User login
Dermatomyositis Cancer Screening Guidelines Get Real-World Validation
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
Newly issued guidelines for cancer screening in patients with dermatomyositis had 100% sensitivity in a single institution’s cohort, though most of the cancers found would have been detected with standard cancer screenings recommended for the general population, according to a research letter published in JAMA Dermatology.
“These early results emphasize the continued need to refine risk assessment and cancer screening for patients with dermatomyositis while balancing resource use and outcomes,” concluded Caroline J. Stone and her colleagues at the Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Patients with dermatomyositis have approximately a 4.7 times greater risk for cancer than those without it, according to a 2016 meta-analysis. Despite the well-established link between cancer and dermatomyositis, cancer in people with idiopathic inflammatory myopathies is commonly diagnosed at a later stage and is the leading cause of death in people with these conditions.
Guidelines First Presented in 2022 and Published in 2023
A wide variability in screening practices eventually led the International Myositis Assessment & Clinical Studies Group (IMACS) to present the first evidence-based and consensus-based guidelines for cancer screening of patients with idiopathic inflammatory myopathies, including those with dermatomyositis, at the 2022 annual meeting of the American College of Rheumatology and publish them in 2023 in Nature Reviews Rheumatology. The guidelines advise low-risk patients to undergo basic cancer screening with routine blood and urine studies, liver function tests, plain chest radiography, and age- and sex-appropriate cancer screening.
Intermediate- and high-risk patients are recommended to undergo enhanced screening that can include mammography, Pap tests, endoscopy/colonoscopy, pelvic and transvaginal ultrasonography, prostate-specific antigen or cancer antigen 125 blood tests, fecal occult blood tests, and CT of the neck, thorax, abdomen, and pelvis.
But because the guidelines are new, little evidence exists regarding their validation in real-world cohorts. Researchers, therefore, assessed the IMACS guidelines in 370 patients, aged 18-80 years, who visited the University of Pennsylvania rheumatology-dermatology specialty clinic between July 2008 and January 2024. All participants had dermatomyositis and at least 3 years of follow-up and were an average 48 years old. The vast majority were women (87%) and White participants (89%).
Most (68.6%) had myositis-specific autoantibody test results, one of the factors included in the guidelines for determining whether the patient should be classified as low, intermediate, or high risk. Other factors for risk stratification included myositis subtype, age at disease onset, and clinical features. About half (49.2%) had classic dermatomyositis, 42.4% had amyopathic dermatomyositis, 3.8% had juvenile dermatomyositis, 3.2% had hypomyopathic dermatomyositis, 0.8% had antisynthetase syndrome, and 0.5% had immune-mediated necrotizing myopathy.
Just over half the patients (54%) were classified as high risk, while 37.3% were classified as intermediate risk and 8.9% as low risk using the guidelines. Among the 18 patients (4.9%) with paraneoplastic dermatomyositis, 15 were classified as high risk and 3 as intermediate risk.
Of the patients diagnosed with cancer, 55% of cases were diagnosed about a year before their dermatomyositis diagnosis. In three patients, symptoms “suggestive of cancer at the time of dermatomyositis diagnosis, including lymphadenopathy and unexplained weight loss,” led to diagnostic testing that found an underlying cancer.
In the eight patients diagnosed with cancer after their dermatomyositis diagnosis, 75% of the cancers were identified during the first year of follow-up and 25% in the second year. Five were identified based on basic cancer screening and three on enhanced screening.
A total of 11 patients (3%) developed intravenous contrast allergies, and no other adverse events were reported to be associated with cancer screening, but the study was not designed to capture other types of adverse screening effects, such as cost, quality of life, or risk from radiation exposure.
The most common neoplasm identified was breast cancer, found in nine (50%) of the patients using mammography. Two patients had lung cancer identified with chest radiography and two had ovarian cancer identified with abdominal radiography and CT. The remaining five patients included one each with bladder cancer, papillary thyroid cancer, renal cell carcinoma, non-Hodgkin lymphoma, and adenocarcinoma with unknown primary.
The sensitivity of the guidelines in detecting cancer related to dermatomyositis was 100%, though the authors noted that the “IMACS risk-stratification scheme may overestimate cancer risk and encourage enhanced screening protocols of unclear benefit.” Most of the cancers found after dermatomyositis diagnosis were detected with routine age- and sex-related screening that already falls under basic cancer screening recommendations for the general population. Nonetheless, 90% of the participants fell into the intermediate- and high-risk groups, warranting a more comprehensive and costly enhanced screening protocol.
Will the Guidelines Lead to Overscreening?
The 4.9% cancer prevalence is considerably lower than the typical 15%-25% prevalence among patients with dermatomyositis, but the findings, regardless, suggest the guidelines will lead to overscreening, wrote Andrea D. Maderal, MD, University of Miami Miller School of Medicine in Florida, and Alisa Femia, MD, New York University Grossman School of Medicine, New York City, in an accompanying editorial. Given that the median age in patients with cancer in the study was 58 years — 18 years older than the age cutoff for high-risk criteria — one way to refine the guidelines may be to increase the age for the high-risk category, they suggested.
“While these guidelines led to many ultimately unnecessary screening tests based on currently recommended designations of intermediate-risk and high-risk patients, these guidelines reflect a more conservative approach to screening than was previously performed,” Dr. Maderal and Dr. Femia wrote.
Jeff Gehlhausen, MD, PhD, an assistant professor of dermatology at Yale School of Medicine, New Haven, Connecticut, said he is not concerned about overscreening in patients, however, and is “very enthusiastic” about the findings.
“Patients are very anxious for good reason,” given the typical cancer prevalence of 25% in this population, he said in an interview. “I think therein lies the challenge — with that risk, what is ‘enough’ screening?” Yet this “incredibly impressive” study “provides real insights into the applicability of the IMACS screenings to our dermatomyositis management,” including relevance to his own patients. “Their findings are instructive for how to better evaluate these patients in a more mindful fashion,” he said, and they are particularly welcome, given how widely variable practice has historically been before the guidelines were issued.
“This question has been an outstanding one for decades, and nearly every doctor has a different answer,” Dr. Gehlhausen said. “The introduction of the guidelines alone are now much more actionable with this study, and that’s why it’s such an important one for our community.”
Benedict Wu, DO, PhD, director of Inpatient Dermatology and an assistant professor at Montefiore Einstein and a member of the Montefiore Einstein Comprehensive Cancer Center in New York City, similarly regarded the findings as reassuring, though he was surprised at the low prevalence of cancer in the patients.
“The most reassuring finding was that the detection of most malignancies was possible by using routine age- and sex-related screening combined with basic cancer screening,” Wu said in an interview. “Basic cancer screening can reduce costs while keeping patients safe.”
He also found it reassuring that all the paraneoplastic dermatomyositis was in intermediate- or high-risk patients, and while he does not see the IMACS guidelines as overestimating cancer risk, he does think “the risk stratification and recommended screening tests could be revised to be less ‘aggressive.’ ”
The overall low rate of cancer in the group “calls into question the need for stringent and annual cancer screening,” he said. “In this large cohort of patients, the fact that malignancy was detected within 2 years of dermatomyositis diagnosis will help guide us with long-term screening recommendations.”
Despite the study’s small size and single-center design, the demographics of the patients nearly represents exactly what is found in the United States more broadly, Wu noted. He also drew attention to how many patients lacked the myositis antibody profile performed, and he agreed with the authors that more extensive and prospective studies need to be conducted. He also emphasized the need to keep in mind that “the primary goal of dermatomyositis management should focus on controlling/reducing the disease burden.”
The research was funded by the National Institutes of Health and the US Department of Veterans Affairs. The authors had no disclosures. Dr. Maderal reported personal fees from argenx. No disclosures were noted for Dr. Gehlhausen and Dr. Wu.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
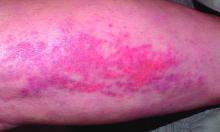
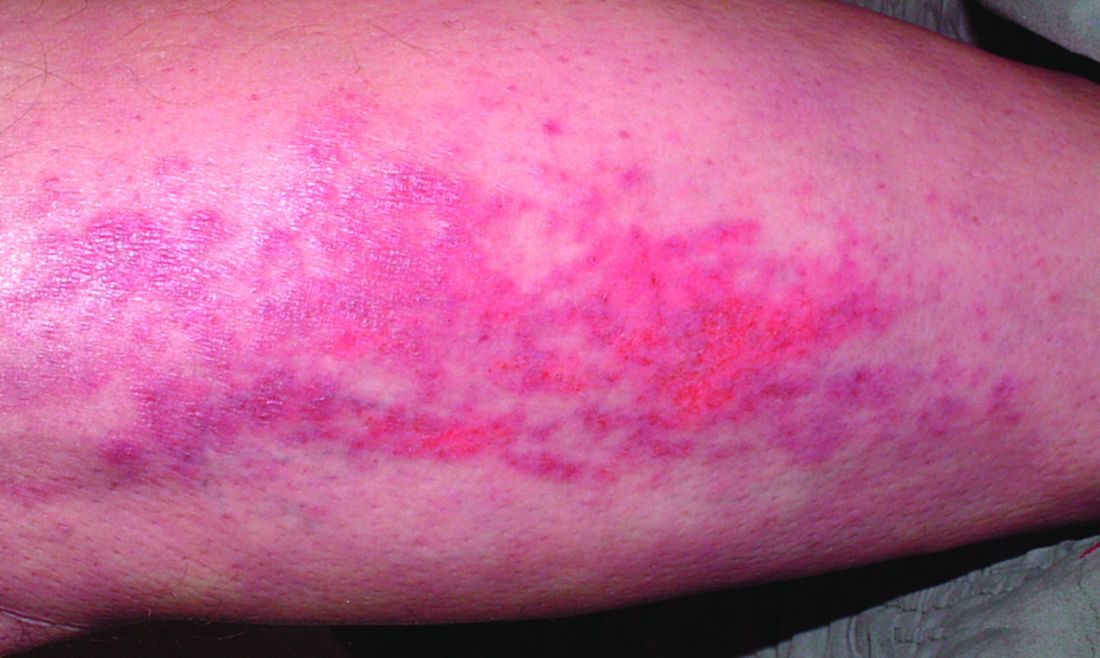
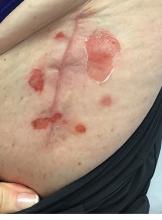
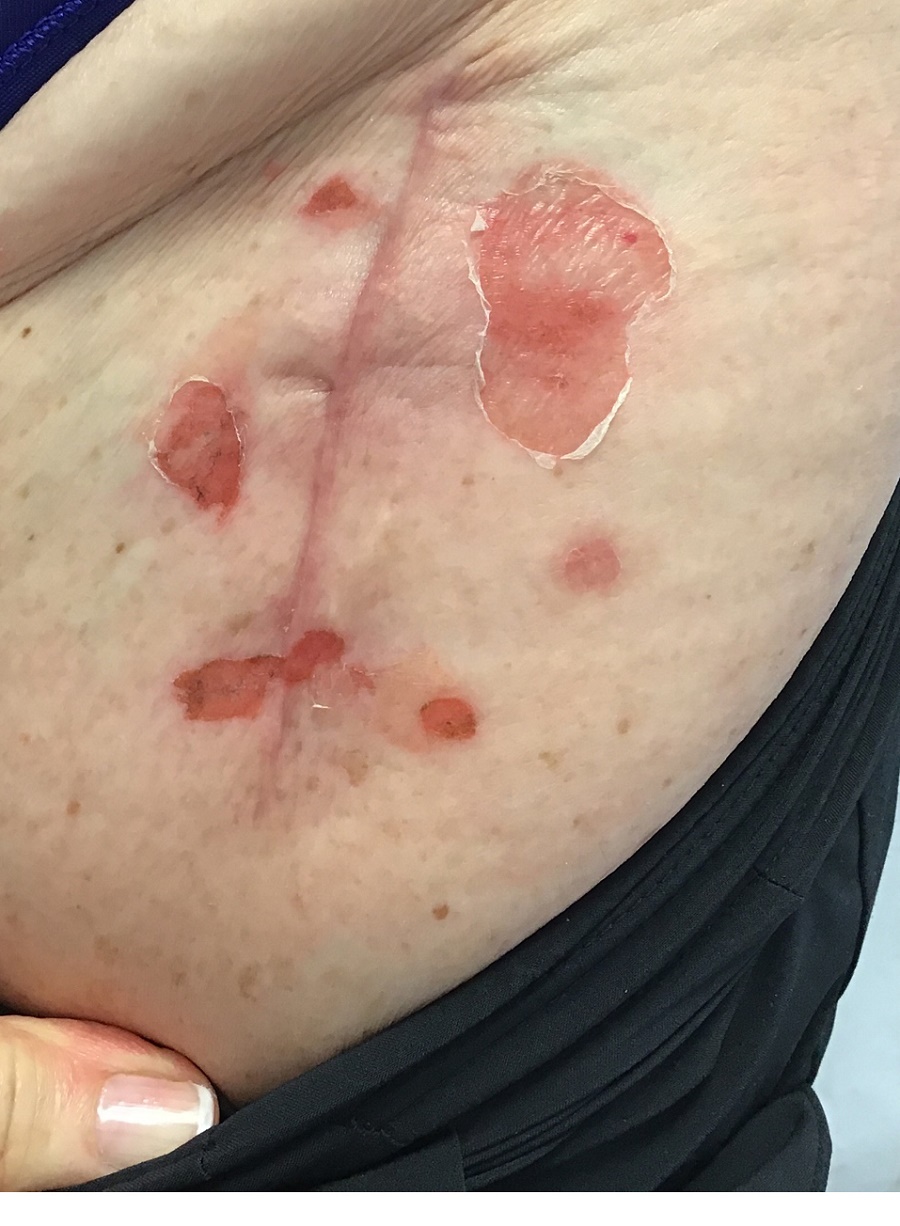
A 71-year-old White female developed erosions after hip replacement surgery 2 months prior to presentation
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
Trial Looks at Early Use of Mycophenolate to Reduce Flares, Nephritis
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Hypnosis May Offer Relief During Sharp Debridement of Skin Ulcers
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Belimumab Hits Newer Remission, Low Disease Activity Metrics
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Current Hydroxychloroquine Use in Lupus May Provide Protection Against Cardiovascular Events
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Managing Vitiligo: Combination Therapies, New Treatments
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients with vitiligo see Jessica Shiu, MD, PhD, for the first time, some mention that prior healthcare providers have told them that vitiligo is merely a cosmetic issue — much to her dismay.
“Vitiligo is not a cosmetic disease,” Dr. Shiu, assistant professor of dermatology at the University of California, Irvine, said at the annual meeting of the Pacific Dermatologic Association. “It is associated with significant depression, stigmatization, and low self-esteem. I have patients who say that vitiligo has affected their marriage ... In certain cultures, it also affects their job prospects.”
As the most common pigmentary disorder, vitiligo is an autoimmune condition that often results in the recruitment of CD8+ T cells into the skin. These cells destroy melanocytes, depleting melanocytes in the epidermis. “Over time, this results in milky white patches of skin that we often see in our patients,” Dr. Shiu said.
“Depending on the site that is involved, the nonsegmental form can be further divided into focal, acrofacial, mucosal, generalized, and universal subtypes,” she said. The first step in your initial management is to determine if the vitiligo is active or stable, which can be challenging. Clinical signs of active disease include the presence of trichome vitiligo, confetti vitiligo, and koebnerization.
“Another sign of active disease is when patients tell you that their vitiligo is expanding rapidly,” Dr. Shiu added. “Stable vitiligo is more difficult to define. Many patients think their lesions don’t change, but we’re now appreciating that there can be some sites in those patients such as the hands and feet that are more susceptible to change in activity.” In general, she noted, vitiligo is considered stable when there is no change in activity for at least 12 months, and “lesions are usually completely depigmented with sharp borders.”
The level of vitiligo disease activity drives medical management. For patients with nonsegmental vitiligo who have clinical signs of active disease, the first goal is to stabilize the active disease and stop further spread of depigmentation. “This is key because losing pigment can occur very quickly, but gaining pigment back is a very slow process,” she said. Stabilization involves suppressing immune responses with topical steroids, topical calcineurin inhibitors, or 1.5% ruxolitinib cream, a JAK inhibitor that became the first Food and Drug Administration (FDA)–approved pharmacologic treatment for nonsegmental vitiligo, in 2022, for patients aged 12 years or older.
“The choice here depends somewhat on insurance coverage and shared decision-making with the patient,” Dr. Shiu said. Meanwhile, clinical trials evaluating the effect of the oral JAK inhibitors ritlecitinib, upadacitinib, povorcitinib, and baricitinib on vitiligo are underway.
Combining Phototherapy With Topical Treatment
A mainstay therapy for nonsegmental vitiligo is phototherapy, which can induce the migration of melanocyte stem cells from hair follicles. “There’s good data to show that combining topical treatment with phototherapy can augment the repigmentation that you see,” she said. “So if it’s possible, try to add phototherapy for your vitiligo patients, but sometimes, logistics for that are a challenge.”
Discussing treatment expectations with patients is key because it can take up to 1 year to see a significant response with topical immunosuppressants and narrowband ultraviolet B treatment. The head and neck areas are often the first sites to repigment, she said, followed by the extremities or the trunk. “The hands and feet are generally last; they are usually the most stubborn areas,” Dr. Shiu said. “Even when you do see repigmentation, it usually happens on the dorsal surfaces. The tips of the fingers and toes are difficult to repigment. Luckily, the face is one of the top responders, so that helps a lot.”
While some treatment efforts result in “complete and beautiful” repigmentation, she added, many yield uneven and incomplete results. “We don’t understand why repigmentation occurs in some areas but not in others,” she said. “We don’t have any biomarkers for treatment response. That is something we are looking into.”
For a patient with rapidly progressing active disease, consider an oral steroid mini-pulse 2 consecutive days per week for a maximum of 3-6 months. “I usually recommend that patients do this on Saturday and Sunday,” Dr. Shiu said. “Studies have shown this strategy can halt progression in 85%-91% of cases if patients are on it for at least 3 months.”
Relapse after successful repigmentation occurs in about 40% of cases following discontinuation of treatment, so she recommends biweekly application of 0.1% tacrolimus ointment as maintenance therapy. “Studies have shown this is enough to decrease the relapse rate to around 9%,” she said.
Tissue, Cellular Grafts
Surgical repigmentation strategies rely on transplanting normal skin to areas affected by vitiligo. In general, more than 50% of patients achieve more than 80% repigmentation. Options are divided into tissue grafts vs cellular grafts. “The old methods are tissue grafting such as punch grafting, tissue blister grafting, and spit thickness grafting, which can treat limited areas of skin,” Dr. Shiu said. Newer approaches include cellular grafting using the melanocyte-keratinocyte transplantation procedure, which can treat larger areas of skin.
The main drawback of this approach is that it is expensive and there is no insurance code for it, “but I hope that this becomes an option for our patients in the future because data indicate that repigmentation is maintained for up to 72 months after treatment,” she said.
In June 2023, an autologous cell harvesting device known as RECELL received FDA approval for repigmentation of stable vitiligo lesions. According to a press release from the manufacturer, AVITA Medical, a clinician “prepares and delivers autologous skin cells from pigmented skin to stable depigmented areas, offering a safe and effective treatment for vitiligo.”
Dr. Shiu disclosed that she received research support from AbbVie.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
What’s Causing Raynaud Phenomenon Severity to Rise With High Temperatures?
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In systemic sclerosis, Raynaud phenomenon is more severe at both high and low temperature extremes, according to new research.
BACKGROUND:
- Raynaud phenomenon, a condition that causes decreased blood flow to extremities, occurs in about 95% of individuals with systemic sclerosis.
- Episodes of Raynaud phenomenon can be triggered by cold exposure and ambient temperature changes.
- In severe cases, it can cause permanent damage to tissues of the fingers and toes.
METHODOLOGY:
- Researchers analyzed data from 2243 participants with Raynaud phenomenon secondary to systemic sclerosis from the Scleroderma Patient-centered Intervention Network (SPIN) Cohort.
- Participants completed past-week Raynaud phenomenon severity assessments using a 0-10 numerical rating scale at enrollment and every 3 months.
- The study included data from 20,233 Raynaud phenomenon severity assessments between April 15, 2014, and August 1, 2023.
- Researchers used average daily temperature from a weather site close to the participant’s recruiting center and mapped these ambient temperature changes to Raynaud’s phenomenon outcomes.
TAKEAWAY:
- Raynaud’s phenomenon severity was highest at –25 °C (–13 °F), with assessment scores at 6.8 points out of 10.0, and lowest at 25 °C (77 °F), with scores at 2.6.
- Severity scores increased again at temperatures above 35 °C (95 °F), reaching a high of 5.6 out of 10 at 40 °C (104 °F).
- This spike at higher temperatures is presumably due to air conditioning, the authors said.
- In an accompanying commentary, Cutolo et al. posited that increased sweating and hypotension could also lead to a relative hypovolemic state in patients, causing Raynaud-like symptoms.
IN PRACTICE:
“Temperature-related variations in Raynaud’s phenomenon severity scores should be considered in clinical trials to account for normal within-season temperature fluctuations, enhancing the accuracy of treatment outcomes,” wrote Cutolo and colleagues in their commentary.
SOURCE:
The study was led by Gabrielle Virgili-Gervais, MSc, McGill University Health Centre in Montreal, Quebec, Canada. It was published online on August 28 in The Lancet Rheumatology. The accompanying commentary, also published on August 28, was authored by Maurizio Cutolo, MD, and Elvis Hysa, MD, both of University of Genova, Italy, as well as Vanessa Smith, MD, PhD, of Ghent University in Ghent, Belgium.
LIMITATIONS:
The lower number of assessments at extreme temperatures (–25 °C and 40 °C) may affect the robustness of the findings at these ranges. The study did not account for vasodilator use, which could influence participants’ response to temperature. The study also did not account for other potential confounding factors such as sex, smoking status, psychosocial factors, and comorbid conditions like cardiovascular disease.
DISCLOSURES:
A variety of scleroderma-related patient advocacy groups helped to fund research on the SPIN cohort, in addition to the Canadian Institutes of Health Research, the Arthritis Society, the Lady Davis Institute for Medical Research of the Jewish General Hospital, the Jewish General Hospital Foundation, and McGill University. Two authors reported having financial ties with pharmaceutical companies. Dr. Cutolo, Dr. Smith, and Dr. Hysa had no disclosures.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Systemic Sclerosis Without Scleroderma Has Unique Severity, Prognosis
TOPLINE:
Systemic sclerosis sine scleroderma (ssSSc) affects nearly 10% of patients with systemic sclerosis (SSc), with substantial internal organ involvement. Despite lacking skin fibrosis, patients with ssSSc are at a risk for interstitial lung disease, pulmonary arterial hypertension, and cardiac dysfunction.
METHODOLOGY:
- Driven by a fatal case of ssSSc with cardiac involvement, researchers aimed to evaluate its prevalence, severity, and prognosis.
- They conducted a systematic literature and qualitative synthesis of 35 studies on SSc cohorts from databases published between 1976 and 2023 that comprised data on the prevalence of SSc with or without organ involvement.
- A total of 25,455 patients with SSc were included, with 2437 identified as having ssSSc.
- Studies used various classification criteria for SSc, including the 1980 American Rheumatism Association criteria, 2001 LeRoy and Medsger criteria, and 2013 American College of Rheumatology/European League Against Rheumatism criteria, while ssSSc was classified on the basis of the definitions provided by Rodnan and Fennell and also Poormoghim.
- The analysis focused on ssSSc prevalence, reclassification rates, and internal organ involvement, including interstitial lung disease, pulmonary arterial hypertension, scleroderma renal crisis, and cardiac dysfunction.
TAKEAWAY:
- The overall mean prevalence of ssSSc was 9.6%, with a range of 0%-22.9% across different studies.
- Reclassification rates of ssSSc into limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc) varied substantially, with some studies reporting rates as high as 27.8% over a 4-year follow-up period.
- The mean frequency of internal organ involvement in patients with ssSSc was 46% for interstitial lung disease, 15% for pulmonary arterial hypertension, 5% for scleroderma renal crisis, and 26.5% for cardiac dysfunction — mainly diastolic dysfunction.
- The survival rates in patients with ssSSc were similar to those with lcSSc and better than those with dcSSc.
IN PRACTICE:
“The results presented herein suggest a slightly more severe yet similar clinical picture of ssSSc compared to lcSSc [limited cutaneous SSc], while dcSSc [diffuse cutaneous SSc] remains the most severe disease form,” the authors wrote. “Although classification criteria should not impact appropriate management of patients, updated ssSSc subclassification criteria, which will take into account time from disease onset, should be considered,” they further added.
SOURCE:
The study was led by Anastasios Makris, MD, First Department of Propaedeutic & Internal Medicine, National and Kapodistrian University of Athens, Medical School, Athens, Greece. It was published online on August 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The variability in the classification criteria across different studies may affect the comparability of results. The included studies lacked data on cardiac MRI, restricting the identification of myocardial fibrosis patterns and characterization of cardiac disease activity.
DISCLOSURES:
The study did not receive any specific funding. Some authors disclosed having a consultancy relationship, serving as speakers, and receiving funding for research from multiple companies. One author reported having a patent and being a cofounder of CITUS AG.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Systemic sclerosis sine scleroderma (ssSSc) affects nearly 10% of patients with systemic sclerosis (SSc), with substantial internal organ involvement. Despite lacking skin fibrosis, patients with ssSSc are at a risk for interstitial lung disease, pulmonary arterial hypertension, and cardiac dysfunction.
METHODOLOGY:
- Driven by a fatal case of ssSSc with cardiac involvement, researchers aimed to evaluate its prevalence, severity, and prognosis.
- They conducted a systematic literature and qualitative synthesis of 35 studies on SSc cohorts from databases published between 1976 and 2023 that comprised data on the prevalence of SSc with or without organ involvement.
- A total of 25,455 patients with SSc were included, with 2437 identified as having ssSSc.
- Studies used various classification criteria for SSc, including the 1980 American Rheumatism Association criteria, 2001 LeRoy and Medsger criteria, and 2013 American College of Rheumatology/European League Against Rheumatism criteria, while ssSSc was classified on the basis of the definitions provided by Rodnan and Fennell and also Poormoghim.
- The analysis focused on ssSSc prevalence, reclassification rates, and internal organ involvement, including interstitial lung disease, pulmonary arterial hypertension, scleroderma renal crisis, and cardiac dysfunction.
TAKEAWAY:
- The overall mean prevalence of ssSSc was 9.6%, with a range of 0%-22.9% across different studies.
- Reclassification rates of ssSSc into limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc) varied substantially, with some studies reporting rates as high as 27.8% over a 4-year follow-up period.
- The mean frequency of internal organ involvement in patients with ssSSc was 46% for interstitial lung disease, 15% for pulmonary arterial hypertension, 5% for scleroderma renal crisis, and 26.5% for cardiac dysfunction — mainly diastolic dysfunction.
- The survival rates in patients with ssSSc were similar to those with lcSSc and better than those with dcSSc.
IN PRACTICE:
“The results presented herein suggest a slightly more severe yet similar clinical picture of ssSSc compared to lcSSc [limited cutaneous SSc], while dcSSc [diffuse cutaneous SSc] remains the most severe disease form,” the authors wrote. “Although classification criteria should not impact appropriate management of patients, updated ssSSc subclassification criteria, which will take into account time from disease onset, should be considered,” they further added.
SOURCE:
The study was led by Anastasios Makris, MD, First Department of Propaedeutic & Internal Medicine, National and Kapodistrian University of Athens, Medical School, Athens, Greece. It was published online on August 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The variability in the classification criteria across different studies may affect the comparability of results. The included studies lacked data on cardiac MRI, restricting the identification of myocardial fibrosis patterns and characterization of cardiac disease activity.
DISCLOSURES:
The study did not receive any specific funding. Some authors disclosed having a consultancy relationship, serving as speakers, and receiving funding for research from multiple companies. One author reported having a patent and being a cofounder of CITUS AG.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Systemic sclerosis sine scleroderma (ssSSc) affects nearly 10% of patients with systemic sclerosis (SSc), with substantial internal organ involvement. Despite lacking skin fibrosis, patients with ssSSc are at a risk for interstitial lung disease, pulmonary arterial hypertension, and cardiac dysfunction.
METHODOLOGY:
- Driven by a fatal case of ssSSc with cardiac involvement, researchers aimed to evaluate its prevalence, severity, and prognosis.
- They conducted a systematic literature and qualitative synthesis of 35 studies on SSc cohorts from databases published between 1976 and 2023 that comprised data on the prevalence of SSc with or without organ involvement.
- A total of 25,455 patients with SSc were included, with 2437 identified as having ssSSc.
- Studies used various classification criteria for SSc, including the 1980 American Rheumatism Association criteria, 2001 LeRoy and Medsger criteria, and 2013 American College of Rheumatology/European League Against Rheumatism criteria, while ssSSc was classified on the basis of the definitions provided by Rodnan and Fennell and also Poormoghim.
- The analysis focused on ssSSc prevalence, reclassification rates, and internal organ involvement, including interstitial lung disease, pulmonary arterial hypertension, scleroderma renal crisis, and cardiac dysfunction.
TAKEAWAY:
- The overall mean prevalence of ssSSc was 9.6%, with a range of 0%-22.9% across different studies.
- Reclassification rates of ssSSc into limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc) varied substantially, with some studies reporting rates as high as 27.8% over a 4-year follow-up period.
- The mean frequency of internal organ involvement in patients with ssSSc was 46% for interstitial lung disease, 15% for pulmonary arterial hypertension, 5% for scleroderma renal crisis, and 26.5% for cardiac dysfunction — mainly diastolic dysfunction.
- The survival rates in patients with ssSSc were similar to those with lcSSc and better than those with dcSSc.
IN PRACTICE:
“The results presented herein suggest a slightly more severe yet similar clinical picture of ssSSc compared to lcSSc [limited cutaneous SSc], while dcSSc [diffuse cutaneous SSc] remains the most severe disease form,” the authors wrote. “Although classification criteria should not impact appropriate management of patients, updated ssSSc subclassification criteria, which will take into account time from disease onset, should be considered,” they further added.
SOURCE:
The study was led by Anastasios Makris, MD, First Department of Propaedeutic & Internal Medicine, National and Kapodistrian University of Athens, Medical School, Athens, Greece. It was published online on August 15, 2024, in The Journal of Rheumatology.
LIMITATIONS:
The variability in the classification criteria across different studies may affect the comparability of results. The included studies lacked data on cardiac MRI, restricting the identification of myocardial fibrosis patterns and characterization of cardiac disease activity.
DISCLOSURES:
The study did not receive any specific funding. Some authors disclosed having a consultancy relationship, serving as speakers, and receiving funding for research from multiple companies. One author reported having a patent and being a cofounder of CITUS AG.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patients With Immune-Mediated Inflammatory Diseases, Type 2 Diabetes Reap GLP-1 Receptor Agonist Benefits, Too
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.