User login
Statins Raise Diabetes Risk, but CV Benefit Outweighs It
Statins raise the risks for increased glucose levels and the development of type 2 diabetes among people who don’t have it at baseline, but those risks are outweighed by the cardiovascular benefit, new data suggested.
The findings come from an analysis of individual participant data from a total of 23 randomized trials of statin therapy involving 154,664 individuals. In people without diabetes at baseline, statin therapy produces a dose-dependent increase in the risk for diabetes diagnosis, particularly among those whose glycemia marker levels are already at the diagnostic threshold.
Statins also tend to raise glucose levels in people who already have diabetes, but “the diabetes-related risks arising from the small changes in glycemia resulting from statin therapy are greatly outweighed by the benefits of statins on major vascular events when the direct clinical consequences of these outcomes are taken into consideration,” wrote the authors of the Cholesterol Treatment Trialists’ (CTT) Collaboration in their paper, published online in The Lancet Diabetes & Endocrinology.
Moreover, they say, “since the effect of statin therapy on measures of glycemia within an individual is small, there is likely to be little clinical benefit in measuring glucose concentrations and A1c values routinely after starting statin therapy with the aim of making comparisons to values taken before the initiation of a statin. However, people should continue to be screened for diabetes and associated risk factors and have their glycemic control monitored in accordance with current clinical guidelines.”
The CTT is co-led by Christina Reith, MBChB, PhD, and David Preiss, PhD, FRCPath, MRCP, both of the Nuffield Department of Population Health, University of Oxford, England.
In an accompanying editorial,
Dr. Gerstein and Dr. Pigeyre also said “these findings emphasize the importance of holistic care. As people at risk for cardiovascular outcomes are also at risk for type 2 diabetes, any prescription of a statin should be accompanied by promoting proven strategies to prevent or delay diabetes, such as modest weight reduction and increased physical activity. Finally, these findings emphasize the importance of always being alert for harmful adverse effects, even with the most beneficial and successful preventive therapies.”
Statins Raise Diabetes Risk, Glucose Levels Slightly
The meta-analysis of trials in the CTT Collaboration included individual participant data from 19 double-blind randomized, controlled trials with a median follow-up of 4.3 years comparing statins with placebo in a total of 123,940 participants, including 18% who had known type 2 diabetes at randomization. Also analyzed were another four double-blind trials of lower- vs higher-intensity statins involving a total of 30,724 participants followed for a median of 4.9 years, with 15% having diabetes at baseline.
In the 19 trials of low- or moderate-intensity statins vs placebo, statins resulted in a significant 10% increase in new-onset diabetes compared with placebo (rate ratio, 1.10), while high-intensity statins raised the risk by an also significant 36% (1.36). This translated to a mean absolute excess of 0.12% per year of treatment.
Compared with less intensive statin therapy, more intensive statin therapy resulted in a significant 10% proportional increase in new-onset diabetes (1.10), giving an absolute annual excess of 0.22%.
In the statin vs placebo trials, differences in A1c values from placebo were 0.06 percentage points higher for low- or moderate-intensity statins and 0.08 points greater for high-intensity statins.
Nearly two thirds (62%) of the excess cases of new-onset diabetes occurred among participants in the highest quarter of the baseline glycemia distribution for both low-intensity or moderate-intensity and high-intensity statin therapy.
And among participants who already had diabetes at baseline, there was a significant 10% relative increase in worsening glycemia (defined by adverse glycemic event, A1c increase of ≥ 0.5 percentage points, or medication escalation) with low- or moderate-intensity statins compared with placebo and a 24% relative increase in the high-intensity trials.
The Nuffield Department of Population Health has an explicit policy of not accepting any personal honoraria payments directly or indirectly from the pharmaceutical and food industries. It seeks reimbursement to the University of Oxford for the costs of travel and accommodation to participate in scientific meetings. Dr. Reith reported receiving funding to the University of Oxford from the UK National Institute for Health and Care Research Health Technology Assessment Programme and holding unpaid roles on the Clinical Data Interchange Standards Consortium as a board member and WHO as a scientific advisor. Dr. Preiss reported receiving funding to his research institution (but no personal funding) from Novartis for the ORION 4 trial of inclisiran, Novo Nordisk for the ASCEND PLUS trial of semaglutide, and Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial and being a committee member for a National Institute for Health and Care Excellence guideline.
Dr. Gerstein holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reported research grants from Eli Lilly, AstraZeneca, Novo Nordisk, Hanmi, and Merck; continuing medical education grants to McMaster University from Eli Lilly, Abbott, Sanofi, Novo Nordisk, and Boehringer Ingelheim; honoraria for speaking from AstraZeneca, Eli Lilly, Novo Nordisk, DKSH, Zuellig Pharma, Sanofi, and Jiangsu Hanson; and consulting fees from Abbott, Eli Lilly, Novo Nordisk, Pfizer, Carbon Brand, Sanofi, Kowa, and Hanmi. Pigeyre had no disclosures.
A version of this article appeared on Medscape.com.
Statins raise the risks for increased glucose levels and the development of type 2 diabetes among people who don’t have it at baseline, but those risks are outweighed by the cardiovascular benefit, new data suggested.
The findings come from an analysis of individual participant data from a total of 23 randomized trials of statin therapy involving 154,664 individuals. In people without diabetes at baseline, statin therapy produces a dose-dependent increase in the risk for diabetes diagnosis, particularly among those whose glycemia marker levels are already at the diagnostic threshold.
Statins also tend to raise glucose levels in people who already have diabetes, but “the diabetes-related risks arising from the small changes in glycemia resulting from statin therapy are greatly outweighed by the benefits of statins on major vascular events when the direct clinical consequences of these outcomes are taken into consideration,” wrote the authors of the Cholesterol Treatment Trialists’ (CTT) Collaboration in their paper, published online in The Lancet Diabetes & Endocrinology.
Moreover, they say, “since the effect of statin therapy on measures of glycemia within an individual is small, there is likely to be little clinical benefit in measuring glucose concentrations and A1c values routinely after starting statin therapy with the aim of making comparisons to values taken before the initiation of a statin. However, people should continue to be screened for diabetes and associated risk factors and have their glycemic control monitored in accordance with current clinical guidelines.”
The CTT is co-led by Christina Reith, MBChB, PhD, and David Preiss, PhD, FRCPath, MRCP, both of the Nuffield Department of Population Health, University of Oxford, England.
In an accompanying editorial,
Dr. Gerstein and Dr. Pigeyre also said “these findings emphasize the importance of holistic care. As people at risk for cardiovascular outcomes are also at risk for type 2 diabetes, any prescription of a statin should be accompanied by promoting proven strategies to prevent or delay diabetes, such as modest weight reduction and increased physical activity. Finally, these findings emphasize the importance of always being alert for harmful adverse effects, even with the most beneficial and successful preventive therapies.”
Statins Raise Diabetes Risk, Glucose Levels Slightly
The meta-analysis of trials in the CTT Collaboration included individual participant data from 19 double-blind randomized, controlled trials with a median follow-up of 4.3 years comparing statins with placebo in a total of 123,940 participants, including 18% who had known type 2 diabetes at randomization. Also analyzed were another four double-blind trials of lower- vs higher-intensity statins involving a total of 30,724 participants followed for a median of 4.9 years, with 15% having diabetes at baseline.
In the 19 trials of low- or moderate-intensity statins vs placebo, statins resulted in a significant 10% increase in new-onset diabetes compared with placebo (rate ratio, 1.10), while high-intensity statins raised the risk by an also significant 36% (1.36). This translated to a mean absolute excess of 0.12% per year of treatment.
Compared with less intensive statin therapy, more intensive statin therapy resulted in a significant 10% proportional increase in new-onset diabetes (1.10), giving an absolute annual excess of 0.22%.
In the statin vs placebo trials, differences in A1c values from placebo were 0.06 percentage points higher for low- or moderate-intensity statins and 0.08 points greater for high-intensity statins.
Nearly two thirds (62%) of the excess cases of new-onset diabetes occurred among participants in the highest quarter of the baseline glycemia distribution for both low-intensity or moderate-intensity and high-intensity statin therapy.
And among participants who already had diabetes at baseline, there was a significant 10% relative increase in worsening glycemia (defined by adverse glycemic event, A1c increase of ≥ 0.5 percentage points, or medication escalation) with low- or moderate-intensity statins compared with placebo and a 24% relative increase in the high-intensity trials.
The Nuffield Department of Population Health has an explicit policy of not accepting any personal honoraria payments directly or indirectly from the pharmaceutical and food industries. It seeks reimbursement to the University of Oxford for the costs of travel and accommodation to participate in scientific meetings. Dr. Reith reported receiving funding to the University of Oxford from the UK National Institute for Health and Care Research Health Technology Assessment Programme and holding unpaid roles on the Clinical Data Interchange Standards Consortium as a board member and WHO as a scientific advisor. Dr. Preiss reported receiving funding to his research institution (but no personal funding) from Novartis for the ORION 4 trial of inclisiran, Novo Nordisk for the ASCEND PLUS trial of semaglutide, and Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial and being a committee member for a National Institute for Health and Care Excellence guideline.
Dr. Gerstein holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reported research grants from Eli Lilly, AstraZeneca, Novo Nordisk, Hanmi, and Merck; continuing medical education grants to McMaster University from Eli Lilly, Abbott, Sanofi, Novo Nordisk, and Boehringer Ingelheim; honoraria for speaking from AstraZeneca, Eli Lilly, Novo Nordisk, DKSH, Zuellig Pharma, Sanofi, and Jiangsu Hanson; and consulting fees from Abbott, Eli Lilly, Novo Nordisk, Pfizer, Carbon Brand, Sanofi, Kowa, and Hanmi. Pigeyre had no disclosures.
A version of this article appeared on Medscape.com.
Statins raise the risks for increased glucose levels and the development of type 2 diabetes among people who don’t have it at baseline, but those risks are outweighed by the cardiovascular benefit, new data suggested.
The findings come from an analysis of individual participant data from a total of 23 randomized trials of statin therapy involving 154,664 individuals. In people without diabetes at baseline, statin therapy produces a dose-dependent increase in the risk for diabetes diagnosis, particularly among those whose glycemia marker levels are already at the diagnostic threshold.
Statins also tend to raise glucose levels in people who already have diabetes, but “the diabetes-related risks arising from the small changes in glycemia resulting from statin therapy are greatly outweighed by the benefits of statins on major vascular events when the direct clinical consequences of these outcomes are taken into consideration,” wrote the authors of the Cholesterol Treatment Trialists’ (CTT) Collaboration in their paper, published online in The Lancet Diabetes & Endocrinology.
Moreover, they say, “since the effect of statin therapy on measures of glycemia within an individual is small, there is likely to be little clinical benefit in measuring glucose concentrations and A1c values routinely after starting statin therapy with the aim of making comparisons to values taken before the initiation of a statin. However, people should continue to be screened for diabetes and associated risk factors and have their glycemic control monitored in accordance with current clinical guidelines.”
The CTT is co-led by Christina Reith, MBChB, PhD, and David Preiss, PhD, FRCPath, MRCP, both of the Nuffield Department of Population Health, University of Oxford, England.
In an accompanying editorial,
Dr. Gerstein and Dr. Pigeyre also said “these findings emphasize the importance of holistic care. As people at risk for cardiovascular outcomes are also at risk for type 2 diabetes, any prescription of a statin should be accompanied by promoting proven strategies to prevent or delay diabetes, such as modest weight reduction and increased physical activity. Finally, these findings emphasize the importance of always being alert for harmful adverse effects, even with the most beneficial and successful preventive therapies.”
Statins Raise Diabetes Risk, Glucose Levels Slightly
The meta-analysis of trials in the CTT Collaboration included individual participant data from 19 double-blind randomized, controlled trials with a median follow-up of 4.3 years comparing statins with placebo in a total of 123,940 participants, including 18% who had known type 2 diabetes at randomization. Also analyzed were another four double-blind trials of lower- vs higher-intensity statins involving a total of 30,724 participants followed for a median of 4.9 years, with 15% having diabetes at baseline.
In the 19 trials of low- or moderate-intensity statins vs placebo, statins resulted in a significant 10% increase in new-onset diabetes compared with placebo (rate ratio, 1.10), while high-intensity statins raised the risk by an also significant 36% (1.36). This translated to a mean absolute excess of 0.12% per year of treatment.
Compared with less intensive statin therapy, more intensive statin therapy resulted in a significant 10% proportional increase in new-onset diabetes (1.10), giving an absolute annual excess of 0.22%.
In the statin vs placebo trials, differences in A1c values from placebo were 0.06 percentage points higher for low- or moderate-intensity statins and 0.08 points greater for high-intensity statins.
Nearly two thirds (62%) of the excess cases of new-onset diabetes occurred among participants in the highest quarter of the baseline glycemia distribution for both low-intensity or moderate-intensity and high-intensity statin therapy.
And among participants who already had diabetes at baseline, there was a significant 10% relative increase in worsening glycemia (defined by adverse glycemic event, A1c increase of ≥ 0.5 percentage points, or medication escalation) with low- or moderate-intensity statins compared with placebo and a 24% relative increase in the high-intensity trials.
The Nuffield Department of Population Health has an explicit policy of not accepting any personal honoraria payments directly or indirectly from the pharmaceutical and food industries. It seeks reimbursement to the University of Oxford for the costs of travel and accommodation to participate in scientific meetings. Dr. Reith reported receiving funding to the University of Oxford from the UK National Institute for Health and Care Research Health Technology Assessment Programme and holding unpaid roles on the Clinical Data Interchange Standards Consortium as a board member and WHO as a scientific advisor. Dr. Preiss reported receiving funding to his research institution (but no personal funding) from Novartis for the ORION 4 trial of inclisiran, Novo Nordisk for the ASCEND PLUS trial of semaglutide, and Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial and being a committee member for a National Institute for Health and Care Excellence guideline.
Dr. Gerstein holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reported research grants from Eli Lilly, AstraZeneca, Novo Nordisk, Hanmi, and Merck; continuing medical education grants to McMaster University from Eli Lilly, Abbott, Sanofi, Novo Nordisk, and Boehringer Ingelheim; honoraria for speaking from AstraZeneca, Eli Lilly, Novo Nordisk, DKSH, Zuellig Pharma, Sanofi, and Jiangsu Hanson; and consulting fees from Abbott, Eli Lilly, Novo Nordisk, Pfizer, Carbon Brand, Sanofi, Kowa, and Hanmi. Pigeyre had no disclosures.
A version of this article appeared on Medscape.com.
Early Olezarsen Results Show 50% Reduction in Triglycerides
ATLANTA — A novel antisense therapy called olezarsen reduced triglycerides (TGs) by approximately 50% with either of the two study doses relative to placebo and did so with a low relative risk for adverse events, new data from a phase 2b trial showed.
“The reduction in triglycerides was greater than that currently possible with any available therapy,” reported Brian A. Bergmark, MD, an interventional cardiologist at Brigham and Women’s Hospital, Boston.
The drug also produced meaningful improvements in multiple other lipid subfractions associated with increased cardiovascular (CV) risk, including ApoC-III, very low–density lipoprotein (VLDL) cholesterol, ApoB, and non-LDL cholesterol. High-density lipoprotein (HDL) cholesterol levels were significantly raised.
The results were presented on April 7 as a late breaker at the American College of Cardiology (ACC) Scientific Session 2024 and published online simultaneously in The New England Journal of Medicine.
No Major Subgroup Failed to Respond
The effect was seen across all the key subgroups evaluated, including women and patients with diabetes, obesity, and severe as well as moderate elevations in TGs at baseline, Dr. Bergmark reported.
Olezarsen is a N-acetylgalactosamine–conjugated antisense oligonucleotide targeting APOC3 RNA.
In this study, 154 patients at 24 sites in North America were randomized in a 1:1 ratio to 50 or 80 mg olezarsen. Those in each of these cohorts were then randomized in a 3:1 ratio to active therapy or placebo. All therapies were administered by subcutaneous injection once per month.
Patients were eligible for the trial if they had moderate hypertriglyceridemia, defined as a level of 150-499 mg/dL, and elevated CV risk or if they had severe hypertriglyceridemia (≥ 500 mg/dL) with or without other evidence of elevated CV risk. The primary endpoint was a change in TGs at 6 months. Complete follow-up was available in about 97% of patients regardless of treatment assignment.
With a slight numerical advantage for the higher dose, the TG reductions were 49.1% for the 50-mg dose and 53.1% for the 80-mg dose relative to no significant change in the placebo group (P < .001 for both olezarsen doses). The reductions in ApoC-III, an upstream driver of TG production and a CV risk factor, were 64.2% and 73.2% relative to placebo (both P < .001), respectively, Dr. Bergmark reported.
In those with moderate hypertriglyceridemia, normal TG levels, defined as < 150 mg/dL, were reached at 6 months in 85.7% and 93.3% in the 40-mg and 80-mg dose groups, respectively. Relative to these reductions, normalization was seen in only 11.8% of placebo patients (P < .001).
TG Lowering Might Not Be Best Endpoint
The primary endpoint in this trial was a change in TGs, but this target was questioned by an invited ACC discussant, Daniel Soffer, MD, who is both an adjunct professor assistant professor of medicine at Penn Medicine, Philadelphia, and current president of the National Lipid Association.
Dr. Soffer noted that highly elevated TGs are a major risk factor for acute pancreatitis, so this predicts a clinical benefit for this purpose, but he thought the other lipid subfractions are far more important for the goal of reducing atherosclerotic cardiovascular disease (ASCVD).
Indeed, he said categorically that it is not TGs that drive ASCVD risk and therefore not what is the real importance of these data. Rather, “it is the non-HDL cholesterol and ApoB lowering” that will drive the likely benefits from this therapy in CV disease.
In addition to the TG reductions, olezarsen did, in fact, produce significant reductions in many of the lipid subfractions associated with increased CV risk. While slightly more favorable in most cases with the higher dose of olezarsen, even the lower dose reduced Apo C-III from baseline by 64.2% (P < .001), VLDL by 46.2% (P < .001), remnant cholesterol by 46.6% (P < .001), ApoB by 18.2% (P < .001), and non-HDL cholesterol by 25.4% (P < .001). HDL cholesterol was increased by 39.6% (P < .001).
These favorable effects on TG and other lipid subfractions were achieved with a safety profile that was reassuring, Dr. Bergmark said. Serious adverse events leading to discontinuation occurred in 0%, 1.7%, and 1.8% of the placebo, lower-dose, and higher-dose arms, respectively. These rates did not differ significantly.
Increased Liver Enzymes Is Common
Liver enzymes were significantly elevated (P < .001) for both doses of olezarsen vs placebo, but liver enzymes > 3× the upper limit of normal did not reach significance on either dose of olezarsen relative to placebo. Low platelet counts and reductions in kidney function were observed in a minority of patients but were generally manageable, according to Dr. Bergmark. There was no impact on hemoglobin A1c levels.
Further evaluation of change in hepatic function is planned in the ongoing extension studies.
Characterizing these results as “exciting,” Neha J. Pagidipati, MD, a member of the Duke Clinical Research Institute and an assistant professor at the Duke School of Medicine, Durham, North Carolina, said that identifying a drug effective for hypertriglyceridemia is likely to be a major advance. While elevated TGs are “one of the toughest” lipid abnormalities to manage, “there is not much out there to offer for treatment.”
She, like Dr. Soffer, was encouraged by the favorable effects on multiple lipid abnormalities associated with increased CV risk, but she said the ultimate clinical utility of this or other agents that lower TGs for ASCVD requires a study showing a change in CV events.
Dr. Bergmark reported financial relationships with 15 pharmaceutical companies, including Ionis, which provided funding for the BRIDGE-TIMI 73a trial. Soffer had financial relationships with Akcea, Amgen, Amryt, AstraZeneca, Ionis, Novartis, Regeneron, and Verve. Dr. Pagidipati had financial relationships with more than 10 pharmaceutical companies but was not involved in the design of management of the BRIDGE-TIMI 73a trial.
A version of this article first appeared on Medscape.com.
ATLANTA — A novel antisense therapy called olezarsen reduced triglycerides (TGs) by approximately 50% with either of the two study doses relative to placebo and did so with a low relative risk for adverse events, new data from a phase 2b trial showed.
“The reduction in triglycerides was greater than that currently possible with any available therapy,” reported Brian A. Bergmark, MD, an interventional cardiologist at Brigham and Women’s Hospital, Boston.
The drug also produced meaningful improvements in multiple other lipid subfractions associated with increased cardiovascular (CV) risk, including ApoC-III, very low–density lipoprotein (VLDL) cholesterol, ApoB, and non-LDL cholesterol. High-density lipoprotein (HDL) cholesterol levels were significantly raised.
The results were presented on April 7 as a late breaker at the American College of Cardiology (ACC) Scientific Session 2024 and published online simultaneously in The New England Journal of Medicine.
No Major Subgroup Failed to Respond
The effect was seen across all the key subgroups evaluated, including women and patients with diabetes, obesity, and severe as well as moderate elevations in TGs at baseline, Dr. Bergmark reported.
Olezarsen is a N-acetylgalactosamine–conjugated antisense oligonucleotide targeting APOC3 RNA.
In this study, 154 patients at 24 sites in North America were randomized in a 1:1 ratio to 50 or 80 mg olezarsen. Those in each of these cohorts were then randomized in a 3:1 ratio to active therapy or placebo. All therapies were administered by subcutaneous injection once per month.
Patients were eligible for the trial if they had moderate hypertriglyceridemia, defined as a level of 150-499 mg/dL, and elevated CV risk or if they had severe hypertriglyceridemia (≥ 500 mg/dL) with or without other evidence of elevated CV risk. The primary endpoint was a change in TGs at 6 months. Complete follow-up was available in about 97% of patients regardless of treatment assignment.
With a slight numerical advantage for the higher dose, the TG reductions were 49.1% for the 50-mg dose and 53.1% for the 80-mg dose relative to no significant change in the placebo group (P < .001 for both olezarsen doses). The reductions in ApoC-III, an upstream driver of TG production and a CV risk factor, were 64.2% and 73.2% relative to placebo (both P < .001), respectively, Dr. Bergmark reported.
In those with moderate hypertriglyceridemia, normal TG levels, defined as < 150 mg/dL, were reached at 6 months in 85.7% and 93.3% in the 40-mg and 80-mg dose groups, respectively. Relative to these reductions, normalization was seen in only 11.8% of placebo patients (P < .001).
TG Lowering Might Not Be Best Endpoint
The primary endpoint in this trial was a change in TGs, but this target was questioned by an invited ACC discussant, Daniel Soffer, MD, who is both an adjunct professor assistant professor of medicine at Penn Medicine, Philadelphia, and current president of the National Lipid Association.
Dr. Soffer noted that highly elevated TGs are a major risk factor for acute pancreatitis, so this predicts a clinical benefit for this purpose, but he thought the other lipid subfractions are far more important for the goal of reducing atherosclerotic cardiovascular disease (ASCVD).
Indeed, he said categorically that it is not TGs that drive ASCVD risk and therefore not what is the real importance of these data. Rather, “it is the non-HDL cholesterol and ApoB lowering” that will drive the likely benefits from this therapy in CV disease.
In addition to the TG reductions, olezarsen did, in fact, produce significant reductions in many of the lipid subfractions associated with increased CV risk. While slightly more favorable in most cases with the higher dose of olezarsen, even the lower dose reduced Apo C-III from baseline by 64.2% (P < .001), VLDL by 46.2% (P < .001), remnant cholesterol by 46.6% (P < .001), ApoB by 18.2% (P < .001), and non-HDL cholesterol by 25.4% (P < .001). HDL cholesterol was increased by 39.6% (P < .001).
These favorable effects on TG and other lipid subfractions were achieved with a safety profile that was reassuring, Dr. Bergmark said. Serious adverse events leading to discontinuation occurred in 0%, 1.7%, and 1.8% of the placebo, lower-dose, and higher-dose arms, respectively. These rates did not differ significantly.
Increased Liver Enzymes Is Common
Liver enzymes were significantly elevated (P < .001) for both doses of olezarsen vs placebo, but liver enzymes > 3× the upper limit of normal did not reach significance on either dose of olezarsen relative to placebo. Low platelet counts and reductions in kidney function were observed in a minority of patients but were generally manageable, according to Dr. Bergmark. There was no impact on hemoglobin A1c levels.
Further evaluation of change in hepatic function is planned in the ongoing extension studies.
Characterizing these results as “exciting,” Neha J. Pagidipati, MD, a member of the Duke Clinical Research Institute and an assistant professor at the Duke School of Medicine, Durham, North Carolina, said that identifying a drug effective for hypertriglyceridemia is likely to be a major advance. While elevated TGs are “one of the toughest” lipid abnormalities to manage, “there is not much out there to offer for treatment.”
She, like Dr. Soffer, was encouraged by the favorable effects on multiple lipid abnormalities associated with increased CV risk, but she said the ultimate clinical utility of this or other agents that lower TGs for ASCVD requires a study showing a change in CV events.
Dr. Bergmark reported financial relationships with 15 pharmaceutical companies, including Ionis, which provided funding for the BRIDGE-TIMI 73a trial. Soffer had financial relationships with Akcea, Amgen, Amryt, AstraZeneca, Ionis, Novartis, Regeneron, and Verve. Dr. Pagidipati had financial relationships with more than 10 pharmaceutical companies but was not involved in the design of management of the BRIDGE-TIMI 73a trial.
A version of this article first appeared on Medscape.com.
ATLANTA — A novel antisense therapy called olezarsen reduced triglycerides (TGs) by approximately 50% with either of the two study doses relative to placebo and did so with a low relative risk for adverse events, new data from a phase 2b trial showed.
“The reduction in triglycerides was greater than that currently possible with any available therapy,” reported Brian A. Bergmark, MD, an interventional cardiologist at Brigham and Women’s Hospital, Boston.
The drug also produced meaningful improvements in multiple other lipid subfractions associated with increased cardiovascular (CV) risk, including ApoC-III, very low–density lipoprotein (VLDL) cholesterol, ApoB, and non-LDL cholesterol. High-density lipoprotein (HDL) cholesterol levels were significantly raised.
The results were presented on April 7 as a late breaker at the American College of Cardiology (ACC) Scientific Session 2024 and published online simultaneously in The New England Journal of Medicine.
No Major Subgroup Failed to Respond
The effect was seen across all the key subgroups evaluated, including women and patients with diabetes, obesity, and severe as well as moderate elevations in TGs at baseline, Dr. Bergmark reported.
Olezarsen is a N-acetylgalactosamine–conjugated antisense oligonucleotide targeting APOC3 RNA.
In this study, 154 patients at 24 sites in North America were randomized in a 1:1 ratio to 50 or 80 mg olezarsen. Those in each of these cohorts were then randomized in a 3:1 ratio to active therapy or placebo. All therapies were administered by subcutaneous injection once per month.
Patients were eligible for the trial if they had moderate hypertriglyceridemia, defined as a level of 150-499 mg/dL, and elevated CV risk or if they had severe hypertriglyceridemia (≥ 500 mg/dL) with or without other evidence of elevated CV risk. The primary endpoint was a change in TGs at 6 months. Complete follow-up was available in about 97% of patients regardless of treatment assignment.
With a slight numerical advantage for the higher dose, the TG reductions were 49.1% for the 50-mg dose and 53.1% for the 80-mg dose relative to no significant change in the placebo group (P < .001 for both olezarsen doses). The reductions in ApoC-III, an upstream driver of TG production and a CV risk factor, were 64.2% and 73.2% relative to placebo (both P < .001), respectively, Dr. Bergmark reported.
In those with moderate hypertriglyceridemia, normal TG levels, defined as < 150 mg/dL, were reached at 6 months in 85.7% and 93.3% in the 40-mg and 80-mg dose groups, respectively. Relative to these reductions, normalization was seen in only 11.8% of placebo patients (P < .001).
TG Lowering Might Not Be Best Endpoint
The primary endpoint in this trial was a change in TGs, but this target was questioned by an invited ACC discussant, Daniel Soffer, MD, who is both an adjunct professor assistant professor of medicine at Penn Medicine, Philadelphia, and current president of the National Lipid Association.
Dr. Soffer noted that highly elevated TGs are a major risk factor for acute pancreatitis, so this predicts a clinical benefit for this purpose, but he thought the other lipid subfractions are far more important for the goal of reducing atherosclerotic cardiovascular disease (ASCVD).
Indeed, he said categorically that it is not TGs that drive ASCVD risk and therefore not what is the real importance of these data. Rather, “it is the non-HDL cholesterol and ApoB lowering” that will drive the likely benefits from this therapy in CV disease.
In addition to the TG reductions, olezarsen did, in fact, produce significant reductions in many of the lipid subfractions associated with increased CV risk. While slightly more favorable in most cases with the higher dose of olezarsen, even the lower dose reduced Apo C-III from baseline by 64.2% (P < .001), VLDL by 46.2% (P < .001), remnant cholesterol by 46.6% (P < .001), ApoB by 18.2% (P < .001), and non-HDL cholesterol by 25.4% (P < .001). HDL cholesterol was increased by 39.6% (P < .001).
These favorable effects on TG and other lipid subfractions were achieved with a safety profile that was reassuring, Dr. Bergmark said. Serious adverse events leading to discontinuation occurred in 0%, 1.7%, and 1.8% of the placebo, lower-dose, and higher-dose arms, respectively. These rates did not differ significantly.
Increased Liver Enzymes Is Common
Liver enzymes were significantly elevated (P < .001) for both doses of olezarsen vs placebo, but liver enzymes > 3× the upper limit of normal did not reach significance on either dose of olezarsen relative to placebo. Low platelet counts and reductions in kidney function were observed in a minority of patients but were generally manageable, according to Dr. Bergmark. There was no impact on hemoglobin A1c levels.
Further evaluation of change in hepatic function is planned in the ongoing extension studies.
Characterizing these results as “exciting,” Neha J. Pagidipati, MD, a member of the Duke Clinical Research Institute and an assistant professor at the Duke School of Medicine, Durham, North Carolina, said that identifying a drug effective for hypertriglyceridemia is likely to be a major advance. While elevated TGs are “one of the toughest” lipid abnormalities to manage, “there is not much out there to offer for treatment.”
She, like Dr. Soffer, was encouraged by the favorable effects on multiple lipid abnormalities associated with increased CV risk, but she said the ultimate clinical utility of this or other agents that lower TGs for ASCVD requires a study showing a change in CV events.
Dr. Bergmark reported financial relationships with 15 pharmaceutical companies, including Ionis, which provided funding for the BRIDGE-TIMI 73a trial. Soffer had financial relationships with Akcea, Amgen, Amryt, AstraZeneca, Ionis, Novartis, Regeneron, and Verve. Dr. Pagidipati had financial relationships with more than 10 pharmaceutical companies but was not involved in the design of management of the BRIDGE-TIMI 73a trial.
A version of this article first appeared on Medscape.com.
EBER-Negative, Double-Hit High-Grade B-Cell Lymphoma Responding to Methotrexate Discontinuation
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
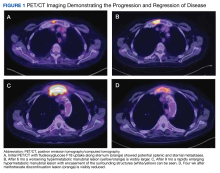
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
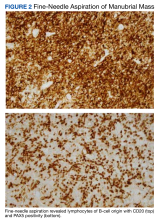
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
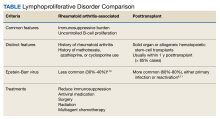
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
Study Shows Nirmatrelvir–Ritonavir No More Effective Than Placebo for COVID-19 Symptom Relief
Paxlovid does not significantly alleviate symptoms of COVID-19 compared with placebo among nonhospitalized adults, a new study published April 3 in The New England Journal of Medicine found.
The results suggest that the drug, a combination of nirmatrelvir and ritonavir, may not be particularly helpful for patients who are not at high risk for severe COVID-19. However, although the rate of hospitalization and death from any cause was low overall, the group that received Paxlovid had a reduced rate compared with people in the placebo group, according to the researchers.
“Clearly, the benefit observed among unvaccinated high-risk persons does not extend to those at lower risk for severe COVID-19,” Rajesh T. Gandhi, MD, and Martin Hirsch, MD, of Massachusetts General Hospital in Boston, wrote in an editorial accompanying the journal article. “This result supports guidelines that recommend nirmatrelvir–ritonavir only for persons who are at high risk for disease progression.”
The time from onset to relief of COVID-19 symptoms — including cough, shortness of breath, body aches, and chills — did not differ significantly between the two study groups, the researchers reported. The median time to sustained alleviation of symptoms was 12 days for the Paxlovid group compared with 13 days in the placebo group (P = .60).
However, the phase 2/3 trial found a 57.6% relative reduction in the risk for hospitalizations or death among people who took Paxlovid and were vaccinated but were at high risk for poor outcomes, according to Jennifer Hammond, PhD, head of antiviral development for Pfizer, which makes the drug, and the corresponding author on the study.
Paxlovid has “an increasing body of evidence supporting the strong clinical value of the treatment in preventing hospitalization and death among eligible patients across age groups, vaccination status, and predominant variants,” Dr. Hammond said.
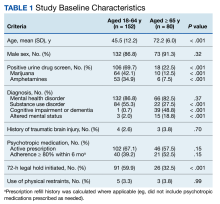
She and her colleagues analyzed data from 1250 adults with symptomatic COVID-19. Participants were fully vaccinated and had a high risk for progression to severe disease or were never vaccinated or had not been in the previous year and had no risk factors for progression to severe disease.
More than half of participants were women, 78.5% were White and 41.4% identified as Hispanic or Latinx. Almost three quarters underwent randomization within 3 days of the start of symptoms, and a little over half had previously received a COVID-19 vaccination. Almost half had one risk factor for severe illness, the most common of these being hypertension (12.3%).
In a subgroup analysis of high-risk participants, hospitalization or death occurred in 0.9% of patients in the Paxlovid group and 2.2% in the placebo group (95% CI, -3.3 to 0.7).
The study’s limitations include that the statistical analysis of COVID-19–related hospitalizations or death from any cause was only descriptive, “because the results for the primary efficacy end point were not significant,” the authors wrote.
Participants who were vaccinated and at high risk were also enrolled regardless of when they had last had a vaccine dose. Furthermore, Paxlovid has a telltale taste, which may have affected the blinding. Finally, the trial was started when the B.1.617.2 (Delta) variant was predominant.
Dr. Gandhi and Dr. Hirsch pointed out that only 5% of participants in the trial were older than 65 years and that other than risk factors such as obesity and smoking, just 2% of people had heart or lung disease.
“As with many medical interventions, there is likely to be a gradient of benefit for nirmatrelvir–ritonavir, with the patients at highest risk for progression most likely to derive the greatest benefit,” Dr. Gandhi and Dr. Hirsch wrote in the editorial. “Thus, it appears reasonable to recommend nirmatrelvir–ritonavir primarily for the treatment of COVID-19 in older patients (particularly those ≥ 65 years of age), those who are immunocompromised, and those who have conditions that substantially increase the risk of severe COVID-19, regardless of previous vaccination or infection status.”
The study was supported by Pfizer.
A version of this article appeared on Medscape.com .
Paxlovid does not significantly alleviate symptoms of COVID-19 compared with placebo among nonhospitalized adults, a new study published April 3 in The New England Journal of Medicine found.
The results suggest that the drug, a combination of nirmatrelvir and ritonavir, may not be particularly helpful for patients who are not at high risk for severe COVID-19. However, although the rate of hospitalization and death from any cause was low overall, the group that received Paxlovid had a reduced rate compared with people in the placebo group, according to the researchers.
“Clearly, the benefit observed among unvaccinated high-risk persons does not extend to those at lower risk for severe COVID-19,” Rajesh T. Gandhi, MD, and Martin Hirsch, MD, of Massachusetts General Hospital in Boston, wrote in an editorial accompanying the journal article. “This result supports guidelines that recommend nirmatrelvir–ritonavir only for persons who are at high risk for disease progression.”
The time from onset to relief of COVID-19 symptoms — including cough, shortness of breath, body aches, and chills — did not differ significantly between the two study groups, the researchers reported. The median time to sustained alleviation of symptoms was 12 days for the Paxlovid group compared with 13 days in the placebo group (P = .60).
However, the phase 2/3 trial found a 57.6% relative reduction in the risk for hospitalizations or death among people who took Paxlovid and were vaccinated but were at high risk for poor outcomes, according to Jennifer Hammond, PhD, head of antiviral development for Pfizer, which makes the drug, and the corresponding author on the study.
Paxlovid has “an increasing body of evidence supporting the strong clinical value of the treatment in preventing hospitalization and death among eligible patients across age groups, vaccination status, and predominant variants,” Dr. Hammond said.
She and her colleagues analyzed data from 1250 adults with symptomatic COVID-19. Participants were fully vaccinated and had a high risk for progression to severe disease or were never vaccinated or had not been in the previous year and had no risk factors for progression to severe disease.
More than half of participants were women, 78.5% were White and 41.4% identified as Hispanic or Latinx. Almost three quarters underwent randomization within 3 days of the start of symptoms, and a little over half had previously received a COVID-19 vaccination. Almost half had one risk factor for severe illness, the most common of these being hypertension (12.3%).
In a subgroup analysis of high-risk participants, hospitalization or death occurred in 0.9% of patients in the Paxlovid group and 2.2% in the placebo group (95% CI, -3.3 to 0.7).
The study’s limitations include that the statistical analysis of COVID-19–related hospitalizations or death from any cause was only descriptive, “because the results for the primary efficacy end point were not significant,” the authors wrote.
Participants who were vaccinated and at high risk were also enrolled regardless of when they had last had a vaccine dose. Furthermore, Paxlovid has a telltale taste, which may have affected the blinding. Finally, the trial was started when the B.1.617.2 (Delta) variant was predominant.
Dr. Gandhi and Dr. Hirsch pointed out that only 5% of participants in the trial were older than 65 years and that other than risk factors such as obesity and smoking, just 2% of people had heart or lung disease.
“As with many medical interventions, there is likely to be a gradient of benefit for nirmatrelvir–ritonavir, with the patients at highest risk for progression most likely to derive the greatest benefit,” Dr. Gandhi and Dr. Hirsch wrote in the editorial. “Thus, it appears reasonable to recommend nirmatrelvir–ritonavir primarily for the treatment of COVID-19 in older patients (particularly those ≥ 65 years of age), those who are immunocompromised, and those who have conditions that substantially increase the risk of severe COVID-19, regardless of previous vaccination or infection status.”
The study was supported by Pfizer.
A version of this article appeared on Medscape.com .
Paxlovid does not significantly alleviate symptoms of COVID-19 compared with placebo among nonhospitalized adults, a new study published April 3 in The New England Journal of Medicine found.
The results suggest that the drug, a combination of nirmatrelvir and ritonavir, may not be particularly helpful for patients who are not at high risk for severe COVID-19. However, although the rate of hospitalization and death from any cause was low overall, the group that received Paxlovid had a reduced rate compared with people in the placebo group, according to the researchers.
“Clearly, the benefit observed among unvaccinated high-risk persons does not extend to those at lower risk for severe COVID-19,” Rajesh T. Gandhi, MD, and Martin Hirsch, MD, of Massachusetts General Hospital in Boston, wrote in an editorial accompanying the journal article. “This result supports guidelines that recommend nirmatrelvir–ritonavir only for persons who are at high risk for disease progression.”
The time from onset to relief of COVID-19 symptoms — including cough, shortness of breath, body aches, and chills — did not differ significantly between the two study groups, the researchers reported. The median time to sustained alleviation of symptoms was 12 days for the Paxlovid group compared with 13 days in the placebo group (P = .60).
However, the phase 2/3 trial found a 57.6% relative reduction in the risk for hospitalizations or death among people who took Paxlovid and were vaccinated but were at high risk for poor outcomes, according to Jennifer Hammond, PhD, head of antiviral development for Pfizer, which makes the drug, and the corresponding author on the study.
Paxlovid has “an increasing body of evidence supporting the strong clinical value of the treatment in preventing hospitalization and death among eligible patients across age groups, vaccination status, and predominant variants,” Dr. Hammond said.
She and her colleagues analyzed data from 1250 adults with symptomatic COVID-19. Participants were fully vaccinated and had a high risk for progression to severe disease or were never vaccinated or had not been in the previous year and had no risk factors for progression to severe disease.
More than half of participants were women, 78.5% were White and 41.4% identified as Hispanic or Latinx. Almost three quarters underwent randomization within 3 days of the start of symptoms, and a little over half had previously received a COVID-19 vaccination. Almost half had one risk factor for severe illness, the most common of these being hypertension (12.3%).
In a subgroup analysis of high-risk participants, hospitalization or death occurred in 0.9% of patients in the Paxlovid group and 2.2% in the placebo group (95% CI, -3.3 to 0.7).
The study’s limitations include that the statistical analysis of COVID-19–related hospitalizations or death from any cause was only descriptive, “because the results for the primary efficacy end point were not significant,” the authors wrote.
Participants who were vaccinated and at high risk were also enrolled regardless of when they had last had a vaccine dose. Furthermore, Paxlovid has a telltale taste, which may have affected the blinding. Finally, the trial was started when the B.1.617.2 (Delta) variant was predominant.
Dr. Gandhi and Dr. Hirsch pointed out that only 5% of participants in the trial were older than 65 years and that other than risk factors such as obesity and smoking, just 2% of people had heart or lung disease.
“As with many medical interventions, there is likely to be a gradient of benefit for nirmatrelvir–ritonavir, with the patients at highest risk for progression most likely to derive the greatest benefit,” Dr. Gandhi and Dr. Hirsch wrote in the editorial. “Thus, it appears reasonable to recommend nirmatrelvir–ritonavir primarily for the treatment of COVID-19 in older patients (particularly those ≥ 65 years of age), those who are immunocompromised, and those who have conditions that substantially increase the risk of severe COVID-19, regardless of previous vaccination or infection status.”
The study was supported by Pfizer.
A version of this article appeared on Medscape.com .
Evaluation of Anti-Agitation Medication Prescribing Patterns by Age in the Emergency Department
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Magnesium Spray for Better Sleep? Experts Weigh In
As your patient’s scheduled bedtime is approaching, they begin to worry another restless night is looming. Could magnesium oil spray actually help them sleep? Some — even doctors — are sharing testimonials about how this simple tactic transformed their sleep quality. Experts suggest some sleep improvement is possible, though it does not negate the need for treatment, and should not be used in patients with cardiovascular disease.
Take Daniel Barrett, MD, a board-certified plastic surgeon and owner of Barrett Plastic Surgery in Beverly Hills, as an example. He decided to test whether magnesium oil could indeed give him a sleepy sensation and shared his experience. Dr. Barrett sprayed magnesium oil on his feet — until they felt “slippery and wet,” he said — and put his socks back on. (He said magnesium is absorbed more easily through the skin. Putting it on the skin helps this mineral get into the lymphatics and circulatory system, offering a way to get a higher concentration of magnesium in the bloodstream. The pores on the feet are also said to be the largest on the body, making them an ideal place for absorption.)
“My central nervous system had calmed down a bit — it’s similar to what I feel when I take oral magnesium as well. It took about 15 minutes to feel the effect,” Dr. Barrett said.
Research shows that magnesium blocks N-methyl-D-aspartate (a receptor that can hinder sleep) and stimulates gamma-aminobutyric acid (a receptor that can promote good sleep), said Dennis Auckley, MD, director of MetroHealth’s Center for Sleep Medicine. And studies looking at the effects of oral magnesium have shown that taking it may be linked to better self-reported sleep quality and less daytime sleepiness, he said. But traditional magnesium supplements taken orally can sometimes come with side effects in your gut, so putting magnesium on the skin could help to avoid this.
Magnesium oil on the feet could also help with certain sleep disturbances, such as nocturnal leg cramps and restless legs syndrome, said Sam Kashani, MD, a sleep medicine specialist and assistant clinical professor at UCLA Medical School. (Nocturnal leg cramps – one of the most common secondary factors of insomnia and sleep disturbances in older adults – includes sudden, painful contractions in the lower leg muscles while sleeping. Restless legs syndrome, on the other hand, is like nocturnal leg cramps, but minus the painful contractions, said Dr. Kashani.)
“Magnesium is a mineral that does have some benefit with regard to reducing the muscle tightness and promoting a little bit more of relaxation of the muscles,” Dr. Kashani said. “This [magnesium oil on your soles] could be beneficial for these types of sleep problems.”
Still, sleep medicine experts stressed that putting magnesium oil on your feet should not be viewed a cure-all for sleep troubles.
“High-quality scientific evidence supporting magnesium as a sleep remedy is severely limited,” said Emerson Wickwire, PhD, an American Academy of Sleep Medicine spokesperson and section head of sleep medicine at the University of Maryland Medical School. “Certainly, magnesium is not supported as a treatment for sleep disorders.”
If your patients plan to use magnesium oil on their feet to help them sleep, make sure they carefully follow the directions to make sure they are taking the proper dosage. Most importantly, patients with a history of cardiovascular complications, or issues with the heart and blood vessels should consult their doctor.
“Magnesium is an electrolyte that has multiple roles and functions in the body, including within our cardiovascular system,” Dr. Kashani said. “So, if you are somebody who has heart troubles, you definitely want to talk to your primary doctor about any kind of supplements that you are taking, including magnesium.”
A version of this article appeared on WebMD.com.
As your patient’s scheduled bedtime is approaching, they begin to worry another restless night is looming. Could magnesium oil spray actually help them sleep? Some — even doctors — are sharing testimonials about how this simple tactic transformed their sleep quality. Experts suggest some sleep improvement is possible, though it does not negate the need for treatment, and should not be used in patients with cardiovascular disease.
Take Daniel Barrett, MD, a board-certified plastic surgeon and owner of Barrett Plastic Surgery in Beverly Hills, as an example. He decided to test whether magnesium oil could indeed give him a sleepy sensation and shared his experience. Dr. Barrett sprayed magnesium oil on his feet — until they felt “slippery and wet,” he said — and put his socks back on. (He said magnesium is absorbed more easily through the skin. Putting it on the skin helps this mineral get into the lymphatics and circulatory system, offering a way to get a higher concentration of magnesium in the bloodstream. The pores on the feet are also said to be the largest on the body, making them an ideal place for absorption.)
“My central nervous system had calmed down a bit — it’s similar to what I feel when I take oral magnesium as well. It took about 15 minutes to feel the effect,” Dr. Barrett said.
Research shows that magnesium blocks N-methyl-D-aspartate (a receptor that can hinder sleep) and stimulates gamma-aminobutyric acid (a receptor that can promote good sleep), said Dennis Auckley, MD, director of MetroHealth’s Center for Sleep Medicine. And studies looking at the effects of oral magnesium have shown that taking it may be linked to better self-reported sleep quality and less daytime sleepiness, he said. But traditional magnesium supplements taken orally can sometimes come with side effects in your gut, so putting magnesium on the skin could help to avoid this.
Magnesium oil on the feet could also help with certain sleep disturbances, such as nocturnal leg cramps and restless legs syndrome, said Sam Kashani, MD, a sleep medicine specialist and assistant clinical professor at UCLA Medical School. (Nocturnal leg cramps – one of the most common secondary factors of insomnia and sleep disturbances in older adults – includes sudden, painful contractions in the lower leg muscles while sleeping. Restless legs syndrome, on the other hand, is like nocturnal leg cramps, but minus the painful contractions, said Dr. Kashani.)
“Magnesium is a mineral that does have some benefit with regard to reducing the muscle tightness and promoting a little bit more of relaxation of the muscles,” Dr. Kashani said. “This [magnesium oil on your soles] could be beneficial for these types of sleep problems.”
Still, sleep medicine experts stressed that putting magnesium oil on your feet should not be viewed a cure-all for sleep troubles.
“High-quality scientific evidence supporting magnesium as a sleep remedy is severely limited,” said Emerson Wickwire, PhD, an American Academy of Sleep Medicine spokesperson and section head of sleep medicine at the University of Maryland Medical School. “Certainly, magnesium is not supported as a treatment for sleep disorders.”
If your patients plan to use magnesium oil on their feet to help them sleep, make sure they carefully follow the directions to make sure they are taking the proper dosage. Most importantly, patients with a history of cardiovascular complications, or issues with the heart and blood vessels should consult their doctor.
“Magnesium is an electrolyte that has multiple roles and functions in the body, including within our cardiovascular system,” Dr. Kashani said. “So, if you are somebody who has heart troubles, you definitely want to talk to your primary doctor about any kind of supplements that you are taking, including magnesium.”
A version of this article appeared on WebMD.com.
As your patient’s scheduled bedtime is approaching, they begin to worry another restless night is looming. Could magnesium oil spray actually help them sleep? Some — even doctors — are sharing testimonials about how this simple tactic transformed their sleep quality. Experts suggest some sleep improvement is possible, though it does not negate the need for treatment, and should not be used in patients with cardiovascular disease.
Take Daniel Barrett, MD, a board-certified plastic surgeon and owner of Barrett Plastic Surgery in Beverly Hills, as an example. He decided to test whether magnesium oil could indeed give him a sleepy sensation and shared his experience. Dr. Barrett sprayed magnesium oil on his feet — until they felt “slippery and wet,” he said — and put his socks back on. (He said magnesium is absorbed more easily through the skin. Putting it on the skin helps this mineral get into the lymphatics and circulatory system, offering a way to get a higher concentration of magnesium in the bloodstream. The pores on the feet are also said to be the largest on the body, making them an ideal place for absorption.)
“My central nervous system had calmed down a bit — it’s similar to what I feel when I take oral magnesium as well. It took about 15 minutes to feel the effect,” Dr. Barrett said.
Research shows that magnesium blocks N-methyl-D-aspartate (a receptor that can hinder sleep) and stimulates gamma-aminobutyric acid (a receptor that can promote good sleep), said Dennis Auckley, MD, director of MetroHealth’s Center for Sleep Medicine. And studies looking at the effects of oral magnesium have shown that taking it may be linked to better self-reported sleep quality and less daytime sleepiness, he said. But traditional magnesium supplements taken orally can sometimes come with side effects in your gut, so putting magnesium on the skin could help to avoid this.
Magnesium oil on the feet could also help with certain sleep disturbances, such as nocturnal leg cramps and restless legs syndrome, said Sam Kashani, MD, a sleep medicine specialist and assistant clinical professor at UCLA Medical School. (Nocturnal leg cramps – one of the most common secondary factors of insomnia and sleep disturbances in older adults – includes sudden, painful contractions in the lower leg muscles while sleeping. Restless legs syndrome, on the other hand, is like nocturnal leg cramps, but minus the painful contractions, said Dr. Kashani.)
“Magnesium is a mineral that does have some benefit with regard to reducing the muscle tightness and promoting a little bit more of relaxation of the muscles,” Dr. Kashani said. “This [magnesium oil on your soles] could be beneficial for these types of sleep problems.”
Still, sleep medicine experts stressed that putting magnesium oil on your feet should not be viewed a cure-all for sleep troubles.
“High-quality scientific evidence supporting magnesium as a sleep remedy is severely limited,” said Emerson Wickwire, PhD, an American Academy of Sleep Medicine spokesperson and section head of sleep medicine at the University of Maryland Medical School. “Certainly, magnesium is not supported as a treatment for sleep disorders.”
If your patients plan to use magnesium oil on their feet to help them sleep, make sure they carefully follow the directions to make sure they are taking the proper dosage. Most importantly, patients with a history of cardiovascular complications, or issues with the heart and blood vessels should consult their doctor.
“Magnesium is an electrolyte that has multiple roles and functions in the body, including within our cardiovascular system,” Dr. Kashani said. “So, if you are somebody who has heart troubles, you definitely want to talk to your primary doctor about any kind of supplements that you are taking, including magnesium.”
A version of this article appeared on WebMD.com.
No Increased Stroke Risk After COVID-19 Bivalent Vaccine
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
The Truth About Compounded GLP-1s That Doctors Need to Know
As a cardiologist specializing in obesity medicine, I often encounter patients who would greatly benefit from the new generation of weight loss drugs that work as glucagon-like peptide 1 (GLP-1) agonists. In the recently published SELECT trial results, for example, semaglutide (marketed by Novo Nordisk as Wegovy for weight loss and Ozempic for type 2 diabetes) demonstrated a 20% risk reduction of heart attacks and strokes in overweight and obese individuals without diabetes and with cardiovascular disease, establishing it as a cardiovascular disease–modifying medication in people without type 2 diabetes.
Unfortunately, the high demand for these new weight loss medications has resulted in a frustrating, long-lasting shortage.
To ensure continuation of patient care, federal law allows compounding pharmacies to make “essentially a copy” of the medications that are listed as “currently in shortage” on the US Food and Drug Administration (FDA) drug shortage list. Both semaglutide and tirzepatide are on that list. For Americans who suffer from obesity and other weight-related diseases, these drugs could be a lifeline.
Despite this, the medical community has broadly criticized the utilization of compounded GLP-1 agonists, even those obtained from reputable and legitimate compounding pharmacies.
Yes, high demand has led to the emergence of unregulated companies and scammers producing substandard or counterfeit versions of these medications.
The FDA has found fraudulent products (masquerading as the weight loss drugs) and has issued warning letters to stop the distribution of illegally marketed semaglutide. “These drugs may be counterfeit, which means they could contain the wrong ingredients, contain too little, too much or no active ingredient at all, or contain other harmful ingredients,” it cautions. Some products use a similar-sounding semaglutide sodium salt, which has uncertain safety and efficacy, and had generated warnings from the FDA and state boards of pharmacy.
Many of these products are marketed directly to consumers online through websites and social media, with little to no medical oversight. This practice is a significant concern, as it may affect patient safety, and should be discouraged.
However, according to a statement from the Alliance for Pharmacy Compounding (APC), legitimate compounding pharmacies aren’t the ones selling these dubious products on the black market, particularly online. This illegal practice has garnered media attention and is sometimes incorrectly associated with legitimate pharmacy compounding.
In contrast, legal and certified versions of GLP-1 agonist medications can be obtained from well-regulated and reputable compounding pharmacies. These pharmacies must adhere to all federal and state regulations and dispense medications only with a valid prescription from a licensed physician.
Meanwhile, the APC statement notes, Novo Nordisk and Eli Lilly have sued compounding companies in several states, questioning, among other things, the purity and potency of some compounded products.
There are different designations for compounding pharmacies: 503A and 503B. 503As are state-licensed pharmacies and physicians, and 503B pharmacies are federally regulated outsourcing facilities that are strictly regulated by the FDA. This regulation, established following a 2012 fungal meningitis outbreak linked to a compounding pharmacy, ensures higher-quality control and oversight, especially for medications intended for intravenous or epidural use. These standards exceed those required for subcutaneous injections like GLP-1 analogs.
In the face of this Wild West climate, where compounded drugs may vary in their source, formulation, potency, and purity, The Obesity Society, the Obesity Medical Association, and the Obesity Action Coalition published a joint statement that advised against the use of compounded GLP-1 agonists, citing safety concerns and lack of regulatory oversight.
This stance, while aimed at ensuring patient safety, inadvertently raises a critical issue.
By completely dismissing compounded medications, experts may unintentionally bolster the black market and overlook the needs of patients who could benefit from these medications, contrary to the intentions of the exemption provided in federal law for compounding during a drug shortage. In fact, the presence of unreliable suppliers highlights the need to direct the public toward trustworthy sources, rather than imposing a total ban on medically appropriate alternatives.
The joint statement calls compounded GLP-1 agonists “counterfeit.” This inaccurate overgeneralization probably stems from a misunderstanding of the compounding process and its regulations. Legitimate and regulated pharmacies compound base GLP-1 agonists, which are “essentially a copy” of FDA-approved medications, not counterfeits. Recognizing this is crucial for maintaining trust in both compounding pharmacies and regulatory bodies.
It is correct that “the only FDA-approved manufacturers of these medications are the companies that created the active pharmaceutical ingredients — Novo Nordisk and Eli Lilly,” but the joint statement fails to mention the exemptions provided by law that allow compounding copies of the branded medications if they are on the shortage list.
Compounding pharmacies must obtain active pharmaceutical ingredients (APIs) from FDA-registered facilities, which are required to adhere to Current Good Manufacturing Practices (cGMP). This ensures the APIs’ quality, potency, and purity, crucial for the safety and efficacy of compounded medications.
Compounded drugs are not FDA approved, but they aren’t inherently unsafe. Compounded medications include critical drugs such as resuscitation medications and antibiotics, and are often used in healthcare settings, especially when there’s a shortage. This raises the question of why compounded GLP-1 agonists would be treated any differently in such scenarios.
And in the case of alternative drugs for individuals with obesity who have a higher risk for cardiovascular disease, the brand-name FDA-approved alternative may be of more concern than the compounded GLP-1 agonist. The obesity societies advise: “If you cannot find or get access to a GLP-1-based treatment now, there are other treatments available,” echoing experts. While the statement doesn’t specify the names of the alternatives, experts have advised using alternatives such as Qsymia and Contrave, despite their potential cardiovascular concerns. This recommendation to the public may not represent a responsible risk-benefit analysis.
Rather than outright banning compounded GLP-1 medications, expert associations can contribute to the solution by creating a “seal of approval,” recognizing high-quality compounded medications. This would contribute to informed decision-making for clinicians and patients.
Possible Solutions
When prescribing GLP-1 agonists for obesity treatment, doctors should consider all of the following steps to ensure patient safety and effective treatment:
Preference for FDA-approved brands: FDA-approved branded GLP-1 agonist medications should be the primary choice because of their established safety and efficacy.
Risk-benefit analysis for non–FDA-approved products: In cases where FDA-approved options are not available, doctors may consider prescribing a non–FDA-approved copy of the branded medication. Prior to this, conduct a thorough risk-benefit analysis with the patient, ensuring that they are fully informed about the potential risks and benefits of using a non–FDA-approved product.
Choosing semaglutide copies for specific cases: In patients with obesity and cardiovascular disease, the benefits of using a compounded copy of semaglutide, with its cardiovascular disease–modifying properties, may outweigh the risks compared with other FDA-approved antiobesity drugs that might pose cardiovascular risks or compared with no antiobesity treatment at all.
Informed consent and monitoring: When prescribing a non–FDA-approved version of a GLP-1 agonist, obtaining informed consent from the patient is advised. They should be made aware of the differences between the FDA-approved and nonapproved versions.
Choosing between 503A and 503B pharmacies: Prescriptions for non–FDA-approved GLP-1 agonists can be directed to either 503A or 503B compounding pharmacies. However, it’s advisable to check whether the product can be compounded by a 503B pharmacy, which is subject to an additional layer of FDA regulation, offering greater quality assurance.
Clear prescription specifications: Ensure that the prescription explicitly states that the compounded GLP-1 agonist should be the base compound without additives.
Requesting a Certificate of Analysis: To further ensure safety, request a Certificate of Analysis from the compounding pharmacy. This provides detailed quality and composition information about the product.
Ongoing monitoring: Continuously monitor the patient’s response to the medication and adjust the treatment plan as necessary, maintaining regular follow-ups.
By adhering to these guidelines, doctors can navigate the complexities of prescribing GLP-1 agonists in a way that prioritizes patient well-being, particularly in scenarios where conventional treatment options are limited.
Dr. Einav is a board-certified cardiologist and a Diplomate of the American Board of Obesity Medicine. He is a fellow of the American College of Cardiology and a member of the Obesity Medicine Association. He serves as the medical director of cardiometabolic health in Guthrie Lourdes in Binghamton, New York, and is the founder of myW8/Cardiometabolic Health located in Beverly Hills, California. This article solely reflects the personal views of Dr. Einav and should not be considered as representing the official stance of Guthrie Lourdes. Dr. Einav served as a promotional speaker for Novo Nordisk in 2022. As of now, he has not prescribed any compounded GLP-1 agonist medications in his medical practice.
A version of this article appeared on Medscape.com.
As a cardiologist specializing in obesity medicine, I often encounter patients who would greatly benefit from the new generation of weight loss drugs that work as glucagon-like peptide 1 (GLP-1) agonists. In the recently published SELECT trial results, for example, semaglutide (marketed by Novo Nordisk as Wegovy for weight loss and Ozempic for type 2 diabetes) demonstrated a 20% risk reduction of heart attacks and strokes in overweight and obese individuals without diabetes and with cardiovascular disease, establishing it as a cardiovascular disease–modifying medication in people without type 2 diabetes.
Unfortunately, the high demand for these new weight loss medications has resulted in a frustrating, long-lasting shortage.
To ensure continuation of patient care, federal law allows compounding pharmacies to make “essentially a copy” of the medications that are listed as “currently in shortage” on the US Food and Drug Administration (FDA) drug shortage list. Both semaglutide and tirzepatide are on that list. For Americans who suffer from obesity and other weight-related diseases, these drugs could be a lifeline.
Despite this, the medical community has broadly criticized the utilization of compounded GLP-1 agonists, even those obtained from reputable and legitimate compounding pharmacies.
Yes, high demand has led to the emergence of unregulated companies and scammers producing substandard or counterfeit versions of these medications.
The FDA has found fraudulent products (masquerading as the weight loss drugs) and has issued warning letters to stop the distribution of illegally marketed semaglutide. “These drugs may be counterfeit, which means they could contain the wrong ingredients, contain too little, too much or no active ingredient at all, or contain other harmful ingredients,” it cautions. Some products use a similar-sounding semaglutide sodium salt, which has uncertain safety and efficacy, and had generated warnings from the FDA and state boards of pharmacy.
Many of these products are marketed directly to consumers online through websites and social media, with little to no medical oversight. This practice is a significant concern, as it may affect patient safety, and should be discouraged.
However, according to a statement from the Alliance for Pharmacy Compounding (APC), legitimate compounding pharmacies aren’t the ones selling these dubious products on the black market, particularly online. This illegal practice has garnered media attention and is sometimes incorrectly associated with legitimate pharmacy compounding.
In contrast, legal and certified versions of GLP-1 agonist medications can be obtained from well-regulated and reputable compounding pharmacies. These pharmacies must adhere to all federal and state regulations and dispense medications only with a valid prescription from a licensed physician.
Meanwhile, the APC statement notes, Novo Nordisk and Eli Lilly have sued compounding companies in several states, questioning, among other things, the purity and potency of some compounded products.
There are different designations for compounding pharmacies: 503A and 503B. 503As are state-licensed pharmacies and physicians, and 503B pharmacies are federally regulated outsourcing facilities that are strictly regulated by the FDA. This regulation, established following a 2012 fungal meningitis outbreak linked to a compounding pharmacy, ensures higher-quality control and oversight, especially for medications intended for intravenous or epidural use. These standards exceed those required for subcutaneous injections like GLP-1 analogs.
In the face of this Wild West climate, where compounded drugs may vary in their source, formulation, potency, and purity, The Obesity Society, the Obesity Medical Association, and the Obesity Action Coalition published a joint statement that advised against the use of compounded GLP-1 agonists, citing safety concerns and lack of regulatory oversight.
This stance, while aimed at ensuring patient safety, inadvertently raises a critical issue.
By completely dismissing compounded medications, experts may unintentionally bolster the black market and overlook the needs of patients who could benefit from these medications, contrary to the intentions of the exemption provided in federal law for compounding during a drug shortage. In fact, the presence of unreliable suppliers highlights the need to direct the public toward trustworthy sources, rather than imposing a total ban on medically appropriate alternatives.
The joint statement calls compounded GLP-1 agonists “counterfeit.” This inaccurate overgeneralization probably stems from a misunderstanding of the compounding process and its regulations. Legitimate and regulated pharmacies compound base GLP-1 agonists, which are “essentially a copy” of FDA-approved medications, not counterfeits. Recognizing this is crucial for maintaining trust in both compounding pharmacies and regulatory bodies.
It is correct that “the only FDA-approved manufacturers of these medications are the companies that created the active pharmaceutical ingredients — Novo Nordisk and Eli Lilly,” but the joint statement fails to mention the exemptions provided by law that allow compounding copies of the branded medications if they are on the shortage list.
Compounding pharmacies must obtain active pharmaceutical ingredients (APIs) from FDA-registered facilities, which are required to adhere to Current Good Manufacturing Practices (cGMP). This ensures the APIs’ quality, potency, and purity, crucial for the safety and efficacy of compounded medications.
Compounded drugs are not FDA approved, but they aren’t inherently unsafe. Compounded medications include critical drugs such as resuscitation medications and antibiotics, and are often used in healthcare settings, especially when there’s a shortage. This raises the question of why compounded GLP-1 agonists would be treated any differently in such scenarios.
And in the case of alternative drugs for individuals with obesity who have a higher risk for cardiovascular disease, the brand-name FDA-approved alternative may be of more concern than the compounded GLP-1 agonist. The obesity societies advise: “If you cannot find or get access to a GLP-1-based treatment now, there are other treatments available,” echoing experts. While the statement doesn’t specify the names of the alternatives, experts have advised using alternatives such as Qsymia and Contrave, despite their potential cardiovascular concerns. This recommendation to the public may not represent a responsible risk-benefit analysis.
Rather than outright banning compounded GLP-1 medications, expert associations can contribute to the solution by creating a “seal of approval,” recognizing high-quality compounded medications. This would contribute to informed decision-making for clinicians and patients.
Possible Solutions
When prescribing GLP-1 agonists for obesity treatment, doctors should consider all of the following steps to ensure patient safety and effective treatment:
Preference for FDA-approved brands: FDA-approved branded GLP-1 agonist medications should be the primary choice because of their established safety and efficacy.
Risk-benefit analysis for non–FDA-approved products: In cases where FDA-approved options are not available, doctors may consider prescribing a non–FDA-approved copy of the branded medication. Prior to this, conduct a thorough risk-benefit analysis with the patient, ensuring that they are fully informed about the potential risks and benefits of using a non–FDA-approved product.
Choosing semaglutide copies for specific cases: In patients with obesity and cardiovascular disease, the benefits of using a compounded copy of semaglutide, with its cardiovascular disease–modifying properties, may outweigh the risks compared with other FDA-approved antiobesity drugs that might pose cardiovascular risks or compared with no antiobesity treatment at all.
Informed consent and monitoring: When prescribing a non–FDA-approved version of a GLP-1 agonist, obtaining informed consent from the patient is advised. They should be made aware of the differences between the FDA-approved and nonapproved versions.
Choosing between 503A and 503B pharmacies: Prescriptions for non–FDA-approved GLP-1 agonists can be directed to either 503A or 503B compounding pharmacies. However, it’s advisable to check whether the product can be compounded by a 503B pharmacy, which is subject to an additional layer of FDA regulation, offering greater quality assurance.
Clear prescription specifications: Ensure that the prescription explicitly states that the compounded GLP-1 agonist should be the base compound without additives.
Requesting a Certificate of Analysis: To further ensure safety, request a Certificate of Analysis from the compounding pharmacy. This provides detailed quality and composition information about the product.
Ongoing monitoring: Continuously monitor the patient’s response to the medication and adjust the treatment plan as necessary, maintaining regular follow-ups.
By adhering to these guidelines, doctors can navigate the complexities of prescribing GLP-1 agonists in a way that prioritizes patient well-being, particularly in scenarios where conventional treatment options are limited.
Dr. Einav is a board-certified cardiologist and a Diplomate of the American Board of Obesity Medicine. He is a fellow of the American College of Cardiology and a member of the Obesity Medicine Association. He serves as the medical director of cardiometabolic health in Guthrie Lourdes in Binghamton, New York, and is the founder of myW8/Cardiometabolic Health located in Beverly Hills, California. This article solely reflects the personal views of Dr. Einav and should not be considered as representing the official stance of Guthrie Lourdes. Dr. Einav served as a promotional speaker for Novo Nordisk in 2022. As of now, he has not prescribed any compounded GLP-1 agonist medications in his medical practice.
A version of this article appeared on Medscape.com.
As a cardiologist specializing in obesity medicine, I often encounter patients who would greatly benefit from the new generation of weight loss drugs that work as glucagon-like peptide 1 (GLP-1) agonists. In the recently published SELECT trial results, for example, semaglutide (marketed by Novo Nordisk as Wegovy for weight loss and Ozempic for type 2 diabetes) demonstrated a 20% risk reduction of heart attacks and strokes in overweight and obese individuals without diabetes and with cardiovascular disease, establishing it as a cardiovascular disease–modifying medication in people without type 2 diabetes.
Unfortunately, the high demand for these new weight loss medications has resulted in a frustrating, long-lasting shortage.
To ensure continuation of patient care, federal law allows compounding pharmacies to make “essentially a copy” of the medications that are listed as “currently in shortage” on the US Food and Drug Administration (FDA) drug shortage list. Both semaglutide and tirzepatide are on that list. For Americans who suffer from obesity and other weight-related diseases, these drugs could be a lifeline.
Despite this, the medical community has broadly criticized the utilization of compounded GLP-1 agonists, even those obtained from reputable and legitimate compounding pharmacies.
Yes, high demand has led to the emergence of unregulated companies and scammers producing substandard or counterfeit versions of these medications.
The FDA has found fraudulent products (masquerading as the weight loss drugs) and has issued warning letters to stop the distribution of illegally marketed semaglutide. “These drugs may be counterfeit, which means they could contain the wrong ingredients, contain too little, too much or no active ingredient at all, or contain other harmful ingredients,” it cautions. Some products use a similar-sounding semaglutide sodium salt, which has uncertain safety and efficacy, and had generated warnings from the FDA and state boards of pharmacy.
Many of these products are marketed directly to consumers online through websites and social media, with little to no medical oversight. This practice is a significant concern, as it may affect patient safety, and should be discouraged.
However, according to a statement from the Alliance for Pharmacy Compounding (APC), legitimate compounding pharmacies aren’t the ones selling these dubious products on the black market, particularly online. This illegal practice has garnered media attention and is sometimes incorrectly associated with legitimate pharmacy compounding.
In contrast, legal and certified versions of GLP-1 agonist medications can be obtained from well-regulated and reputable compounding pharmacies. These pharmacies must adhere to all federal and state regulations and dispense medications only with a valid prescription from a licensed physician.
Meanwhile, the APC statement notes, Novo Nordisk and Eli Lilly have sued compounding companies in several states, questioning, among other things, the purity and potency of some compounded products.
There are different designations for compounding pharmacies: 503A and 503B. 503As are state-licensed pharmacies and physicians, and 503B pharmacies are federally regulated outsourcing facilities that are strictly regulated by the FDA. This regulation, established following a 2012 fungal meningitis outbreak linked to a compounding pharmacy, ensures higher-quality control and oversight, especially for medications intended for intravenous or epidural use. These standards exceed those required for subcutaneous injections like GLP-1 analogs.
In the face of this Wild West climate, where compounded drugs may vary in their source, formulation, potency, and purity, The Obesity Society, the Obesity Medical Association, and the Obesity Action Coalition published a joint statement that advised against the use of compounded GLP-1 agonists, citing safety concerns and lack of regulatory oversight.
This stance, while aimed at ensuring patient safety, inadvertently raises a critical issue.
By completely dismissing compounded medications, experts may unintentionally bolster the black market and overlook the needs of patients who could benefit from these medications, contrary to the intentions of the exemption provided in federal law for compounding during a drug shortage. In fact, the presence of unreliable suppliers highlights the need to direct the public toward trustworthy sources, rather than imposing a total ban on medically appropriate alternatives.
The joint statement calls compounded GLP-1 agonists “counterfeit.” This inaccurate overgeneralization probably stems from a misunderstanding of the compounding process and its regulations. Legitimate and regulated pharmacies compound base GLP-1 agonists, which are “essentially a copy” of FDA-approved medications, not counterfeits. Recognizing this is crucial for maintaining trust in both compounding pharmacies and regulatory bodies.
It is correct that “the only FDA-approved manufacturers of these medications are the companies that created the active pharmaceutical ingredients — Novo Nordisk and Eli Lilly,” but the joint statement fails to mention the exemptions provided by law that allow compounding copies of the branded medications if they are on the shortage list.
Compounding pharmacies must obtain active pharmaceutical ingredients (APIs) from FDA-registered facilities, which are required to adhere to Current Good Manufacturing Practices (cGMP). This ensures the APIs’ quality, potency, and purity, crucial for the safety and efficacy of compounded medications.
Compounded drugs are not FDA approved, but they aren’t inherently unsafe. Compounded medications include critical drugs such as resuscitation medications and antibiotics, and are often used in healthcare settings, especially when there’s a shortage. This raises the question of why compounded GLP-1 agonists would be treated any differently in such scenarios.
And in the case of alternative drugs for individuals with obesity who have a higher risk for cardiovascular disease, the brand-name FDA-approved alternative may be of more concern than the compounded GLP-1 agonist. The obesity societies advise: “If you cannot find or get access to a GLP-1-based treatment now, there are other treatments available,” echoing experts. While the statement doesn’t specify the names of the alternatives, experts have advised using alternatives such as Qsymia and Contrave, despite their potential cardiovascular concerns. This recommendation to the public may not represent a responsible risk-benefit analysis.
Rather than outright banning compounded GLP-1 medications, expert associations can contribute to the solution by creating a “seal of approval,” recognizing high-quality compounded medications. This would contribute to informed decision-making for clinicians and patients.
Possible Solutions
When prescribing GLP-1 agonists for obesity treatment, doctors should consider all of the following steps to ensure patient safety and effective treatment:
Preference for FDA-approved brands: FDA-approved branded GLP-1 agonist medications should be the primary choice because of their established safety and efficacy.
Risk-benefit analysis for non–FDA-approved products: In cases where FDA-approved options are not available, doctors may consider prescribing a non–FDA-approved copy of the branded medication. Prior to this, conduct a thorough risk-benefit analysis with the patient, ensuring that they are fully informed about the potential risks and benefits of using a non–FDA-approved product.
Choosing semaglutide copies for specific cases: In patients with obesity and cardiovascular disease, the benefits of using a compounded copy of semaglutide, with its cardiovascular disease–modifying properties, may outweigh the risks compared with other FDA-approved antiobesity drugs that might pose cardiovascular risks or compared with no antiobesity treatment at all.
Informed consent and monitoring: When prescribing a non–FDA-approved version of a GLP-1 agonist, obtaining informed consent from the patient is advised. They should be made aware of the differences between the FDA-approved and nonapproved versions.
Choosing between 503A and 503B pharmacies: Prescriptions for non–FDA-approved GLP-1 agonists can be directed to either 503A or 503B compounding pharmacies. However, it’s advisable to check whether the product can be compounded by a 503B pharmacy, which is subject to an additional layer of FDA regulation, offering greater quality assurance.
Clear prescription specifications: Ensure that the prescription explicitly states that the compounded GLP-1 agonist should be the base compound without additives.
Requesting a Certificate of Analysis: To further ensure safety, request a Certificate of Analysis from the compounding pharmacy. This provides detailed quality and composition information about the product.
Ongoing monitoring: Continuously monitor the patient’s response to the medication and adjust the treatment plan as necessary, maintaining regular follow-ups.
By adhering to these guidelines, doctors can navigate the complexities of prescribing GLP-1 agonists in a way that prioritizes patient well-being, particularly in scenarios where conventional treatment options are limited.
Dr. Einav is a board-certified cardiologist and a Diplomate of the American Board of Obesity Medicine. He is a fellow of the American College of Cardiology and a member of the Obesity Medicine Association. He serves as the medical director of cardiometabolic health in Guthrie Lourdes in Binghamton, New York, and is the founder of myW8/Cardiometabolic Health located in Beverly Hills, California. This article solely reflects the personal views of Dr. Einav and should not be considered as representing the official stance of Guthrie Lourdes. Dr. Einav served as a promotional speaker for Novo Nordisk in 2022. As of now, he has not prescribed any compounded GLP-1 agonist medications in his medical practice.
A version of this article appeared on Medscape.com.
Statins Tied to Lower Mortality, Even With Comorbid Dementia
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Study Sounds Alert About GLP-1 RA Use and Aspiration Risk
TOPLINE:
Patients on weekly glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have high residual gastric content, a major risk factor for aspiration under anesthesia, despite following fasting guidelines before undergoing elective procedures.
METHODOLOGY:
- The increasing use of GLP-1 RAs to manage weight and hyperglycemia has sparked safety concerns because of the drugs’ association with slow gastric emptying, a major risk factor for aspiration under anesthesia.
- This cross-sectional study used gastric ultrasonography to examine the link between GLP-1 RA use and the prevalence of increased residual gastric content.
- All 124 participants (median age, 56 years; 60% women) — half of whom received once-weekly GLP-1 RAs such as semaglutide, dulaglutide, or tirzepatide — adhered to the guideline-recommended fasting duration before undergoing elective procedures under anesthesia.
- The primary outcome focused on identifying increased residual gastric content, defined by the presence of solids, thick liquids, or > 1.5 mL/kg of clear liquids on ultrasound.
- An exploratory analysis examined the association between the duration of GLP-1 RA discontinuation and increased residual gastric content.
TAKEAWAY:
- The adjusted prevalence of increased residual gastric content was 30.5% (95% CI, 9.9%-51.2%) higher in participants who received GLP-1 RA than those who did not.
- Most patients took their last dose of GLP-1 RA within 5 days before their procedure, but elevated residual gastric content persisted even after 7 days of GLP-1 RA discontinuation.
- There was also no significant association between the type of GLP-1 RA used and the prevalence of increased residual gastric content.
IN PRACTICE:
“We expect healthcare professionals will encounter these classes of drugs with increasing frequency in the perioperative period. Perioperative physicians, including anesthesiologists, surgeons, and primary care physicians, should be well-informed about the safety implications of GLP-1 RA drugs,” the authors wrote.
SOURCE:
The study was led by Sudipta Sen, MD, from the Department of Anesthesiology, Critical Care and Pain Medicine, McGovern Medical School, University of Texas Health Center at Houston, Houston, Texas, and published online in JAMA Surgery.
LIMITATIONS:
Residual gastric content, the primary outcome, served as a proxy for aspiration risk and does not have an exact threshold of volume associated with increased risk. The study did not directly evaluate aspiration events. The authors also acknowledged potential bias from unmeasured confounders owing to the observational nature of this study. A small sample size limited the ability to detect a risk difference for each additional day of drug discontinuation before surgery.
DISCLOSURES:
One of the authors reported receiving a grant from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients on weekly glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have high residual gastric content, a major risk factor for aspiration under anesthesia, despite following fasting guidelines before undergoing elective procedures.
METHODOLOGY:
- The increasing use of GLP-1 RAs to manage weight and hyperglycemia has sparked safety concerns because of the drugs’ association with slow gastric emptying, a major risk factor for aspiration under anesthesia.
- This cross-sectional study used gastric ultrasonography to examine the link between GLP-1 RA use and the prevalence of increased residual gastric content.
- All 124 participants (median age, 56 years; 60% women) — half of whom received once-weekly GLP-1 RAs such as semaglutide, dulaglutide, or tirzepatide — adhered to the guideline-recommended fasting duration before undergoing elective procedures under anesthesia.
- The primary outcome focused on identifying increased residual gastric content, defined by the presence of solids, thick liquids, or > 1.5 mL/kg of clear liquids on ultrasound.
- An exploratory analysis examined the association between the duration of GLP-1 RA discontinuation and increased residual gastric content.
TAKEAWAY:
- The adjusted prevalence of increased residual gastric content was 30.5% (95% CI, 9.9%-51.2%) higher in participants who received GLP-1 RA than those who did not.
- Most patients took their last dose of GLP-1 RA within 5 days before their procedure, but elevated residual gastric content persisted even after 7 days of GLP-1 RA discontinuation.
- There was also no significant association between the type of GLP-1 RA used and the prevalence of increased residual gastric content.
IN PRACTICE:
“We expect healthcare professionals will encounter these classes of drugs with increasing frequency in the perioperative period. Perioperative physicians, including anesthesiologists, surgeons, and primary care physicians, should be well-informed about the safety implications of GLP-1 RA drugs,” the authors wrote.
SOURCE:
The study was led by Sudipta Sen, MD, from the Department of Anesthesiology, Critical Care and Pain Medicine, McGovern Medical School, University of Texas Health Center at Houston, Houston, Texas, and published online in JAMA Surgery.
LIMITATIONS:
Residual gastric content, the primary outcome, served as a proxy for aspiration risk and does not have an exact threshold of volume associated with increased risk. The study did not directly evaluate aspiration events. The authors also acknowledged potential bias from unmeasured confounders owing to the observational nature of this study. A small sample size limited the ability to detect a risk difference for each additional day of drug discontinuation before surgery.
DISCLOSURES:
One of the authors reported receiving a grant from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients on weekly glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have high residual gastric content, a major risk factor for aspiration under anesthesia, despite following fasting guidelines before undergoing elective procedures.
METHODOLOGY:
- The increasing use of GLP-1 RAs to manage weight and hyperglycemia has sparked safety concerns because of the drugs’ association with slow gastric emptying, a major risk factor for aspiration under anesthesia.
- This cross-sectional study used gastric ultrasonography to examine the link between GLP-1 RA use and the prevalence of increased residual gastric content.
- All 124 participants (median age, 56 years; 60% women) — half of whom received once-weekly GLP-1 RAs such as semaglutide, dulaglutide, or tirzepatide — adhered to the guideline-recommended fasting duration before undergoing elective procedures under anesthesia.
- The primary outcome focused on identifying increased residual gastric content, defined by the presence of solids, thick liquids, or > 1.5 mL/kg of clear liquids on ultrasound.
- An exploratory analysis examined the association between the duration of GLP-1 RA discontinuation and increased residual gastric content.
TAKEAWAY:
- The adjusted prevalence of increased residual gastric content was 30.5% (95% CI, 9.9%-51.2%) higher in participants who received GLP-1 RA than those who did not.
- Most patients took their last dose of GLP-1 RA within 5 days before their procedure, but elevated residual gastric content persisted even after 7 days of GLP-1 RA discontinuation.
- There was also no significant association between the type of GLP-1 RA used and the prevalence of increased residual gastric content.
IN PRACTICE:
“We expect healthcare professionals will encounter these classes of drugs with increasing frequency in the perioperative period. Perioperative physicians, including anesthesiologists, surgeons, and primary care physicians, should be well-informed about the safety implications of GLP-1 RA drugs,” the authors wrote.
SOURCE:
The study was led by Sudipta Sen, MD, from the Department of Anesthesiology, Critical Care and Pain Medicine, McGovern Medical School, University of Texas Health Center at Houston, Houston, Texas, and published online in JAMA Surgery.
LIMITATIONS:
Residual gastric content, the primary outcome, served as a proxy for aspiration risk and does not have an exact threshold of volume associated with increased risk. The study did not directly evaluate aspiration events. The authors also acknowledged potential bias from unmeasured confounders owing to the observational nature of this study. A small sample size limited the ability to detect a risk difference for each additional day of drug discontinuation before surgery.
DISCLOSURES:
One of the authors reported receiving a grant from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.