User login
Hematology News welcomes Dr. Ify Osunkwo as editor in chief
Hematology News welcomes Ifeyinwa (Ify) Osunkwo, MD, MPH, as the new editor in chief.
Dr. Osunkwo is a professor of medicine at Atrium Health and the director of the Sickle Cell Enterprise at the Levine Cancer Institute, part of Atrium Health, in Charlotte, N.C.
She has made it her personal mission to improve the quality of life for patients with sickle cell disease, a passion that began during time spent in Nigeria as a child, where 150,000 children are born each year with the condition. In 2014, Dr. Osunkwo created a comprehensive sickle cell center in Charlotte with a multidisciplinary team of providers that includes physicians, nurses, social workers, psychologists, and nurse managers. She has also been an instrumental part of the Carolinas Sickle Cell Collaborative, which seeks to match sickle cell patients in the community with blood donors who have similar blood characteristics.
“As a practicing hematologist and researcher, I have a deep appreciation for the timely and relevant content provided by Hematology News,” Dr. Osunkwo said. “I hope to use my experience to help make this publication even better.”
She is a member of the National Adult Sickle Cell Provider Network and leads the Transition/Medical Home Committee for the Southeast Regional Genetics Network. Her interests include health literacy, adolescent transition of care, and chronic pain management.
Dr. Osunkwo graduated from medical school at the University of Nigeria, Enugu, performed her residency at the New Jersey Medical School, Newark, and completed her fellowship training at Columbia University, New York.
Dr. Osunkwo takes the reigns at Hematology News from Matt Kalaycio, MD, of the Cleveland Clinic Taussig Cancer Center. Dr. Kalaycio was the first editor in chief of Hematology News and held the post for 3 years.
Hematology News welcomes Ifeyinwa (Ify) Osunkwo, MD, MPH, as the new editor in chief.
Dr. Osunkwo is a professor of medicine at Atrium Health and the director of the Sickle Cell Enterprise at the Levine Cancer Institute, part of Atrium Health, in Charlotte, N.C.
She has made it her personal mission to improve the quality of life for patients with sickle cell disease, a passion that began during time spent in Nigeria as a child, where 150,000 children are born each year with the condition. In 2014, Dr. Osunkwo created a comprehensive sickle cell center in Charlotte with a multidisciplinary team of providers that includes physicians, nurses, social workers, psychologists, and nurse managers. She has also been an instrumental part of the Carolinas Sickle Cell Collaborative, which seeks to match sickle cell patients in the community with blood donors who have similar blood characteristics.
“As a practicing hematologist and researcher, I have a deep appreciation for the timely and relevant content provided by Hematology News,” Dr. Osunkwo said. “I hope to use my experience to help make this publication even better.”
She is a member of the National Adult Sickle Cell Provider Network and leads the Transition/Medical Home Committee for the Southeast Regional Genetics Network. Her interests include health literacy, adolescent transition of care, and chronic pain management.
Dr. Osunkwo graduated from medical school at the University of Nigeria, Enugu, performed her residency at the New Jersey Medical School, Newark, and completed her fellowship training at Columbia University, New York.
Dr. Osunkwo takes the reigns at Hematology News from Matt Kalaycio, MD, of the Cleveland Clinic Taussig Cancer Center. Dr. Kalaycio was the first editor in chief of Hematology News and held the post for 3 years.
Hematology News welcomes Ifeyinwa (Ify) Osunkwo, MD, MPH, as the new editor in chief.
Dr. Osunkwo is a professor of medicine at Atrium Health and the director of the Sickle Cell Enterprise at the Levine Cancer Institute, part of Atrium Health, in Charlotte, N.C.
She has made it her personal mission to improve the quality of life for patients with sickle cell disease, a passion that began during time spent in Nigeria as a child, where 150,000 children are born each year with the condition. In 2014, Dr. Osunkwo created a comprehensive sickle cell center in Charlotte with a multidisciplinary team of providers that includes physicians, nurses, social workers, psychologists, and nurse managers. She has also been an instrumental part of the Carolinas Sickle Cell Collaborative, which seeks to match sickle cell patients in the community with blood donors who have similar blood characteristics.
“As a practicing hematologist and researcher, I have a deep appreciation for the timely and relevant content provided by Hematology News,” Dr. Osunkwo said. “I hope to use my experience to help make this publication even better.”
She is a member of the National Adult Sickle Cell Provider Network and leads the Transition/Medical Home Committee for the Southeast Regional Genetics Network. Her interests include health literacy, adolescent transition of care, and chronic pain management.
Dr. Osunkwo graduated from medical school at the University of Nigeria, Enugu, performed her residency at the New Jersey Medical School, Newark, and completed her fellowship training at Columbia University, New York.
Dr. Osunkwo takes the reigns at Hematology News from Matt Kalaycio, MD, of the Cleveland Clinic Taussig Cancer Center. Dr. Kalaycio was the first editor in chief of Hematology News and held the post for 3 years.
Vermont tops America’s Health Rankings for 2019
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.
Moffitt announces new chief digital innovation officer
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
Edmondo Robinson, MD, is the new senior vice president and chief digital innovation officer at Moffitt Cancer Center in Tampa, Fla. In this newly created position, Dr. Robinson will “oversee Moffitt’s portfolio of digital innovation, including the development and commercialization of health products, tools, and technology.”
Dr. Robinson is also associate professor of medicine at Thomas Jefferson University’s Sidney Kimmel Medical College in Philadelphia. He was previously the chief transformation officer and senior vice-president of consumerism at ChristianaCare, a health system based in Wilmington, Del. Dr. Robinson’s research is focused on health services, particularly care transitions and how technology impacts care delivery.
In other news, Elizabeth Fox, MD, has been named senior vice president of clinical trials research at St. Jude Children’s Research Hospital in Memphis, Tenn. She will also serve as the associate director for clinical research in the St. Jude Comprehensive Cancer Center. Dr. Fox will take on these roles in January 2020.
Dr. Fox was previously director of developmental therapeutics in oncology and professor of pediatrics at Children’s Hospital of Philadelphia. According to St. Jude, Dr. Fox is an expert in integrating clinical and preclinical pharmacology in clinical trial design.
The International Society of Gastrointestinal Oncology has appointed Weijing Sun, MD, as its president-elect. After his 2-year term as president-elect, Dr. Sun will take over as president from Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Sun is director of the division of medical oncology and associate director for clinical research at the University of Kansas Cancer Center in Kansas City. He is a gastrointestinal medical oncologist with a research focus on the development of new treatments for pancreatic, gastroesophageal, hepatobiliary, and colorectal cancers.
Finally, Elizabeth Plimack, MD, has been elected to the American Society of Clinical Oncology’s board of directors for a term of 4 years. She will begin this appointment in June 2020.
Dr. Plimack is a professor and chief of the division of genitourinary medical oncology at Fox Chase Cancer Center in Philadelphia. Dr. Plimack’s clinical practice is focused on the treatment of kidney, bladder, prostate, and testicular cancer. Her research is focused on developing new therapies for bladder and kidney cancers.
HealthCare.gov enrollment ends with unexpected extension
The 2020 open enrollment period on HealthCare.gov ended on Dec. 18 after an unplanned extension, but Centers for Medicare & Medicaid Services Administrator Seema Verma touted the system’s stability.
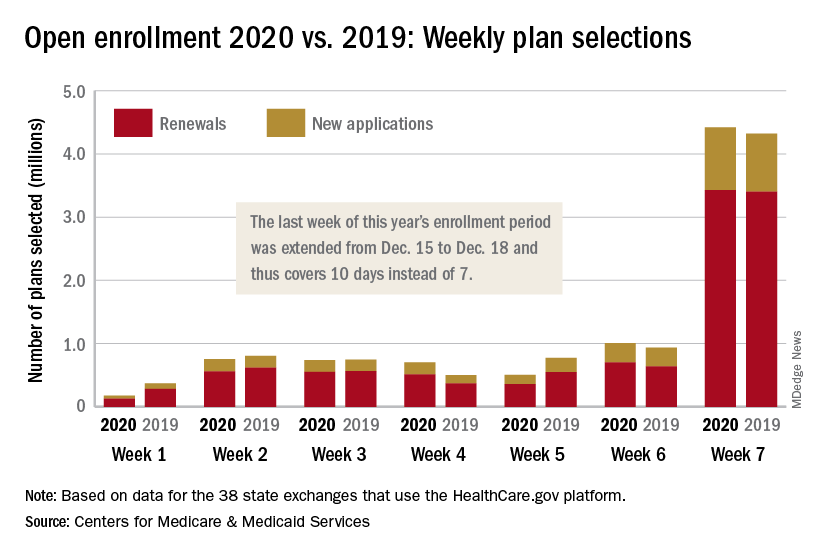
“We are reporting that for the third year in a row enrollment in the Federal Exchange remained stable,” she said in a statement. “For all our successes, too many Americans who do not qualify for subsidies still cannot afford premiums that remain in the stratosphere – constituting a new class of uninsured. The Affordable Care Act remains fundamentally broken and nothing less than wholesale reforms can fix it.”
The open enrollment period was scheduled to end on Dec. 15, but some individuals had problems signing up for coverage that day so the CMS extended the deadline to Dec. 18. During that last “week,” consumers selected over 4.4 million plans – 3.4 million were renewals and just under 1 million were new – bringing the cumulative total for the 2020 enrollment period to 8.3 million plans selected from Nov. 1 to Dec. 17, CMS reported.
Plans selected during the last 3 hours of open enrollment – 12:00 a.m. to 3:00 a.m. on Dec. 18 – are not included in the weekly or final counts, so it’s still possible that the 2020 enrollment could surpass last year’s total of 8.45 million plan selections. The fully updated enrollment data will be released during the second week of January, CMS said.
The 2020 open enrollment period on HealthCare.gov ended on Dec. 18 after an unplanned extension, but Centers for Medicare & Medicaid Services Administrator Seema Verma touted the system’s stability.

“We are reporting that for the third year in a row enrollment in the Federal Exchange remained stable,” she said in a statement. “For all our successes, too many Americans who do not qualify for subsidies still cannot afford premiums that remain in the stratosphere – constituting a new class of uninsured. The Affordable Care Act remains fundamentally broken and nothing less than wholesale reforms can fix it.”
The open enrollment period was scheduled to end on Dec. 15, but some individuals had problems signing up for coverage that day so the CMS extended the deadline to Dec. 18. During that last “week,” consumers selected over 4.4 million plans – 3.4 million were renewals and just under 1 million were new – bringing the cumulative total for the 2020 enrollment period to 8.3 million plans selected from Nov. 1 to Dec. 17, CMS reported.
Plans selected during the last 3 hours of open enrollment – 12:00 a.m. to 3:00 a.m. on Dec. 18 – are not included in the weekly or final counts, so it’s still possible that the 2020 enrollment could surpass last year’s total of 8.45 million plan selections. The fully updated enrollment data will be released during the second week of January, CMS said.
The 2020 open enrollment period on HealthCare.gov ended on Dec. 18 after an unplanned extension, but Centers for Medicare & Medicaid Services Administrator Seema Verma touted the system’s stability.

“We are reporting that for the third year in a row enrollment in the Federal Exchange remained stable,” she said in a statement. “For all our successes, too many Americans who do not qualify for subsidies still cannot afford premiums that remain in the stratosphere – constituting a new class of uninsured. The Affordable Care Act remains fundamentally broken and nothing less than wholesale reforms can fix it.”
The open enrollment period was scheduled to end on Dec. 15, but some individuals had problems signing up for coverage that day so the CMS extended the deadline to Dec. 18. During that last “week,” consumers selected over 4.4 million plans – 3.4 million were renewals and just under 1 million were new – bringing the cumulative total for the 2020 enrollment period to 8.3 million plans selected from Nov. 1 to Dec. 17, CMS reported.
Plans selected during the last 3 hours of open enrollment – 12:00 a.m. to 3:00 a.m. on Dec. 18 – are not included in the weekly or final counts, so it’s still possible that the 2020 enrollment could surpass last year’s total of 8.45 million plan selections. The fully updated enrollment data will be released during the second week of January, CMS said.
Appropriations bill, now law, eliminates ACA taxes, raises tobacco age
Congress took steps to permanently eliminate three taxes from within the Affordable Care Act that were enacted to help offset the law’s cost, but have been sporadically implemented.
The appropriations bill, H.R. 1865, signed into law Dec. 20 by President Trump, includes a number of other health-related provisions, including increasing the minimum age for purchasing tobacco to 21.
The repealed ACA-related taxes include the medical device tax (which previously had been delayed twice); the health insurance tax (which taxed insurers that offered fully insured health coverage in the individual market and has been under sporadic moratorium); and the so-called Cadillac tax on high-cost health plans, which is currently under suspension until the end of 2022.
The appropriations bill offers no offset for the lost revenue.
The tax repeals come on the heels of the U.S. Fifth Circuit Court of Appeals’ ruling that the ACA’s individual mandate is unconstitutional, which is putting the ACA in its entirety in jeopardy should the district court rule that the individual mandate is not severable from the rest of the law, which would invalidate the ACA.
Other key provisions in H.R. 1865 include the short-term extension of a number of federal programs, including a delay in Medicaid disproportionate share hospital payment reductions, payments to community health centers, funding for teaching health centers, and the special diabetes program. Funding for these extenders will go through May 22, 2020.
H.R. 1865 is also notable for what is missing, including any broad provisions that address the price of prescription drugs and surprise billing.
The House of Representatives earlier this month passed a bill, H.R. 3, aimed at lowering the cost of prescription drugs, but that bill was essentially dead on arrival in the Senate, with Speaker Mitch McConnell (R-Ky.) saying he would not bring it to the floor for consideration. There was also a veto threat from the White House hanging over it on the off chance it got past the upper chamber.
There was some optimism that surprise billing would be addressed in the appropriations bill after a bipartisan agreement was reached with the House Energy and Commerce Committee and the Senate Health, Education, Labor, and Pensions Committee, but that stalled after a different bipartisan agreement forged in the House Ways and Means Committee was introduced. More work is expected on surprise billing in the coming year.
One portion of H.R. 1865 that does address the cost of drugs is the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act, which is designed to allow generic manufacturers easier access to brand-name samples to help bring more generic drugs to market.
Another provision that gained applause from the American College of Physicians is the funding for research into gun violence.
“We are particularly encouraged that the legislation authorizes funding for the Centers for Disease Control and Prevention and the National Institutes of Health to study gun violence and safety for the first time in decades,” ACP President Robert McLean, MD, said in a statement. “The key to solving any public health crisis is knowledge, and our efforts to prevent firearms-related injuries and deaths have been hampered by inadequate research. This funding is a promising first step.
However, ACP called for more action in this area.
“Congress should do more to reduce injuries and deaths from firearms,” Dr. McLean said. “The Senate should pass the Bipartisan Background Check Act and reauthorize the Violence Against Women Act, which would close the ‘domestic violence’ loophole in the background check system, as passed by the House of Representatives.”
H.R. 1865 also reauthorizes the Patient-Centered Outcomes Research Institute for 10 additional years.
Congress took steps to permanently eliminate three taxes from within the Affordable Care Act that were enacted to help offset the law’s cost, but have been sporadically implemented.
The appropriations bill, H.R. 1865, signed into law Dec. 20 by President Trump, includes a number of other health-related provisions, including increasing the minimum age for purchasing tobacco to 21.
The repealed ACA-related taxes include the medical device tax (which previously had been delayed twice); the health insurance tax (which taxed insurers that offered fully insured health coverage in the individual market and has been under sporadic moratorium); and the so-called Cadillac tax on high-cost health plans, which is currently under suspension until the end of 2022.
The appropriations bill offers no offset for the lost revenue.
The tax repeals come on the heels of the U.S. Fifth Circuit Court of Appeals’ ruling that the ACA’s individual mandate is unconstitutional, which is putting the ACA in its entirety in jeopardy should the district court rule that the individual mandate is not severable from the rest of the law, which would invalidate the ACA.
Other key provisions in H.R. 1865 include the short-term extension of a number of federal programs, including a delay in Medicaid disproportionate share hospital payment reductions, payments to community health centers, funding for teaching health centers, and the special diabetes program. Funding for these extenders will go through May 22, 2020.
H.R. 1865 is also notable for what is missing, including any broad provisions that address the price of prescription drugs and surprise billing.
The House of Representatives earlier this month passed a bill, H.R. 3, aimed at lowering the cost of prescription drugs, but that bill was essentially dead on arrival in the Senate, with Speaker Mitch McConnell (R-Ky.) saying he would not bring it to the floor for consideration. There was also a veto threat from the White House hanging over it on the off chance it got past the upper chamber.
There was some optimism that surprise billing would be addressed in the appropriations bill after a bipartisan agreement was reached with the House Energy and Commerce Committee and the Senate Health, Education, Labor, and Pensions Committee, but that stalled after a different bipartisan agreement forged in the House Ways and Means Committee was introduced. More work is expected on surprise billing in the coming year.
One portion of H.R. 1865 that does address the cost of drugs is the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act, which is designed to allow generic manufacturers easier access to brand-name samples to help bring more generic drugs to market.
Another provision that gained applause from the American College of Physicians is the funding for research into gun violence.
“We are particularly encouraged that the legislation authorizes funding for the Centers for Disease Control and Prevention and the National Institutes of Health to study gun violence and safety for the first time in decades,” ACP President Robert McLean, MD, said in a statement. “The key to solving any public health crisis is knowledge, and our efforts to prevent firearms-related injuries and deaths have been hampered by inadequate research. This funding is a promising first step.
However, ACP called for more action in this area.
“Congress should do more to reduce injuries and deaths from firearms,” Dr. McLean said. “The Senate should pass the Bipartisan Background Check Act and reauthorize the Violence Against Women Act, which would close the ‘domestic violence’ loophole in the background check system, as passed by the House of Representatives.”
H.R. 1865 also reauthorizes the Patient-Centered Outcomes Research Institute for 10 additional years.
Congress took steps to permanently eliminate three taxes from within the Affordable Care Act that were enacted to help offset the law’s cost, but have been sporadically implemented.
The appropriations bill, H.R. 1865, signed into law Dec. 20 by President Trump, includes a number of other health-related provisions, including increasing the minimum age for purchasing tobacco to 21.
The repealed ACA-related taxes include the medical device tax (which previously had been delayed twice); the health insurance tax (which taxed insurers that offered fully insured health coverage in the individual market and has been under sporadic moratorium); and the so-called Cadillac tax on high-cost health plans, which is currently under suspension until the end of 2022.
The appropriations bill offers no offset for the lost revenue.
The tax repeals come on the heels of the U.S. Fifth Circuit Court of Appeals’ ruling that the ACA’s individual mandate is unconstitutional, which is putting the ACA in its entirety in jeopardy should the district court rule that the individual mandate is not severable from the rest of the law, which would invalidate the ACA.
Other key provisions in H.R. 1865 include the short-term extension of a number of federal programs, including a delay in Medicaid disproportionate share hospital payment reductions, payments to community health centers, funding for teaching health centers, and the special diabetes program. Funding for these extenders will go through May 22, 2020.
H.R. 1865 is also notable for what is missing, including any broad provisions that address the price of prescription drugs and surprise billing.
The House of Representatives earlier this month passed a bill, H.R. 3, aimed at lowering the cost of prescription drugs, but that bill was essentially dead on arrival in the Senate, with Speaker Mitch McConnell (R-Ky.) saying he would not bring it to the floor for consideration. There was also a veto threat from the White House hanging over it on the off chance it got past the upper chamber.
There was some optimism that surprise billing would be addressed in the appropriations bill after a bipartisan agreement was reached with the House Energy and Commerce Committee and the Senate Health, Education, Labor, and Pensions Committee, but that stalled after a different bipartisan agreement forged in the House Ways and Means Committee was introduced. More work is expected on surprise billing in the coming year.
One portion of H.R. 1865 that does address the cost of drugs is the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act, which is designed to allow generic manufacturers easier access to brand-name samples to help bring more generic drugs to market.
Another provision that gained applause from the American College of Physicians is the funding for research into gun violence.
“We are particularly encouraged that the legislation authorizes funding for the Centers for Disease Control and Prevention and the National Institutes of Health to study gun violence and safety for the first time in decades,” ACP President Robert McLean, MD, said in a statement. “The key to solving any public health crisis is knowledge, and our efforts to prevent firearms-related injuries and deaths have been hampered by inadequate research. This funding is a promising first step.
However, ACP called for more action in this area.
“Congress should do more to reduce injuries and deaths from firearms,” Dr. McLean said. “The Senate should pass the Bipartisan Background Check Act and reauthorize the Violence Against Women Act, which would close the ‘domestic violence’ loophole in the background check system, as passed by the House of Representatives.”
H.R. 1865 also reauthorizes the Patient-Centered Outcomes Research Institute for 10 additional years.
Four new programs, new slots added to rheumatology Specialty Match Day
This year’s Specialty Match Day was highlighted by the addition of four new programs and 13 new fellowship positions for future rheumatologists.
For the 2020 appointment year, there were 249 fellowships in rheumatology available, up from 236 in the previous year. It continues a trend of an increasing number of fellowship slots. There were 221 slots available in the 2018 appointment year.
There were 242 slots filled in the 2020 appointment year, up from 233 in the previous year and 218 from two appointment years ago.
“The overall message from the match this year in rheumatology is that it really was a very good, very successful match,” Beth Jonas, MD, chair of the American College of Rheumatology’s Committee on Training and Workforce Issues, said in an interview. “Rheumatology continues to be extremely strong in terms of its ability to attract excellent physicians to the subspecialty, so we are really very, very happy about that.”
She also applauded the addition of the four new programs and 13 new fellowship slots.
“We’ve been working very hard to try and figure out ways to increase slots,” said Dr. Jonas, a professor at the University of North Carolina, Chapel Hill. She noted that while more needs to be done to address future workforce needs, even the small increases “are meaningful. There is the knowledge out there that there is a workforce shortage in rheumatology. There certainly are a lot of people who would like to become rheumatologists. There is a little bit of a bottleneck here at the level of training slots to get interested and qualified applicants into the match and matched with programs, so we’ve had some marginal increases there.”
One number that decreased this year was the number of specialty applicants who stated they preferred to be in a rheumatology program, which decreased to 335 applicants in the 2020 appointment year from 358 in the 2019 application year. Of the 335 applicants that stated rheumatology as the preferred specialty, 239 received fellowships in rheumatology, 2 were matched to other specialties, and 94 did not match into any program.
The decrease in applicants did not concern Dr. Jonas.
“It is still pretty robust and our match rate is in the low 70s, which makes it one of the most competitive subspecialties in internal medicine, up there with cardiology and gastroenterology,” she said. “It doesn’t really worry me.”
She opined that the reason could be that the “people who might have been not strong candidates might have just not applied because it is so competitive now,” but she cautioned that it is just musings with no specific data to say exactly what is causing the decrease in applications.
She also was not concerned that the number of unfulfilled slots increased this year compared with the previous 2 years.
“The programs that did not fill tended to be the ones that were highly focused on research,” she said. “It is not surprising that there were a couple of slots left empty. We know for certain that of all of the people who applied to rheumatology fellowships, the vast majority are interested in clinical rheumatology, in clinical care, so there are fewer applicants out there that are really interested in research slots.”
Overall across all specialties, there were 6,286 applicants with rank for 5,576 positions in appointment year 2020, of which 4,909 were matched to a specialty program. The fill rate increased slightly to 88% from 87.8% in the previous year, when there were 5,881 applicants for 5,125 program slots with 4,579 positions filled.
SOURCE: National Resident Matching Program.
This year’s Specialty Match Day was highlighted by the addition of four new programs and 13 new fellowship positions for future rheumatologists.
For the 2020 appointment year, there were 249 fellowships in rheumatology available, up from 236 in the previous year. It continues a trend of an increasing number of fellowship slots. There were 221 slots available in the 2018 appointment year.
There were 242 slots filled in the 2020 appointment year, up from 233 in the previous year and 218 from two appointment years ago.
“The overall message from the match this year in rheumatology is that it really was a very good, very successful match,” Beth Jonas, MD, chair of the American College of Rheumatology’s Committee on Training and Workforce Issues, said in an interview. “Rheumatology continues to be extremely strong in terms of its ability to attract excellent physicians to the subspecialty, so we are really very, very happy about that.”
She also applauded the addition of the four new programs and 13 new fellowship slots.
“We’ve been working very hard to try and figure out ways to increase slots,” said Dr. Jonas, a professor at the University of North Carolina, Chapel Hill. She noted that while more needs to be done to address future workforce needs, even the small increases “are meaningful. There is the knowledge out there that there is a workforce shortage in rheumatology. There certainly are a lot of people who would like to become rheumatologists. There is a little bit of a bottleneck here at the level of training slots to get interested and qualified applicants into the match and matched with programs, so we’ve had some marginal increases there.”
One number that decreased this year was the number of specialty applicants who stated they preferred to be in a rheumatology program, which decreased to 335 applicants in the 2020 appointment year from 358 in the 2019 application year. Of the 335 applicants that stated rheumatology as the preferred specialty, 239 received fellowships in rheumatology, 2 were matched to other specialties, and 94 did not match into any program.
The decrease in applicants did not concern Dr. Jonas.
“It is still pretty robust and our match rate is in the low 70s, which makes it one of the most competitive subspecialties in internal medicine, up there with cardiology and gastroenterology,” she said. “It doesn’t really worry me.”
She opined that the reason could be that the “people who might have been not strong candidates might have just not applied because it is so competitive now,” but she cautioned that it is just musings with no specific data to say exactly what is causing the decrease in applications.
She also was not concerned that the number of unfulfilled slots increased this year compared with the previous 2 years.
“The programs that did not fill tended to be the ones that were highly focused on research,” she said. “It is not surprising that there were a couple of slots left empty. We know for certain that of all of the people who applied to rheumatology fellowships, the vast majority are interested in clinical rheumatology, in clinical care, so there are fewer applicants out there that are really interested in research slots.”
Overall across all specialties, there were 6,286 applicants with rank for 5,576 positions in appointment year 2020, of which 4,909 were matched to a specialty program. The fill rate increased slightly to 88% from 87.8% in the previous year, when there were 5,881 applicants for 5,125 program slots with 4,579 positions filled.
SOURCE: National Resident Matching Program.
This year’s Specialty Match Day was highlighted by the addition of four new programs and 13 new fellowship positions for future rheumatologists.
For the 2020 appointment year, there were 249 fellowships in rheumatology available, up from 236 in the previous year. It continues a trend of an increasing number of fellowship slots. There were 221 slots available in the 2018 appointment year.
There were 242 slots filled in the 2020 appointment year, up from 233 in the previous year and 218 from two appointment years ago.
“The overall message from the match this year in rheumatology is that it really was a very good, very successful match,” Beth Jonas, MD, chair of the American College of Rheumatology’s Committee on Training and Workforce Issues, said in an interview. “Rheumatology continues to be extremely strong in terms of its ability to attract excellent physicians to the subspecialty, so we are really very, very happy about that.”
She also applauded the addition of the four new programs and 13 new fellowship slots.
“We’ve been working very hard to try and figure out ways to increase slots,” said Dr. Jonas, a professor at the University of North Carolina, Chapel Hill. She noted that while more needs to be done to address future workforce needs, even the small increases “are meaningful. There is the knowledge out there that there is a workforce shortage in rheumatology. There certainly are a lot of people who would like to become rheumatologists. There is a little bit of a bottleneck here at the level of training slots to get interested and qualified applicants into the match and matched with programs, so we’ve had some marginal increases there.”
One number that decreased this year was the number of specialty applicants who stated they preferred to be in a rheumatology program, which decreased to 335 applicants in the 2020 appointment year from 358 in the 2019 application year. Of the 335 applicants that stated rheumatology as the preferred specialty, 239 received fellowships in rheumatology, 2 were matched to other specialties, and 94 did not match into any program.
The decrease in applicants did not concern Dr. Jonas.
“It is still pretty robust and our match rate is in the low 70s, which makes it one of the most competitive subspecialties in internal medicine, up there with cardiology and gastroenterology,” she said. “It doesn’t really worry me.”
She opined that the reason could be that the “people who might have been not strong candidates might have just not applied because it is so competitive now,” but she cautioned that it is just musings with no specific data to say exactly what is causing the decrease in applications.
She also was not concerned that the number of unfulfilled slots increased this year compared with the previous 2 years.
“The programs that did not fill tended to be the ones that were highly focused on research,” she said. “It is not surprising that there were a couple of slots left empty. We know for certain that of all of the people who applied to rheumatology fellowships, the vast majority are interested in clinical rheumatology, in clinical care, so there are fewer applicants out there that are really interested in research slots.”
Overall across all specialties, there were 6,286 applicants with rank for 5,576 positions in appointment year 2020, of which 4,909 were matched to a specialty program. The fill rate increased slightly to 88% from 87.8% in the previous year, when there were 5,881 applicants for 5,125 program slots with 4,579 positions filled.
SOURCE: National Resident Matching Program.
Out-of-network billing in in-network hospitals adds $40 billion in spending
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
gtwachtman@mdedge.com
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
gtwachtman@mdedge.com
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
As the debate over how best to address surprise billing continues, new research shows that billing from out-of-network physicians at in-network facilities is adding $40 billion in costs.
Researchers focused on four different types of physicians that account for out-of-network billing: anesthesiologists, pathologists, radiologists, and cases involving an assistant surgeon, which had out-of-network bills in about 10% of claims that were examined as part of the research.
“To give a rough estimate of the savings that could be achieved by eliminating the ability of these four types of specialists to readily bill out of network, we simulated what would happen if all of these specialists received the same average payments as orthopedic surgeons did (164% of Medicare rates),” Zack Cooper, PhD, associate professor of health policy at Yale University, New Haven, Conn., and colleagues wrote in a research report published in Health Affairs.
“We estimated that if these physicians were paid the same average rate as orthopedists for all of the services that they delivered in our sample, spending would be lowered on anesthesiologists by 53.5%, on pathologists by 47.4%, on radiologists by 16.3%, and on assistant surgeons by 46.2%,” the authors wrote.
Researchers said that physician spending for these four specialties would be lowered by 13.4% and would lower total spending for people with employer-sponsored insurance by about 3.4%, or $40 billion. If spending on these four specialties were lowered to 150% of Medicare rates, it would lower spending on physicians by 15.3%.
To help combat the issues of surprise billing in a way that lowers total commercial health care spending and helps to preserve a competitive price for physician services, Dr. Cooper and colleagues recommended an approach that would regulate the contracts of physicians who work in hospitals and are not chosen by patients. It would establish a bundled package for services that include the emergency department physicians and the four specialists examined as part of the research and would use the fee associated with the package of services to recruit specialists to work at the hospital.
The authors said this kind of policy would eliminate the possibility of patients seeing out-of-network providers at in-network hospitals and, unlike arbitration (a favored solution among physician groups if it is set up in an agreeable manner), patients are protected without being required to take any action. The policy also sets a competitive rate for these services.
“Under this bundled care approach, physicians would compete to offer their services on the basis of price and quality,” Dr. Cooper and colleagues stated. “Hospitals would compete with one another on the price and quality of their care, including the services provided by the physicians they recruited. Hospitals would also need to compete to retain physicians.”
This approach is not included in any current surprise billing legislation. There was hope that surprise billing would be addressed in a government spending bill that would be signed before year’s end. But a second bipartisan plan was introduced in the House Ways and Means Committee after a bipartisan compromise was reached by the House Energy and Commerce and the Senate Health, Education, Labor, and Pensions committees. This has postponed a decision on surprise billing legislation into the coming year.
gtwachtman@mdedge.com
SOURCE: Cooper Z et al. Health Aff. 2019 Dec 16. doi: 10.1377/hlthaff.2019.00507.
FROM HEALTH AFFAIRS
Appeals court rules ACA’s individual mandate is unconstitutional
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
A version of this story first appeared on Medscape.com.
Seven things I am grateful for
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
It’s almost a New Year and .
The wildlife in my backyard provides endless entertainment. Not only the birds, rabbits, squirrels, raccoons, and skunks, but turkey, red fox, coyote, hawk, and even a mink. At some point, I quit buying koi for the pond and substituted dime gold fish. Of course, we cannot overlook the deer, who trash my shrubs, eat and grind the bark off my baby trees, consume my garden (don’t they know tomato plants are related to deadly nightshade?), and shed ticks. They watch me with calm indifference, even when I shout at them.
Most folks hate cold weather, but it kills the mosquitoes and stink bugs, and allows me to build mesmerizing fires. It is time to clean the yard, turn over the garden, plant new things, all without breaking much of a sweat. It is almost time to empty the compost pile onto the garden mixed with the ashes from the fire pit.
Last year’s tomato crop started late but was a blockbuster. I plant heirlooms grafted onto resistant rootstock (territorial seed company) placed under walls of water in April. I cage them up high. I like to stand in the tomato jungle in high summer, invisible for a few minutes, and eat the little cherry tomatoes and think about nothing but how perfectly the sweetness and tartness is balanced. I still have a few on the kitchen counter making that crucial, very late, decision on whether to ripen or rot.
The U.S. Navy cannot be thanked enough for taking my defiant teenage boy and molding him into what is starting to resemble a fine young man. The Navy is what he needed.
I give much professional credit to my office staff and my patients. I really haven’t run the office for years; it has its own rhythm and knowledge. You spend more waking hours there than at home, so being fun and entertaining is important. That said, the hiring and management of employees is the most difficult part of running a small office. The patients generally know to come in sooner rather than later if they start growing something ugly. And I have also been blessed with good health, mandatory for maintaining a small office. It’s been a good ride.
My wife is quiet when I am loud, reserved when I am bombastic, an only child matched with a middle. She wears a child’s size bicycle helmet, but her head is packed with brains. She knows millions of things I don’t. She is terribly organized. I float my crazy ideas past her daily and leave with punctured remnants to patch together into a better weave. We spend all our free time together, which is the way it ought to be.
Finally, of course, I am grateful for my specialty of dermatology. I kind of wandered into dermatology after internal medicine, and after seriously considering cardiology. It is a happy and joyous specialty with enough cures and successes to keep gloom and hopelessness at bay. I look forward to going to work and get great satisfaction from my work. I have continued to improve in this field, which gives so much more than it takes.
So enjoy the New Year! Take time to build a roaring fire, buy quirky gifts for your staff, get your spouses or significant others whatever they want, and enjoy your specialty as a dermatologist. You are in one of the best places in the world.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
HHS drug importation proposals aim to address high costs
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.














