User login
Collateral damage in the war on obesity
In a recent New York Times opinion article, author Aubrey Gordon claims that since a visit to her pediatrician in fourth grade she has felt like an “enemy combatant in the nation’s war on childhood obesity.” (“Leave Fat Kids Alone,” Nov. 13, 2020).
At that unfortunate encounter, she recalls being told that “You’ll be thin and beautiful ... If you can just stay the same weight.” In retrospect she feels that the comment by her well-meaning but misguided physician “planted the seeds of depression” that have plagued her ever since.
Ms. Gordon goes on to list the many national and local initiatives that have done little to bend the curve in this country’s obesity trajectory but have succeeded in targeting bodies like hers as an epidemic and have resulted in her and thousands of other children being treated as “its virus personified.”
It is deeply troubling to read of her journey through life as collateral damage in a failed war effort, but Ms. Gordon offers little advice to us other than that we stop doing what we have been doing. It hasn’t been helping and it’s not working.
I suspect she would agree that obesity is one of our nation’s most serious public health problems. There is voluminous evidence of the association of obesity with cardiac disease, cancer, mental health challenges, and more recently COVID-19 – just to name a few. If blaming obese children who are the victims is counterproductive where do we point the finger? It is tempting to blame parents and certainly they deserve some culpability. Some parents could have created less obesity-enabling environments through healthier menu choices and done a better job discouraging sedentary behaviors. However, some families lack the access to, or the resources to, provide less calorie-dense food options. We know that many obese children have parents who have been obese themselves since childhood and we know that breaking the obesity cycle can be extremely difficult. Do we extend the sweep of our finger-pointing to include grandparents and great grandparents?
While guilt can be a powerful motivating force, obesity seems to be one of those conditions in which by the time it becomes obvious to a family, the die is cast and blaming the victim or her parents is going to do little more than engender bad feelings. We have done more than enough. In fact, Ms. Gordon’s commentary suggests we have gone too far in creating public opinion that being lean is healthy and being overweight is bad. More motivational testimonials will merely add to the shaming.
Obesity is clearly a societal problem and selectively targeting the victims is not the answer. A famine would certainly lower our national body mass index, but not even the most callous among us would include it on the list of options. Attempts at levying a hefty tax on sweetened beverages have been attempted sporadically around the country without much success. We are a nation that cherishes our personal freedoms and unfortunately this includes the freedom to do some things the aren’t in our own best interests.
You could argue that this leaves us with education as our only hope of turning the tide. However, educating without characterizing the obese among us as bad, ugly, and undisciplined people is a public relations challenge of heroic proportions. Choosing language and images that somehow convey the idea that although obesity is bad being obese doesn’t make you a bad or ugly person is walking along a fine semantic edge.
If I sound discouraged, you are reading me correctly. As pediatricians, we are left doing the few things that have been shown to make a difference. This means promoting breastfeeding and encouraging thoughtful introduction of solid foods; both strategies can be done before the child can hear our well-intentioned but misguided words of encouragement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In a recent New York Times opinion article, author Aubrey Gordon claims that since a visit to her pediatrician in fourth grade she has felt like an “enemy combatant in the nation’s war on childhood obesity.” (“Leave Fat Kids Alone,” Nov. 13, 2020).
At that unfortunate encounter, she recalls being told that “You’ll be thin and beautiful ... If you can just stay the same weight.” In retrospect she feels that the comment by her well-meaning but misguided physician “planted the seeds of depression” that have plagued her ever since.
Ms. Gordon goes on to list the many national and local initiatives that have done little to bend the curve in this country’s obesity trajectory but have succeeded in targeting bodies like hers as an epidemic and have resulted in her and thousands of other children being treated as “its virus personified.”
It is deeply troubling to read of her journey through life as collateral damage in a failed war effort, but Ms. Gordon offers little advice to us other than that we stop doing what we have been doing. It hasn’t been helping and it’s not working.
I suspect she would agree that obesity is one of our nation’s most serious public health problems. There is voluminous evidence of the association of obesity with cardiac disease, cancer, mental health challenges, and more recently COVID-19 – just to name a few. If blaming obese children who are the victims is counterproductive where do we point the finger? It is tempting to blame parents and certainly they deserve some culpability. Some parents could have created less obesity-enabling environments through healthier menu choices and done a better job discouraging sedentary behaviors. However, some families lack the access to, or the resources to, provide less calorie-dense food options. We know that many obese children have parents who have been obese themselves since childhood and we know that breaking the obesity cycle can be extremely difficult. Do we extend the sweep of our finger-pointing to include grandparents and great grandparents?
While guilt can be a powerful motivating force, obesity seems to be one of those conditions in which by the time it becomes obvious to a family, the die is cast and blaming the victim or her parents is going to do little more than engender bad feelings. We have done more than enough. In fact, Ms. Gordon’s commentary suggests we have gone too far in creating public opinion that being lean is healthy and being overweight is bad. More motivational testimonials will merely add to the shaming.
Obesity is clearly a societal problem and selectively targeting the victims is not the answer. A famine would certainly lower our national body mass index, but not even the most callous among us would include it on the list of options. Attempts at levying a hefty tax on sweetened beverages have been attempted sporadically around the country without much success. We are a nation that cherishes our personal freedoms and unfortunately this includes the freedom to do some things the aren’t in our own best interests.
You could argue that this leaves us with education as our only hope of turning the tide. However, educating without characterizing the obese among us as bad, ugly, and undisciplined people is a public relations challenge of heroic proportions. Choosing language and images that somehow convey the idea that although obesity is bad being obese doesn’t make you a bad or ugly person is walking along a fine semantic edge.
If I sound discouraged, you are reading me correctly. As pediatricians, we are left doing the few things that have been shown to make a difference. This means promoting breastfeeding and encouraging thoughtful introduction of solid foods; both strategies can be done before the child can hear our well-intentioned but misguided words of encouragement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In a recent New York Times opinion article, author Aubrey Gordon claims that since a visit to her pediatrician in fourth grade she has felt like an “enemy combatant in the nation’s war on childhood obesity.” (“Leave Fat Kids Alone,” Nov. 13, 2020).
At that unfortunate encounter, she recalls being told that “You’ll be thin and beautiful ... If you can just stay the same weight.” In retrospect she feels that the comment by her well-meaning but misguided physician “planted the seeds of depression” that have plagued her ever since.
Ms. Gordon goes on to list the many national and local initiatives that have done little to bend the curve in this country’s obesity trajectory but have succeeded in targeting bodies like hers as an epidemic and have resulted in her and thousands of other children being treated as “its virus personified.”
It is deeply troubling to read of her journey through life as collateral damage in a failed war effort, but Ms. Gordon offers little advice to us other than that we stop doing what we have been doing. It hasn’t been helping and it’s not working.
I suspect she would agree that obesity is one of our nation’s most serious public health problems. There is voluminous evidence of the association of obesity with cardiac disease, cancer, mental health challenges, and more recently COVID-19 – just to name a few. If blaming obese children who are the victims is counterproductive where do we point the finger? It is tempting to blame parents and certainly they deserve some culpability. Some parents could have created less obesity-enabling environments through healthier menu choices and done a better job discouraging sedentary behaviors. However, some families lack the access to, or the resources to, provide less calorie-dense food options. We know that many obese children have parents who have been obese themselves since childhood and we know that breaking the obesity cycle can be extremely difficult. Do we extend the sweep of our finger-pointing to include grandparents and great grandparents?
While guilt can be a powerful motivating force, obesity seems to be one of those conditions in which by the time it becomes obvious to a family, the die is cast and blaming the victim or her parents is going to do little more than engender bad feelings. We have done more than enough. In fact, Ms. Gordon’s commentary suggests we have gone too far in creating public opinion that being lean is healthy and being overweight is bad. More motivational testimonials will merely add to the shaming.
Obesity is clearly a societal problem and selectively targeting the victims is not the answer. A famine would certainly lower our national body mass index, but not even the most callous among us would include it on the list of options. Attempts at levying a hefty tax on sweetened beverages have been attempted sporadically around the country without much success. We are a nation that cherishes our personal freedoms and unfortunately this includes the freedom to do some things the aren’t in our own best interests.
You could argue that this leaves us with education as our only hope of turning the tide. However, educating without characterizing the obese among us as bad, ugly, and undisciplined people is a public relations challenge of heroic proportions. Choosing language and images that somehow convey the idea that although obesity is bad being obese doesn’t make you a bad or ugly person is walking along a fine semantic edge.
If I sound discouraged, you are reading me correctly. As pediatricians, we are left doing the few things that have been shown to make a difference. This means promoting breastfeeding and encouraging thoughtful introduction of solid foods; both strategies can be done before the child can hear our well-intentioned but misguided words of encouragement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Physicians react: Doctors worry about patients reading their clinical notes
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
Patients will soon be able to read the notes that physicians make during an episode of care, as well as information about diagnostic testing and imaging results, tests for STDs, fetal ultrasounds, and cancer biopsies. This open access is raising concerns among physicians.
As part of the 21st Century Cures Act, patients have the right to see their medical notes. Known as Open Notes, the policy will go into effect on April 5, 2021. The Department of Health & Human Services recently changed the original start date, which was to be Nov. 2, 2020.
The mandate has some physicians worrying about potential legal risks and possible violation of doctor-patient confidentiality. When asked to share their views on the new Open Notes mandate, many physicians expressed their concerns but also cited some of the positive effects that could come from this.
Potentially more legal woes for physicians?
A key concern raised by one physician commenter is that patients could misunderstand legitimate medical terminology or even put a physician in legal crosshairs. For example, a medical term such as “spontaneous abortion” could be misconstrued by patients. A physician might write notes with the idea that a patient is reading them and thus might alter those notes in a way that creates legal trouble.
“This layers another level of censorship and legal liability onto physicians, who in attempting to be [politically correct], may omit critical information or have to use euphemisms in order to avoid conflict,” one physician said.
She also questioned whether notes might now have to be run through legal counsel before being posted to avoid potential liability.
Another doctor questioned how physicians would be able to document patients suspected of faking injuries for pain medication, for example. Could such documentation lead to lawsuits for the doctor?
As one physician noted, some patients “are drug seekers. Some refuse to aid in their own care. Some are malingerers. Not documenting that is bad medicine.”
The possibility of violating doctor-patient confidentiality laws, particularly for teenagers, could be another negative effect of Open Notes, said one physician.
“Won’t this violate the statutes that teenagers have the right to confidential evaluations?” the commenter mused. “If charts are to be immediately available, then STDs and pregnancies they weren’t ready to talk about will now be suddenly known by their parents.”
One doctor has already faced this issue. “I already ran into this problem once,” he noted. “Now I warn those on their parents’ insurance before I start the visit. I have literally had a patient state, ‘well then we are done,’ and leave without being seen due to it.”
Another physician questioned the possibility of having to write notes differently than they do now, especially if the patients have lower reading comprehension abilities.
One physician who uses Open Notes said he receives patient requests for changes that have little to do with the actual diagnosis and relate to ancillary issues. He highlighted patients who “don’t want psych diagnosis in their chart or are concerned a diagnosis will raise their insurance premium, so they ask me to delete it.”
Will Open Notes erode patient communication?
One physician questioned whether it would lead to patients being less open and forthcoming about their medical concerns with doctors.
“The main problem I see is the patient not telling me the whole story, or worse, telling me the story, and then asking me not to document it (as many have done in the past) because they don’t want their spouse, family, etc. to read the notes and they have already given their permission for them to do so, for a variety of reasons,” he commented. “This includes topics of STDs, infidelity, depression, suicidal thoughts, and other symptoms the patient doesn’t want their family to read about.”
Some physicians envision positive developments
Many physicians are unconcerned by the new mandate. “I see some potential good in this, such as improving doctor-patient communication and more scrupulous charting,” one physician said.
A doctor working in the U.S. federal health care system noted that open access has been a part of that system for decades.
“Since health care providers work in this unveiled setting for their entire career, they usually know how to write appropriate clinical notes and what information needs to be included in them,” he wrote. “Now it’s time for the rest of the medical community to catch up to a reality that we have worked within for decades now.
“The world did not end, malpractice complaints did not increase, and physician/patient relationships were not damaged. Living in the information age, archaic practices like private notes were surely going to end at some point.”
One doctor who has been using Open Notes has had experiences in which the patient noted an error in the medical chart that needed correcting. “I have had one patient correct me on a timeline in the HPI which was helpful and I made the requested correction in that instance,” he said.
Another physician agreed. “I’ve had patients add or correct valuable information I’ve missed. Good probably outweighs the bad if we set limits on behaviors expressed by the personality disordered group. The majority of people don’t seem to care and still ask me ‘what would you do’ or ‘tell me what to do.’ It’s all about patient/physician trust.”
Another talked about how Open Notes should have little or no impact. “Here’s a novel concept – talking to our patients,” he commented. “There is nothing in every one of my chart notes that has not already been discussed with my patients and I dictate (speech to text) my findings and plan in front of them. So, if they are reviewing my office notes, it will only serve to reinforce what we have already discussed.”
“I don’t intend to change anything,” he added. “Chances are if they were to see a test result before I have a chance to discuss it with them, they will have already ‘Googled’ its meaning and we can have more meaningful interaction if they have a basic understanding of the test.”
“I understand that this is anxiety provoking, but in general I think it is appropriate for patients to have access to their notes,” said another physician. “If physicians write lousy notes that say they did things they didn’t do, that fail to actually state a diagnosis and a plan (and they often do), that is the doc’s problem, not the patient’s.”
A version of this article first appeared on Medscape.com.
The importance of community pediatric hospital medicine
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
ADHD through the retrospectoscope
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Isolation in response to COVID-19 pandemic has driven many people to reestablish long forgotten connections between old friends and geographically distant relatives. Fed by the ease in which Zoom and other electronic miracles can bring once familiar voices and faces into our homes, we no longer need to wait until our high school or college reunions to reconnect.
The Class of 1962 at Pleasantville (N.Y.) High School has always attracted an unusually large number of attendees at its reunions, and its exuberant response to pandemic-fueled mini Zoom reunions is not surprising. With each virtual gathering we learn and relearn more about each other. I had always felt that because my birthday was in December that I was among the very youngest in my class. (New York’s school enrollment calendar cutoff is in December.) However, I recently learned that some of my classmates were even younger, having been born in the following spring.
This revelation prompted a discussion among the younger septuagenarians about whether we felt that our relative immaturity, at least as measured by the calendar, affected us. It was generally agreed that for the women, being younger seemed to present little problem. For, the men there were a few for whom immaturity put them at an athletic disadvantage. But, there was uniform agreement that social immaturity made dating an uncomfortable adventure. No one felt that his or her immaturity placed them at an academic disadvantage. Of course, all of these observations are heavily colored by the bias of those who have chosen to maintain contact with classmates.
A recent flurry of papers and commentaries about relative age at school entry and the diagnosis of attention deficit/hyperactivity disorder prompted me to ask my Zoom mates if they could recall anyone whom they would label as having exhibited the behavior we have all come to associate with ADHD (Vuori M et al. Children’s relative age and ADHD medication use: A Finnish population-based study. Pediatrics 2020 Oct. doi: 10.1542/peds.2019-4046, and Butter EM. Keeping relative age effects and ADHD care in context. Pediatrics. 2020;146[4]:e2020022798).
We could all recall classmates who struggled academically and seemed to not be paying attention. However, when one includes the hyperactivity descriptor we couldn’t recall anyone whose in-classroom physical activity drew our attention. Of course, there were many shared anecdotes about note passing, spitball throwing, and out-of-class shenanigans. But, from the perspective of behavior that disrupted the classroom there were very few. And, not surprisingly, given the intervening 6 decades, none of us could make an association between immaturity and the behavior.
While I have very few memories of what happened when I was in grade school, many of my classmates have vivid recollections of events both mundane and dramatic even as far back as first and second grade. Why do none of them recall classmates whose behavior would in current terminology be labeled as ADHD?
Were most of us that age bouncing off the walls and so there were no outliers? Were the teachers more tolerant because they expected that many children, particularly the younger ones, would be more physically active? Or, maybe we arrived at school, even those who were chronologically less mature, having already been settled down by home environments that neither fostered nor tolerated hyperactivity?
If you ask a pediatrician over the age of 70 if he or she recalls being taught anything about ADHD in medical school or seeing any children in his or her first years of practice who would fit the current diagnostic criteria, you will see them simply shrug. ADHD was simply not on our radar in the 1970s and 1980s. And it’s not because radar hadn’t been invented. We pediatricians were paying attention, and I trust in my high school classmates’ observations. I am sure there were isolated cases that could easily have been labeled as ADHD if the term had existed. But, the volume of hyperactive children a pediatrician sees today in the course of a normal office day just didn’t exist.
I have trouble believing that this dramatic increase in frequency is the result of accumulating genetic damage from Teflon cookware or climate change or air pollution. Although I am open to any serious attempt to explain the phenomenon I think we should look first into the home environment in which children are being raised. Sleep schedules, activity, and amusement opportunities as well as discipline styles – just to name a few – are far different now than before the ADHD diagnosis overtook the landscape.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Social isolation at the time of social distancing
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Reducing COVID-19 opioid deaths
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.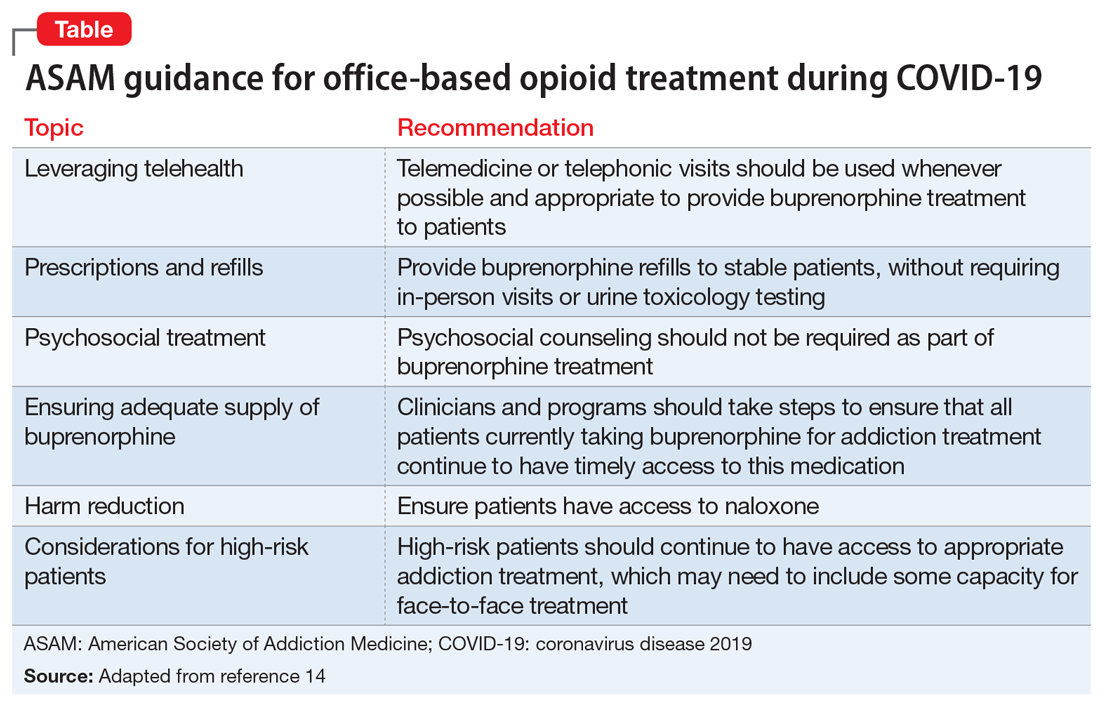
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Should your practice be acquired by private equity?
Dear colleagues and friends,
The Perspectives series continues! Few current issues in Gastroenterology practice are as passionately debated as those associated with private equity. In this edition, our own Dr. John Allen and Dr. Marc Sonenshine explain private equity’s evolution in the GI field, dispel misconceptions, and dissect the central question of whether it is right for your practice. Thank you for your support, and I hope you will find the discussions enlightening and relevant to your practices. As always, I welcome your comments and suggestions for future topics at ginews@gastro.org.
Charles Kahi, MD, MS, AGAF, is a professor of medicine, Indiana University School of Medicine, Indianapolis. He is also an Associate Editor for GI & Hepatology News.
Yes
But, at a minimum, you should absolutely lean-in, listen, and learn.
The physician leadership team at Atlanta Gastroenterology Associates has been focused on developing strategies and partnerships that strengthen our ability to thrive in our marketplace while also fending off threats to our mission. The path to forming the managed services organization (MSO) United Digestive (UD) through our agreement with the private equity firm Frazier Healthcare Partners was arduous and required a significant investment of resources and time. Like at Atlanta Gastroenterology Associates, many influential leaders within our field, also supported by their physician partners, have concluded that the investment of a private equity firm to build an MSO led by professional business executives will reduce the administrative stresses looming over the traditional independent gastroenterologist business model. Now, and after almost 2 years as a member of UD, I unequivocally believe my ability to provide timely, high-quality, and affordable care to my community is currently more stable and in a stronger position for the future.
Like we did in deciding whether to establish a formal relationship with a private equity–backed platform group, here are some critical questions you should explore and answer:
-What advantages and disadvantages will being a part of a private equity–backed MSO group bring to our patients, our practice, our team, and our providers?
-What forces threaten our practice’s ability to remain viable and pertinent in both the near and long term? And, how can our group ward off these threats?
-There are many private equity firms interested in our practice as well as already established platform groups. How do we decide which is best?
-If remaining completely independent is not a sustainable long-term option, why not just become employed by a hospital, join a strategic partner, or form/join a multi-specialty group or accountable care organization (ACO)?
In the first 2 years, UD has answered many of the questions and executed on desired priorities. Our management team helped us to navigate the chaos of COVID, and UD still remains on target to meet many annual budget goals as well as end of the year financial targets. Processes and enhanced technologies like real-time dashboards provide immediate insight into all aspects of our business, allowing for more analytical decision-making. Our payor and vendor negotiations yielded stronger returns than anticipated leading to material earnings. The revamped patient services center improved clinic utilization rates, reduced patient call wait times, deployed an online patient scheduling option, and employed medical assistants for handling clinical phone matters.
Most importantly, not one change at UD has negatively affected our clinical autonomy and decision-making. The MSO and its management team has steered all medical-related issues to our chief medical officer and physician executive committee. Moreover, there was much less consternation amongst partners when the time arose for significant capital expenditures (i.e., upgrading our endoscopic equipment, instituting a new electronic health record and practice management system, or surviving the cash-flow crunch during the beginning of COVID), as our annual compensation was not affected.
A few broader points to consider that pertain to private equity activity in physician services (i.e., not specific to gastroenterology or UD):
- Private equity firms invest money in private companies with the expectation of superior financial returns. Their principals are searching for opportunities with significant upside and potential to generate the necessary earnings for such returns. In fragmented fields, there is potential to use MSO relationships to consolidate providers into a larger organization. Then, economies of scale will create benefits through sharing and saving costs, increased leverage in contract negotiations, and augmenting organic, de-novo growth through the addition of new lines of services. Make sure you understand the overall business strategy, how your addition impacts the overall MSO, and how you may personally benefit.
- It appears that many groups are overly-focused on the deal multiple, yet understanding the comprehensive value of a deal goes far beyond the multiple. A complete evaluation must also explore the principles of the compensation model, rollover equity, compounding interest, tax deferral strategies, utilization of debt, and potential earn-out terms. Experienced legal counsel can shed light on these issues.
- The timing in one’s professional career may cloud the perspective of whether partnering with a private equity group through an MSO is wise. However, I would argue the more important perspective is the judgement of the trajectory of your current practice versus adopting a new business model. If a practice can skillfully withstand the headwinds of the regulatory challenges, fierce competition for patient referrals from hospitals plus new provider entrants, and continued downward pressure from payors, then remaining independent may be reasonable. On the other hand, there is great value, security and protection of being within an organization with sizeable financial and experiential capital with like-minded colleagues.
- Many independent practices are also often approached by local and national hospitals. Relationships with hospitals are popular as they too offer professional management teams lessening administrative burdens, often secure referral networks and higher contractual rates for services rendered. Unlike with a PE deal, these partnerships may limit patient choice, almost inevitably increase patient cost, and do not include equity for the provider.
While there are many questions that need to be answered for each practice considering joining a PE backed MSO, what is clear from my experience is that there are enough benefits to such a partnership that it should be explored to understand how it might improve your ability to serve your patients and secure a long term “home” for your practice, providers, and employees.
Marc Sonenshine, MD, is a partner in United Digestive and the chairman of medicine at Northside Hospital, Atlanta.
A note of caution
Is private equity good for gastroenterology? The answer is not a definitive “yes” or “no”; it is “depends”. That said, private equity is here so you must understand the implications.
Private equity is an alternative investment strategy focused on assets not listed on a public exchange. Capital usually is derived from investors who can tolerate risk with the hope of a high return such as pension funds, university endowments, and high net-worth individuals. Capital is collected within a fund (or funds) managed by a professional team who invests in, or buys private companies using internal capital leveraged with debt (leveraged buy-out or LBO). Assets and governance both are sold to fund managers, who restructure operations, centralize or standardize workflows, acquire similar companies to achieve economies of scale, and eventually resell the new company to another entity (usually a larger private equity fund). Typically, the resale (second bite) occurs 5-7 years after initial acquisition and during that 5-7-year period, Private equity funds expect a substantial (10%-20%) annual return on investment resulting from revenue enhancement, new service lines, and overhead reduction.
Since 2016, private equity has actively courted GI practices and there now have been over 20 closed deals. Private equity fund managers have specific expertise in valuing GI practices, enhancing revenue, reducing overhead, collecting other regional (and sometimes distant) practices, centralizing operations, converting all practices to a single EMR, payer negotiations, and other practice functions, while leaving clinical care decisions to providers. Although this postacquisition scenario sounds attractive, there are downsides.
First, let’s review the upsides. As a mature partner in a highly valued practice, you could expect an acquisition payment in the range of $1 million (subject to capital gains tax). You receive a gross distribution based on a purchase multiple (9-12 times EBITA – a measure of your annual profit), minus investment in the new company, and annual payments to the Management Services Organization or MSO. Your income going forward will be reduced by annual obligations to the private equity fund, about 10%-40% of your production. Typically, a second sale occurs between 5 and 7 years after acquisition (yet to occur in GI), where the new company sells for another EBITA multiple (so it is in your financial interest to keep increasing practice value). Even with a modest EBITA multiple, you might net an amount that is double the initial acquisition payout. A senior partner could benefit financially in ways not readily available through other avenues of retirement.
Another benefit is access to capital to acquire more practices, bring new technology, improve facilities, integrate clinical and practice information, and weather reduced demand (like occurred with COVID-19). Independent practices are struggling to incorporate digital technologies that patients now expect, enhanced (and more expensive) endoscopes, new service lines, and the demand for real patient outcomes data during payer or health system negotiations.
So, what takes private equity from a clear “yes” to a “depends”? During COVID-19, physician incomes dropped substantially, since any revenue went first to pay bank debt, then fund fees, payment of overhead (leases, vendor commitments, residual staff), and finally to the doctors. A recent Medscape survey of 5,000 US physicians, revealed that 62% of MDs saw their income drop (23% by more than 50%). Physicians employed by health systems did not see nearly that income drop.
Once a practice is sold, physicians lose autonomy. When you are acquired by a private equity fund, the primary goal of the fund is a financial target. Long-term staff may be downsized, you may be asked to use equipment or supplies that are not to your standard, relationships with regional payers or health systems may become adversarial, productivity targets may alter your patient care decisions (more procedures, less external referrals), and relations with your partners may be strained (younger versus older).
A young physician who enters a private equity–acquired practice may work for decades at an income level discounted from preacquisition levels. They face a substantial buy-in if they hope to benefit from the second sale. Of course, one might argue that future salaries for all gastroenterologists will be reduced by increasing technology costs (endoscope companies are adding AI – can’t wait to see their pricing), reduced reimbursements, and increasing labor and supply costs. Serious threats to colonoscopy-based cancer screening are here, a development that makes future values of GI practices more tenuous. Finally, our payer mix will be worse than before COVID-19 because of long-term financial strains on the US economy.
We have to reflect on a similar practice acquisition trend that occurred in the 1990s, where practice management companies bought independent practices. While times are different now (for many reasons), all but one of those companies went bankrupt and the acquired practices had to rebuild from the ground up. Private equity funds that are heavily leveraged are especially vulnerable, as can be seen by current bankruptcies of large established companies (Hertz, Neiman-Marcus, and others).
Finally, we have to ask ourselves how patients will view your practice as more of us become acquired by financially driven partners. No matter how we paint private equity acquisitions, people understand that these funds are financially driven and practice sales are an income enhancement play for physicians. In 1986, Arnold Relman (Editor of the New England Journal of Medicine) gave two Tanner Lectures on human values at the University of Utah. He asked the following question:
“Is medical care a consumer good like any other, a commercial service provided by skilled vendors for consumers willing to pay the market price, or is there something fundamentally different about the relation between doctor and patient?”
I am not a Luddite, nor am I Don Quixote jousting at windmills. I do, however, want you to consider carefully before giving up on the traditional practice models that made our specialty what it is.
John I. Allen, MD, MBA, AGAF, is clinical professor of medicine, department of internal medicine, division of gastroenterology and hepatology, Institute for Healthcare Policy and Innovation, University School of Medicine, Chief Clinical Officer, University of Michigan Medical Group, Ann Arbor. He has no disclosures and takes full responsibility for the content.
Dear colleagues and friends,
The Perspectives series continues! Few current issues in Gastroenterology practice are as passionately debated as those associated with private equity. In this edition, our own Dr. John Allen and Dr. Marc Sonenshine explain private equity’s evolution in the GI field, dispel misconceptions, and dissect the central question of whether it is right for your practice. Thank you for your support, and I hope you will find the discussions enlightening and relevant to your practices. As always, I welcome your comments and suggestions for future topics at ginews@gastro.org.
Charles Kahi, MD, MS, AGAF, is a professor of medicine, Indiana University School of Medicine, Indianapolis. He is also an Associate Editor for GI & Hepatology News.
Yes
But, at a minimum, you should absolutely lean-in, listen, and learn.
The physician leadership team at Atlanta Gastroenterology Associates has been focused on developing strategies and partnerships that strengthen our ability to thrive in our marketplace while also fending off threats to our mission. The path to forming the managed services organization (MSO) United Digestive (UD) through our agreement with the private equity firm Frazier Healthcare Partners was arduous and required a significant investment of resources and time. Like at Atlanta Gastroenterology Associates, many influential leaders within our field, also supported by their physician partners, have concluded that the investment of a private equity firm to build an MSO led by professional business executives will reduce the administrative stresses looming over the traditional independent gastroenterologist business model. Now, and after almost 2 years as a member of UD, I unequivocally believe my ability to provide timely, high-quality, and affordable care to my community is currently more stable and in a stronger position for the future.
Like we did in deciding whether to establish a formal relationship with a private equity–backed platform group, here are some critical questions you should explore and answer:
-What advantages and disadvantages will being a part of a private equity–backed MSO group bring to our patients, our practice, our team, and our providers?
-What forces threaten our practice’s ability to remain viable and pertinent in both the near and long term? And, how can our group ward off these threats?
-There are many private equity firms interested in our practice as well as already established platform groups. How do we decide which is best?
-If remaining completely independent is not a sustainable long-term option, why not just become employed by a hospital, join a strategic partner, or form/join a multi-specialty group or accountable care organization (ACO)?
In the first 2 years, UD has answered many of the questions and executed on desired priorities. Our management team helped us to navigate the chaos of COVID, and UD still remains on target to meet many annual budget goals as well as end of the year financial targets. Processes and enhanced technologies like real-time dashboards provide immediate insight into all aspects of our business, allowing for more analytical decision-making. Our payor and vendor negotiations yielded stronger returns than anticipated leading to material earnings. The revamped patient services center improved clinic utilization rates, reduced patient call wait times, deployed an online patient scheduling option, and employed medical assistants for handling clinical phone matters.
Most importantly, not one change at UD has negatively affected our clinical autonomy and decision-making. The MSO and its management team has steered all medical-related issues to our chief medical officer and physician executive committee. Moreover, there was much less consternation amongst partners when the time arose for significant capital expenditures (i.e., upgrading our endoscopic equipment, instituting a new electronic health record and practice management system, or surviving the cash-flow crunch during the beginning of COVID), as our annual compensation was not affected.
A few broader points to consider that pertain to private equity activity in physician services (i.e., not specific to gastroenterology or UD):
- Private equity firms invest money in private companies with the expectation of superior financial returns. Their principals are searching for opportunities with significant upside and potential to generate the necessary earnings for such returns. In fragmented fields, there is potential to use MSO relationships to consolidate providers into a larger organization. Then, economies of scale will create benefits through sharing and saving costs, increased leverage in contract negotiations, and augmenting organic, de-novo growth through the addition of new lines of services. Make sure you understand the overall business strategy, how your addition impacts the overall MSO, and how you may personally benefit.
- It appears that many groups are overly-focused on the deal multiple, yet understanding the comprehensive value of a deal goes far beyond the multiple. A complete evaluation must also explore the principles of the compensation model, rollover equity, compounding interest, tax deferral strategies, utilization of debt, and potential earn-out terms. Experienced legal counsel can shed light on these issues.
- The timing in one’s professional career may cloud the perspective of whether partnering with a private equity group through an MSO is wise. However, I would argue the more important perspective is the judgement of the trajectory of your current practice versus adopting a new business model. If a practice can skillfully withstand the headwinds of the regulatory challenges, fierce competition for patient referrals from hospitals plus new provider entrants, and continued downward pressure from payors, then remaining independent may be reasonable. On the other hand, there is great value, security and protection of being within an organization with sizeable financial and experiential capital with like-minded colleagues.
- Many independent practices are also often approached by local and national hospitals. Relationships with hospitals are popular as they too offer professional management teams lessening administrative burdens, often secure referral networks and higher contractual rates for services rendered. Unlike with a PE deal, these partnerships may limit patient choice, almost inevitably increase patient cost, and do not include equity for the provider.
While there are many questions that need to be answered for each practice considering joining a PE backed MSO, what is clear from my experience is that there are enough benefits to such a partnership that it should be explored to understand how it might improve your ability to serve your patients and secure a long term “home” for your practice, providers, and employees.
Marc Sonenshine, MD, is a partner in United Digestive and the chairman of medicine at Northside Hospital, Atlanta.
A note of caution
Is private equity good for gastroenterology? The answer is not a definitive “yes” or “no”; it is “depends”. That said, private equity is here so you must understand the implications.
Private equity is an alternative investment strategy focused on assets not listed on a public exchange. Capital usually is derived from investors who can tolerate risk with the hope of a high return such as pension funds, university endowments, and high net-worth individuals. Capital is collected within a fund (or funds) managed by a professional team who invests in, or buys private companies using internal capital leveraged with debt (leveraged buy-out or LBO). Assets and governance both are sold to fund managers, who restructure operations, centralize or standardize workflows, acquire similar companies to achieve economies of scale, and eventually resell the new company to another entity (usually a larger private equity fund). Typically, the resale (second bite) occurs 5-7 years after initial acquisition and during that 5-7-year period, Private equity funds expect a substantial (10%-20%) annual return on investment resulting from revenue enhancement, new service lines, and overhead reduction.
Since 2016, private equity has actively courted GI practices and there now have been over 20 closed deals. Private equity fund managers have specific expertise in valuing GI practices, enhancing revenue, reducing overhead, collecting other regional (and sometimes distant) practices, centralizing operations, converting all practices to a single EMR, payer negotiations, and other practice functions, while leaving clinical care decisions to providers. Although this postacquisition scenario sounds attractive, there are downsides.
First, let’s review the upsides. As a mature partner in a highly valued practice, you could expect an acquisition payment in the range of $1 million (subject to capital gains tax). You receive a gross distribution based on a purchase multiple (9-12 times EBITA – a measure of your annual profit), minus investment in the new company, and annual payments to the Management Services Organization or MSO. Your income going forward will be reduced by annual obligations to the private equity fund, about 10%-40% of your production. Typically, a second sale occurs between 5 and 7 years after acquisition (yet to occur in GI), where the new company sells for another EBITA multiple (so it is in your financial interest to keep increasing practice value). Even with a modest EBITA multiple, you might net an amount that is double the initial acquisition payout. A senior partner could benefit financially in ways not readily available through other avenues of retirement.
Another benefit is access to capital to acquire more practices, bring new technology, improve facilities, integrate clinical and practice information, and weather reduced demand (like occurred with COVID-19). Independent practices are struggling to incorporate digital technologies that patients now expect, enhanced (and more expensive) endoscopes, new service lines, and the demand for real patient outcomes data during payer or health system negotiations.
So, what takes private equity from a clear “yes” to a “depends”? During COVID-19, physician incomes dropped substantially, since any revenue went first to pay bank debt, then fund fees, payment of overhead (leases, vendor commitments, residual staff), and finally to the doctors. A recent Medscape survey of 5,000 US physicians, revealed that 62% of MDs saw their income drop (23% by more than 50%). Physicians employed by health systems did not see nearly that income drop.
Once a practice is sold, physicians lose autonomy. When you are acquired by a private equity fund, the primary goal of the fund is a financial target. Long-term staff may be downsized, you may be asked to use equipment or supplies that are not to your standard, relationships with regional payers or health systems may become adversarial, productivity targets may alter your patient care decisions (more procedures, less external referrals), and relations with your partners may be strained (younger versus older).
A young physician who enters a private equity–acquired practice may work for decades at an income level discounted from preacquisition levels. They face a substantial buy-in if they hope to benefit from the second sale. Of course, one might argue that future salaries for all gastroenterologists will be reduced by increasing technology costs (endoscope companies are adding AI – can’t wait to see their pricing), reduced reimbursements, and increasing labor and supply costs. Serious threats to colonoscopy-based cancer screening are here, a development that makes future values of GI practices more tenuous. Finally, our payer mix will be worse than before COVID-19 because of long-term financial strains on the US economy.
We have to reflect on a similar practice acquisition trend that occurred in the 1990s, where practice management companies bought independent practices. While times are different now (for many reasons), all but one of those companies went bankrupt and the acquired practices had to rebuild from the ground up. Private equity funds that are heavily leveraged are especially vulnerable, as can be seen by current bankruptcies of large established companies (Hertz, Neiman-Marcus, and others).
Finally, we have to ask ourselves how patients will view your practice as more of us become acquired by financially driven partners. No matter how we paint private equity acquisitions, people understand that these funds are financially driven and practice sales are an income enhancement play for physicians. In 1986, Arnold Relman (Editor of the New England Journal of Medicine) gave two Tanner Lectures on human values at the University of Utah. He asked the following question:
“Is medical care a consumer good like any other, a commercial service provided by skilled vendors for consumers willing to pay the market price, or is there something fundamentally different about the relation between doctor and patient?”
I am not a Luddite, nor am I Don Quixote jousting at windmills. I do, however, want you to consider carefully before giving up on the traditional practice models that made our specialty what it is.
John I. Allen, MD, MBA, AGAF, is clinical professor of medicine, department of internal medicine, division of gastroenterology and hepatology, Institute for Healthcare Policy and Innovation, University School of Medicine, Chief Clinical Officer, University of Michigan Medical Group, Ann Arbor. He has no disclosures and takes full responsibility for the content.
Dear colleagues and friends,
The Perspectives series continues! Few current issues in Gastroenterology practice are as passionately debated as those associated with private equity. In this edition, our own Dr. John Allen and Dr. Marc Sonenshine explain private equity’s evolution in the GI field, dispel misconceptions, and dissect the central question of whether it is right for your practice. Thank you for your support, and I hope you will find the discussions enlightening and relevant to your practices. As always, I welcome your comments and suggestions for future topics at ginews@gastro.org.
Charles Kahi, MD, MS, AGAF, is a professor of medicine, Indiana University School of Medicine, Indianapolis. He is also an Associate Editor for GI & Hepatology News.
Yes
But, at a minimum, you should absolutely lean-in, listen, and learn.
The physician leadership team at Atlanta Gastroenterology Associates has been focused on developing strategies and partnerships that strengthen our ability to thrive in our marketplace while also fending off threats to our mission. The path to forming the managed services organization (MSO) United Digestive (UD) through our agreement with the private equity firm Frazier Healthcare Partners was arduous and required a significant investment of resources and time. Like at Atlanta Gastroenterology Associates, many influential leaders within our field, also supported by their physician partners, have concluded that the investment of a private equity firm to build an MSO led by professional business executives will reduce the administrative stresses looming over the traditional independent gastroenterologist business model. Now, and after almost 2 years as a member of UD, I unequivocally believe my ability to provide timely, high-quality, and affordable care to my community is currently more stable and in a stronger position for the future.
Like we did in deciding whether to establish a formal relationship with a private equity–backed platform group, here are some critical questions you should explore and answer:
-What advantages and disadvantages will being a part of a private equity–backed MSO group bring to our patients, our practice, our team, and our providers?
-What forces threaten our practice’s ability to remain viable and pertinent in both the near and long term? And, how can our group ward off these threats?
-There are many private equity firms interested in our practice as well as already established platform groups. How do we decide which is best?
-If remaining completely independent is not a sustainable long-term option, why not just become employed by a hospital, join a strategic partner, or form/join a multi-specialty group or accountable care organization (ACO)?
In the first 2 years, UD has answered many of the questions and executed on desired priorities. Our management team helped us to navigate the chaos of COVID, and UD still remains on target to meet many annual budget goals as well as end of the year financial targets. Processes and enhanced technologies like real-time dashboards provide immediate insight into all aspects of our business, allowing for more analytical decision-making. Our payor and vendor negotiations yielded stronger returns than anticipated leading to material earnings. The revamped patient services center improved clinic utilization rates, reduced patient call wait times, deployed an online patient scheduling option, and employed medical assistants for handling clinical phone matters.
Most importantly, not one change at UD has negatively affected our clinical autonomy and decision-making. The MSO and its management team has steered all medical-related issues to our chief medical officer and physician executive committee. Moreover, there was much less consternation amongst partners when the time arose for significant capital expenditures (i.e., upgrading our endoscopic equipment, instituting a new electronic health record and practice management system, or surviving the cash-flow crunch during the beginning of COVID), as our annual compensation was not affected.
A few broader points to consider that pertain to private equity activity in physician services (i.e., not specific to gastroenterology or UD):
- Private equity firms invest money in private companies with the expectation of superior financial returns. Their principals are searching for opportunities with significant upside and potential to generate the necessary earnings for such returns. In fragmented fields, there is potential to use MSO relationships to consolidate providers into a larger organization. Then, economies of scale will create benefits through sharing and saving costs, increased leverage in contract negotiations, and augmenting organic, de-novo growth through the addition of new lines of services. Make sure you understand the overall business strategy, how your addition impacts the overall MSO, and how you may personally benefit.
- It appears that many groups are overly-focused on the deal multiple, yet understanding the comprehensive value of a deal goes far beyond the multiple. A complete evaluation must also explore the principles of the compensation model, rollover equity, compounding interest, tax deferral strategies, utilization of debt, and potential earn-out terms. Experienced legal counsel can shed light on these issues.
- The timing in one’s professional career may cloud the perspective of whether partnering with a private equity group through an MSO is wise. However, I would argue the more important perspective is the judgement of the trajectory of your current practice versus adopting a new business model. If a practice can skillfully withstand the headwinds of the regulatory challenges, fierce competition for patient referrals from hospitals plus new provider entrants, and continued downward pressure from payors, then remaining independent may be reasonable. On the other hand, there is great value, security and protection of being within an organization with sizeable financial and experiential capital with like-minded colleagues.
- Many independent practices are also often approached by local and national hospitals. Relationships with hospitals are popular as they too offer professional management teams lessening administrative burdens, often secure referral networks and higher contractual rates for services rendered. Unlike with a PE deal, these partnerships may limit patient choice, almost inevitably increase patient cost, and do not include equity for the provider.
While there are many questions that need to be answered for each practice considering joining a PE backed MSO, what is clear from my experience is that there are enough benefits to such a partnership that it should be explored to understand how it might improve your ability to serve your patients and secure a long term “home” for your practice, providers, and employees.
Marc Sonenshine, MD, is a partner in United Digestive and the chairman of medicine at Northside Hospital, Atlanta.
A note of caution
Is private equity good for gastroenterology? The answer is not a definitive “yes” or “no”; it is “depends”. That said, private equity is here so you must understand the implications.
Private equity is an alternative investment strategy focused on assets not listed on a public exchange. Capital usually is derived from investors who can tolerate risk with the hope of a high return such as pension funds, university endowments, and high net-worth individuals. Capital is collected within a fund (or funds) managed by a professional team who invests in, or buys private companies using internal capital leveraged with debt (leveraged buy-out or LBO). Assets and governance both are sold to fund managers, who restructure operations, centralize or standardize workflows, acquire similar companies to achieve economies of scale, and eventually resell the new company to another entity (usually a larger private equity fund). Typically, the resale (second bite) occurs 5-7 years after initial acquisition and during that 5-7-year period, Private equity funds expect a substantial (10%-20%) annual return on investment resulting from revenue enhancement, new service lines, and overhead reduction.
Since 2016, private equity has actively courted GI practices and there now have been over 20 closed deals. Private equity fund managers have specific expertise in valuing GI practices, enhancing revenue, reducing overhead, collecting other regional (and sometimes distant) practices, centralizing operations, converting all practices to a single EMR, payer negotiations, and other practice functions, while leaving clinical care decisions to providers. Although this postacquisition scenario sounds attractive, there are downsides.
First, let’s review the upsides. As a mature partner in a highly valued practice, you could expect an acquisition payment in the range of $1 million (subject to capital gains tax). You receive a gross distribution based on a purchase multiple (9-12 times EBITA – a measure of your annual profit), minus investment in the new company, and annual payments to the Management Services Organization or MSO. Your income going forward will be reduced by annual obligations to the private equity fund, about 10%-40% of your production. Typically, a second sale occurs between 5 and 7 years after acquisition (yet to occur in GI), where the new company sells for another EBITA multiple (so it is in your financial interest to keep increasing practice value). Even with a modest EBITA multiple, you might net an amount that is double the initial acquisition payout. A senior partner could benefit financially in ways not readily available through other avenues of retirement.
Another benefit is access to capital to acquire more practices, bring new technology, improve facilities, integrate clinical and practice information, and weather reduced demand (like occurred with COVID-19). Independent practices are struggling to incorporate digital technologies that patients now expect, enhanced (and more expensive) endoscopes, new service lines, and the demand for real patient outcomes data during payer or health system negotiations.
So, what takes private equity from a clear “yes” to a “depends”? During COVID-19, physician incomes dropped substantially, since any revenue went first to pay bank debt, then fund fees, payment of overhead (leases, vendor commitments, residual staff), and finally to the doctors. A recent Medscape survey of 5,000 US physicians, revealed that 62% of MDs saw their income drop (23% by more than 50%). Physicians employed by health systems did not see nearly that income drop.
Once a practice is sold, physicians lose autonomy. When you are acquired by a private equity fund, the primary goal of the fund is a financial target. Long-term staff may be downsized, you may be asked to use equipment or supplies that are not to your standard, relationships with regional payers or health systems may become adversarial, productivity targets may alter your patient care decisions (more procedures, less external referrals), and relations with your partners may be strained (younger versus older).
A young physician who enters a private equity–acquired practice may work for decades at an income level discounted from preacquisition levels. They face a substantial buy-in if they hope to benefit from the second sale. Of course, one might argue that future salaries for all gastroenterologists will be reduced by increasing technology costs (endoscope companies are adding AI – can’t wait to see their pricing), reduced reimbursements, and increasing labor and supply costs. Serious threats to colonoscopy-based cancer screening are here, a development that makes future values of GI practices more tenuous. Finally, our payer mix will be worse than before COVID-19 because of long-term financial strains on the US economy.
We have to reflect on a similar practice acquisition trend that occurred in the 1990s, where practice management companies bought independent practices. While times are different now (for many reasons), all but one of those companies went bankrupt and the acquired practices had to rebuild from the ground up. Private equity funds that are heavily leveraged are especially vulnerable, as can be seen by current bankruptcies of large established companies (Hertz, Neiman-Marcus, and others).
Finally, we have to ask ourselves how patients will view your practice as more of us become acquired by financially driven partners. No matter how we paint private equity acquisitions, people understand that these funds are financially driven and practice sales are an income enhancement play for physicians. In 1986, Arnold Relman (Editor of the New England Journal of Medicine) gave two Tanner Lectures on human values at the University of Utah. He asked the following question:
“Is medical care a consumer good like any other, a commercial service provided by skilled vendors for consumers willing to pay the market price, or is there something fundamentally different about the relation between doctor and patient?”
I am not a Luddite, nor am I Don Quixote jousting at windmills. I do, however, want you to consider carefully before giving up on the traditional practice models that made our specialty what it is.
John I. Allen, MD, MBA, AGAF, is clinical professor of medicine, department of internal medicine, division of gastroenterology and hepatology, Institute for Healthcare Policy and Innovation, University School of Medicine, Chief Clinical Officer, University of Michigan Medical Group, Ann Arbor. He has no disclosures and takes full responsibility for the content.
DART trial hits the target in angiosarcoma
Rare cancers comprise about 20% of all cancers in the United States and Europe, according to recent estimates, but patients with rare cancers are vastly underrepresented in clinical trials.
Recently, there has been a focus on immune checkpoint blockade (ICB) in common cancer types. Since several rare tumor types share similar biologic features with the more common tumors, there is a need to test ICB in rare tumors, particularly because remissions with ICB can be durable.
Enter the DART trial, a phase 2, single-arm study of combinatorial ICB with ipilimumab plus nivolumab in patients with unresectable or metastatic rare cancers.
Results from DART were recently presented at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting. Michael J. Wagner, MD, of the University of Washington, Seattle, reported results in patients with advanced or unresectable angiosarcoma, one of the rare tumor types included in DART.
About angiosarcomas
Angiosarcomas account for less than 3% of all adult soft-tissue sarcomas, according to a review published in The Lancet Oncology. Angiosarcomas may arise in any part of the body, especially the head and neck (27%), breast (19.7%), and extremities (15.3%). These cancers can be primary or secondary (i.e., associated with prior radiation therapy or chronic lymphedema).
Angiosarcomas are aggressive, difficult to treat, and confer high mortality. The tumors are responsive to chemotherapy, but responses are brief. The estimated 5-year survival rate for all patients with angiosarcoma, including those who present with localized disease, is 30%-40%.
According to Dr. Wagner, a subset of angiosarcomas are characterized by high tumor mutational burden (TMB) and COSMIC signature 7, a DNA mutational signature that is consistent with other cancers caused by ultraviolet light exposure.
The high TMB subset of angiosarcomas is comparable with other cancer types that are responsive to ICB. Indeed, patients with angiosarcoma treated with ICB have shown responses, according to research published in the Journal for Immunotherapy of Cancer. However, no prospective studies of ICB in angiosarcoma have been published.
About DART
The DART trial includes more than 50 cohorts of rare cancer subtypes. Patients receive IV ipilimumab at 1 mg/kg every 6 weeks and IV nivolumab at 240 mg every 2 weeks.
The primary endpoint is objective response rate, as assessed by RECIST v1.1. Secondary endpoints include progression-free survival, overall survival, stable disease at 6 months, and toxicity.
The trial has a two-stage design. Six patients are enrolled in the first stage, and, if at least one patient responds to treatment, an additional 10 patients are enrolled in the second stage.
If at least two responses are seen among the 16 patients enrolled, further study of ICB is considered warranted.
Results in angiosarcoma
Dr. Wagner reported on the 16 angiosarcoma patients enrolled in DART. Nine patients had cutaneous primary tumors, seven had noncutaneous primary tumors, and three patients had radiation-associated angiosarcoma of the breast or chest wall.
Patients had received a median of two (range, zero to five) prior lines of therapy.
Adverse events (AEs) were consistent with prior safety results of the ipilimumab-nivolumab combination. Three-quarters of patients experienced an AE of any grade. The most common AEs were transaminase elevation, anemia, diarrhea, fatigue, hypothyroidism, pneumonitis, pruritus, and rash.
A quarter of patients had a grade 3-4 AE, and 12.5% of AEs led to premature treatment discontinuation. There were no fatal AEs.
The ORR was 25%. Responses occurred in 4 of the 16 patients, including 3 of 5 patients with primary cutaneous tumors of the scalp or face and 1 of 3 patients with radiation-associated breast angiosarcoma.
Two of the four responses and one case of stable disease have persisted for almost a year, and these patients remain on treatment. To put these results into perspective, Dr. Wagner noted that responses to cytotoxic chemotherapy rarely last 6 months.
The 6-month progression-free survival rate was 38%. The median overall survival has not yet been reached.
Dr. Wagner concluded that the combinatorial ICB regimen employed in DART was well tolerated and had an ORR of 25% in angiosarcoma regardless of primary site. Per the criteria of the DART trial, further investigation of ICB in angiosarcoma is warranted.
Molecular insights
Although correlative analyses of tumor tissue and peripheral blood are embedded in the DART trial, those analyses have not yet been performed. Eight of the 16 angiosarcoma patients had diagnostic molecular studies performed at their parent institutions, utilizing a variety of commercial platforms.
All eight patients for whom molecular data were available had at least two deleterious genomic alterations detected, but each had a distinct molecular profile.
Seven patients had TMB analyzed, including two partial responders to ICB. One of the seven patients had a high TMB, and this patient was one of the two responders. The other responder had an intermediate TMB.
Three patients had programmed death–ligand 1 staining on their tumors. Two of the three had high expression of PD-L1, including the responder with an intermediate TMB.
The real impact of DART
The DART trial is a “basket trial,” employing a similar treatment regimen for multiple tumor types. It provides a uniform framework for studying tumors that have been neglected in clinical trials heretofore.
Although the cohort of angiosarcoma patients is small, central pathology review was not required, and the treatment regimen was not compared directly with other potential therapies, the reported results of the ipilimumab-nivolumab regimen justify further study.
The biospecimens collected in DART will provide a rich source of data to identify common themes among responders and nonresponders, among patients who experience durable remissions and those who do not.
Angiosarcoma is not the only rare cancer for which combinatorial ICB has been valuable under the auspices of the DART trial. In Clinical Cancer Research, investigators reported an ORR of 44% among patients with high-grade neuroendocrine cancers, independent of primary site of origin. Progression-free survival at 6 months was 31%.
The DART trial is available at more than 800 sites, providing access to potentially promising treatment in a rigorous, scientifically valuable study for geographically underserved populations, including patients who live in rural areas.
The key message for practicing oncologists and clinical investigators is that clinical trials in rare tumors are feasible and can yield hope for patients who might lack it otherwise.
DART is funded by the National Cancer Institute and Bristol-Myers Squibb. Dr. Wagner disclosed relationships with Deciphera, Adaptimmune, GlaxoSmithKline, Athenex, and Incyte.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Wagner M et al. SITC 2020, Abstract 795.
Rare cancers comprise about 20% of all cancers in the United States and Europe, according to recent estimates, but patients with rare cancers are vastly underrepresented in clinical trials.
Recently, there has been a focus on immune checkpoint blockade (ICB) in common cancer types. Since several rare tumor types share similar biologic features with the more common tumors, there is a need to test ICB in rare tumors, particularly because remissions with ICB can be durable.
Enter the DART trial, a phase 2, single-arm study of combinatorial ICB with ipilimumab plus nivolumab in patients with unresectable or metastatic rare cancers.
Results from DART were recently presented at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting. Michael J. Wagner, MD, of the University of Washington, Seattle, reported results in patients with advanced or unresectable angiosarcoma, one of the rare tumor types included in DART.
About angiosarcomas
Angiosarcomas account for less than 3% of all adult soft-tissue sarcomas, according to a review published in The Lancet Oncology. Angiosarcomas may arise in any part of the body, especially the head and neck (27%), breast (19.7%), and extremities (15.3%). These cancers can be primary or secondary (i.e., associated with prior radiation therapy or chronic lymphedema).
Angiosarcomas are aggressive, difficult to treat, and confer high mortality. The tumors are responsive to chemotherapy, but responses are brief. The estimated 5-year survival rate for all patients with angiosarcoma, including those who present with localized disease, is 30%-40%.
According to Dr. Wagner, a subset of angiosarcomas are characterized by high tumor mutational burden (TMB) and COSMIC signature 7, a DNA mutational signature that is consistent with other cancers caused by ultraviolet light exposure.
The high TMB subset of angiosarcomas is comparable with other cancer types that are responsive to ICB. Indeed, patients with angiosarcoma treated with ICB have shown responses, according to research published in the Journal for Immunotherapy of Cancer. However, no prospective studies of ICB in angiosarcoma have been published.
About DART
The DART trial includes more than 50 cohorts of rare cancer subtypes. Patients receive IV ipilimumab at 1 mg/kg every 6 weeks and IV nivolumab at 240 mg every 2 weeks.
The primary endpoint is objective response rate, as assessed by RECIST v1.1. Secondary endpoints include progression-free survival, overall survival, stable disease at 6 months, and toxicity.
The trial has a two-stage design. Six patients are enrolled in the first stage, and, if at least one patient responds to treatment, an additional 10 patients are enrolled in the second stage.
If at least two responses are seen among the 16 patients enrolled, further study of ICB is considered warranted.
Results in angiosarcoma
Dr. Wagner reported on the 16 angiosarcoma patients enrolled in DART. Nine patients had cutaneous primary tumors, seven had noncutaneous primary tumors, and three patients had radiation-associated angiosarcoma of the breast or chest wall.
Patients had received a median of two (range, zero to five) prior lines of therapy.
Adverse events (AEs) were consistent with prior safety results of the ipilimumab-nivolumab combination. Three-quarters of patients experienced an AE of any grade. The most common AEs were transaminase elevation, anemia, diarrhea, fatigue, hypothyroidism, pneumonitis, pruritus, and rash.
A quarter of patients had a grade 3-4 AE, and 12.5% of AEs led to premature treatment discontinuation. There were no fatal AEs.
The ORR was 25%. Responses occurred in 4 of the 16 patients, including 3 of 5 patients with primary cutaneous tumors of the scalp or face and 1 of 3 patients with radiation-associated breast angiosarcoma.
Two of the four responses and one case of stable disease have persisted for almost a year, and these patients remain on treatment. To put these results into perspective, Dr. Wagner noted that responses to cytotoxic chemotherapy rarely last 6 months.
The 6-month progression-free survival rate was 38%. The median overall survival has not yet been reached.
Dr. Wagner concluded that the combinatorial ICB regimen employed in DART was well tolerated and had an ORR of 25% in angiosarcoma regardless of primary site. Per the criteria of the DART trial, further investigation of ICB in angiosarcoma is warranted.
Molecular insights
Although correlative analyses of tumor tissue and peripheral blood are embedded in the DART trial, those analyses have not yet been performed. Eight of the 16 angiosarcoma patients had diagnostic molecular studies performed at their parent institutions, utilizing a variety of commercial platforms.
All eight patients for whom molecular data were available had at least two deleterious genomic alterations detected, but each had a distinct molecular profile.
Seven patients had TMB analyzed, including two partial responders to ICB. One of the seven patients had a high TMB, and this patient was one of the two responders. The other responder had an intermediate TMB.
Three patients had programmed death–ligand 1 staining on their tumors. Two of the three had high expression of PD-L1, including the responder with an intermediate TMB.
The real impact of DART
The DART trial is a “basket trial,” employing a similar treatment regimen for multiple tumor types. It provides a uniform framework for studying tumors that have been neglected in clinical trials heretofore.
Although the cohort of angiosarcoma patients is small, central pathology review was not required, and the treatment regimen was not compared directly with other potential therapies, the reported results of the ipilimumab-nivolumab regimen justify further study.
The biospecimens collected in DART will provide a rich source of data to identify common themes among responders and nonresponders, among patients who experience durable remissions and those who do not.
Angiosarcoma is not the only rare cancer for which combinatorial ICB has been valuable under the auspices of the DART trial. In Clinical Cancer Research, investigators reported an ORR of 44% among patients with high-grade neuroendocrine cancers, independent of primary site of origin. Progression-free survival at 6 months was 31%.
The DART trial is available at more than 800 sites, providing access to potentially promising treatment in a rigorous, scientifically valuable study for geographically underserved populations, including patients who live in rural areas.
The key message for practicing oncologists and clinical investigators is that clinical trials in rare tumors are feasible and can yield hope for patients who might lack it otherwise.
DART is funded by the National Cancer Institute and Bristol-Myers Squibb. Dr. Wagner disclosed relationships with Deciphera, Adaptimmune, GlaxoSmithKline, Athenex, and Incyte.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Wagner M et al. SITC 2020, Abstract 795.
Rare cancers comprise about 20% of all cancers in the United States and Europe, according to recent estimates, but patients with rare cancers are vastly underrepresented in clinical trials.
Recently, there has been a focus on immune checkpoint blockade (ICB) in common cancer types. Since several rare tumor types share similar biologic features with the more common tumors, there is a need to test ICB in rare tumors, particularly because remissions with ICB can be durable.
Enter the DART trial, a phase 2, single-arm study of combinatorial ICB with ipilimumab plus nivolumab in patients with unresectable or metastatic rare cancers.
Results from DART were recently presented at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting. Michael J. Wagner, MD, of the University of Washington, Seattle, reported results in patients with advanced or unresectable angiosarcoma, one of the rare tumor types included in DART.
About angiosarcomas
Angiosarcomas account for less than 3% of all adult soft-tissue sarcomas, according to a review published in The Lancet Oncology. Angiosarcomas may arise in any part of the body, especially the head and neck (27%), breast (19.7%), and extremities (15.3%). These cancers can be primary or secondary (i.e., associated with prior radiation therapy or chronic lymphedema).
Angiosarcomas are aggressive, difficult to treat, and confer high mortality. The tumors are responsive to chemotherapy, but responses are brief. The estimated 5-year survival rate for all patients with angiosarcoma, including those who present with localized disease, is 30%-40%.
According to Dr. Wagner, a subset of angiosarcomas are characterized by high tumor mutational burden (TMB) and COSMIC signature 7, a DNA mutational signature that is consistent with other cancers caused by ultraviolet light exposure.
The high TMB subset of angiosarcomas is comparable with other cancer types that are responsive to ICB. Indeed, patients with angiosarcoma treated with ICB have shown responses, according to research published in the Journal for Immunotherapy of Cancer. However, no prospective studies of ICB in angiosarcoma have been published.
About DART
The DART trial includes more than 50 cohorts of rare cancer subtypes. Patients receive IV ipilimumab at 1 mg/kg every 6 weeks and IV nivolumab at 240 mg every 2 weeks.
The primary endpoint is objective response rate, as assessed by RECIST v1.1. Secondary endpoints include progression-free survival, overall survival, stable disease at 6 months, and toxicity.
The trial has a two-stage design. Six patients are enrolled in the first stage, and, if at least one patient responds to treatment, an additional 10 patients are enrolled in the second stage.
If at least two responses are seen among the 16 patients enrolled, further study of ICB is considered warranted.
Results in angiosarcoma
Dr. Wagner reported on the 16 angiosarcoma patients enrolled in DART. Nine patients had cutaneous primary tumors, seven had noncutaneous primary tumors, and three patients had radiation-associated angiosarcoma of the breast or chest wall.
Patients had received a median of two (range, zero to five) prior lines of therapy.
Adverse events (AEs) were consistent with prior safety results of the ipilimumab-nivolumab combination. Three-quarters of patients experienced an AE of any grade. The most common AEs were transaminase elevation, anemia, diarrhea, fatigue, hypothyroidism, pneumonitis, pruritus, and rash.
A quarter of patients had a grade 3-4 AE, and 12.5% of AEs led to premature treatment discontinuation. There were no fatal AEs.
The ORR was 25%. Responses occurred in 4 of the 16 patients, including 3 of 5 patients with primary cutaneous tumors of the scalp or face and 1 of 3 patients with radiation-associated breast angiosarcoma.
Two of the four responses and one case of stable disease have persisted for almost a year, and these patients remain on treatment. To put these results into perspective, Dr. Wagner noted that responses to cytotoxic chemotherapy rarely last 6 months.
The 6-month progression-free survival rate was 38%. The median overall survival has not yet been reached.
Dr. Wagner concluded that the combinatorial ICB regimen employed in DART was well tolerated and had an ORR of 25% in angiosarcoma regardless of primary site. Per the criteria of the DART trial, further investigation of ICB in angiosarcoma is warranted.
Molecular insights
Although correlative analyses of tumor tissue and peripheral blood are embedded in the DART trial, those analyses have not yet been performed. Eight of the 16 angiosarcoma patients had diagnostic molecular studies performed at their parent institutions, utilizing a variety of commercial platforms.
All eight patients for whom molecular data were available had at least two deleterious genomic alterations detected, but each had a distinct molecular profile.
Seven patients had TMB analyzed, including two partial responders to ICB. One of the seven patients had a high TMB, and this patient was one of the two responders. The other responder had an intermediate TMB.
Three patients had programmed death–ligand 1 staining on their tumors. Two of the three had high expression of PD-L1, including the responder with an intermediate TMB.
The real impact of DART
The DART trial is a “basket trial,” employing a similar treatment regimen for multiple tumor types. It provides a uniform framework for studying tumors that have been neglected in clinical trials heretofore.
Although the cohort of angiosarcoma patients is small, central pathology review was not required, and the treatment regimen was not compared directly with other potential therapies, the reported results of the ipilimumab-nivolumab regimen justify further study.
The biospecimens collected in DART will provide a rich source of data to identify common themes among responders and nonresponders, among patients who experience durable remissions and those who do not.
Angiosarcoma is not the only rare cancer for which combinatorial ICB has been valuable under the auspices of the DART trial. In Clinical Cancer Research, investigators reported an ORR of 44% among patients with high-grade neuroendocrine cancers, independent of primary site of origin. Progression-free survival at 6 months was 31%.
The DART trial is available at more than 800 sites, providing access to potentially promising treatment in a rigorous, scientifically valuable study for geographically underserved populations, including patients who live in rural areas.
The key message for practicing oncologists and clinical investigators is that clinical trials in rare tumors are feasible and can yield hope for patients who might lack it otherwise.
DART is funded by the National Cancer Institute and Bristol-Myers Squibb. Dr. Wagner disclosed relationships with Deciphera, Adaptimmune, GlaxoSmithKline, Athenex, and Incyte.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Wagner M et al. SITC 2020, Abstract 795.
FROM SITC 2020
Leading in crisis
Lessons from the trail
I have learned a lot about crisis management and leadership in the rapidly changing COVID health care environment. I have learned how to make quick and imperfect decisions with limited information, and how to move on swiftly. I have learned how to quickly fade out memories of how we used to run our business, and pivot to unknown and untested delivery modalities. I have learned how to take regulatory standards as guidance, not doctrine. And I have learned how tell longstanding loyal colleagues that they are being laid off.
Many of these leadership challenges are not new, but the rapidity of change and the weight and magnitude of decision making is unparalleled in my relatively short career. In some ways, it reminds me of some solid lessons I have learned over time as a lifetime runner, with many analogies and applications to leadership.
Some people ask me why I run. “You must get a runner’s high.” The truth is, I have never had a runner’s high. I feel every step. In fact, the very nature of running makes a person feel like they are being pulled under water. Runners are typically tachycardic and short of breath the whole time they are running. But what running does allow for is to ignore some of the signals your body is sending, and wholly and completely focus on other things. I often have my most creative and innovative thoughts while running. So that is why I run. But back to the point of what running and leadership have in common – and how lessons learned can translate between the two:
They are both really hard. As I mentioned above, running literally makes you feel like you are drowning. But when you finish running, it is amazing how easy everything else feels! Similar to leadership, it should feel hard, but not too hard. I have seen firsthand the effects of under- and over-delegating, and both are dysfunctional. Good leadership is a blend of being humble and servant, but also ensuring self-care and endurance. It is also important to acknowledge the difficulty of leadership. Dr. Tom Lee, currently chief medical officer at Press Ganey, is a leader I have always admired. He once said, “Leadership can be very lonely.” At the time, I did not quite understand that, but I have come to experience that feeling occasionally. The other aspect of leadership that I find really hard is that often, people’s anger is misdirected at leaders as a natural outlet for that anger. Part of being a leader is enduring such anger, gaining an understanding for it, and doing what you can to help people through it.
They both work better when you are restored. It sounds generic and cliché, but you can’t be a good runner or a good leader when you are totally depleted.
They both require efficiency. When I was running my first marathon, a complete stranger ran up beside me and started giving me advice. I thought it was sort of strange advice at the time, but it turned out to be sound and useful. He noticed my running pattern of “sticking to the road,” and he told me I should rather “run as the crow flies.” What he meant was to run in as straight of a line as possible, regardless of the road, to preserve energy and save steps. He recommended picking a point on the horizon and running toward that point as straight as possible. As he sped off ahead of me in the next mile, his parting words were, “You’ll thank me at mile 24…” To this day, I still use that tactic, which I find very steadying and calming during running. The same can be said for leadership; as you pick a point on the horizon, keep yourself and your team heading toward that point with intense focus, and before you realize it, you’ve reached your destination.
They both require having a goal. That same stranger who gave me advice on running efficiently also asked what my goal was. It caught me off guard a bit, as I realized my only goal was to finish. He encouraged me to make a goal for the run, which could serve as a motivator when the going got tough. This was another piece of lasting advice I have used for both running and for leadership.
They both can be endured by committing to continuous forward motion. Running and leadership both become psychologically much easier when you realize all you really have to do is maintain continuous forward motion. Some days require less effort than others, but I can always convince myself I am capable of some forward motion.
They both are easier if you don’t overthink things. When I first started in a leadership position, I would have moments of anxiety if I thought too hard about what I was responsible for. Similar to running, it works best if you don’t overthink what difficulties it may bring; rather, just put on your shoes and get going.
In the end, leading during COVID is like stepping onto a new trail. Despite the new terrain and foreign path, my prior training and trusty pair of sneakers – like my leadership skills and past experiences – will get me through this journey, one step at a time.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Lessons from the trail
Lessons from the trail
I have learned a lot about crisis management and leadership in the rapidly changing COVID health care environment. I have learned how to make quick and imperfect decisions with limited information, and how to move on swiftly. I have learned how to quickly fade out memories of how we used to run our business, and pivot to unknown and untested delivery modalities. I have learned how to take regulatory standards as guidance, not doctrine. And I have learned how tell longstanding loyal colleagues that they are being laid off.
Many of these leadership challenges are not new, but the rapidity of change and the weight and magnitude of decision making is unparalleled in my relatively short career. In some ways, it reminds me of some solid lessons I have learned over time as a lifetime runner, with many analogies and applications to leadership.
Some people ask me why I run. “You must get a runner’s high.” The truth is, I have never had a runner’s high. I feel every step. In fact, the very nature of running makes a person feel like they are being pulled under water. Runners are typically tachycardic and short of breath the whole time they are running. But what running does allow for is to ignore some of the signals your body is sending, and wholly and completely focus on other things. I often have my most creative and innovative thoughts while running. So that is why I run. But back to the point of what running and leadership have in common – and how lessons learned can translate between the two:
They are both really hard. As I mentioned above, running literally makes you feel like you are drowning. But when you finish running, it is amazing how easy everything else feels! Similar to leadership, it should feel hard, but not too hard. I have seen firsthand the effects of under- and over-delegating, and both are dysfunctional. Good leadership is a blend of being humble and servant, but also ensuring self-care and endurance. It is also important to acknowledge the difficulty of leadership. Dr. Tom Lee, currently chief medical officer at Press Ganey, is a leader I have always admired. He once said, “Leadership can be very lonely.” At the time, I did not quite understand that, but I have come to experience that feeling occasionally. The other aspect of leadership that I find really hard is that often, people’s anger is misdirected at leaders as a natural outlet for that anger. Part of being a leader is enduring such anger, gaining an understanding for it, and doing what you can to help people through it.
They both work better when you are restored. It sounds generic and cliché, but you can’t be a good runner or a good leader when you are totally depleted.
They both require efficiency. When I was running my first marathon, a complete stranger ran up beside me and started giving me advice. I thought it was sort of strange advice at the time, but it turned out to be sound and useful. He noticed my running pattern of “sticking to the road,” and he told me I should rather “run as the crow flies.” What he meant was to run in as straight of a line as possible, regardless of the road, to preserve energy and save steps. He recommended picking a point on the horizon and running toward that point as straight as possible. As he sped off ahead of me in the next mile, his parting words were, “You’ll thank me at mile 24…” To this day, I still use that tactic, which I find very steadying and calming during running. The same can be said for leadership; as you pick a point on the horizon, keep yourself and your team heading toward that point with intense focus, and before you realize it, you’ve reached your destination.
They both require having a goal. That same stranger who gave me advice on running efficiently also asked what my goal was. It caught me off guard a bit, as I realized my only goal was to finish. He encouraged me to make a goal for the run, which could serve as a motivator when the going got tough. This was another piece of lasting advice I have used for both running and for leadership.
They both can be endured by committing to continuous forward motion. Running and leadership both become psychologically much easier when you realize all you really have to do is maintain continuous forward motion. Some days require less effort than others, but I can always convince myself I am capable of some forward motion.
They both are easier if you don’t overthink things. When I first started in a leadership position, I would have moments of anxiety if I thought too hard about what I was responsible for. Similar to running, it works best if you don’t overthink what difficulties it may bring; rather, just put on your shoes and get going.
In the end, leading during COVID is like stepping onto a new trail. Despite the new terrain and foreign path, my prior training and trusty pair of sneakers – like my leadership skills and past experiences – will get me through this journey, one step at a time.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
I have learned a lot about crisis management and leadership in the rapidly changing COVID health care environment. I have learned how to make quick and imperfect decisions with limited information, and how to move on swiftly. I have learned how to quickly fade out memories of how we used to run our business, and pivot to unknown and untested delivery modalities. I have learned how to take regulatory standards as guidance, not doctrine. And I have learned how tell longstanding loyal colleagues that they are being laid off.
Many of these leadership challenges are not new, but the rapidity of change and the weight and magnitude of decision making is unparalleled in my relatively short career. In some ways, it reminds me of some solid lessons I have learned over time as a lifetime runner, with many analogies and applications to leadership.
Some people ask me why I run. “You must get a runner’s high.” The truth is, I have never had a runner’s high. I feel every step. In fact, the very nature of running makes a person feel like they are being pulled under water. Runners are typically tachycardic and short of breath the whole time they are running. But what running does allow for is to ignore some of the signals your body is sending, and wholly and completely focus on other things. I often have my most creative and innovative thoughts while running. So that is why I run. But back to the point of what running and leadership have in common – and how lessons learned can translate between the two:
They are both really hard. As I mentioned above, running literally makes you feel like you are drowning. But when you finish running, it is amazing how easy everything else feels! Similar to leadership, it should feel hard, but not too hard. I have seen firsthand the effects of under- and over-delegating, and both are dysfunctional. Good leadership is a blend of being humble and servant, but also ensuring self-care and endurance. It is also important to acknowledge the difficulty of leadership. Dr. Tom Lee, currently chief medical officer at Press Ganey, is a leader I have always admired. He once said, “Leadership can be very lonely.” At the time, I did not quite understand that, but I have come to experience that feeling occasionally. The other aspect of leadership that I find really hard is that often, people’s anger is misdirected at leaders as a natural outlet for that anger. Part of being a leader is enduring such anger, gaining an understanding for it, and doing what you can to help people through it.
They both work better when you are restored. It sounds generic and cliché, but you can’t be a good runner or a good leader when you are totally depleted.
They both require efficiency. When I was running my first marathon, a complete stranger ran up beside me and started giving me advice. I thought it was sort of strange advice at the time, but it turned out to be sound and useful. He noticed my running pattern of “sticking to the road,” and he told me I should rather “run as the crow flies.” What he meant was to run in as straight of a line as possible, regardless of the road, to preserve energy and save steps. He recommended picking a point on the horizon and running toward that point as straight as possible. As he sped off ahead of me in the next mile, his parting words were, “You’ll thank me at mile 24…” To this day, I still use that tactic, which I find very steadying and calming during running. The same can be said for leadership; as you pick a point on the horizon, keep yourself and your team heading toward that point with intense focus, and before you realize it, you’ve reached your destination.
They both require having a goal. That same stranger who gave me advice on running efficiently also asked what my goal was. It caught me off guard a bit, as I realized my only goal was to finish. He encouraged me to make a goal for the run, which could serve as a motivator when the going got tough. This was another piece of lasting advice I have used for both running and for leadership.
They both can be endured by committing to continuous forward motion. Running and leadership both become psychologically much easier when you realize all you really have to do is maintain continuous forward motion. Some days require less effort than others, but I can always convince myself I am capable of some forward motion.
They both are easier if you don’t overthink things. When I first started in a leadership position, I would have moments of anxiety if I thought too hard about what I was responsible for. Similar to running, it works best if you don’t overthink what difficulties it may bring; rather, just put on your shoes and get going.
In the end, leading during COVID is like stepping onto a new trail. Despite the new terrain and foreign path, my prior training and trusty pair of sneakers – like my leadership skills and past experiences – will get me through this journey, one step at a time.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Paul Summergrad, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.

















