when insulin doses exceed 200 units per day or 2 units/kg per day.3-5 Treating this condition demands large volumes of U-100 insulin and a high frequency of injections (usually 4-7 per day), which can lead to reduced patient adherence.8-10 Likewise, large injected volumes are more painful and can lead to altered absorption.3,9-11
U-500 insulin (500 units/mL) is 5 times more concentrated than U-100 insulin and has advantages in the management of severe insulin-resistant DM.11-13 Its pharmacokinetic profile is unique, for the clinical effect can last for up to 24 hours.4-6 U-500 can replace basal-bolus and other complex insulin regimens, offering convenient, effective glycemic control with 2 or 3 injections per day.11,14-20 U-500 can also improve the quality of life and adherence compared with formulations that require more frequent injections.7,14,21 Historically, only exceptional or “special” cases were treated with U-500, but demand for concentrated insulins has increased in the last decade as clinicians adjust their care for this growing patient population.17
The purpose of this study was to determine whether a population of subjects with severe insulin-resistant T2DM would benefit from the use of U-500 vs U-100 insulin regimens. The hypothesis was that this population would obtain equal or better glycemic control while achieving improved adherence. Other studies have demonstrated that U-500 yields improvements in glycemic control but also potentially increases hypoglycemic episodes.15-18,22-24 To our knowledge, this study is the first to evaluate the clinical outcomes of subjects with severe insulin-resistant T2DM who changed from U-100 to U-500 vs subjects who remained on high-dose U-100 insulin.
Methods
This was a single-site, retrospective chart review of subjects with T2DM who attended the endocrinology specialty clinic at the James A. Haley Veterans’ Hospital (JAHVA) in Tampa, Florida, between July 2002 and June 2011. The study included a group of subjects using U-500 insulin and a comparison group using U-100 insulin. The study was approved by the JAHVA Research & Development Committee and by the University of South Florida Institutional Review Board.
Inclusion criteria included diagnosis of T2DM, body mass index (BMI) of more than 30, use of U-500 insulin, or > 200 units daily of U-100 insulin. Exclusion criteria included hypoglycemia unawareness, type 1 DM, and use of an insulin pump. A total of 142 subjects met the inclusion criteria (68 in the U-500 group and 74 in the U-100 group).
All study subjects had at least 1 DM education session. U-500 subjects used insulin vials and 1-mL volumetric hypodermal syringes. All U-500 prescriptions were issued electronically in units and volume (U-500 insulin was available exclusively in vials during the time frame from which data were collected). Subjects in the U-100 group used insulin vials or pen devices. Laboratory studies were processed in house by the institution using high-pressure liquid chromatography to determine hemoglobin A1C (Hb A1C) levels. All study subjects required at least 2 Hb A1C measurements over the observed 12 months for inclusion.
Transition to U-500 Insulin
U-500 transition was considered routinely and presented as an option for patients requiring > 200 units of insulin daily. The transition criteria included adherence to medications, follow-up appointments, and glucose monitoring recommendations, and ability to learn and apply insulin self-adjustment instructions. All subjects were given an additional U-500 insulin education session before transition. The endocrinologist calculated all starting doses by reducing the total daily dose by 20%.
Data Collection
Data were collected using the automatic data mining tools within the JAHVA Computerized Patient Record System and confirmed individually by clinical staff. Demographic data included age, race, and sex. Other parameters were weight; BMI; Hb A1C; estimated glomerular filtration rate (eGFR); duration of DM; use of metformin and other oral agents; total daily insulin dose; number of daily injections; prior history of atherosclerotic cardiovascular disease (ASCVD), including coronary artery disease (CAD), cerebrovascular accident (CVA), or peripheral vascular disease (PVD); occurrence of severe hypoglycemia (symptomatic hypoglycemia requiring treatment assistance from another individual) and of new cardiovascular events, classified as CAD, CVA, or PVD.
For the U-500 group, data were collected and analyzed for the 3 months before (baseline) and the 12 months after the initiation of concentrated insulin. For the U-100 group, data were collected and analyzed for the comparable 3 months before (baseline) and the 12 months after the first clinic visit in which the subject started using more than 200 units per day of U-100. Frequency of follow-up visits was individualized according to clinical needs.
Clinical Endpoints
Primary outcomes included changes in Hb A1C from baseline to the following 12 months, and the occurrence of severe hypoglycemia. Secondary outcomes included the occurrence of new ASCVD events during the study, and changes in weight, BMI, and number of injections.
Statistical Analysis
The primary and secondary outcomes were assessed through univariate and multivariate general linear models. Multivariate models were used to compare differences in the variation of Hb A1C over time. Data were incomplete for the Hb A1C in 27 subjects, 6% of the dataset (Each subject had more than one variable or observation). Therefore, a multiple imputation was used to account for the incompleteness on Hb A1C (value substitutions by the mean and by the prior Hb A1C and models were balanced against the unaltered data). A P value of ≤ .05 was used to determine statistical significance. The statistical analyses were performed using IBM SPSS Statistics 21.
Results
Most patients were male (94%) of white race (86%), with a mean age of 57 years and comparable duration of DM (Table 1). Demographics were balanced between the groups, except for weight and BMI, both higher in the U-500 group (P < .001). Use of oral antidiabetic agents was not significantly different between groups, nor were comorbid conditions, with nearly 50% of subjects in each group affected by CKD and ASCVD, of which CAD was the most common (approximately 40% of both groups). Only about one-third of subjects used metformin and/or other oral agents, likely due to the high prevalence of CKD (contraindicating metformin) and high insulin requirements (due to correlation with β cell failure). A subgroup analysis of subjects on metformin did not demonstrate significant differences in risk of severe hypoglycemia or in Hb A1C levels (data not shown).
Both groups had similar initial Hb A1C baselines (> 9%) and both improved glycemic control during the study period. However, the Hb A1C reduction was greater in the U-500 group (P= .034), 0.84% vs 0.56% for U-100 and the between-groups difference was 0.4%. (Figure 1, Tables 2 and 3).
The univariate general linear model shows a statistically significant difference in the levels of Hb A1C within each treatment group, regardless of the imputation strategy. However, the differences were not significant when comparing postintervention Hb A1C means between groups with unaltered data (P = .23), because the U-500 group Hb A1C improvement gap narrowed at the end of study. In the multivariate analysis, irrespective of imputation method, the differences in Hb A1C between group treated with U-100 and U-500 were statistically significant (Table 3).
Overall, more subjects in the U-500 group than in the U-100 group achieved Hb A1C levels < 8.5% (56% vs 46%, respectively, P = .003) and the proportion of subjects achieving Hb A1C levels < 7.5% doubled that of the U-100 group (26% vs 12%; Figure 2). Five subjects in the U-500 group experienced severe hypoglycemia, compared with 1 in the U-100 group (P = .08). The total daily insulin dose was significantly higher in the U-500 group (296 units daily) than in the U-100 group (209 units daily) (P < .001) (Table 2). Baseline weight and BMI differences were also significant for the U-500 and U-100 groups (P < .001). Weight gain of approximately 2 kg occurred in both groups, a change that was not statistically significant (P = .79)
There were 21 new ASCVD events in the U-100 and 16 in the U-500 group (P = .51) and there were no statistically significant differences in the incidence of new CAD, PVD or CVA events. The U-500 group required significantly fewer injections than U-100 insulin users (2 vs 4; P < .001).
Discussion
The purpose of the study was to compare subjects with obesity and T2DM using U-500 concentrated insulin with similarly matched subjects using U-100 insulin. Available studies using U-500 insulin, including prospective trials, have reported the experience after transitioning patients who “failed” U-100 regimens.13-16,18,21-24 This failure is a relative and transient condition that, in theory, could be improved with medical intervention and lifestyle changes. Such changes cannot be easily quantified in a clinical trial or retrospective study without a control group. This study was an attempt to fill this knowledge gap.
The U-500 intervention resulted in a 0.8% overall reduction in Hb A1C and a significant 0.4% reduction compared to subjects using U-100. While both groups had improvement in Hb A1C , U-500 was associated with superior reductions in Hb A1C . This finding confirms prior assertions that U-500, compared with U-100, is associated with larger Hb A1C improvement.14-16
The preintervention and postintervention Hb A1C means were > 8% in both groups. This finding suggests that lowering Hb A1C is challenging, similar to published results demonstrating that Hb A1C levels < 7% are achieved by fewer than one-third of U-500 users.16-18 The explanation for this finding remains elusive, due to the methodologic limitations of a retrospective analysis. A possible explanation is the high prevalence of CKD and ASCVD among the study population, conditions which, according to guidelines justify less aggressive glycemic control efforts.25 Multiple prior studies using retrospective data8,13-16 and 2 prospective trials18,22 demonstrated similar Hb A1C reductions after failure of U-100 regimens.
In this study, U-500 resulted in a nominal increase in the risk of severe hypoglycemic episodes. A detailed review of the events found that most of these patients had preestablished CKD and ASCVD, and half of the subjects with sever hypoglycemic episodes had new vascular events during the study (Appendix). These findings suggest a possible correlation between CKD and ASCVD complications and the risk of severe hypoglycemic events. Pharmacokinetic profiles for U-500 have not been studied in subjects with CKD, but the clinical effect of CKD is likely prolonged by the expected reduction in insulin clearance. Similarly, the frailty associated with preexisting ASCVD, or the related polypharmacy, could be factors increasing the risk of hypoglycemia and deserve further study.
Most of the U-500 subjects used it twice daily in this study, which could have contributed to the higher hypoglycemia rate. In a prospective randomized trial Hood and colleagues reported a rate of symptomatic hypoglycemia exceeding 90% in the 2 study groups, and 8 subjects (of 325 total) had severe hypoglycemia during the 6-month observation. The group assigned to 2 daily injections had a significantly higher rate of hypoglycemic events compared with a group that had 3 injections per day.18 Additional studies are required to ascertain whether U-500, compared with specific U-100 regimens (basal-bolus vs premixed; human vs insulin analogs), results in a higher risk of severe hypoglycemia.
This study also investigated the incidence of new cardiovascular events, and no difference was found between the 2 groups. A longer observation would be required to better assess whether U-500 therapy can reduce the incidence of microvascular and macrovascular complications. The similar incidence of complications is further evidence of the similarity between the 2 studied groups. It was also reassuring to find that weight gains were small and nearly identical in both insulin groups.
Strengths and Limitations
This study has several limitations. Data about hospitalizations for congestive heart failure, amputations, progression of diabetic retinopathy, neuropathy, and nephropathy were not collected for this analysis. As both groups of subjects were relatively small, statistical power to assess outcomes is a concern. Retrospective chart reviews may also be affected by incomplete data collections and multiple biases. No data were available for other hypoglycemic episodes, especially to calculate the rate of the more common forms of hypoglycemia. The period of data analyzed spanned only about 15 months. A longer, longitudinal assessment of the differences between these 2 groups may yield more differences, and clearer results and conclusions. Moreover, the data set had aged before publication of this report; however, the authors think that the analysis and information remain highly clinically relevant. The uncommon use of U-500, and prominence as a “special case” insulin may also lead to a detection bias for severe hypoglycemia in the U-500 group. In contrast, lapses in documentation of hypoglycemia in subjects using U-100 could have occurred. Finally, the differences in total daily dose and body weight among groups were significant and may reflect on important physiologic differences between the 2 groups that may affect the reproducibility of our results.
Nevertheless, this study had notable strengths. Comparing U-500 insulin users with similar subjects using U-100 over a period of time provides head-to-head data with potentially important clinical utility. Also, we collected and analyzed a sizable number of clinically important variables, including cardiovascular risk factors, the occurrence of new cardiovascular events, and prevalence of renal disease. The use of linear regression and multivariate analysis using multiple models also strengthened the results. Previous studies compared the outcomes in subjects using U-500 insulin with only their historical selves.8,13-16,18,19,22-25 Therefore, these studies analyzed the data for preconversion and postconversion of U-500 only and consistently favored U-500. This design in a retrospective study cannot eliminate the selection and/or intervention biases, as the subjects of study had inevitably “failed” prior therapies. Similarly, there is no prospective clinical trial data comparing patients on U-500 with patients on high doses of U-100 insulin. Finally, the patients in our study had high rates of comorbidities, which may have increased the applicability of our results to those of “real-life” patients in the community. To our knowledge, no other study has attempted a similar study design approach either prospectively or retrospectively.
Conclusions
In this population of elderly veterans with severely insulin-resistant T2DM, with a high incidence of CKD and ASCVD, U-500 insulin was associated with significantly greater reductions in Hb A1C than U-100 insulin-based regimens, while requiring fewer injections. No difference was noted in the incidence of new ASCVD events. More studies are needed to assess whether U-500 may increase the risk of severe hypoglycemic episodes.
Acknowledgments
The authors recognize the invaluable help provided by the editorial staff of University of South Florida IMpact, the Intramural Review to Support Research and Scientific Publication, and especially to Richard F. Lockey, MD, who has mentored us in this beautiful journey of scientific writing and for his editorial assistance. A portion of this study preliminary data was presented as an abstract at ENDO 2013, The Endocrine Society Annual meeting in San Francisco, CA, June 15-18, 2013.
Appendix. Severe Hypoglycemic Events
Subject 1: U-500 user, 61-year-old African American male. Hypoglycemia occurred during fasting and was associated with a seizure-like event 9 months after transition to concentrated insulin. He was taken by ambulance to a local hospital. No additional data were obtained. Hb A1C was 8.2% in the month before the episode (lowest of the studied period) and increased to 9.1% in the last segment of the study.
Subject 2: U-500 user, 57-year-old white male. The severe hypoglycemic episode occurred approximately 8 months after transition. His Hb A1C was 5.6% around the time of the event, the lowest of the studied period, and increased to 6.8% over the next 4 months. No other data were available.
Subject 3: U-500 user, 67-year-old white male. The event occurred at home in the morning while fasting, 3 months after transition. He was assisted by his family. Hb A1C was 7.1% 10 weeks after the event and was 7% at the end of the studied period. He had a history of CKD and PVD.
Subject 4: U-500 user, 68-year-old white male. He presented with altered consciousness, hypoglycemia, and elevated troponin levels, which was later confirmed as a non-ST elevation myocardial infarction (NSTEMI), 7 months after transition. Hb A1C during the events was 7.1% and was followed by a 9.3% level 9 weeks later. He had history of CKD and PVD.
Subject 5: U-500 user, 67-year-old white man. Hypoglycemia occurred 6 months after transition to U-500. Hb A1C was 8.4% 2 months prior, and was followed by a 7% during the admission for severe hypoglycemia. 3 months later, his HbA1c rose to 8.2%. He had an extensive history of CAD and had a NSTEMI during the study period.
Subject 6: U-100 user, 65-year-old white man. He was found unconscious in the morning while fasting, 6 months after his first clinic visit. He had CKD and advanced ASCVD with prior CAD, PVD, and CVA. He had also had a recent CVA that had affected his movement and cognition.
More than 70% of Americans are overweight or obese and 1 in 10 has type 2 diabetes mellitus (T2DM). In the last 20 years, the prevalence of obesity and DM has each increased drastically according to the Centers for Disease Control and Prevention.1,2 Thus, an increase in severe insulin-resistant DM is predicted. Severe insulin resistance occurs when insulin doses exceed 200 units per day or 2 units/kg per day.3-5 Treating this condition demands large volumes of U-100 insulin and a high frequency of injections (usually 4-7 per day), which can lead to reduced patient adherence.8-10 Likewise, large injected volumes are more painful and can lead to altered absorption.3,9-11
U-500 insulin (500 units/mL) is 5 times more concentrated than U-100 insulin and has advantages in the management of severe insulin-resistant DM.11-13 Its pharmacokinetic profile is unique, for the clinical effect can last for up to 24 hours.4-6 U-500 can replace basal-bolus and other complex insulin regimens, offering convenient, effective glycemic control with 2 or 3 injections per day.11,14-20 U-500 can also improve the quality of life and adherence compared with formulations that require more frequent injections.7,14,21 Historically, only exceptional or “special” cases were treated with U-500, but demand for concentrated insulins has increased in the last decade as clinicians adjust their care for this growing patient population.17
The purpose of this study was to determine whether a population of subjects with severe insulin-resistant T2DM would benefit from the use of U-500 vs U-100 insulin regimens. The hypothesis was that this population would obtain equal or better glycemic control while achieving improved adherence. Other studies have demonstrated that U-500 yields improvements in glycemic control but also potentially increases hypoglycemic episodes.15-18,22-24 To our knowledge, this study is the first to evaluate the clinical outcomes of subjects with severe insulin-resistant T2DM who changed from U-100 to U-500 vs subjects who remained on high-dose U-100 insulin.
Methods
This was a single-site, retrospective chart review of subjects with T2DM who attended the endocrinology specialty clinic at the James A. Haley Veterans’ Hospital (JAHVA) in Tampa, Florida, between July 2002 and June 2011. The study included a group of subjects using U-500 insulin and a comparison group using U-100 insulin. The study was approved by the JAHVA Research & Development Committee and by the University of South Florida Institutional Review Board.
Inclusion criteria included diagnosis of T2DM, body mass index (BMI) of more than 30, use of U-500 insulin, or > 200 units daily of U-100 insulin. Exclusion criteria included hypoglycemia unawareness, type 1 DM, and use of an insulin pump. A total of 142 subjects met the inclusion criteria (68 in the U-500 group and 74 in the U-100 group).
All study subjects had at least 1 DM education session. U-500 subjects used insulin vials and 1-mL volumetric hypodermal syringes. All U-500 prescriptions were issued electronically in units and volume (U-500 insulin was available exclusively in vials during the time frame from which data were collected). Subjects in the U-100 group used insulin vials or pen devices. Laboratory studies were processed in house by the institution using high-pressure liquid chromatography to determine hemoglobin A1C (Hb A1C) levels. All study subjects required at least 2 Hb A1C measurements over the observed 12 months for inclusion.
Transition to U-500 Insulin
U-500 transition was considered routinely and presented as an option for patients requiring > 200 units of insulin daily. The transition criteria included adherence to medications, follow-up appointments, and glucose monitoring recommendations, and ability to learn and apply insulin self-adjustment instructions. All subjects were given an additional U-500 insulin education session before transition. The endocrinologist calculated all starting doses by reducing the total daily dose by 20%.
Data Collection
Data were collected using the automatic data mining tools within the JAHVA Computerized Patient Record System and confirmed individually by clinical staff. Demographic data included age, race, and sex. Other parameters were weight; BMI; Hb A1C; estimated glomerular filtration rate (eGFR); duration of DM; use of metformin and other oral agents; total daily insulin dose; number of daily injections; prior history of atherosclerotic cardiovascular disease (ASCVD), including coronary artery disease (CAD), cerebrovascular accident (CVA), or peripheral vascular disease (PVD); occurrence of severe hypoglycemia (symptomatic hypoglycemia requiring treatment assistance from another individual) and of new cardiovascular events, classified as CAD, CVA, or PVD.
For the U-500 group, data were collected and analyzed for the 3 months before (baseline) and the 12 months after the initiation of concentrated insulin. For the U-100 group, data were collected and analyzed for the comparable 3 months before (baseline) and the 12 months after the first clinic visit in which the subject started using more than 200 units per day of U-100. Frequency of follow-up visits was individualized according to clinical needs.
Clinical Endpoints
Primary outcomes included changes in Hb A1C from baseline to the following 12 months, and the occurrence of severe hypoglycemia. Secondary outcomes included the occurrence of new ASCVD events during the study, and changes in weight, BMI, and number of injections.
Statistical Analysis
The primary and secondary outcomes were assessed through univariate and multivariate general linear models. Multivariate models were used to compare differences in the variation of Hb A1C over time. Data were incomplete for the Hb A1C in 27 subjects, 6% of the dataset (Each subject had more than one variable or observation). Therefore, a multiple imputation was used to account for the incompleteness on Hb A1C (value substitutions by the mean and by the prior Hb A1C and models were balanced against the unaltered data). A P value of ≤ .05 was used to determine statistical significance. The statistical analyses were performed using IBM SPSS Statistics 21.
Results
Most patients were male (94%) of white race (86%), with a mean age of 57 years and comparable duration of DM (Table 1). Demographics were balanced between the groups, except for weight and BMI, both higher in the U-500 group (P < .001). Use of oral antidiabetic agents was not significantly different between groups, nor were comorbid conditions, with nearly 50% of subjects in each group affected by CKD and ASCVD, of which CAD was the most common (approximately 40% of both groups). Only about one-third of subjects used metformin and/or other oral agents, likely due to the high prevalence of CKD (contraindicating metformin) and high insulin requirements (due to correlation with β cell failure). A subgroup analysis of subjects on metformin did not demonstrate significant differences in risk of severe hypoglycemia or in Hb A1C levels (data not shown).
Both groups had similar initial Hb A1C baselines (> 9%) and both improved glycemic control during the study period. However, the Hb A1C reduction was greater in the U-500 group (P= .034), 0.84% vs 0.56% for U-100 and the between-groups difference was 0.4%. (Figure 1, Tables 2 and 3).
The univariate general linear model shows a statistically significant difference in the levels of Hb A1C within each treatment group, regardless of the imputation strategy. However, the differences were not significant when comparing postintervention Hb A1C means between groups with unaltered data (P = .23), because the U-500 group Hb A1C improvement gap narrowed at the end of study. In the multivariate analysis, irrespective of imputation method, the differences in Hb A1C between group treated with U-100 and U-500 were statistically significant (Table 3).
Overall, more subjects in the U-500 group than in the U-100 group achieved Hb A1C levels < 8.5% (56% vs 46%, respectively, P = .003) and the proportion of subjects achieving Hb A1C levels < 7.5% doubled that of the U-100 group (26% vs 12%; Figure 2). Five subjects in the U-500 group experienced severe hypoglycemia, compared with 1 in the U-100 group (P = .08). The total daily insulin dose was significantly higher in the U-500 group (296 units daily) than in the U-100 group (209 units daily) (P < .001) (Table 2). Baseline weight and BMI differences were also significant for the U-500 and U-100 groups (P < .001). Weight gain of approximately 2 kg occurred in both groups, a change that was not statistically significant (P = .79)
There were 21 new ASCVD events in the U-100 and 16 in the U-500 group (P = .51) and there were no statistically significant differences in the incidence of new CAD, PVD or CVA events. The U-500 group required significantly fewer injections than U-100 insulin users (2 vs 4; P < .001).
Discussion
The purpose of the study was to compare subjects with obesity and T2DM using U-500 concentrated insulin with similarly matched subjects using U-100 insulin. Available studies using U-500 insulin, including prospective trials, have reported the experience after transitioning patients who “failed” U-100 regimens.13-16,18,21-24 This failure is a relative and transient condition that, in theory, could be improved with medical intervention and lifestyle changes. Such changes cannot be easily quantified in a clinical trial or retrospective study without a control group. This study was an attempt to fill this knowledge gap.
The U-500 intervention resulted in a 0.8% overall reduction in Hb A1C and a significant 0.4% reduction compared to subjects using U-100. While both groups had improvement in Hb A1C , U-500 was associated with superior reductions in Hb A1C . This finding confirms prior assertions that U-500, compared with U-100, is associated with larger Hb A1C improvement.14-16
The preintervention and postintervention Hb A1C means were > 8% in both groups. This finding suggests that lowering Hb A1C is challenging, similar to published results demonstrating that Hb A1C levels < 7% are achieved by fewer than one-third of U-500 users.16-18 The explanation for this finding remains elusive, due to the methodologic limitations of a retrospective analysis. A possible explanation is the high prevalence of CKD and ASCVD among the study population, conditions which, according to guidelines justify less aggressive glycemic control efforts.25 Multiple prior studies using retrospective data8,13-16 and 2 prospective trials18,22 demonstrated similar Hb A1C reductions after failure of U-100 regimens.
In this study, U-500 resulted in a nominal increase in the risk of severe hypoglycemic episodes. A detailed review of the events found that most of these patients had preestablished CKD and ASCVD, and half of the subjects with sever hypoglycemic episodes had new vascular events during the study (Appendix). These findings suggest a possible correlation between CKD and ASCVD complications and the risk of severe hypoglycemic events. Pharmacokinetic profiles for U-500 have not been studied in subjects with CKD, but the clinical effect of CKD is likely prolonged by the expected reduction in insulin clearance. Similarly, the frailty associated with preexisting ASCVD, or the related polypharmacy, could be factors increasing the risk of hypoglycemia and deserve further study.
Most of the U-500 subjects used it twice daily in this study, which could have contributed to the higher hypoglycemia rate. In a prospective randomized trial Hood and colleagues reported a rate of symptomatic hypoglycemia exceeding 90% in the 2 study groups, and 8 subjects (of 325 total) had severe hypoglycemia during the 6-month observation. The group assigned to 2 daily injections had a significantly higher rate of hypoglycemic events compared with a group that had 3 injections per day.18 Additional studies are required to ascertain whether U-500, compared with specific U-100 regimens (basal-bolus vs premixed; human vs insulin analogs), results in a higher risk of severe hypoglycemia.
This study also investigated the incidence of new cardiovascular events, and no difference was found between the 2 groups. A longer observation would be required to better assess whether U-500 therapy can reduce the incidence of microvascular and macrovascular complications. The similar incidence of complications is further evidence of the similarity between the 2 studied groups. It was also reassuring to find that weight gains were small and nearly identical in both insulin groups.
Strengths and Limitations
This study has several limitations. Data about hospitalizations for congestive heart failure, amputations, progression of diabetic retinopathy, neuropathy, and nephropathy were not collected for this analysis. As both groups of subjects were relatively small, statistical power to assess outcomes is a concern. Retrospective chart reviews may also be affected by incomplete data collections and multiple biases. No data were available for other hypoglycemic episodes, especially to calculate the rate of the more common forms of hypoglycemia. The period of data analyzed spanned only about 15 months. A longer, longitudinal assessment of the differences between these 2 groups may yield more differences, and clearer results and conclusions. Moreover, the data set had aged before publication of this report; however, the authors think that the analysis and information remain highly clinically relevant. The uncommon use of U-500, and prominence as a “special case” insulin may also lead to a detection bias for severe hypoglycemia in the U-500 group. In contrast, lapses in documentation of hypoglycemia in subjects using U-100 could have occurred. Finally, the differences in total daily dose and body weight among groups were significant and may reflect on important physiologic differences between the 2 groups that may affect the reproducibility of our results.
Nevertheless, this study had notable strengths. Comparing U-500 insulin users with similar subjects using U-100 over a period of time provides head-to-head data with potentially important clinical utility. Also, we collected and analyzed a sizable number of clinically important variables, including cardiovascular risk factors, the occurrence of new cardiovascular events, and prevalence of renal disease. The use of linear regression and multivariate analysis using multiple models also strengthened the results. Previous studies compared the outcomes in subjects using U-500 insulin with only their historical selves.8,13-16,18,19,22-25 Therefore, these studies analyzed the data for preconversion and postconversion of U-500 only and consistently favored U-500. This design in a retrospective study cannot eliminate the selection and/or intervention biases, as the subjects of study had inevitably “failed” prior therapies. Similarly, there is no prospective clinical trial data comparing patients on U-500 with patients on high doses of U-100 insulin. Finally, the patients in our study had high rates of comorbidities, which may have increased the applicability of our results to those of “real-life” patients in the community. To our knowledge, no other study has attempted a similar study design approach either prospectively or retrospectively.
Conclusions
In this population of elderly veterans with severely insulin-resistant T2DM, with a high incidence of CKD and ASCVD, U-500 insulin was associated with significantly greater reductions in Hb A1C than U-100 insulin-based regimens, while requiring fewer injections. No difference was noted in the incidence of new ASCVD events. More studies are needed to assess whether U-500 may increase the risk of severe hypoglycemic episodes.
Acknowledgments
The authors recognize the invaluable help provided by the editorial staff of University of South Florida IMpact, the Intramural Review to Support Research and Scientific Publication, and especially to Richard F. Lockey, MD, who has mentored us in this beautiful journey of scientific writing and for his editorial assistance. A portion of this study preliminary data was presented as an abstract at ENDO 2013, The Endocrine Society Annual meeting in San Francisco, CA, June 15-18, 2013.
Appendix. Severe Hypoglycemic Events
Subject 1: U-500 user, 61-year-old African American male. Hypoglycemia occurred during fasting and was associated with a seizure-like event 9 months after transition to concentrated insulin. He was taken by ambulance to a local hospital. No additional data were obtained. Hb A1C was 8.2% in the month before the episode (lowest of the studied period) and increased to 9.1% in the last segment of the study.
Subject 2: U-500 user, 57-year-old white male. The severe hypoglycemic episode occurred approximately 8 months after transition. His Hb A1C was 5.6% around the time of the event, the lowest of the studied period, and increased to 6.8% over the next 4 months. No other data were available.
Subject 3: U-500 user, 67-year-old white male. The event occurred at home in the morning while fasting, 3 months after transition. He was assisted by his family. Hb A1C was 7.1% 10 weeks after the event and was 7% at the end of the studied period. He had a history of CKD and PVD.
Subject 4: U-500 user, 68-year-old white male. He presented with altered consciousness, hypoglycemia, and elevated troponin levels, which was later confirmed as a non-ST elevation myocardial infarction (NSTEMI), 7 months after transition. Hb A1C during the events was 7.1% and was followed by a 9.3% level 9 weeks later. He had history of CKD and PVD.
Subject 5: U-500 user, 67-year-old white man. Hypoglycemia occurred 6 months after transition to U-500. Hb A1C was 8.4% 2 months prior, and was followed by a 7% during the admission for severe hypoglycemia. 3 months later, his HbA1c rose to 8.2%. He had an extensive history of CAD and had a NSTEMI during the study period.
Subject 6: U-100 user, 65-year-old white man. He was found unconscious in the morning while fasting, 6 months after his first clinic visit. He had CKD and advanced ASCVD with prior CAD, PVD, and CVA. He had also had a recent CVA that had affected his movement and cognition.
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief no. 288. Published October 2017. Accessed January 29, 2021. https://www.cdc.gov/nchs/products/databriefs/db288.htm
2. Centers for Disease Control and Prevention. Diabetes and prediabetes: CDC works to prevent type 2 diabetes and improve the health of all people with diabetes. Updated November 30, 2020. Accessed February 17, 2021. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm
3. Cochran E, Gorden P. Use of U-500 insulin in the treatment of severe insulin resistance. Insulin. 2008;3(4):211-218 [Published correction appears in Insulin. 2009;4(1):81]. doi:10.1016/S1557-0843(08)80049-8
4. Shrestha RT, Kumar AF, Taddese A, et al. Duration and onset of action of high dose U-500 regular insulin in severely insulin resistant subjects with type 2 diabetes. Endocrinol Diabetes Metab. 2018;1(4):e00041. Published 2018 Sep 10. doi:10.1002/edm2.41
5. Dailey AM, Tannock LR. Extreme insulin resistance: indications and approaches to the use of U-500 insulin in type 2 diabetes mellitus. Curr Diab Rep. 2011;11(2):77-82. doi:10.1007/s11892-010-0167-6
6. de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects [published correction appears in Diabetes Care. 2014 Aug;37(8):2414]. Diabetes Care. 2011;34(12):2496-2501. doi:10.2337/dc11-0721
7. Brusko C, Jackson JA, de la Peña A. Comparative properties of U-500 and U-100 regular human insulin. Am J Health Syst Pharm. 2013;70(15):1283-1284. doi:10.2146/130117
8. Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88(3):259-264. doi:10.1016/j.diabres.2010.02.012
9. Wysham C, Hood RC, Warren ML, Wang T, Morwick TM, Jackson JA. Effect of total daily dose on efficacy, dosing, and safety of 2 dose titration regimens of human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes. Endocr Pract. 2010;22(6):653-665. doi:10.4158/EP15959.OR
10. Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care. 2010;33(12):2502-2507. doi:10.2337/dc10-1126
11. Schloot NC, Hood RC, Corrigan SM, Panek RL, Heise T. Concentrated insulins in current clinical practice. Diabetes Res Clin Pract. 2019;148:93-101. doi:10.1016/j.diabres.2018.12.007
12. Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin?. Endocr Pract. 2009;15(1):71-79. doi:10.4158/EP.15.1.71
13. Boldo A, Comi RJ. Clinical experience with U500 insulin: risks and benefits. Endocr Pract. 2012;18(1):56-61. doi:10.4158/EP11163.OR
14. Granata JA, Nawarskas AD, Resch ND, Vigil JM. Evaluating the effect of u-500 insulin therapy on glycemic control in veterans with type 2 diabetes. Clin Diabetes. 2015;33(1):14-19. doi:10.2337/diaclin.33.1.14
15. Eby EL, Zagar AJ, Wang P, et al. Healthcare costs and adherence associated with human regular U-500 versus high-dose U-100 insulin in patients with diabetes. Endocr Pract. 2014;20(7):663-670. doi:10.4158/EP13407.OR
16. Eby EL, Curtis BH, Gelwicks SC, et al. Initiation of human regular U-500 insulin use is associated with improved glycemic control: a real-world US cohort study. BMJ Open Diabetes Res Care. 2015;3(1):e000074. Published 2015 Apr 30. doi:10.1136/bmjdrc-2014-000074
17. Jones P, Idris I. The use of U-500 regular insulin in the management of patients with obesity and insulin resistance. Diabetes Obes Metab. 2013;15(10):882-887. doi:10.1111/dom.12094
18. Hood RC, Arakaki RF, Wysham C, Li YG, Settles JA, Jackson JA. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: a randomized, titration-to-target clinical trial. Endocr Pract. 2015;21(7):782-793. doi: 10.4158/EP15612.OR
19. Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients [published correction appears in Diabetes Care. 2007 Feb;30(2):455]. Diabetes Care. 2006;29(11):2504-2505. doi:10.2337/dc06-1478
20. Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2010;33(2):281-283. doi:10.2337/dc09-1490
21. Bulchandani DG, Konrady T, Hamburg MS. Clinical efficacy and patient satisfaction with U-500 insulin pump therapy in patients with type 2 diabetes. Endocr Pract. 2007;13(7):721-725. doi:10.4158/EP.13.7.721
22. Lane WS, Weinrib SL, Rappaport JM, Przestrzelski T. A prospective trial of U500 insulin delivered by Omnipod in patients with type 2 diabetes mellitus and severe insulin resistance [published correction appears in Endocr Pract. 2010 Nov-Dec;16(6):1082]. Endocr Pract. 2010;16(5):778-784. doi:10.4158/EP10014.OR
23. Martin C, Perez-Molinar D, Shah M, Billington C. U500 Disposable Patch Insulin Pump: Results and Discussion of a Veterans Affairs Pilot Study. J Endocr Soc. 2018;2(11):1275-1283. Published 2018 Sep 17. doi:10.1210/js.2018-00198
24. Ziesmer AE, Kelly KC, Guerra PA, George KG, Dunn FL. U500 regular insulin use in insulin-resistant type 2 diabetic veteran patients. Endocr Pract. 2012;18(1):34-38. doi:10.4158/EP11043.OR
25. American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61-S70. doi:10.2337/dc19-S006
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief no. 288. Published October 2017. Accessed January 29, 2021. https://www.cdc.gov/nchs/products/databriefs/db288.htm
2. Centers for Disease Control and Prevention. Diabetes and prediabetes: CDC works to prevent type 2 diabetes and improve the health of all people with diabetes. Updated November 30, 2020. Accessed February 17, 2021. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm
3. Cochran E, Gorden P. Use of U-500 insulin in the treatment of severe insulin resistance. Insulin. 2008;3(4):211-218 [Published correction appears in Insulin. 2009;4(1):81]. doi:10.1016/S1557-0843(08)80049-8
4. Shrestha RT, Kumar AF, Taddese A, et al. Duration and onset of action of high dose U-500 regular insulin in severely insulin resistant subjects with type 2 diabetes. Endocrinol Diabetes Metab. 2018;1(4):e00041. Published 2018 Sep 10. doi:10.1002/edm2.41
5. Dailey AM, Tannock LR. Extreme insulin resistance: indications and approaches to the use of U-500 insulin in type 2 diabetes mellitus. Curr Diab Rep. 2011;11(2):77-82. doi:10.1007/s11892-010-0167-6
6. de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects [published correction appears in Diabetes Care. 2014 Aug;37(8):2414]. Diabetes Care. 2011;34(12):2496-2501. doi:10.2337/dc11-0721
7. Brusko C, Jackson JA, de la Peña A. Comparative properties of U-500 and U-100 regular human insulin. Am J Health Syst Pharm. 2013;70(15):1283-1284. doi:10.2146/130117
8. Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88(3):259-264. doi:10.1016/j.diabres.2010.02.012
9. Wysham C, Hood RC, Warren ML, Wang T, Morwick TM, Jackson JA. Effect of total daily dose on efficacy, dosing, and safety of 2 dose titration regimens of human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes. Endocr Pract. 2010;22(6):653-665. doi:10.4158/EP15959.OR
10. Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care. 2010;33(12):2502-2507. doi:10.2337/dc10-1126
11. Schloot NC, Hood RC, Corrigan SM, Panek RL, Heise T. Concentrated insulins in current clinical practice. Diabetes Res Clin Pract. 2019;148:93-101. doi:10.1016/j.diabres.2018.12.007
12. Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin?. Endocr Pract. 2009;15(1):71-79. doi:10.4158/EP.15.1.71
13. Boldo A, Comi RJ. Clinical experience with U500 insulin: risks and benefits. Endocr Pract. 2012;18(1):56-61. doi:10.4158/EP11163.OR
14. Granata JA, Nawarskas AD, Resch ND, Vigil JM. Evaluating the effect of u-500 insulin therapy on glycemic control in veterans with type 2 diabetes. Clin Diabetes. 2015;33(1):14-19. doi:10.2337/diaclin.33.1.14
15. Eby EL, Zagar AJ, Wang P, et al. Healthcare costs and adherence associated with human regular U-500 versus high-dose U-100 insulin in patients with diabetes. Endocr Pract. 2014;20(7):663-670. doi:10.4158/EP13407.OR
16. Eby EL, Curtis BH, Gelwicks SC, et al. Initiation of human regular U-500 insulin use is associated with improved glycemic control: a real-world US cohort study. BMJ Open Diabetes Res Care. 2015;3(1):e000074. Published 2015 Apr 30. doi:10.1136/bmjdrc-2014-000074
17. Jones P, Idris I. The use of U-500 regular insulin in the management of patients with obesity and insulin resistance. Diabetes Obes Metab. 2013;15(10):882-887. doi:10.1111/dom.12094
18. Hood RC, Arakaki RF, Wysham C, Li YG, Settles JA, Jackson JA. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: a randomized, titration-to-target clinical trial. Endocr Pract. 2015;21(7):782-793. doi: 10.4158/EP15612.OR
19. Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients [published correction appears in Diabetes Care. 2007 Feb;30(2):455]. Diabetes Care. 2006;29(11):2504-2505. doi:10.2337/dc06-1478
20. Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2010;33(2):281-283. doi:10.2337/dc09-1490
21. Bulchandani DG, Konrady T, Hamburg MS. Clinical efficacy and patient satisfaction with U-500 insulin pump therapy in patients with type 2 diabetes. Endocr Pract. 2007;13(7):721-725. doi:10.4158/EP.13.7.721
22. Lane WS, Weinrib SL, Rappaport JM, Przestrzelski T. A prospective trial of U500 insulin delivered by Omnipod in patients with type 2 diabetes mellitus and severe insulin resistance [published correction appears in Endocr Pract. 2010 Nov-Dec;16(6):1082]. Endocr Pract. 2010;16(5):778-784. doi:10.4158/EP10014.OR
23. Martin C, Perez-Molinar D, Shah M, Billington C. U500 Disposable Patch Insulin Pump: Results and Discussion of a Veterans Affairs Pilot Study. J Endocr Soc. 2018;2(11):1275-1283. Published 2018 Sep 17. doi:10.1210/js.2018-00198
24. Ziesmer AE, Kelly KC, Guerra PA, George KG, Dunn FL. U500 regular insulin use in insulin-resistant type 2 diabetic veteran patients. Endocr Pract. 2012;18(1):34-38. doi:10.4158/EP11043.OR
25. American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61-S70. doi:10.2337/dc19-S006
Who Receives Care in VA Medical Foster Homes?
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
New models are needed for delivering long-term care (LTC) that are home-based, cost-effective, and appropriate for older adults with a range of care needs.1,2 In fiscal year (FY) 2015, the US Department of Veterans Affairs (VA) spent $7.4 billion on LTC, accounting for 13% of total VA health care spending. Overall, 71% of LTC spending in FY 2015 was allocated to institutional care.3 Beyond cost, 95% of older adults prefer to remain in community rather than institutional LTC settings, such as nursing homes.4 The COVID-19 pandemic created additional concerns related to the spread of infectious disease, with > 37% of COVID-19 deaths in the United States occurring in nursing homes irrespective of facility quality.5,6
One community-based LTC alternative developed within the VA is the Medical Foster Home (MFH) program. The MFH program is an adult foster care program in which veterans who are unable to live independently receive round-the-clock care in the home of a community-based caregiver.7 MFH caregivers usually have previous experience caring for family, working in a nursing home, or working as a caregiver in another capacity. These caregivers are responsible for providing 24-hour supervision and support to residents in their MFH and can care for up to 3 adults. In the MFH program, VA home-based primary care (HBPC) teams composed of physicians, registered nurses, physical and occupational therapists, social workers, pharmacists, dieticians, and psychologists, provide primary care for MFH veterans and oversee care in the caregiver’s home.
The goal of the VA HBPC program is to improve veterans’ access to medical care and shift LTC services from institutional to noninstitutional settings by providing in-home care for those who are too sick or disabled to go to a clinic for care. On average, veterans pay the MFH caregiver $2,500 out-of-pocket per month for their care.8 In 2016, there were 992 veterans residing in MFHs across the country.9 Since MFH program implementation expanded nationwide in 2008, more than 4,000 veterans have resided in MFHs in 45 states and territories.10
The VA is required to pay for nursing home care for veterans who have a qualifying VA service-connected disability or who meet a specific threshold of disability.11 Currently, the VA is not authorized to pay for MFH care for veterans who meet the eligibility criteria for VA-paid nursing home care. Over the past decade, the VA has introduced and expanded several initiatives and programs to help veterans who require LTC remain in their homes and communities. These include but are not limited to the Veteran Directed Care program, the Choose Home Initiative, and the Caregiver Support Program.12-14 Additionally, attempts have been made to pass legislation to authorize the VA to pay for MFH for veterans’ care whose military benefits include coverage for nursing home care.15 This legislation and VA initiatives are clear signs that the VA is committed to supporting programs such as the MFH program. Given this commitment, demand for the MFH program will likely increase.
Therefore, VA practitioners need to better identify which veterans are currently in the MFH program. While veterans are expected to need nursing home level care to qualify for MFH enrollment, little has been published about the physical and mental health care needs of veterans currently receiving MFH care. One previous study compared the demographics, diagnostic characteristics, and care utilization of MFH veterans with that of veterans receiving LTC in VA community living centers (CLCs), and found that veterans in MFHs had similar levels of frailty and comorbidity and had a higher mean age when compared with veterans in CLCs.16
Our study assessed a sample of veterans living in MFHs and describes these veterans’ clinical and functional characteristics. We used the Minimum Data Set 3.0 (MDS) to complete the assessments to allow comparisons with other populations residing in long-term care.17,18 While MDS assessments are required for Medicare/Medicaid-certified nursing home residents and for residents in VA CLCs, this study was the first attempt to perform in-home MDS data assessments in MFHs. This collection of descriptive clinical data is an important first step in providing VA practitioners with information about the characteristics of veterans currently cared for in MFHs and policymakers with data to think critically about which veterans are willing to pay for the MFH program.
Methods
This study was part of a larger research project assessing the impact of the MFH program on veterans’ outcomes and health care spending as well as factors influencing program growth.7,9,10,16,19-23 We report on the characteristics of veterans staying in MFHs, using data from the MDS, including a clinical assessment of patients’ cognitive, function, and health care–related needs, collected from participants recruited for this study.
Five research nurses were trained to administer the MDS assessment to veterans in MFHs. Data were collected between April 2014 and December 2015 from veterans at MFH sites associated with 4 urban VA medical centers in 4 different Veterans Integrated Service Networks (58 total homes). While the VA medical centers (VAMCs)were urban, many of the MFHs were in rural areas, given that MFHs can be up to 50 miles from the associated VAMC. We selected MFH sites for this study based on MFH program veteran census. Specifically, we identified MFH sites with high veteran enrollment to ensure we would have a sufficiently large sample for participant recruitment.
Veterans who had resided in an MFH for at least 90 days were eligible to participate. Of the 155 veterans mailed a letter of invitation to participate, 92 (59%) completed the in-home MDS assessment. Reasons for not participating included: 13 veterans died prior to data collection, 18 veterans declined to participate, 18 family members or legal guardians of cognitively impaired veterans did not want the veteran to participate, and 14 veterans left the MFH program or were hospitalized at the time of data collection.
Family members and legal guardians who declined participation on behalf of a veteran reported that they felt the veteran was too frail to participate or that participating would be an added burden on the veteran. Based on the census of veterans residing in all MFHs nationally in November 2015 (N = 972), 9.5% of MFH veterans were included in this study.7This study was approved by the VA Central Institutional Review Board (CIRB #12–31), in addition to the local VA research and development review boards where MFH MDS assessments were collected.
Assessment Instrument and Variables
The MDS 3.0 assesses numerous aspects of clinical and functional status. Several resident-level characteristics from the MDS 3.0 were included in this study. The Cognitive Function Scale (CFS) was used to categorize cognitive function. The CFS is a categorical variable that is created from MDS 3.0 data. The CFS integrates self- and staff-reported data to classify individuals as cognitively intact, mildly impaired, moderately impaired, or severely impaired based on respondents’ Brief Interview for Mental Status (BIMS) assessment or staff-reported cognitive function collected as part of the MDS 3.0.24 We explored depression by calculating a mean summary severity score for all respondents from the Patient Health Questionnaire-9 item interview (PHQ-9).25 PHQ-9 summary scores range from 0 to 27, with mean scores of ≤ 4 indicating no or minimal depression, and higher scores corresponding to more severe depression as scores increase. For respondents who were unable to complete the PHQ-9, we calculated mean PHQ Observational Version (PHQ-9-OV) scores.
We included 2 variables to characterize behaviors: wandering frequency and presence and frequency of aggressive behaviors. We summarized aggressive behaviors using the Aggressive and Reactive Behavior Scale, which characterizes whether a resident has none, mild, moderate, or severe behavioral symptoms based on the presence and frequency of physical and verbal behaviors and resistance to care.26,27 We included items that described pain, number of falls since admission or prior assessment, degree of urinary and bowel continence (always continent vs not always continent) and mobility device use to describe respondents’ health conditions and functional status. To characterize pain, we used veteran’s self-reported frequency and intensity of pain experienced in the prior 5 days and classified the experienced pain as none, mild, moderate, or severe. Finally, demographic characteristics included age and gender.
To determine functional status, we included measures of needing help to perform activities of daily living (ADLs). The MDS allows us to understand functional status ranging from ADLs lost early in the trajectory of functional decline (ie, bathing, hygiene) to those lost in the middle (ie, walking, dressing, toileting, transferring) to those lost late in the trajectory of functional decline (ie, bed mobility and eating).28,29 To assess MFH veterans’ independence in mobility, we considered the veteran’s ability to walk without supervision or assistance in the hallway outside of their room, ability to move between their room and hallway, and ability to move throughout the house. Mobility includes use of an assistive device such as a cane, walker, or wheelchair if the veteran can use it without assistance. We summarized dependency in ADLs, using a combined score of dependence in bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene that ranges from 0 (independent) to 28 (completely dependent).30 Additionally, we created 3-category variables to indicate the degree of dependence in performing ADLs (independent, supervision or assistance, and completely dependent).
Finally, we included diagnoses identified as active to explore differences in neurologic, mood, psychiatric, and chronic disease morbidity. In the MDS 3.0 assessment, an active diagnosis is defined as a diagnosis documented by a licensed independent practitioner in the prior 60 days that has affected the resident or their care in the prior 7 days.
Analysis
We conducted statistical analyses using Stata MP version 15.1 (StataCorp). We summarized demographic characteristics, cognitive function scores, depression scores, pain status, behavioral symptoms, incidence of falls, degree of continence, functional status, and comorbidities, using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Results
Of the 92 MFH veterans in our sample, 85% were male and 83% were aged ≥ 65 years (Table 1). Veterans had an average length of stay of 927 days at the time of MDS assessment. More than half (55%) of MFH veterans had cognitive impairment (ranging from mild to severe). The mean (SD) depression score was 3.3 (3.9), indicating minimal depression. For veterans who could not complete the depression questionnaire, the mean (SD) staff-assessed depression score was 5.9 (5.5), suggesting mild depression. Overall, 22% of the sample had aggressive behaviors but only 7 were noted to be severe. Few residents had caregiver-reported wandering. Self-reported pain intensity indicated that 45% of the sample had mild, moderate, or severe pain. While more than half the cohort had complete bowel continence (53%), only 36% had complete urinary continence. Use of mobility devices was common, with 56% of residents using a wheelchair, 42% using a walker, and 14% using a cane. One-fourth of veterans had fallen at least once since admission to the MFH.
Of the 11 ADLs assessed, the percentage of MFH veterans requiring assistance with early and mid-loss ADLs ranged from 63% for transferring to 84% for bathing (Table 2). Even for the late-loss ADL of eating, 57% of the MFH cohort required assistance. Overall, MFH veterans had an average ADL dependency score of 11.
Physicians documented a diagnosis of either Alzheimer disease or non-Alzheimer dementia comorbidity for 65% of the cohort and traumatic brain injury for 9% (Table 3). Based on psychiatric comorbidities recorded in veterans’ health records, over half of MFH residents had depression (52%). Additionally, 1 in 5 MFH veterans had an anxiety disorder diagnosis. Chronic diseases were prevalent among veterans in MFHs, with 33% diagnosed with diabetes mellitus, 30% with asthma, chronic obstructive pulmonary disease, or chronic lung disease, and 16% with heart failure.
Discussion
In this study, we describe the characteristics of veterans receiving LTC in a sample of MFHs. This is the first study to assess veteran health and function across a group of MFHs. To help provide context for the description of MFH residents, we compared demographic characteristics, cognitive impairment, depression, pain, behaviors, functional status, and morbidity of veterans in the MFH program to long-stay residents in community nursing homes (eAppendix 1-3 available at doi:10.12788/fp.0102). A comparison with this reference population suggests that these MFH and nursing home cohorts are similar in terms of age, wandering behavior, incidence of falls, and prevalence of neurologic, psychiatric, and chronic diseases. Compared with nursing home residents, veterans in the MFH cohort had slightly higher mood symptom scores, were more likely to display aggressive behavior, and were more likely to report experiencing moderate and severe pain.
Additionally, MFH veterans displayed a lower level of cognitive impairment, fewer functional impairments, measured by the ADL dependency score, and were less likely to be bowel or bladder incontinent. Despite an overall lower ADL dependency score, a similar proportion of MFH veterans and nursing home residents were totally dependent in performing 7 of 11 ADLs and a higher proportion of MFH veterans were completely dependent for toileting (22% long-stay nursing home vs 31% MFH). The only ADLs for which there was a higher proportion of long-stay nursing home residents who were totally dependent compared with MFH residents were walking in room (54% long-stay nursing home vs 38% MFH), walking in the corridor (57% long-stay nursing home vs 33% MFH), and locomotion off the unit (36% long-stay nursing home vs 22% MFH).
While the rates of total ADL dependence among veterans in MFHs suggest that MFHs are providing care to a subset of veterans with high levels of functional impairment and care needs, MFHs are also providing care to veterans who are more independent in performing ADLs and who resemble low-care nursing home residents. A low-care nursing home resident is broadly defined as an one who does not need assistance performing late-loss ADLs (bed mobility, transferring, toileting, and eating) and who does not have the Resource Utilization Group classification of special rehab or clinically complex.31,32 Due to their overall higher functional capacity, low-care residents, even those with chronic medical care needs, may be more appropriately cared for in less intensive care settings than in nursing homes. About 5% to 30% of long-stay nursing home residents can be classified as low care.31,33-37 Additionally, a majority of newly admitted nursing home patients report a preference for or support community discharge rather than long-stay nursing home care, suggesting that many nursing home residents have the potential and desire to transition to a community-based setting.33
Based on the prevalence of veterans in our sample who are similar to low-care nursing home residents and the national focus on shifting LTC to community-based settings, MFHs may be an ideal setting for both low-care nursing home residents and those seeking community-based alternatives to traditional, institutionalized LTC. Additionally, given that we observed greater behavioral and pain needs and similar rates of comorbidities in MFH veterans relative to long-stay nursing home residents, our results indicate that MFHs also have the capacity to care for veterans with higher care needs who desire community-based LTC.
Previous research identified barriers to program MFH growth that may contribute to referral of veterans with fewer ADL dependencies compared with long-stay nursing home residents. A key barrier to MFH referral is that nursing home referral requires selection of a home, whereas MFH referral involves matching veterans with appropriate caregivers, which requires time to align the veteran’s needs with the right caregiver in the right home.7 Given the rigors of finding a match, VA staff who refer veterans may preferentially refer veterans with greater ADL impairments to nursing homes, assuming that higher levels of care needs will complicate the matching process and reserve MFH referral for only the highest functioning candidates.19 However, the ADL data presented here indicate that many MFH residents with significant levels of ADL dependence are living in MFHs. Meeting the care needs of those who have higher ADL dependencies is possible because MFH coordinators and HBPC providers deliver individual, ongoing education to MFH caregivers about caring for MFH veterans and provide available resources needed to safely care for MFH veterans across the spectrum of ADL dependency.7
Veterans with higher levels of functional dependence may also be referred to nursing homes rather than to MFHs because of payment issues. Independent of the VA, veterans or their families negotiate a contract with their caregiver to pay out-of-pocket for MFH caregiving as well as room and board. Particularly for veterans who have military benefits to cover nursing home care costs, the out-of-pocket payment for veterans with high degrees of functional dependence increase as needs increase. These out-of-pocket payments may serve as a barrier to MFH enrollment. The proposed Long-Term Care Veterans Choice Act, which would allow the VA to pay for MFH care for eligible veterans may address this barrier.15
Another possible explanation for the higher rates of functional independence in the MFH cohort is that veterans with functional impairment are not being referred to MFHs. A previous study of the MFH program found that health care providers were often unaware of the program and as a result did not refer eligible veterans to this alternative LTC option.7 The changes proposed by the Long-Term Care Veterans Choice Act may result in an increase in demand in MFH care and thus increase awareness of the program among VA physicians.15
Limitations
There are several potential limitations in this study. First, there are limits to the generalizability of the MFH sample given that the sample of veterans was not randomly selected and that weights were not applied to account for nonresponse bias. Second, charting requirements in MFHs are less intensive compared with nursing home tracking. While the training for research nurses on how to conduct MDS assessments in MFHs was designed to simulate the process in nursing homes, MDS data were likely impacted by differences in charting practices. In addition, MFH caregivers may report certain items, such as aggressive behaviors, more often because they observe MFH veterans round-the-clock compared with NH caregivers who work in shifts and have a lower caregiver to resident ratio. The current data suggest differences in prevalence of behavioral symptoms.
Future studies should examine whether this reflects differences in the populations served or differences in how MFH caregivers track and manage behavioral symptoms. Third, this study was conducted at only MFH sites associated with 4 VAMCs, thus our findings may not be generalizable to veterans in other areas. Finally, there may be differences in the veterans who agreed to participate in the study compared with those who declined to participate. For example, it is possible that the eligible MFH veterans who declined to participate in this study were more functionally impaired than those who did participate. More than one-third (39%) of the family members of cognitively impaired MFH veterans who did not participate cited concerns about the veteran’s frailty as a primary reason for declining to participate. Consequently, the high level of functional status among veterans included in this study compared to nursing home residents may be in part a result of selection bias from more ADL-impaired veterans declining to participate in the study.
Conclusions
Although the MFH program has provided LTC nationally to veterans for nearly 2 decades, this study is the first to administer in-home MDS assessments to veterans in MFHs, allowing for a detailed description of cognitive, functional, and behavioral characteristics of MFH residents. In this study, we found that veterans currently receiving care in MFHs have a wide range of care needs. Our findings indicate that MFHs are caring for some veterans with high functional impairment as well as those who are completely independent in performing ADLs.
Moreover, these results are a preliminary attempt to assist VA health care providers in determining which veterans can be cared for in an MFH such that they can make informed referrals to this alternative LTC setting. To improve the generalizability of these findings, future studies should collect MDS 3.0 assessments longitudinally from a representative sample of veterans in MFHs. Further research is needed to explore how VA providers make the decision to refer a veteran to an MFH compared to a nursing home. Additionally, the percentage of veterans in this study who reported experiencing pain may indicate the need to identify innovative, integrated pain management programs for home settings.
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
1. Rowe JW, Fulmer T, Fried L. Preparing for better health and health care for an aging population. JAMA. 2016;316(16):1643. doi:10.1001/jama.2016.12335
2. Reaves E, Musumeci M. Medicaid and long-term services and supports: a primer. kaiser family foundation. Published December 15, 2015. Accessed February 12, 2021. https://www.kff.org/medicaid/report/medicaid-and-long-term-services-and-supports-a-primer
3. Collelo KJ, Panangala SV. Long-term care services for veterans. Congressional Research Service Report No. R44697. Published February 14, 2017. Accessed February 12, 2021. https://fas.org/sgp/crs/misc/R44697.pdf
4. American Association of Retired Persons. Beyond 50.05: a report to the nation on livable communities creating environments for successful aging. Published online 2005. Accessed February 12, 2021. https://assets.aarp.org/rgcenter/il/beyond_50_communities.pdf
5. Kaiser Family Foundation. State data and policy actions to address coronavirus. Updated February 11, 2021. Accessed February 12, 2021. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/
6. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID-19 Cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
7. Haverhals LM, Manheim CE, Jones J, Levy C. Launching medical foster home programs: key components to growing this alternative to nursing home placement. J Hous Elderly. 2017;31(1):14-33. doi:10.1080/01634372.2016.1268556
8. US Department of Veterans Affairs. Medical Foster Home Program Procedures- VHA Directive 1141.02(1). Published August 9, 2017. Accessed February 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5447.
9. Haverhals LM, Manheim CE, Gilman CV, Jones J, Levy C. Caregivers create a veteran-centric community in VHA medical foster homes. J Gerontol Soc Work. 2016;59(6):441-457. doi:10.1080/01634372.2016.1231730
10. Jones J, Haverhals LM, Manheim CE, Levy C. Fostering excellence: an examination of high-enrollment VHA Medical Foster Home programs. Home Health Care Manag Pract. 2017;30(1):16-22. doi:10.1177/1084822317736795
11. US Department of Veterans Affairs. Veterans Health Administration. Veterans Health Benefits Handbook. Published 2017. Accessed February 17, 2021. https://www. va.gov/healthbenefits/vhbh/publications/vhbh_sample_handb ook_2014.pdf
12. Duan-Porter W, Ullman K, Rosebush C, McKenzie L, et al; Evidence Synthesis Program. Risk factors and interventions to prevent or delay long term nursing home placement for adults with impairments. Published May 2019. Accessed March 2, 2021. https://www.hsrd.research.va.gov/publications/esp/nursing-home-delay.pdf
13. US Department of Veterans Affairs. Caregiver Support Program- VHA NOTICE 2020-31. Published October 1, 2020. Accessed February 2, 2021. https://www.va.gov/VHApublications/ViewPublication.asp?pub_ID=9048
14. US Department of Veterans Affairs. Geriatrics and extended care. Published June 10, 2020. Accessed February 22, 2021. https://www.va.gov/geriatrics/pages/Veteran-Directed_Care.asp
15. HR 1527, 116th Cong (2019). Accessed March 1, 2021. congress.gov/bill/116th-congress/house-bill/1527
16. Levy C, Whitfield EA. Medical foster homes: can the adult foster care model substitute for nursing home care? J Am Geriatr Soc. 2016;64(12):2585-2592. doi:10.1111/jgs.14517
17. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602-610. doi:10.1016/j.jamda.2012.06.002
18. Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi:10.1016/j.jamda.2012.06.001
19. Gilman C, Haverhals L, Manheim C, Levy C. A qualitative exploration of veteran and family perspectives on medical foster homes. Home Health Care Serv Q. 2018;37(1):1-24. doi:10.1080/01621424.2017.1419156
20. Levy CR, Alemi F, Williams AE, et al. Shared homes as an alternative to nursing home care: impact of VA’s Medical Foster Home program on hospitalization. Gerontologist. 2016;56(1):62-71. doi:10.1093/geront/gnv092
21. Levy CR, Jones J, Haverhals LM, Nowels CT. A qualitative evaluation of a new community living model: medical foster home placement. J Nurs Educ Pract. 2013;4(1):p162. doi:10.5430/jnep.v4n1p162
22. Levy C, Whitfield EA, Gutman R. Medical foster home is less costly than traditional nursing home care. Health Serv Res. 2019;54(6):1346-1356. doi:10.1111/1475-6773.13195
23. Manheim CE, Haverhals LM, Jones J, Levy CR. Allowing family to be family: end-of-life care in Veterans Affairs medical foster homes. J Soc Work End Life Palliat Care. 2016;12(1-2):104-125. doi:10.1080/15524256.2016.1156603
24. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi:10.1097/MLR.0000000000000334
25. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618-625. doi:10.1016/j.jamda.2012.06.003
26. Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298-2303. doi:10.1111/j.1532-5415.2008.02048.x
27. McCreedy E, Ogarek JA, Thomas KS, Mor V. The minimum data set agitated and reactive behavior scale: measuring behaviors in nursing home residents with dementia. J Am Med Dir Assoc. 2019;20(12):1548-1552. doi:10.1016/j.jamda.2019.08.030
28. Levy CR, Zargoush M, Williams AE, et al. Sequence of functional loss and recovery in nursing homes. Gerontologist. 2016;56(1):52-61. doi:10.1093/geront/gnv099
29. Wysocki A, Thomas KS, Mor V. Functional improvement among short-stay nursing home residents in the MDS 3.0. J Am Med Dir Assoc. 2015;16(6):470-474. doi:10.1016/j.jamda.2014.11.018
30. Morris JN, Pries B, Morris’ S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546-M553. doi:10.1093/gerona/54.11.m546
31. Mor V, Zinn J, Gozalo P, Feng Z, Intrator O, Grabowski DC. Prospects for transferring nursing home residents to the community. Health Aff (Millwood). 2007;26(6):1762-1771. doi:10.1377/hlthaff.26.6.1762
32. Ikegami N, Morris JN, Fries BE. Low-care cases in long-term care settings: variation among nations. Age Ageing. 1997;26(suppl 2):67-71. doi:10.1093/ageing/26.suppl_2.67
33. Arling G, Kane RL, Cooke V, Lewis T. Targeting residents for transitions from nursing home to community. Health Serv Res. 2010;45(3):691-711. doi:10.1111/j.1475-6773.2010.01105.x
34. Castle NG. Low-care residents in nursing homes: the impact of market characteristics. J Health Soc Policy. 2002;14(3):41-58. doi:10.1300/J045v14n03_03
35. Grando VT, Rantz MJ, Petroski GF, et al. Prevalence and characteristics of nursing homes residents requiring light-care. Res Nurs Health. 2005;28(3):210-219. doi:10.1002/nur.20079
36. Hahn EA, Thomas KS, Hyer K, Andel R, Meng H. Predictors of low-care prevalence in Florida nursing homes: the role of Medicaid waiver programs. Gerontologist. 2011;51(4):495-503. doi:10.1093/geront/gnr020
37. Thomas KS. The relationship between older Americans act in-home services and low-care residents in nursing homes. J Aging Health. 2014;26(2):250-260. doi:10.1177/0898264313513611
Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
Distribution of Skin-Type Diversity in Photographs in AAD Online Educational Modules
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
PRACTICE POINTS
- Recent studies have highlighted poor representation of darker skin types in textbooks.
- The Basic Dermatology Curriculum of the American Academy of Dermatology has a low (16%) representation of darker skin types in photographs; more than one-quarter of curriculum lectures had no such images.
- Darker skin types were underrepresented for skin cancers and overrepresented for sexually transmitted infections, raising questions about how photographs were chosen.
- Educators should consider using existing resources of photographs of diverse skin types when designing future curricula.
Are There Mobile Applications Related to Nail Disorders?
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
Practice Points
- Patient-targeted mobile applications (apps) might aid with clinical referencing and education.
- There are patient-directed psoriasis and hair loss apps on iOS and Android platforms, but informative apps related to nail disorders are limited.
- There is a need to develop apps related to nail health for patient education.
Readmissions Following Hospitalization for Infection in Children With or Without Medical Complexity
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.
Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.
Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.
Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.
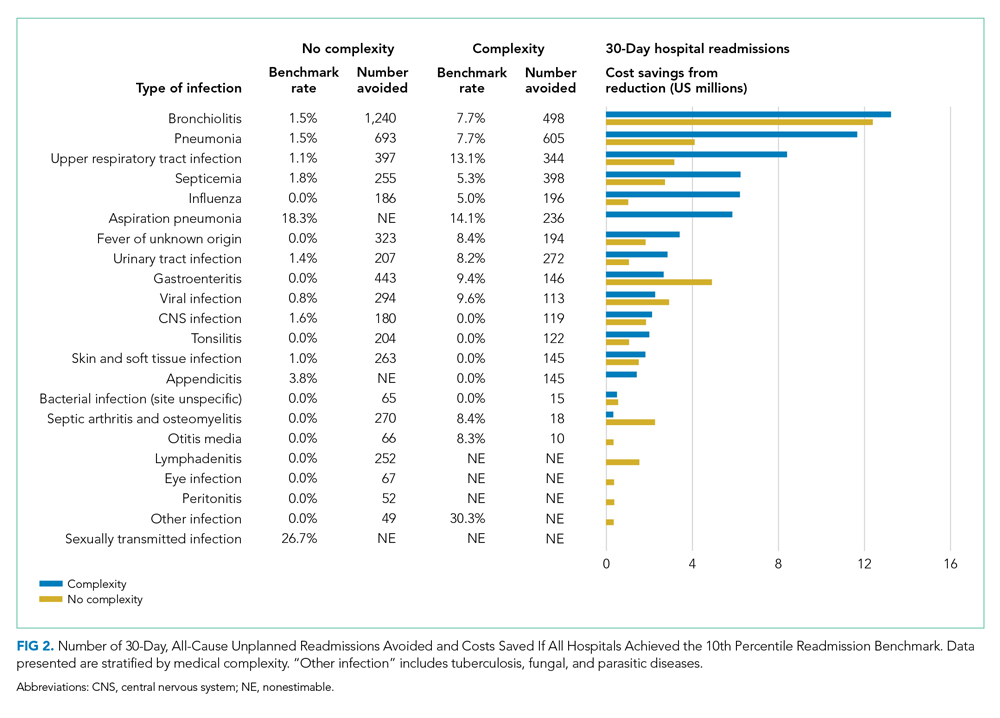
Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
© 2021 Society of Hospital Medicine
Early and Significant Reduction in C-Reactive Protein Levels After Corticosteroid Therapy Is Associated With Reduced Mortality in Patients With COVID-19
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.
After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.
CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.
The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.
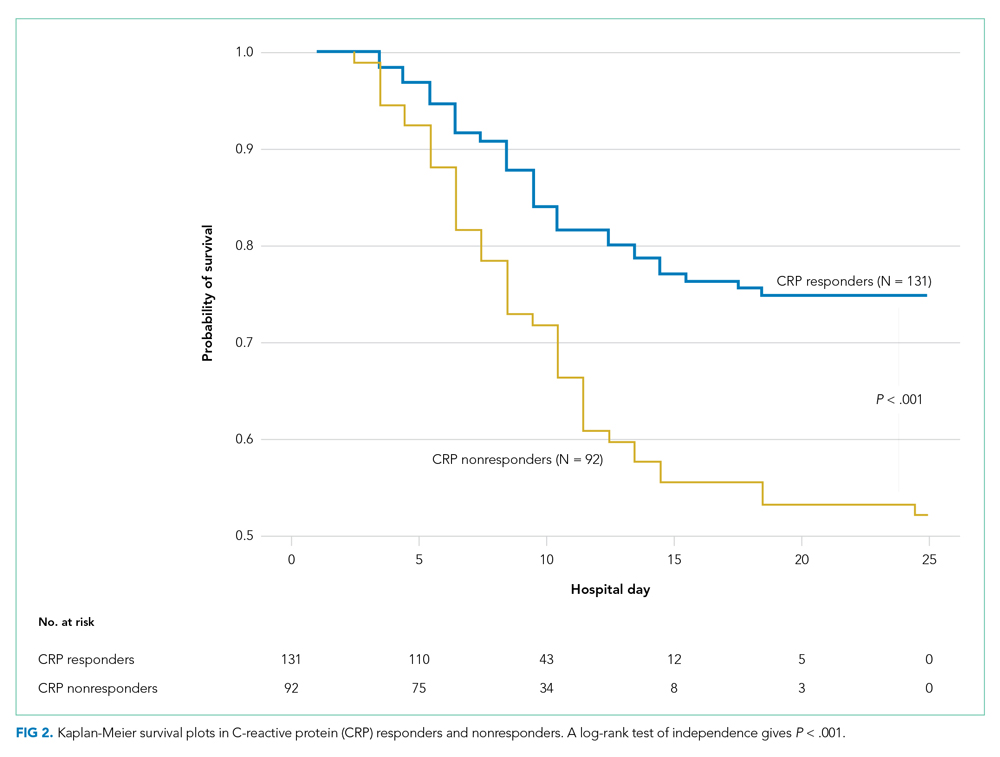
To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.
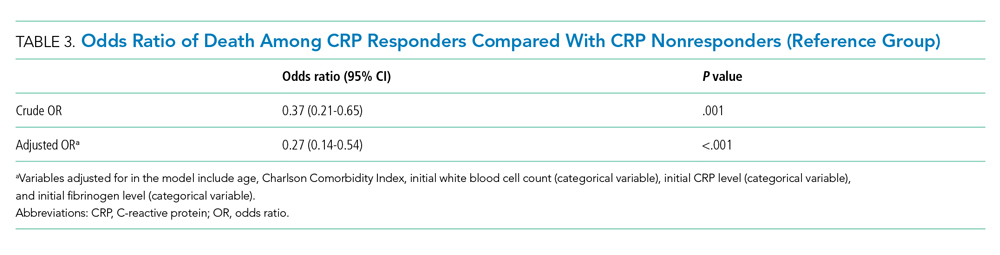
DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Advancing Diversity, Equity, and Inclusion in Hospital Medicine
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.
Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.
Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.
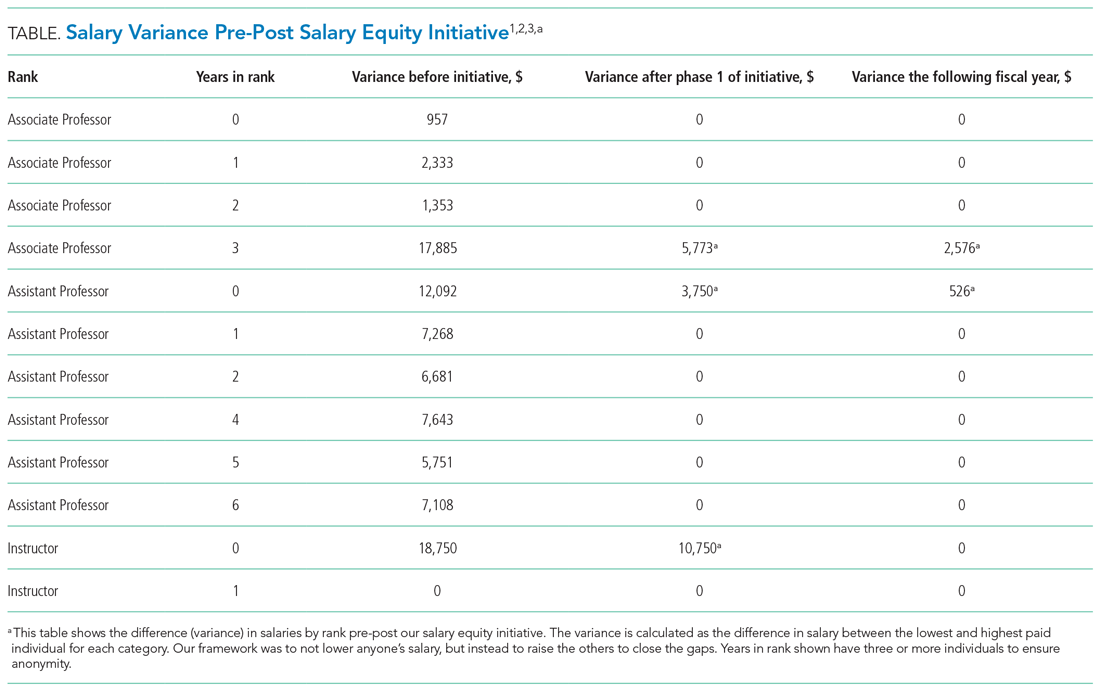
Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.
Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.

Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.

Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
Studies continue to demonstrate persistent gaps in equity for women and underrepresented minorities (URMs)1 throughout nearly all aspects of academic medicine, including rank,2-4 tenure,5 authorship,6,7 funding opportunities,8,9 awards,10 speakership,11 leadership,12,13 and salaries.2,14,15
In this article, we report DEI efforts within our division, focusing on the development of our strategic plan and specific outcomes related to compensation, recruitment, and policies.
METHODS
Our Division’s Framework to DEI—“It Takes a Village”
Our Division of Hospital Medicine (DHM), previously within the Division of General Internal Medicine, was founded in October 2017. The DHM at the University of Colorado Hospital (UCH) is composed of 100 faculty members (70 physicians and 30 advanced-practice providers; 58% women and 42% men). In 2018, we implemented a stepwise approach to critically assess DEI within our group and to build a strategic plan to address the issues.

As a new division, we sought stakeholder feedback from division members. All faculty within the division were invited to attend a meeting in which issues related to DEI were discussed. A literature review that spanned both medical and nonmedical fields was also completed. Search terms included salary equity, gender equity, diverse teams, diversity recruitment and retention, diversifying leadership, and diverse speakers. Salaries, internally funded time, and other processes, such as recruitment, promotion, and hiring for leadership positions, were evaluated during the first year we became a division.
TThrough this work, and with stakeholder engagement, we developed a divisional strategic plan to address DEI globally. Our strategic plan included developing a DEI director role to assist with overseeing DEI efforts. We have highlighted the various methods utilized for each component (Figure 1). This work occurred from October 2017 to December 2018.
Our institutional structures
Using best practices from both medical and nonmedical fields, we developed evidence-based approaches to
Compensation: transparent and consistent approaches based upon benchmarking with a framework of equal pay for equal work and similar advanced training/academic rank. In conjunction with efforts within the School of Medicine (SOM), Department of Medicine (DOM), and the UCH, our division sought to study salaries across DHM faculty members. We had an open call for faculty to participate in a newly developed DHM Compensation Committee, with the intent of rigorously examining our compensation practices and goals. Through faculty feedback and committee work, salary equity was defined as equal pay (ie, base salary for one clinical full-time equivalent [FTE]) for equal work based on academic rank and/or years of practice/advanced training. We also compared DHM salaries to regional academic hospital medicine groups and concluded that DHM salaries were lower than local and national benchmarks. This information was used to create a two-phase approach to increasing salaries for all individuals below the American Association of Medical Colleges (AAMC) benchmarks33 for academic hospitalists. We also developed a stipend system for external roles that came with additional compensation and roles within our own division that came with additional pay (ie, nocturnist). Phase 1 focused on those whose salaries were furthest away from and below benchmark, and phase 2 targeted all remaining individuals below benchmark.
A similar review of FTEs (based on required number of shifts for a full-time hospitalist) tied to our internal DHM leadership positions was completed by the division head and director of DEI. Specifically, the mission for each of our internally funded roles, job descriptions, and responsibilities was reviewed to ensure equity in funding.
Recruitment and advancement: processes to ensure equity and diversity in recruitment, tracking, and reporting, working to eliminate/mitigate bias. In collaboration with members of the AAMC Group on Women in Medicine and Science (GWIMS) and coauthors from various institutions, we developed toolkits and checklists aimed at achieving equity and diversity within candidate pools and on major committees, including, but not limited to, search and promotion committees.32 Additionally, a checklist was developed to help recruit more diverse speakers, including women and URMs, for local, regional, and national conferences.
Policies: evidence-based approaches, tracking and reporting, standardized approaches to eliminate/mitigate bias, embracing nontraditional paths. In partnership with our departmental efforts, members of our team led data collection and reporting for salary benchmarking, leadership roles, and committee membership. This included developing surveys and reporting templates that can be used to identify disparities and inform future efforts. We worked to ensure that we have faculty representing our field at the department and SOM levels. Specifically, we made sure to nominate division members during open calls for departmental and schoolwide committees, including the promotions committee.
Our People
The faculty and staff within our division have been instrumental in moving efforts forward in the following important areas.
Leadership: develop the position of director of DEI as well as leadership structures to support and increase DEI. One of the first steps in our strategic plan was creating a director of DEI leadership role (Appendix Figure 2). The director is responsible for researching, applying, and promoting a broad scope of DEI initiatives and best practices within the DHM, DOM, and SOM (in collaboration with their leaders), including recruitment, retention, and promotion of medical students, residents, and faculty; educational program development; health disparities research; and community-engaged scholarship.
Support: develop family leave policies/develop flexible work policies. Several members of our division worked on departmental committees and served in leadership roles on staff and faculty council. Estimated costs were assessed. Through collective efforts of department leadership and division head support, the department approved parental leave to employees following the birth of an employee’s child or the placement of a child with an employee in connection with adoption or permanent foster care.
Mentorship/sponsorship: enhance faculty advancement programs/develop pipeline and trainings/collaborate with student groups and organizations/invest in all of our people. Faculty across our divisional sites have held important roles in developing pipeline programs for undergraduate students bound for health professions, as well as programs developed specifically for medical students and internal medicine residents. This includes two programs, the CU Hospitalist Scholars Program (CUHSP) and Leadership Education for Aspiring Doctors (LEAD), in which undergraduate students have the opportunity to round with hospital medicine teams, work on quality-improvement projects, and receive extensive mentorship and advising from a diverse faculty team. Additionally, our faculty advancement team within the DHM has grown and been restructured to include more defined goals and to ensure each faculty member has at least one mentor in their area of interest.
Supportive: lactation space and support/diverse space options/inclusive and diverse environments. We worked closely with hospital leadership to advocate for adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations.
Measures
Our measures focused on (1) development and implementation of our DEI strategic plan, including new policies, processes, and practices related to key components of the DEI program; and (2) assessment of specific DEI programs, including pre-post salary data disparities based on rank and pre-post disparities for protected time for similar roles.
Analysis
Through rapid PDSA cycles, we evaluated salary equity, equity in leadership allotment, and committee membership. We have developed a tracking board to track progress of the multiple projects in the strategic plan.
RESULTS
Strategic Plan Development and Tracking
From October 2017 to December 2018, we developed a robust strategic plan and stepwise approach to DEI (Figure 1 and Figure 2). The director of DEI position was developed (see Appendix Figure 2 for job description) to help oversee these efforts. Figure 3 highlights the specific efforts and the progress made on implementation (ie, high-level dashboard or “tracking board”). While outcomes are still pending in the areas of recruitment and advancement and environment, we have made measurable improvements in compensation, as outlined in the following section.
Compensation
One year after the salary-equity interventions, all of our physician faculty’s salaries were at the goal benchmark (Table), and differences in salary for those in similar years of rank were nearly eliminated. Similarly, after implementing an internally consistent approach to assigning FTE for new and established positions within the division (ie, those that fall within the purview of the division), all faculty in similar types of roles had similar amounts of protected time.

Recruitment and Advancement
Toolkits32 and committee recommendations have been incorporated into division goals, though some aspects are still in implementation phases, as division-wide implicit bias training was delayed secondary to the COVID-19 pandemic. Key goals include: (1) implicit bias training for all members of major committees; (2) aiming for a goal of at least 40% representation of women and 40% URMs on committees; (3) having a diversity expert serve on each committee in order to identify and discuss any potential bias in the search and candidate-selection processes; and (4) careful tracking of diversity metrics in regard to diversity of candidates at each step of the interview and selection process.

Surveys and reporting templates for equity on committees and leadership positions have been developed and deployed. Data dashboards for our division have been developed as well (for compensation, leadership, and committee membership). A divisional dashboard to report recruitment efforts is in progress.
We have successfully nominated several faculty members to the SOM promotions committee and departmental committees during open calls for these positions. At the division level, we have also adapted internal policies to ensure promotion occurs on time and offers alternative pathways for faculty that may primarily focus on clinical pathways. All faculty who have gone up for promotion thus far have been successfully promoted in their desired pathway.
Environment
We successfully advocated and achieved adequately equipped lactation spaces, including equipment such as pumps, refrigerators, and computer workstations. This achievement was possible because of our hospital partners. Our efforts helped us acquire sufficient space and facilities such that nursing mothers can pump and still be able to answer phones, enter orders, and document visits.
Our team members conducted environmental scans and raised concerns when the environment was not inclusive, such as conference rooms with portraits of leadership that do not show diversity. The all-male pictures were removed from one frequently used departmental conference room, which will eventually house a diverse group of pictures and achievements.
We aim to eliminate bias by offering implicit bias training for our faculty. While this is presently required for those who serve on committees, in leadership positions, or those involved in recruitment and interviewing for the DOM, our goal is to eventually provide this training to all faculty and staff in the division. We have also incorporated DEI topics into our educational conferences for faculty, including sessions on recognizing bias in medicine, how to be an upstander/ally, and the impact of race and racism on medicine.
DISCUSSION
The important findings of this work are: (1) that successes in DEI can be achieved with strategic planning and stakeholder engagement; (2) through simple modification of processes, we can improve equity in compensation and FTE allotted to leadership; (3) though it takes time, diversity recruitment can be improved using sound, sustainable, evidence-based processes; (4) this work is time-intensive and challenging, requiring ongoing efforts to improve, modify, and enhance current efforts and future successes.
We have certainly made some progress with DEI initiatives within our division and have also learned a great deal from this experience. First, change is difficult for all parties involved, including those leading change and those affected by the changes. We purposely made an effort to facilitate discussions with all of the DHM faculty and staff to ensure that everyone felt included in this work and that everyone’s voice was heard. This was exemplified by inviting all faculty members to a feedback session in which we discussed DEI within our division and areas that we wanted to improve on. Early on, we were able to define what diversity, equity, and inclusion meant to us as a division and then use these definitions to develop tangible goals for all the areas of highest importance to the group.
By increasing faculty presence on key committees, such as the promotions committee, we now have faculty members who are well versed in promotions processes. We are fortunate to have a promotions process that supports faculty advancement for faculty with diverse interests that spans from supporting highly clinical faculty, clinician educators, as well as more traditional researchers.34 By having hospitalists serve in these roles, we help to add to the diverse perspectives on these committees, including emphasizing the scholarship that is associated with quality improvement, as well as DEI efforts which can often be viewed as service as opposed to scholarship.
Clear communication and transparency were key to all of our DEI initiatives. We had monthly updates on our DEI efforts during business meetings and also held impromptu meetings (also known as flash mobs35) to answer questions and discuss concerns in real time. As with all DEI work, it is important to know where you are starting (having accurate data and a clear understanding of the data) and be able to communicate that data to the group. For example, using AAMC salary benchmarking33 as well as other benchmarks allowed us to accurately calculate variance among salaries and identify the appropriate goal salary for each of our faculty members. Likewise, by completing an in-depth inventory on the work being done by all of our faculty in leadership roles, we were able to standardize the compensation/FTE for each of these roles. Tracking these changes over time, via the use of dashboards in our case, allows for real-time measurements and accountability for all of those involved. Our end goal will be to have all of these initiatives feed into one large dashboard.
Collaborating with leadership and stakeholders in the DOM, SOM, and hospital helped to make our DEI initiatives successful. Much too often, we work in silos when it comes to DEI work. However, we tend to have similar goals and can achieve much more if we work together. Collaboration with multiple stakeholders allowed for wider dissemination and resulted in a larger impact to the campus and community at large. This has been exemplified by the committee composition guidance that has been utilized by the DOM, as well as implementation of campus-wide policies, specifically the parental leave policy, which our faculty members played an important role in creating. Likewise, it is important to look outside of our institutions and work with other hospital medicine groups around the country who are interested in promoting DEI.
We still have much work ahead of us. We are continuing to measure outcomes status postimplementation of the toolkit and checklists being used for diversity recruitment and committee composition. Additionally, we are actively working on several initiatives, including:
- Instituting implicit bias training for all of our faculty
- Partnering with national leaders and our hospital systems to develop zero-tolerance policies regarding abusive behaviors (verbal, physical, and other), racism, and sexism in the hospital and other work settings
- Development of specific recruitment strategies as a means of diversifying our healthcare workforce (of note, based on a 2020 survey of our faculty, in which there was a 70% response rate, 8.5% of our faculty identified as URMs)
- Completion of a diversity dashboard to track our progress in all of these efforts over time
- Development of a more robust pipeline to promotion and leadership for our URM faculty
This study has several strengths. Many of the plans and strategies described here can be used to guide others interested in implementing this work. Figure 2 provides a stepwise
approach to addressing DEI in hospital medicine groups and divisions. We conducted this work at a large academic medical center, and while it may not be generalizable, it does offer some ideas for others to consider in their own work to advance DEI at their institutions. There are also several limitations to this work. Eliminating salary inequities with our approach did take resources. We took advantage of already lower salaries and the need to increase salaries closer to benchmark and paired this effort with our DEI efforts to achieve salary equity. This required partnerships with the department and hospital. Efforts to advance DEI also take a lot of time and effort, and thus commitment from the division, department, and institution as a whole is key. While we have outcomes for our efforts related to salary equity, recruitment efforts should be realized over time, as currently it is too early to tell. We have highlighted the efforts that have been put in place at this time.
CONCLUSION
Using a systematic evidence-based approach with key stakeholder involvement, a division-wide DEI strategy was developed and implemented. While this work is still ongoing, short-term wins are possible, in particular around salary equity and development of policies and structures to promote DEI.
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
1. Underrepresented racial and ethnic groups. National Institutes of Health website. Accessed December 26, 2020. https://extramural-diversity.nih.gov/diversity-matters/underrepresented-groups
2. Ash AS, Carr PL, Goldstein R, Friedman RH. Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141(3):205-212. https://doi.org/10.7326/0003-4819-141-3-200408030-00009
3. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
4. Fang D, Moy E, Colburn L, Hurley J. Racial and ethnic disparities in faculty promotion in academic medicine. JAMA. 2000;284(9):1085-1092. https://doi.org/10.1001/jama.284.9.1085
5. Baptiste D, Fecher AM, Dolejs SC, et al. Gender differences in academic surgery, work-life balance, and satisfaction. J Surg Res. 2017;218:99-107. https://doi.org/10.1016/j.jss.2017.05.075
6. Hart KL, Perlis RH. Trends in proportion of women as authors of medical journal articles, 2008-2018. JAMA Intern Med. 2019;179:1285-1287. https://doi.org/10.1001/jamainternmed.2019.0907
7. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
8. Hechtman LA, Moore NP, Schulkey CE, et al. NIH funding longevity by gender. Proc Natl Acad Sci U S A. 2018;115(31):7943-7948. https://doi.org/10.1073/pnas.1800615115
9. Sege R, Nykiel-Bub L, Selk S. Sex differences in institutional support for junior biomedical researchers. JAMA. 2015;314(11):1175-1177. https://doi.org/10.1001/jama.2015.8517
10. Silver JK, Slocum CS, Bank AM, et al. Where are the women? The underrepresentation of women physicians among recognition award recipients from medical specialty societies. PM R. 2017;9(8):804-815. https://doi.org/10.1016/j.pmrj.2017.06.001
11. Ruzycki SM, Fletcher S, Earp M, Bharwani A, Lithgow KC. Trends in the proportion of female speakers at medical conferences in the United States and in Canada, 2007 to 2017. JAMA Netw Open. 2019;2(4):e192103. https://doi.org/10.1001/jamanetworkopen.2019.2103
12. Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: retention, rank, and leadership comparisons from the National Faculty Survey. Acad Med. 2018;93(11):1694-1699. https://doi.org/10.1097/ACM.0000000000002146
13. Carr PL, Gunn C, Raj A, Kaplan S, Freund KM. Recruitment, promotion, and retention of women in academic medicine: how institutions are addressing gender disparities. Womens Health Issues. 2017;27(3):374-381. https://doi.org/10.1016/j.whi.2016.11.003
14. Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176(9):1294-1304. https://doi.org/10.1001/jamainternmed.2016.3284
15. Lo Sasso AT, Richards MR, Chou CF, Gerber SE. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30(2):193-201. https://doi.org/10.1377/hlthaff.2010.0597
16. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/NEJM199608153350713
17. Weaver AC, Wetterneck TB, Whelan CT, Hinami K. A matter of priorities? Exploring the persistent gender pay gap in hospital medicine. J Hosp Med. 2015;10(8):486-490. https://doi.org/10.1002/jhm.2400
18. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
19. Northcutt N, Papp S, Keniston A, et al, Society of Hospital Medicine Diversity, Equity and Inclusion Special Interest Group. SPEAKers at the National Society of Hospital Medicine Meeting: a follow-up study of gender equity for conference speakers from 2015 to 2019. The SPEAK UP Study. J Hosp Med. 2020;15(4):228-231. https://doi.org/10.12788/jhm.3401
20. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
21. Shah SS, Shaughnessy EE, Spector ND. Promoting gender equity at the Journal of Hospital Medicine [editorial]. J Hosp Med. 2020;15(9):517. https://doi.org/10.12788/jhm.3522
22. Sheehy AM, Kolehmainen C, Carnes M. We specialize in change leadership: a call for hospitalists to lead the quest for workforce gender equity [editorial]. J Hosp Med. 2015;10(8):551-552. https://doi.org/10.1002/jhm.2399
23. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020;383(3):274-276. https://doi.org/10.1056/NEJMe2021693
24. Rock D, Grant H. Why diverse teams are smarter. Harvard Business Review. Published November 4, 2016. Accessed July 24, 2019. https://hbr.org/2016/11/why-diverse-teams-are-smarter
25. Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19(2):101-110. https://doi.org/10.1111/j.1525-1497.2004.30262.x
26. Betancourt JR, Green AR, Carrillo JE, Park ER. Cultural competence and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
27. Acosta D, Ackerman-Barger K. Breaking the silence: time to talk about race and racism [comment]. Acad Med. 2017;92(3):285-288. https://doi.org/10.1097/ACM.0000000000001416
28. Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Aff (Millwood). 2002;21(5):90-102. https://doi.org/10.1377/hlthaff.21.5.90
29. Chang E, Simon M, Dong X. Integrating cultural humility into health care professional education and training. Adv Health Sci Educ Theory Pract. 2012;17(2):269-278. https://doi.org/10.1007/s10459-010-9264-1
30. Foronda C, Baptiste DL, Reinholdt MM, Ousman K. Cultural humility: a concept analysis. J Transcult Nurs. 2016;27(3):210-217. https://doi.org/10.1177/1043659615592677
31. Butkus R, Serchen J, Moyer DV, et al; Health and Public Policy Committee of the American College of Physicians. Achieving gender equity in physician compensation and career advancement: a position paper of the American College of Physicians. Ann Intern Med. 2018;168(10):721-723. https://doi.org/10.7326/M17-3438
32. Burden M, del Pino-Jones A, Shafer M, Sheth S, Rexrode K. GWIMS Equity Recruitment Toolkit. Accessed July 27, 2019. https://www.aamc.org/download/492864/data/equityinrecruitmenttoolkit.pdf
33. AAMC Faculty Salary Report. AAMC website. Accessed September 6, 2020. https://www.aamc.org/data-reports/workforce/report/aamc-faculty-salary-report
34. Promotion process. University of Colorado Anschutz Medical Campus website. Accessed September 7, 2020. https://medschool.cuanschutz.edu/faculty-affairs/for-faculty/promotion-and-tenure/promotion-process
35. Pierce RG, Diaz M, Kneeland P. Optimizing well-being, practice culture, and professional thriving in an era of turbulence. J Hosp Med. 2019;14(2):126-128. https://doi.org/10.12788/jhm.3101
© 2021 Society of Hospital Medicine
Antibiotic Regimens and Associated Outcomes in Children Hospitalized With Staphylococcal Scalded Skin Syndrome
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.
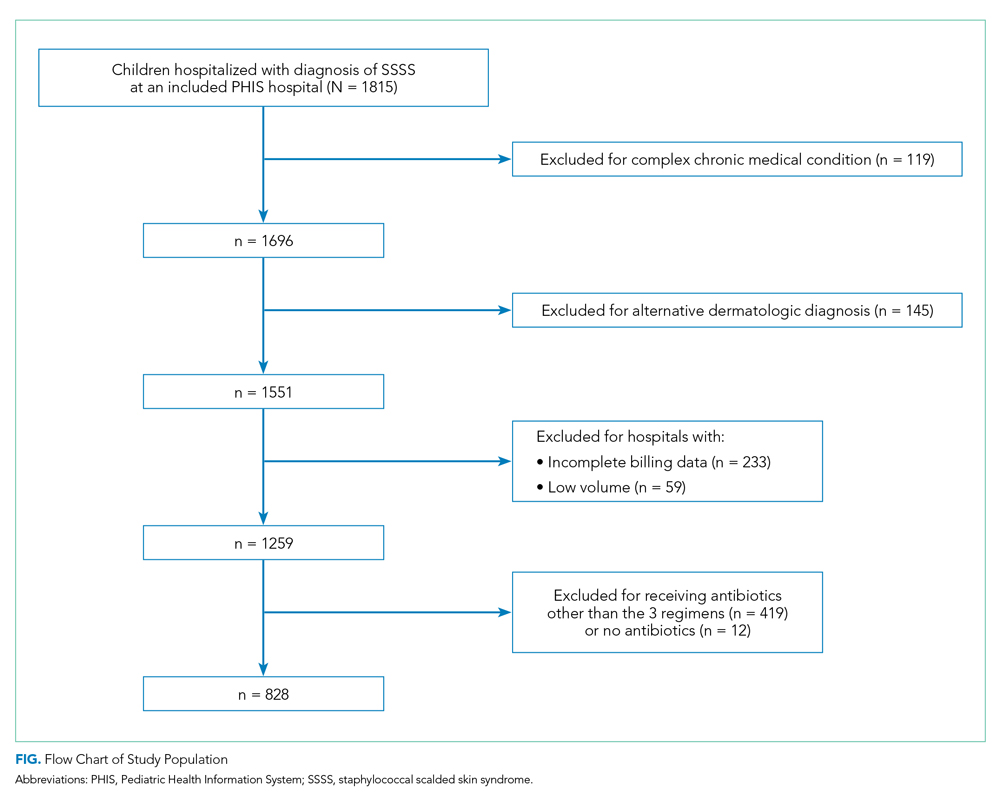
Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.
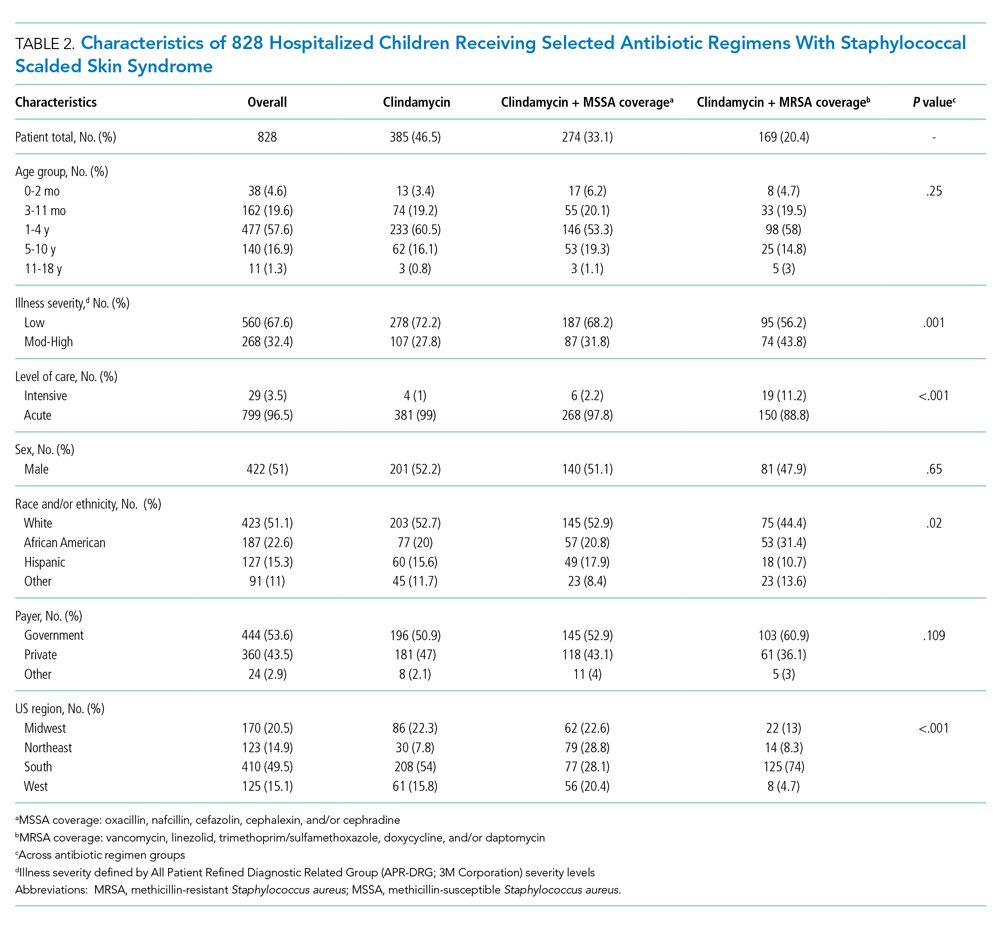
Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.
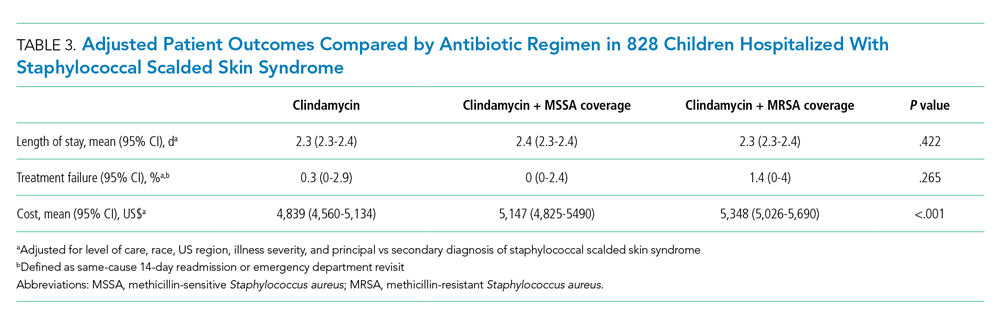
DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.

Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.

Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.

DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
Staphylococcal scalded skin syndrome (SSSS) is an exfoliative toxin-mediated dermatitis that predominantly occurs in young children. Multiple recent reports indicate a rising incidence of this disease.1-4 Recommended treatment for SSSS includes antistaphylococcal antibiotics and supportive care measures.5,6 Elimination or reduction of the toxin-producing Staphylococcus aureus is thought to help limit disease progression and promote recovery. Experts advocate for the use of antibiotics even when there is no apparent focal source of infection, such as an abscess.6,7
Several factors may affect antibiotic selection, including the desire to inhibit toxin production and to target the causative pathogen in a bactericidal fashion. Because SSSS is toxin mediated, clindamycin is often recommended because of its inhibition of toxin synthesis.5,8 The clinical utility of adding other antibiotics to clindamycin for coverage of methicillin-sensitive S aureus (MSSA) or methicillin-resistant S aureus (MRSA) is uncertain. Several studies report MSSA to be the predominant pathogen identified by culture2,9; however, SSSS caused by MRSA has been reported.9-11 Additionally, bactericidal antibiotics (eg, nafcillin) have been considered to hold potential clinical advantage as compared with bacteriostatic antibiotics (eg, clindamycin), even though clinical studies have not clearly demonstrated this advantage in the general population.12,13 Some experts recommend additional MRSA or MSSA coverage (such as vancomycin or nafcillin) in patients with high illness severity or nonresponse to therapy, or in areas where there is high prevalence of staphylococcal resistance to clindamycin.5,7,9,14 Alternatively, for areas with low MRSA prevalence, monotherapy with an anti-MSSA antibiotic is another potential option. No recent studies have compared patient outcomes among antibiotic regimens in children with SSSS.
Knowledge of the outcomes associated with different antibiotic regimens for children hospitalized with SSSS is needed and could be used to improve patient outcomes and potentially promote antibiotic stewardship. In this study, our objectives were to (1) describe antibiotic regimens given to children hospitalized with SSSS, and (2) examine the association of three antibiotic regimens commonly used for SSSS (clindamycin monotherapy, clindamycin plus additional MSSA coverage, and clindamycin plus additional MRSA coverage) with patient outcomes of length of stay (LOS), treatment failure, and cost in a large cohort of children at US children’s hospitals.
METHODS
We conducted a multicenter, retrospective cohort study utilizing data within the Pediatric Health Information System (PHIS) database from July 1, 2011, to June 30, 2016. Thirty-five free-standing tertiary care US children’s hospitals within 24 states were included. The Children’s Hospital Association (Lenexa, Kansas) maintains the PHIS database, which contains de-identified patient information, including diagnoses (with International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]), demographics, procedures, and daily billing records. Data quality and reliability are confirmed by participating institutions and the Children’s Hospital Association.15 The local institutional review board (IRB) deemed the study exempt from formal IRB review, as patient information was de-identified.
Study Population
We included hospitalized children aged newborn to 18 years with a primary or secondary diagnosis of SSSS (ICD-9, 695.81; ICD-10, L00). Children whose primary presentation and admission were to a PHIS hospital were included; children transferred from another hospital were excluded. The following exclusion criteria were based on previously published methodology.16 Children with complex chronic medical conditions as classified by Feudtner et al17 were excluded, since these children may require a different treatment approach than the general pediatric population. In order to decrease diagnostic ambiguity, we excluded children if an alternative dermatologic diagnosis was recorded as a principal or secondary diagnosis (eg, Stevens-Johnson syndrome or scarlet fever).16 Finally, hospitals with fewer than 10 children with SSSS during the study period were excluded.
Antibiotic Regimen Groups
We used PHIS daily billing codes to determine the antibiotics received by the study population. Children were classified into antibiotic regimen groups based on whether they received specific antibiotic combinations. Antibiotics received on any day during the hospitalization, including in the emergency department (ED), were used to assign patients to regimen groups. Antibiotics were classified into regimen groups based on consensus among study investigators, which included two board-certified pediatric infectious diseases specialists (A.C., R.M.). Antibiotic group definitions are listed in Table 1. Oral and intravenous (IV) therapies were grouped together for clindamycin, cephalexin/cefazolin, and linezolid because of good oral bioavailability in most situations.18 The three most common antistaphylococcal groups were chosen for further analysis: clindamycin alone, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage. The clindamycin group was defined as children with receipt of oral or IV clindamycin. Children who received clindamycin with additional MSSA coverage, such as cefazolin or nafcillin, were categorized as the clindamycin plus MSSA group. Children who received clindamycin with additional MRSA coverage, such as vancomycin or linezolid, were categorized as the clindamycin plus MRSA group. We chose not to include children who received the above regimens plus other antibiotics with partial antistaphylococcal activity, such as ampicillin, gentamicin, or ceftriaxone, in the clindamycin plus MSSA and clindamycin plus MRSA groups. We excluded these antibiotics to decrease the heterogeneity in the definition of regimen groups and allow a more direct comparison for effectiveness.

Covariates
Covariates included age, sex, ethnicity and/or race, payer type, level of care, illness severity, and region. The variable definitions below are in keeping with a prior study of SSSS.16 Age was categorized as: birth to 59 days, 2 to 11 months, 1 to 4 years (preschool age), 5 to 10 years (school age), and 11 to 18 years (adolescent). We examined infants younger than 60 days separately from older infants because this population may warrant additional treatment considerations. Race and ethnicity were categorized as White (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types included government, private, or other. Level of care was assigned as either intensive care or acute care. Illness severity was assigned using the All Patient Refined Diagnosis Related Group (APR-DRG; 3M Corporation, St. Paul, Minnesota) severity levels.19 In line with a prior study,16 we defined “low illness severity” as the APR-DRG minor (1) classification. The moderate (2), major (3), and extreme (4) classifications were defined as “moderate to high illness severity,” since there were very few classifications of major or extreme (<5%) illness severity. We categorized hospitals into the following US regions: Northeast, Midwest, South, and West.
Outcome Measures
The primary outcome was hospital LOS in days, and secondary outcomes were treatment failure and hospital costs. Hospital LOS was chosen as the primary outcome to represent the time needed for the child to show clinical improvement. Treatment failure was defined as a same-cause 14-day ED revisit or hospital readmission, and these were determined to be same-cause if a diagnosis for SSSS (ICD-9, 695.81; ICD-10, L00) was documented for the return encounter. The 14-day interval for readmission and ED revisit was chosen to measure any relapse of symptoms after completion of antibiotic therapy, similar to a prior study of treatment failure in skin and soft tissue infections.20 Total costs of the hospitalization were estimated from charges using hospital- and year-specific cost-to-charge ratios. Subcategories of cost, including clinical, pharmacy, imaging, laboratory, supply, and other, were also compared among the three groups.
Statistical Analysis
Demographic and clinical characteristics of children were summarized using frequencies and percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. These were compared across antibiotic groups using chi-square and Kruskal–Wallis tests, respectively. In unadjusted analyses, outcomes were compared across antibiotic regimen groups using these same statistical tests. In order to account for patient clustering within hospitals, generalized linear mixed-effects models were used to model outcomes with a random intercept for each hospital. Models were adjusted for SSSS being listed as a principal or secondary diagnosis, race, illness severity, and level of care. We log-transformed LOS and cost data prior to modeling because of the nonnormal distributions for these data. Owing to the inability to measure the number of antibiotic doses, and to reduce the possibility of including children who received few regimen-defined combination antibiotics, a post hoc sensitivity analysis was performed. This analysis used an alternative definition for antibiotic regimen groups, for which children admitted for 2 or more calendar days must have received regimen-specified antibiotics on at least 2 days of the admission. Additionally, outcomes were stratified by low and moderate/high illness severity and compared across the three antibiotic regimen groups. All analyses were performed with SAS (SAS 9.4; SAS Institute, Cary, North Carolina), and P values of less than .05 were considered statistically significant.
RESULTS
Overall, 1,815 hospitalized children with SSSS were identified in the PHIS database, and after application of the exclusion criteria, 1,259 children remained, with 1,247 (99%) receiving antibiotics (Figure). The antibiotic regimens received by these children are described in Table 1. Of these, 828 children (66%) received one of the three most common antistaphylococcal regimens (clindamycin, clindamycin + MSSA, and clindamycin + MRSA) and were included for further analysis.

Characteristics of the 828 children are presented in Table 2. Most children (82%) were aged 4 years or younger, and distributions of age, sex, and insurance payer were similar among children receiving the three regimens. Thirty-two percent had moderate to high illness severity, and 3.5% required management in the intensive care setting. Of the three antibiotic regimens, clindamycin monotherapy was most common (47%), followed by clindamycin plus MSSA coverage (33%), and clindamycin plus MRSA coverage (20%). A higher proportion of children in the clindamycin plus MRSA group were African American and were hospitalized in the South. Children receiving clindamycin plus MRSA coverage had higher illness severity (44%) as compared with clindamycin monotherapy (28%) and clindamycin plus MSSA coverage (32%) (P = .001). Additionally, a larger proportion of children treated with clindamycin plus MRSA coverage were managed in the intensive care setting as compared with the clindamycin plus MSSA or clindamycin monotherapy groups.

Among the 828 children with SSSS, the median LOS was 2 days (IQR, 2-3), and treatment failure was 1.1% (95% CI, 0.4-1.8). After adjustment for illness severity, race, payer, and region (Table 3), the three antibiotic regimens were not associated with significant differences in LOS or treatment failure. Costs were significantly different among the three antibiotic regimens. Clindamycin plus MRSA coverage was associated with the greatest costs, whereas clindamycin monotherapy was associated with the lowest costs (mean, $5,348 vs $4,839, respectively; P < .001) (Table 3). In a sensitivity analysis using an alternative antibiotic regimen definition, we found results in line with the primary analysis, with no statistically significant differences in LOS (P = .44) or treatment failure (P = .54), but significant differences in cost (P < .001). Additionally, the same findings were present for LOS, treatment failure, and cost when outcomes were stratified by illness severity (Appendix Table). However, significant contributors to the higher cost in the clindamycin plus MRSA group did vary by illness severity stratification. Laboratory, supply, and pharmacy cost categories differed significantly among antibiotic groups for the low illness severity strata, whereas pharmacy was the only significant cost category difference in moderate/high illness severity.

DISCUSSION
Clindamycin monotherapy, clindamycin plus MSSA coverage, and clindamycin plus MRSA coverage are the most commonly administered antistaphylococcal antibiotic regimens for children hospitalized with SSSS at US children’s hospitals. Our multicenter study found that, across these antistaphylococcal antibiotic regimens, there were no associated differences in hospital LOS or treatment failure. However, the antibiotic regimens were associated with significant differences in overall hospital costs. These findings suggest that the use of clindamycin with additional MSSA or MRSA antibiotic coverage for children with SSSS may not be associated with additional clinical benefit, as compared with clindamycin monotherapy, and could potentially be more costly.
Prior literature describing LOS in relation to antibiotic use for children with SSSS is limited. Authors of a recent case series of 21 children in Philadelphia reported approximately 50% of children received clindamycin monotherapy or combination therapy, but patient outcomes such as LOS were not described.9 Clindamycin use and outcomes have been described in smaller studies and case reports of SSSS, which reported positive outcomes such as patient recovery and lack of disease recurrence.2,9,21 A small retrospective, comparative effectiveness study of 30 neonates with SSSS examined beta-lactamase–resistant penicillin use with and without cephalosporins. They found no effect on LOS, but findings were limited by a small sample size.22 Our study cohort included relatively few neonates, and thus our findings may not be applicable to this population subgroup. We chose not to include regimens with third-generation cephalosporins or ampicillin, which may have limited the number of included neonates, because these antibiotics are frequently administered during evaluation for invasive bacterial infections.23 We found a very low occurrence of treatment failure in our study cohort across all three groups, which is consistent with other studies of SSSS that report an overall good prognosis and low recurrence and/or readmission rates.6,16,24 The low prevalence of treatment failure, however, precluded our ability to detect small differences among antibiotic regimen groups that may exist.
We observed that cost differed significantly across antibiotic regimen groups, with lowest cost associated with clindamycin monotherapy in adjusted analysis despite similar LOS. Even with our illness-severity adjustment, there may have been other unmeasured factors resulting in the higher cost associated with the combination groups. Hence, we also examined cost breakdown with a stratified analysis by illness severity. We found that pharmacy costs were significantly different among antibiotic groups in both illness severity strata, whereas those with low illness severity also differed by laboratory and supply costs. Thus, pharmacy cost differences may be the largest driver in the cost differential among groups. Lower cost in the clindamycin monotherapy group is likely due to administration of a single antibiotic. The reason for supply and laboratory cost differences is uncertain, but higher cost in the clindamycin plus MRSA group could possibly be from laboratory testing related to drug monitoring (eg, renal function testing or drug levels). While other studies have reported costs for hospitalized children with SSSS associated with different patient characteristics or diagnostic testing,1,16 to our knowledge, no other studies have reported cost related to antibiotic regimens for SSSS. As healthcare reimbursements shift to value-based models, identifying treatment regimens with equal efficacy but lower cost will become increasingly important. Future studies should also examine other covariates and outcomes, such as oral vs parenteral antibiotic use, use of monitoring laboratories related to antibiotic choice, and adverse drug effects.
Several strengths and additional limitations apply to our study. Our study is one of the few to describe outcomes associated with antibiotic regimens for children with SSSS. With the PHIS database, we were able to include a large number of children with SSSS from children’s hospitals across the United States. Although the PHIS database affords these strengths, there are limitations inherent to administrative data. Children with SSSS were identified by documented ICD-9 and ICD-10 diagnostic codes, which might lead to misclassification. However, misclassification is less likely because only one ICD-9 and ICD-10 code exists for SSSS, and the characteristics of this condition are specific. Also, diagnostic codes for other dermatologic conditions (eg, scarlet fever) were excluded to further reduce the chance of misclassification. A limitation to our use of PHIS billing codes was the inability to confirm the dosage of antibiotics given, the number of doses, or whether antibiotics were prescribed upon discharge. Another limitation is that children whose antibiotic therapy was changed during hospitalization (eg, from clindamycin monotherapy to cefazolin monotherapy) were categorized into the combination groups. However, the sensitivity analysis performed based on a stricter antibiotic group definition (receipt of both antibiotics on at least 2 calendar days) did not alter the outcomes, which is reassuring. We were unable to assess the use of targeted antibiotic therapy because clinical data (eg, microbiology results) were not available. However, this may be less important because some literature suggests that cultures for S aureus are obtained infrequently2 and may be difficult to interpret when obtained,25 since culture growth can represent colonization rather than causative strains. An additional limitation is that administrative data do not include certain clinical outcomes, such as fever duration or degree of skin involvement, which could have differed among the groups. Last, the PHIS database only captures revisits or readmissions to PHIS hospitals, and so we are unable to exclude the possibility of a child being seen at or readmitted to another hospital.
Due to the observational design of this study and potential for incomplete measurement of illness severity, we recommend a future prospective trial with randomization to confirm these findings. One possible reason that LOS did not differ among groups is that the burden of clindamycin-resistant strains in our cohort could be low, and the addition of MSSA or MRSA coverage does not result in a clinically important increase in S aureus coverage. However, pooled pediatric hospital antibiogram data suggest the overall rate of clindamycin resistance is close to 20% in hospitals located in all US regions.26 Limited studies also suggest that MSSA may be the predominant pathogen associated with SSSS.2,9 To address this, future randomized trials could compare the effectiveness of clindamycin monotherapy to MSSA-specific agents like cefazolin or nafcillin. Unfortunately, anti-MSSA monotherapy was not evaluated in our study because very few children received this treatment. Using monotherapy as opposed to multiple antibiotics has the potential to promote antibiotic stewardship for antistaphylococcal antibiotics in the management of SSSS. Reducing unnecessary antibiotic use not only potentially affects antibiotic resistance, but could also benefit patients in reducing possible side effects, cost, and IV catheter complications.27 However, acknowledging our study limitations, our findings should be applied cautiously in clinical settings, in the context of local antibiogram data, individual culture results, and specific patient factors. The local clindamycin resistance rate for both MSSA and MRSA should be considered. Many antibiotics chosen to treat MRSA—such as vancomycin and trimethoprim/sulfamethoxazole—will also have anti-MSSA activity and may have lower local resistance rates than clindamycin. Practitioners may also consider how each antibiotic kills bacteria; for example, beta-lactams rely on bacterial replication, but clindamycin does not. Each factor should influence how empiric treatment, whether monotherapy or combination, is chosen for children with SSSS.
CONCLUSION
In this large, multicenter cohort of hospitalized children with SSSS, we found that the addition of MSSA or MRSA coverage to clindamycin monotherapy was not associated with differences in outcomes of hospital LOS and treatment failure. Furthermore, clindamycin monotherapy was associated with lower overall cost. Prospective randomized studies are needed to confirm these findings and assess whether clindamycin monotherapy, monotherapy with an anti-MSSA antibiotic, or alternative regimens are most effective for treatment of children with SSSS.
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
1. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in United States children. Br J Dermatol. 2018;178(3):704-708. https://doi.org/10.1111/bjd.16097
2. Hulten KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases caused by ST121 in Houston, TX. Pediatr Infect Dis J. 2020;39(1):30-34. https://doi.org/10.1097/INF.0000000000002499
3. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018;78(2):404-406. https://doi.org/10.1016/j.jaad.2017.09.023
4. Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720-726. https://doi.org/10.3201/eid1405.070153
5. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627-633. https://doi.org/10.3928/00904481-20100922-02
6. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224-242.
7. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418-1423. https://doi.org/10.1111/jdv.12541
8. Hodille E, Rose W, Diep BA, Goutelle S, Lina G, Dumitrescu O. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887-917. https://doi.org/10.1128/CMR.00120-16
9. Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305-308. https://doi.org/10.1111/pde.12195
10. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511-1516. https://doi.org/10.1086/340212
11. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006;44(6):2119-2125. https://doi.org/10.1128/JCM.02690-05
12. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864-870. https://doi.org/10.1086/381972
13. Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of “static vs cidal”: a systemic literature review. Clin Infect Dis. 2018;66(9):1470-1474. https://doi.org/10.1093/cid/cix1127
14. Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819-821. https://doi.org/10.1097/00006454-200009000-00002
15. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048
16. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
17. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
18. Sauberan JS, Bradley JS. Antimicrobial agents. In: Long SS, ed. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018:1499-1531.
19. Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965-969. https://doi.org/10.1542/peds.2004-0650
20. Williams DJ, Cooper WO, Kaltenbach LA, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics. 2011;128(3):e479-487. https://doi.org/10.1542/peds.2010-3681
21. Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. J Med Case Rep. 2018;12(1):20. https://doi.org/ 10.1186/s13256-017-1533-7
22. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47. https://doi.org/10.1111/pde.12114
23. Markham JL, Hall M, Queen MA, et al. Variation in antibiotic selection and clinical outcomes in infants <60 days hospitalized with skin and soft tissue infections. Hosp Pediatr. 2019;9(1):30-38. https://doi.org/10.1542/hpeds.2017-0237
24. Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134-e137. https://doi.org/10.1055/s-0037-1603971
25. Ladhani S, Robbie S, Chapple DS, Joannou CL, Evans RW. Isolating Staphylococcus aureus from children with suspected Staphylococcal scalded skin syndrome is not clinically useful. Pediatr Infect Dis J. 2003;22(3):284-286.
26. Tamma PD, Robinson GL, Gerber JS, et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol. 2013;34(12):1244-1251. https://doi.org/10.1086/673974
27. Unbeck M, Forberg U, Ygge BM, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015;104(6):566-574. https://doi.org/10.1111/apa.12963
©2021 Society of Hospital Medicine