User login
Is it time for neurologists to manage high blood pressure?
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Quantifying the EHR connection to burnout
While plenty of anecdotal and other evidence exists to connect the use of electronic health records to physician burnout, new research puts a more standard, quantifiable measure to it in an effort to help measure progress in improving the usability of EHRs.
Researchers used the System Usability Scale (SUS), “favored as an industry standard as a short, simple, and reliable measurement of technology usability with solid benchmarks to easily interpret its results, as the measure in this research, Edward Melnick, MD, of Yale University, New Haven, Conn., and colleagues wrote in Mayo Clinic Proceedings.
“The previous studies have definitely hinted at [the link between EHRs and burnout], but never really quantified it,” Dr. Melnick said in an interview.
Among the 870 physicians evaluating their EHRs’ usability, the mean score on a scale of 0-100 (higher being more usable) was 45.9. As a point of comparison, Microsoft Excel has an SUS score of 57, digital video recorders score 74, Amazon scores 82, microwave ovens score 87, and Google search scores 93.
“A score of 45.9 is in the bottom 9% of usability scores across studies in other industries and is categorized as in the ‘not acceptable’ range with a grade of F,” the authors wrote. “In aggregate, 733 of 870 (84.2%) of respondents rated their EHR less than 68 on the SUS, the average score across industries.”
In tying the SUS results to burnout, which was measured using the Maslach Burnout Inventory, the authors noted that the scores “were strongly and independently associated with physician burnout in a dose-response relationship. The odds of burnout were lower for each 1 point more favorable SUS score, a finding that persisted after adjusting for an extensive array of other personal and professional characteristics. The relationship between SUS score and burnout also persisted when emotional exhaustion and depersonalization were treated as continuous variables.”
The authors did note that, despite the strong relationship, they could not determine a causation given the cross-sectional nature of the data.
“I’m hoping that this paper will spark conversation and drive change and be a way of tracking improvements,” Dr. Melnick said. “So, if you bring in something new and say this is going to be better, how do you know it is going to be better? Well maybe you measure it using the System Usability Scale” to give it a quantifiable measure of improvement. He said it is an advantage “of having a metric that has been standardized and used in other industries,” allowing EHR stakeholders to measure improvement. “Once you can measure it, you can manage it and make improvements faster.”
The findings “will not come as a surprise to anyone who practices medicine,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “It is a national imperative to overhaul the design and use of EHRs and reframe the technology to focus primarily on its most critical function: helping physicians care for patients. Significantly enhancing EHR usability is key and the AMA is working to ensure a new generation of EHRs are designed to prioritize time with patients, rather than overload physicians with type-and-click tasks.”
Funding for the study was provided by the Stanford Medicine WebMD Center, AMA, and the Mayo Clinic Department of Medicine Program on Physician Well-Being. No conflicts of interest were reported by the authors.
SOURCE: Melnick E et al. Mayo Clinic Proceedings 2019 Nov 14. doi: 10.1016/j.mayocp.2019.09.024.
While plenty of anecdotal and other evidence exists to connect the use of electronic health records to physician burnout, new research puts a more standard, quantifiable measure to it in an effort to help measure progress in improving the usability of EHRs.
Researchers used the System Usability Scale (SUS), “favored as an industry standard as a short, simple, and reliable measurement of technology usability with solid benchmarks to easily interpret its results, as the measure in this research, Edward Melnick, MD, of Yale University, New Haven, Conn., and colleagues wrote in Mayo Clinic Proceedings.
“The previous studies have definitely hinted at [the link between EHRs and burnout], but never really quantified it,” Dr. Melnick said in an interview.
Among the 870 physicians evaluating their EHRs’ usability, the mean score on a scale of 0-100 (higher being more usable) was 45.9. As a point of comparison, Microsoft Excel has an SUS score of 57, digital video recorders score 74, Amazon scores 82, microwave ovens score 87, and Google search scores 93.
“A score of 45.9 is in the bottom 9% of usability scores across studies in other industries and is categorized as in the ‘not acceptable’ range with a grade of F,” the authors wrote. “In aggregate, 733 of 870 (84.2%) of respondents rated their EHR less than 68 on the SUS, the average score across industries.”
In tying the SUS results to burnout, which was measured using the Maslach Burnout Inventory, the authors noted that the scores “were strongly and independently associated with physician burnout in a dose-response relationship. The odds of burnout were lower for each 1 point more favorable SUS score, a finding that persisted after adjusting for an extensive array of other personal and professional characteristics. The relationship between SUS score and burnout also persisted when emotional exhaustion and depersonalization were treated as continuous variables.”
The authors did note that, despite the strong relationship, they could not determine a causation given the cross-sectional nature of the data.
“I’m hoping that this paper will spark conversation and drive change and be a way of tracking improvements,” Dr. Melnick said. “So, if you bring in something new and say this is going to be better, how do you know it is going to be better? Well maybe you measure it using the System Usability Scale” to give it a quantifiable measure of improvement. He said it is an advantage “of having a metric that has been standardized and used in other industries,” allowing EHR stakeholders to measure improvement. “Once you can measure it, you can manage it and make improvements faster.”
The findings “will not come as a surprise to anyone who practices medicine,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “It is a national imperative to overhaul the design and use of EHRs and reframe the technology to focus primarily on its most critical function: helping physicians care for patients. Significantly enhancing EHR usability is key and the AMA is working to ensure a new generation of EHRs are designed to prioritize time with patients, rather than overload physicians with type-and-click tasks.”
Funding for the study was provided by the Stanford Medicine WebMD Center, AMA, and the Mayo Clinic Department of Medicine Program on Physician Well-Being. No conflicts of interest were reported by the authors.
SOURCE: Melnick E et al. Mayo Clinic Proceedings 2019 Nov 14. doi: 10.1016/j.mayocp.2019.09.024.
While plenty of anecdotal and other evidence exists to connect the use of electronic health records to physician burnout, new research puts a more standard, quantifiable measure to it in an effort to help measure progress in improving the usability of EHRs.
Researchers used the System Usability Scale (SUS), “favored as an industry standard as a short, simple, and reliable measurement of technology usability with solid benchmarks to easily interpret its results, as the measure in this research, Edward Melnick, MD, of Yale University, New Haven, Conn., and colleagues wrote in Mayo Clinic Proceedings.
“The previous studies have definitely hinted at [the link between EHRs and burnout], but never really quantified it,” Dr. Melnick said in an interview.
Among the 870 physicians evaluating their EHRs’ usability, the mean score on a scale of 0-100 (higher being more usable) was 45.9. As a point of comparison, Microsoft Excel has an SUS score of 57, digital video recorders score 74, Amazon scores 82, microwave ovens score 87, and Google search scores 93.
“A score of 45.9 is in the bottom 9% of usability scores across studies in other industries and is categorized as in the ‘not acceptable’ range with a grade of F,” the authors wrote. “In aggregate, 733 of 870 (84.2%) of respondents rated their EHR less than 68 on the SUS, the average score across industries.”
In tying the SUS results to burnout, which was measured using the Maslach Burnout Inventory, the authors noted that the scores “were strongly and independently associated with physician burnout in a dose-response relationship. The odds of burnout were lower for each 1 point more favorable SUS score, a finding that persisted after adjusting for an extensive array of other personal and professional characteristics. The relationship between SUS score and burnout also persisted when emotional exhaustion and depersonalization were treated as continuous variables.”
The authors did note that, despite the strong relationship, they could not determine a causation given the cross-sectional nature of the data.
“I’m hoping that this paper will spark conversation and drive change and be a way of tracking improvements,” Dr. Melnick said. “So, if you bring in something new and say this is going to be better, how do you know it is going to be better? Well maybe you measure it using the System Usability Scale” to give it a quantifiable measure of improvement. He said it is an advantage “of having a metric that has been standardized and used in other industries,” allowing EHR stakeholders to measure improvement. “Once you can measure it, you can manage it and make improvements faster.”
The findings “will not come as a surprise to anyone who practices medicine,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “It is a national imperative to overhaul the design and use of EHRs and reframe the technology to focus primarily on its most critical function: helping physicians care for patients. Significantly enhancing EHR usability is key and the AMA is working to ensure a new generation of EHRs are designed to prioritize time with patients, rather than overload physicians with type-and-click tasks.”
Funding for the study was provided by the Stanford Medicine WebMD Center, AMA, and the Mayo Clinic Department of Medicine Program on Physician Well-Being. No conflicts of interest were reported by the authors.
SOURCE: Melnick E et al. Mayo Clinic Proceedings 2019 Nov 14. doi: 10.1016/j.mayocp.2019.09.024.
FROM MAYO CLINIC PROCEEDINGS
Ob.Gyn. News welcomes Dr. Angela Martin to the board
Dr. Martin is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City, where she is the director of ultrasound training for the residency program. She also serves on the Pharmacy and Therapeutics Committee at the university.
Dr. Martin has been a primary author or coauthor of many articles published and accepted in refereed medical publications on topics including influenza infection during pregnancy, maternal and fetal risk associated with assisted reproductive technology, hepatitis B in pregnancy, umbilical cord blood banking, pregnancy and obesity, and cell-free fetal DNA. She currently is involved in research on resident operative delivery training, chorionic villus sampling, and preterm birth.
Graduating summa cum laude from Drake University in Des Moines, Iowa, with a bachelor of science degree in biology and a minor in psychology, Dr. Martin received her doctorate of medicine at the University of Missouri, Columbia. She completed her postgraduate training at Emory University in Atlanta with an internship and residency in the department of gynecology and obstetrics, followed by a fellowship in the maternal-fetal medicine division.
Dr. Martin has won numerous honors and awards, including Outstanding Research Proposal by a fellow at Emory University, Excellence in Teaching awards for a fellow for several consecutive years at the university, and a National Faculty Award from the American College of Obstetricians and Gynecologists.*
*This article was updated 2/7/2020.
Dr. Martin is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City, where she is the director of ultrasound training for the residency program. She also serves on the Pharmacy and Therapeutics Committee at the university.
Dr. Martin has been a primary author or coauthor of many articles published and accepted in refereed medical publications on topics including influenza infection during pregnancy, maternal and fetal risk associated with assisted reproductive technology, hepatitis B in pregnancy, umbilical cord blood banking, pregnancy and obesity, and cell-free fetal DNA. She currently is involved in research on resident operative delivery training, chorionic villus sampling, and preterm birth.
Graduating summa cum laude from Drake University in Des Moines, Iowa, with a bachelor of science degree in biology and a minor in psychology, Dr. Martin received her doctorate of medicine at the University of Missouri, Columbia. She completed her postgraduate training at Emory University in Atlanta with an internship and residency in the department of gynecology and obstetrics, followed by a fellowship in the maternal-fetal medicine division.
Dr. Martin has won numerous honors and awards, including Outstanding Research Proposal by a fellow at Emory University, Excellence in Teaching awards for a fellow for several consecutive years at the university, and a National Faculty Award from the American College of Obstetricians and Gynecologists.*
*This article was updated 2/7/2020.
Dr. Martin is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City, where she is the director of ultrasound training for the residency program. She also serves on the Pharmacy and Therapeutics Committee at the university.
Dr. Martin has been a primary author or coauthor of many articles published and accepted in refereed medical publications on topics including influenza infection during pregnancy, maternal and fetal risk associated with assisted reproductive technology, hepatitis B in pregnancy, umbilical cord blood banking, pregnancy and obesity, and cell-free fetal DNA. She currently is involved in research on resident operative delivery training, chorionic villus sampling, and preterm birth.
Graduating summa cum laude from Drake University in Des Moines, Iowa, with a bachelor of science degree in biology and a minor in psychology, Dr. Martin received her doctorate of medicine at the University of Missouri, Columbia. She completed her postgraduate training at Emory University in Atlanta with an internship and residency in the department of gynecology and obstetrics, followed by a fellowship in the maternal-fetal medicine division.
Dr. Martin has won numerous honors and awards, including Outstanding Research Proposal by a fellow at Emory University, Excellence in Teaching awards for a fellow for several consecutive years at the university, and a National Faculty Award from the American College of Obstetricians and Gynecologists.*
*This article was updated 2/7/2020.
ASH to recognize researchers at annual meeting
The American Society of Hematology (ASH) plans to honor 10 researchers with awards and lectures at this year’s annual meeting, scheduled to take place Dec. 7-10 in Orlando.
Richard Aster, MD, will receive the 2019 Wallace H. Coulter Award for Lifetime Achievement in Hematology. Dr. Aster, of the Medical College of Wisconsin and Versiti Blood Center of Wisconsin in Milwaukee, “will be honored for his significant contributions to hematology through research, mentorship, and education throughout his 62-year career,” according to ASH.
Dr. Aster is known for his research on immune diseases that affect blood cells, particularly platelets. His work has led to improvements in platelet preparation, storage, and matching. In addition, he and his team developed the standard techniques for diagnosing immune thrombocytopenia and heparin-induced thrombocytopenia-thrombosis.
Other ASH awardees include William Eaton, MD, PhD, and Richard A. Larson, MD, who will receive the 2019 Henry M. Stratton Medal “for their seminal contributions to basic and clinical/translational hematology research, respectively.”
Dr. Eaton, of the National Institute of Diabetes and Digestive and Kidney Diseases conducts research on sickle cell disease, and his work contributed to the development of hydroxyurea. Dr. Larson, of the University of Chicago is the namesake of the Larson regimen (CALGB 8811) for acute lymphoblastic leukemia, and he played a key role in research that led to the U.S. approval of midostaurin.
Philip Greenberg, MD, will deliver the 2019 E. Donnall Thomas Lecture in recognition of “his outstanding contributions to the field of immunotherapy.” Dr. Greenberg, of theFred Hutchinson Cancer Research Center and the University of Washington in Seattle, is known for contributing to the development of T-cell adoptive immune therapy. His lecture will focus on the engineering of T cells to target acute myeloid leukemia and other malignancies.
Sriram Krishnaswamy, PhD, and Jeffrey I. Weitz, MD, will deliver the 2019 Ernest Beutler Lecture in recognition of “their significant research contributions to the understanding and treatment of blood clots.” The researchers will each deliver one part of the lecture at the meeting, and both will discuss research related to novel anticoagulants.
Dr. Krishnaswamy, of the University of Pennsylvania and Children’s Hospital of Philadelphia, is considered an authority on the function of surface-dependent coagulation complexes. Dr. Weitz, of McMaster University in Hamilton, Ont., has conducted research that led to the development of novel anticoagulants.
Emmanuelle Passegué, PhD, will receive the 2019 William Dameshek Prize “for her outstanding contributions to the understanding of hematopoietic stem cells.” Dr. Passegué, of Columbia University Irving Medical Center in New York, conducts research focused on changes to hematopoietic stem cells in the contexts of myeloid malignancies and physiological aging.
Griffin Rodgers, MD, will receive the ASH Award for Leadership in Promoting Diversity “for his extraordinary commitment to diversity and inclusion in hematology.” As director of the National Institute of Diabetes and Digestive and Kidney Diseases, Dr. Rodgers has worked to promote diversity in the scientific workforce and in clinical trials.
Leonard Zon, MD, and Michael R. DeBaun, MD, will receive the 2019 ASH Mentor Award “for their sustained, outstanding commitment to the training and career development of early career hematologists.”
Dr. DeBaun, of Vanderbilt University School of Medicine in Nashville, Tenn., has mentees ranging from high school students with sickle cell disease to tenured faculty members at medical schools. Dr. Zon, of Harvard University and Boston Children’s Hospital, organizes mentoring events for postdocs, graduate students, and technicians.
The American Society of Hematology (ASH) plans to honor 10 researchers with awards and lectures at this year’s annual meeting, scheduled to take place Dec. 7-10 in Orlando.
Richard Aster, MD, will receive the 2019 Wallace H. Coulter Award for Lifetime Achievement in Hematology. Dr. Aster, of the Medical College of Wisconsin and Versiti Blood Center of Wisconsin in Milwaukee, “will be honored for his significant contributions to hematology through research, mentorship, and education throughout his 62-year career,” according to ASH.
Dr. Aster is known for his research on immune diseases that affect blood cells, particularly platelets. His work has led to improvements in platelet preparation, storage, and matching. In addition, he and his team developed the standard techniques for diagnosing immune thrombocytopenia and heparin-induced thrombocytopenia-thrombosis.
Other ASH awardees include William Eaton, MD, PhD, and Richard A. Larson, MD, who will receive the 2019 Henry M. Stratton Medal “for their seminal contributions to basic and clinical/translational hematology research, respectively.”
Dr. Eaton, of the National Institute of Diabetes and Digestive and Kidney Diseases conducts research on sickle cell disease, and his work contributed to the development of hydroxyurea. Dr. Larson, of the University of Chicago is the namesake of the Larson regimen (CALGB 8811) for acute lymphoblastic leukemia, and he played a key role in research that led to the U.S. approval of midostaurin.
Philip Greenberg, MD, will deliver the 2019 E. Donnall Thomas Lecture in recognition of “his outstanding contributions to the field of immunotherapy.” Dr. Greenberg, of theFred Hutchinson Cancer Research Center and the University of Washington in Seattle, is known for contributing to the development of T-cell adoptive immune therapy. His lecture will focus on the engineering of T cells to target acute myeloid leukemia and other malignancies.
Sriram Krishnaswamy, PhD, and Jeffrey I. Weitz, MD, will deliver the 2019 Ernest Beutler Lecture in recognition of “their significant research contributions to the understanding and treatment of blood clots.” The researchers will each deliver one part of the lecture at the meeting, and both will discuss research related to novel anticoagulants.
Dr. Krishnaswamy, of the University of Pennsylvania and Children’s Hospital of Philadelphia, is considered an authority on the function of surface-dependent coagulation complexes. Dr. Weitz, of McMaster University in Hamilton, Ont., has conducted research that led to the development of novel anticoagulants.
Emmanuelle Passegué, PhD, will receive the 2019 William Dameshek Prize “for her outstanding contributions to the understanding of hematopoietic stem cells.” Dr. Passegué, of Columbia University Irving Medical Center in New York, conducts research focused on changes to hematopoietic stem cells in the contexts of myeloid malignancies and physiological aging.
Griffin Rodgers, MD, will receive the ASH Award for Leadership in Promoting Diversity “for his extraordinary commitment to diversity and inclusion in hematology.” As director of the National Institute of Diabetes and Digestive and Kidney Diseases, Dr. Rodgers has worked to promote diversity in the scientific workforce and in clinical trials.
Leonard Zon, MD, and Michael R. DeBaun, MD, will receive the 2019 ASH Mentor Award “for their sustained, outstanding commitment to the training and career development of early career hematologists.”
Dr. DeBaun, of Vanderbilt University School of Medicine in Nashville, Tenn., has mentees ranging from high school students with sickle cell disease to tenured faculty members at medical schools. Dr. Zon, of Harvard University and Boston Children’s Hospital, organizes mentoring events for postdocs, graduate students, and technicians.
The American Society of Hematology (ASH) plans to honor 10 researchers with awards and lectures at this year’s annual meeting, scheduled to take place Dec. 7-10 in Orlando.
Richard Aster, MD, will receive the 2019 Wallace H. Coulter Award for Lifetime Achievement in Hematology. Dr. Aster, of the Medical College of Wisconsin and Versiti Blood Center of Wisconsin in Milwaukee, “will be honored for his significant contributions to hematology through research, mentorship, and education throughout his 62-year career,” according to ASH.
Dr. Aster is known for his research on immune diseases that affect blood cells, particularly platelets. His work has led to improvements in platelet preparation, storage, and matching. In addition, he and his team developed the standard techniques for diagnosing immune thrombocytopenia and heparin-induced thrombocytopenia-thrombosis.
Other ASH awardees include William Eaton, MD, PhD, and Richard A. Larson, MD, who will receive the 2019 Henry M. Stratton Medal “for their seminal contributions to basic and clinical/translational hematology research, respectively.”
Dr. Eaton, of the National Institute of Diabetes and Digestive and Kidney Diseases conducts research on sickle cell disease, and his work contributed to the development of hydroxyurea. Dr. Larson, of the University of Chicago is the namesake of the Larson regimen (CALGB 8811) for acute lymphoblastic leukemia, and he played a key role in research that led to the U.S. approval of midostaurin.
Philip Greenberg, MD, will deliver the 2019 E. Donnall Thomas Lecture in recognition of “his outstanding contributions to the field of immunotherapy.” Dr. Greenberg, of theFred Hutchinson Cancer Research Center and the University of Washington in Seattle, is known for contributing to the development of T-cell adoptive immune therapy. His lecture will focus on the engineering of T cells to target acute myeloid leukemia and other malignancies.
Sriram Krishnaswamy, PhD, and Jeffrey I. Weitz, MD, will deliver the 2019 Ernest Beutler Lecture in recognition of “their significant research contributions to the understanding and treatment of blood clots.” The researchers will each deliver one part of the lecture at the meeting, and both will discuss research related to novel anticoagulants.
Dr. Krishnaswamy, of the University of Pennsylvania and Children’s Hospital of Philadelphia, is considered an authority on the function of surface-dependent coagulation complexes. Dr. Weitz, of McMaster University in Hamilton, Ont., has conducted research that led to the development of novel anticoagulants.
Emmanuelle Passegué, PhD, will receive the 2019 William Dameshek Prize “for her outstanding contributions to the understanding of hematopoietic stem cells.” Dr. Passegué, of Columbia University Irving Medical Center in New York, conducts research focused on changes to hematopoietic stem cells in the contexts of myeloid malignancies and physiological aging.
Griffin Rodgers, MD, will receive the ASH Award for Leadership in Promoting Diversity “for his extraordinary commitment to diversity and inclusion in hematology.” As director of the National Institute of Diabetes and Digestive and Kidney Diseases, Dr. Rodgers has worked to promote diversity in the scientific workforce and in clinical trials.
Leonard Zon, MD, and Michael R. DeBaun, MD, will receive the 2019 ASH Mentor Award “for their sustained, outstanding commitment to the training and career development of early career hematologists.”
Dr. DeBaun, of Vanderbilt University School of Medicine in Nashville, Tenn., has mentees ranging from high school students with sickle cell disease to tenured faculty members at medical schools. Dr. Zon, of Harvard University and Boston Children’s Hospital, organizes mentoring events for postdocs, graduate students, and technicians.
Black-box warnings: How they can improve your clinical practice
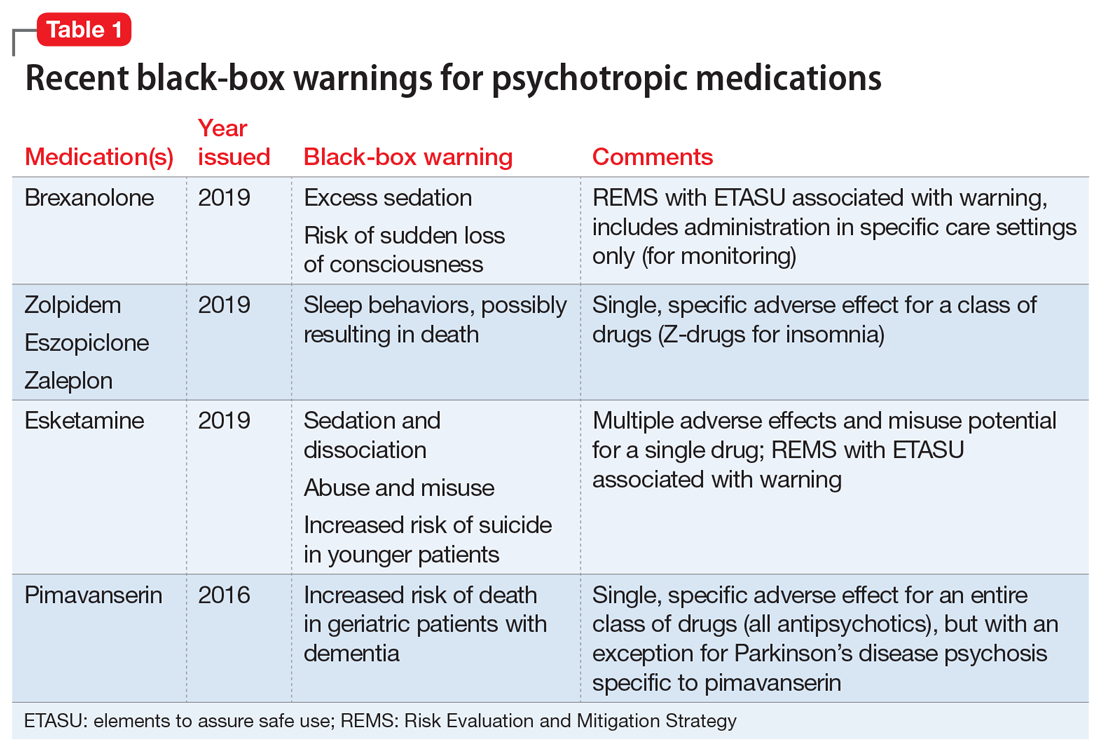
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
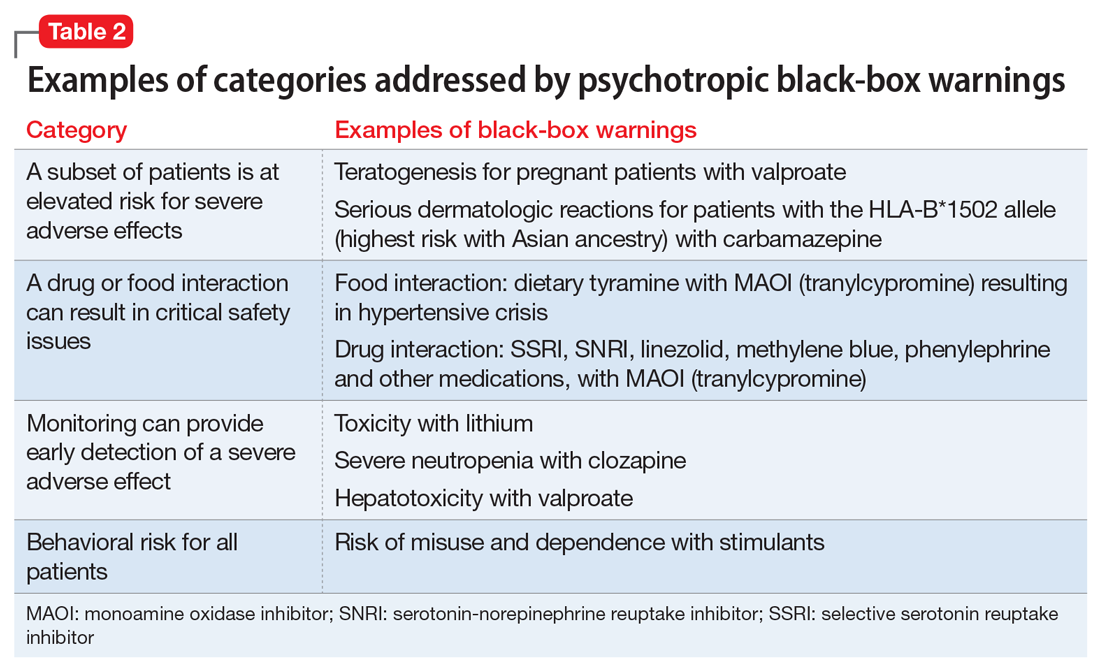
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
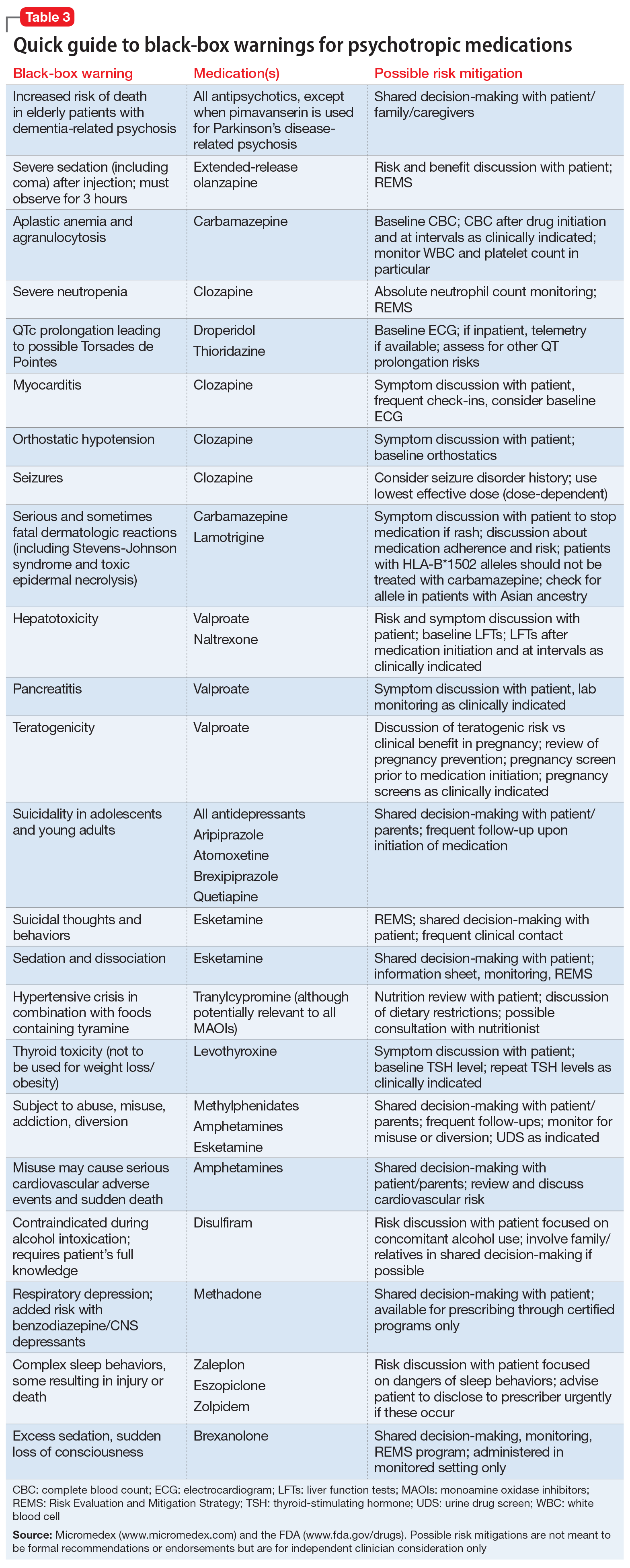
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Resignation
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at kalaycm@ccf.org.
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at kalaycm@ccf.org.
Along with resigning as chairman of the department of hematology and medical oncology at the Cleveland Clinic (Reunion), I am also resigning as editor in chief of Hematology News. In contrast to the drawn out process of choosing the next department chairman, however, I was in the enviable position of being able to hand pick my successor as editor in chief. I am proud to announce that Ifeyinwa (Ify) Osunkwo, MD, MPH, will be the new editor in chief of Hematology News. Dr. Osunkwo’s new perspective and energy will guide the further development of Hematology News for the benefit of our readers.
As editor in chief, I have had the opportunity to write essays for Hematology News that reflect my experience as a leader in an academic medical department. By doing so, I was trying to summarize some of what I learned along my career path. In my final essay, I want to direct some of these nuggets of wisdom directly to aspiring leaders who are closer to the beginning of their career journey than I am.
My junior colleagues are very interested in developing their careers to maximize opportunities in leadership, and I have coached many to try to understand that the path to leadership is not always straight, may be difficult, and does not always end comfortably. While the goal may seem to be in one direction, the path may lead to another. That is what has happened to me.
I did not seek to be Chairman. The opportunity came to me while I was busy doing other things. As I expressed in an earlier editorial (Seeking the chair), those who are diligent about their work without actively trying to rise through the leadership hierarchy are the ones who seem to rise more often.
Ambition is overrated. The ambitious find it harder to accept failure, and some degree of failure is likely. In his book “Falling Upward: A Spirituality for the Two Halves of Life,” Father Richard Rohr suggests that failure is required in order to mature from someone whose life centers on self to someone whose self centers on life.
Junior faculty tend to focus on self. They try to excel at whatever they attempt as they always have. Whether that is teaching, performing research, or treating patients, they try to be the absolute best teacher, researcher, or practitioner they can be. Many try to do all three well. Rare are those who can perfectly balance all three endeavors. Tension results, both at work and at home. Here is where failure often happens. The student disappoints, the paper is rejected, the grant isn’t funded, the patient relapses, and the family wishes you were home more. This confluence of difficulties challenges our concept of self. Maybe we aren’t perfect after all. Perhaps for the first time, failure looms.
In my experience, the usual solution to the possibility of failure is a desire to reduce patient care responsibilities. Academic faculty cherish their protected time and usually look for ways to increase it rather than to balance it (Professional time). Academic careers require thick CVs, not satisfied patients. A talk on leukemia at a major conference is more valued than talking to a patient about their leukemia. The cognitive dissonance between what we think is important and what is actually important challenges our personal sense of identity. The resulting burnout represents the necessary failure required to then mature spiritually and reprioritize our ambitions.
On some level, then, the path most of us are on is the time-honored – but painful – journey that must be traveled in order to attain peace.
I also recommend planning a career path with quality work, not a future title, as the goal. Quality work implies measurable objectives. For teachers, work could be measured by teaching scores and student accomplishments. For researchers, work could be measured by published papers, grants received, and invited lectures. For practitioners, work could be measured by outcomes, particularly patient-reported outcomes. Once work is measured, continuous improvements can be made and tracked. Highly reliable teachers, researchers, and practitioners who value quality work will be rewarded both personally and professionally (Defining high reliability).
There is a difference, however, between trying to be the best and trying to improve. The former implies competition with someone else, while the latter involves only one person. Competition can be motivating, but can also undermine interpersonal relationships while causing unhealthy behaviors like overworking and sleep deprivation. If the position sought requires selfish and destructive behaviors, it is not a position worth seeking (Rat race).
By doing quality work – not just more work – leadership positions will inevitably follow. Once a position is obtained, the work increases because a leader is now responsible for others. There are some easy-to-learn tools that can help with that responsibility. I find them very useful for helping colleagues work through interpersonal struggles and resource issues (Leadership hacks: The drama triangle; Leadership hacks: Structural tension).
Success as a leader is harder to measure, but many institutions employ engagement surveys similar to job satisfaction surveys. Leadership scores are generally accurate reflections of leader effectiveness, as are 360-degree surveys of those who work with you. Of course, being a leader also means holding those in your charge accountable for their behaviors (The white wall; Full disclosure). Leadership is no place for someone unwilling to hold crucial and difficult conversations with colleagues.
Success, of course, begets success and additional leadership roles are offered to successful leaders. Meanwhile, the work you started in order to get to the leadership position will probably need to be scaled back as excellence in teaching, research, patient care, and leadership is daunting, difficult to manage, and threatens work-life balance. The ability to say “no” is a valuable skill to learn as leadership roles increase.
Even though none of us work alone, academic medicine generally rewards only the individual. Yet, the camaraderie developed over time working together helps balance work and life roles. To advance as a leader, learning to work in a team is a critical ability. There is a science behind teamwork and aspiring leaders should acquaint themselves with it (Successful teams). While you may be rewarded as an individual, your success will be dependent on your ability to work on a team.
Finally, at least for clinicians, our obligation to our patients largely supersedes all our other commitments. Knowing the most, or being the most technically gifted, is not what patients value. They value empathy and relationships. We need to develop care designed for them, not us (Timed perfectly). We need to communicate with them on their terms, not ours (Pathologic superstition). We must walk with patients on their path, not ours. A patient-centered approach to care and career can take you far. Good luck on your journey.
Dr. Kalaycio is the outgoing editor in chief of Hematology News. He is a hematologist-oncologist at the Cleveland Clinic Taussig Cancer Institute. Contact him at kalaycm@ccf.org.
CMS announces application process for Direct Contracting model
The Centers for Medicare & Medicaid Services will be accepting letters of intent from physician practices interested in participating in the Direct Contracting model, a new payment model aimed at practices serving at least 5,000 Medicare beneficiaries.
First announced in April 2019, the Direct Contracting program is an advanced alternative payment model designed for organizations that are ready to take on more financial risk and have experience managing large populations through accountable care organizations or working with Medicare Advantage plans.
Interested practices will be able to choose from the Professional population-based payment, which has a lower risk-sharing arrangement (50% savings/losses), and the Global model, which offers a 100% savings/loss risk-sharing arrangement.
“The payment model options available under Direct Contracting aim to reduce expenditures while preserving or enhancing quality of care for beneficiaries,” agency officials said on a web page detailing information for the payment model.
The model offers a prospectively determined and predictable revenue stream for participants and aims to transform risk-sharing arrangements through capitated and partially capitated population-based payments, open up participation in alternative payment models to more physicians, and reduce clinician burden by using smaller sets of quality measures and waivers to help facilitate the delivery of care.
Letters of intent are due to the agency by Dec. 10. CMS noted that submission of a letter of intent will not bind the organization to participating in the program.
The Centers for Medicare & Medicaid Services will be accepting letters of intent from physician practices interested in participating in the Direct Contracting model, a new payment model aimed at practices serving at least 5,000 Medicare beneficiaries.
First announced in April 2019, the Direct Contracting program is an advanced alternative payment model designed for organizations that are ready to take on more financial risk and have experience managing large populations through accountable care organizations or working with Medicare Advantage plans.
Interested practices will be able to choose from the Professional population-based payment, which has a lower risk-sharing arrangement (50% savings/losses), and the Global model, which offers a 100% savings/loss risk-sharing arrangement.
“The payment model options available under Direct Contracting aim to reduce expenditures while preserving or enhancing quality of care for beneficiaries,” agency officials said on a web page detailing information for the payment model.
The model offers a prospectively determined and predictable revenue stream for participants and aims to transform risk-sharing arrangements through capitated and partially capitated population-based payments, open up participation in alternative payment models to more physicians, and reduce clinician burden by using smaller sets of quality measures and waivers to help facilitate the delivery of care.
Letters of intent are due to the agency by Dec. 10. CMS noted that submission of a letter of intent will not bind the organization to participating in the program.
The Centers for Medicare & Medicaid Services will be accepting letters of intent from physician practices interested in participating in the Direct Contracting model, a new payment model aimed at practices serving at least 5,000 Medicare beneficiaries.
First announced in April 2019, the Direct Contracting program is an advanced alternative payment model designed for organizations that are ready to take on more financial risk and have experience managing large populations through accountable care organizations or working with Medicare Advantage plans.
Interested practices will be able to choose from the Professional population-based payment, which has a lower risk-sharing arrangement (50% savings/losses), and the Global model, which offers a 100% savings/loss risk-sharing arrangement.
“The payment model options available under Direct Contracting aim to reduce expenditures while preserving or enhancing quality of care for beneficiaries,” agency officials said on a web page detailing information for the payment model.
The model offers a prospectively determined and predictable revenue stream for participants and aims to transform risk-sharing arrangements through capitated and partially capitated population-based payments, open up participation in alternative payment models to more physicians, and reduce clinician burden by using smaller sets of quality measures and waivers to help facilitate the delivery of care.
Letters of intent are due to the agency by Dec. 10. CMS noted that submission of a letter of intent will not bind the organization to participating in the program.
ACGME deepening its commitment to physician well-being, leader says
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
EXPERT ANALYSIS AT AAP 2019
Is there a (robotic) doctor in the house?
In the 2012 movie “Robot and Frank,” an aging ex-jewel thief named Frank receives a robotic home assistant from his well-meaning son. Frank lives alone and suffers from dementia, and his son hopes that the friendly electronic companion will help keep his father safe, assisting him with housework and improving his cognitive health. Frank initially rejects the idea but changes his mind when he realizes the robot’s talents aren’t limited to domestic chores. He begins teaching the robot new skills, and an unlikely partnership develops. With Frank’s penchant for pilfering and the robot’s digital dexterity, the two of them pull off a multimillion-dollar jewelry heist – and Frank’s outlook improves in ways his son never dreamed possible!
“Robot and Frank” takes place “in the near future,” and while we don’t yet have robotic home companions as capable as the one in the movie, we need not look very far to realize that robotics and artificial intelligence may revolutionize the delivery of health care.
With an aging population and an industry shift toward value-based care, new research has focused on novel ways of avoiding hospitalization and reducing hospital readmission. We have seen a resurgence of home visits and the development of telemedicine and remote monitoring.
To stay healthy, patients need to be safe in their home environment and at a minimum need to be able to navigate their activities of daily living. Research published last year by Washington University’s Center for Advanced Studies in Adaptive Systems (CASAS) describes a technology that aims to help patients in their own homes.
The Robot Activity Support system, or RAS, interacts with intelligent sensors in a home environment “to detect and assist with activity errors that may occur in everyday settings.”1 If sensors in the home indicate that a person is experiencing difficulty completing a certain task such as taking a medication or finding a bathroom, a robot can navigate to the person in need and show an instructional video, or lead the patient to the next step in the process.
Another manufacturer is taking a ‘softer’ approach to activity support in the elderly. Toymaker Hasbro has developed a line of robotic cats that provide companionship and comfort. While currently limited to tactile stimulation and simple responses, the manufacturer is working in collaboration with researchers at Brown University to add artificial intelligence capabilities. The goal of the program – Project ARIES (Affordable Robotic Intelligence for Elderly Support) – is to give the cats useful skills such as being able to provide medication and safety reminders while keeping their price point accessible to all.
Other organizations are attempting to take the robotic home health aide idea to the next level. “RUDY,” a robotic companion developed by INF Robotics, is capable of much more than just educating patients and leading them around the house. About the size of small child and wearing a huge smile, Rudy can detect falls, ensure medication adherence, provide social interaction, and even offer remote patient monitoring. According to the manufacturer, it uses natural language processing, machine learning, and a smart social interface to “facilitate trusting relationships between RUDY and older adults.” The idea is to promote acceptance from patients and offer peace of mind to their loved ones. This can be particularly valuable in assisting those with dementia, as a heavy emphasis is placed on socialization and maintaining cognitive stimulation.
Instead of developing novel artificial intelligence platforms, many groups are attempting to leverage existing technologies to assist patients in their homes. One such technology is Amazon’s Echo smart speakers, which became more attractive to health care providers on April 4 of this year with the launch of the Alexa Healthcare Skills Kit. This is Amazon’s HIPAA-compliant application programming interface (API) that allows developers to create ‘skills’ (apps for Echo devices) that can securely handle protected health information.
At launch, Amazon announced six partner organizations who have already written skills for patients. One organization, Boston Children’s Hospital, developed a skill called My Children’s Enhanced Recovery After Surgery (ERAS). According to John Brownstein, the hospital’s Chief Innovation Officer, it “allows patients and caregivers to easily share recovery progress with their care team post surgery ... it is just one example of how voice technology can extend the care and support of our patients beyond the four walls of the hospital.”
Some companies, such as HealthTap, have been working on artificial intelligence to build a platform to allow physicians and patients to interact online. HealthTap is leveraging the wisdom of those interactions to power a deep learning system called Dr. A.I. Available for Alexa and mobile devices, it attempts to assess patients’ symptoms and provide personalized medical explanations and health recommendations. In the developer’s own words: “Dr. A.I. engages with you in an empathetic conversation about your symptoms and overall health ... then gives you appropriate doctor-recommended insights as well as the best possible courses of action you can take on the road to feeling good.”
Some physicians may find this movement troubling, but we believe it represents an early glimpse of what is to come. with no shortage of companies stepping up to meet the demand. While it’s doubtful the robots they create can be easily reprogrammed to steal jewelry, it won’t stop them from trying to steal our jobs. We as physicians will need to continue to hone our skills in compassion and empathy to provide something a computer never can: true care for our patients.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
1. Robot-enabled support of daily activities in smart home environments. Cogn Syst Res. 2019 May. doi: 10.1016/j.cogsys.2018.10.032.
In the 2012 movie “Robot and Frank,” an aging ex-jewel thief named Frank receives a robotic home assistant from his well-meaning son. Frank lives alone and suffers from dementia, and his son hopes that the friendly electronic companion will help keep his father safe, assisting him with housework and improving his cognitive health. Frank initially rejects the idea but changes his mind when he realizes the robot’s talents aren’t limited to domestic chores. He begins teaching the robot new skills, and an unlikely partnership develops. With Frank’s penchant for pilfering and the robot’s digital dexterity, the two of them pull off a multimillion-dollar jewelry heist – and Frank’s outlook improves in ways his son never dreamed possible!
“Robot and Frank” takes place “in the near future,” and while we don’t yet have robotic home companions as capable as the one in the movie, we need not look very far to realize that robotics and artificial intelligence may revolutionize the delivery of health care.
With an aging population and an industry shift toward value-based care, new research has focused on novel ways of avoiding hospitalization and reducing hospital readmission. We have seen a resurgence of home visits and the development of telemedicine and remote monitoring.
To stay healthy, patients need to be safe in their home environment and at a minimum need to be able to navigate their activities of daily living. Research published last year by Washington University’s Center for Advanced Studies in Adaptive Systems (CASAS) describes a technology that aims to help patients in their own homes.
The Robot Activity Support system, or RAS, interacts with intelligent sensors in a home environment “to detect and assist with activity errors that may occur in everyday settings.”1 If sensors in the home indicate that a person is experiencing difficulty completing a certain task such as taking a medication or finding a bathroom, a robot can navigate to the person in need and show an instructional video, or lead the patient to the next step in the process.
Another manufacturer is taking a ‘softer’ approach to activity support in the elderly. Toymaker Hasbro has developed a line of robotic cats that provide companionship and comfort. While currently limited to tactile stimulation and simple responses, the manufacturer is working in collaboration with researchers at Brown University to add artificial intelligence capabilities. The goal of the program – Project ARIES (Affordable Robotic Intelligence for Elderly Support) – is to give the cats useful skills such as being able to provide medication and safety reminders while keeping their price point accessible to all.
Other organizations are attempting to take the robotic home health aide idea to the next level. “RUDY,” a robotic companion developed by INF Robotics, is capable of much more than just educating patients and leading them around the house. About the size of small child and wearing a huge smile, Rudy can detect falls, ensure medication adherence, provide social interaction, and even offer remote patient monitoring. According to the manufacturer, it uses natural language processing, machine learning, and a smart social interface to “facilitate trusting relationships between RUDY and older adults.” The idea is to promote acceptance from patients and offer peace of mind to their loved ones. This can be particularly valuable in assisting those with dementia, as a heavy emphasis is placed on socialization and maintaining cognitive stimulation.
Instead of developing novel artificial intelligence platforms, many groups are attempting to leverage existing technologies to assist patients in their homes. One such technology is Amazon’s Echo smart speakers, which became more attractive to health care providers on April 4 of this year with the launch of the Alexa Healthcare Skills Kit. This is Amazon’s HIPAA-compliant application programming interface (API) that allows developers to create ‘skills’ (apps for Echo devices) that can securely handle protected health information.
At launch, Amazon announced six partner organizations who have already written skills for patients. One organization, Boston Children’s Hospital, developed a skill called My Children’s Enhanced Recovery After Surgery (ERAS). According to John Brownstein, the hospital’s Chief Innovation Officer, it “allows patients and caregivers to easily share recovery progress with their care team post surgery ... it is just one example of how voice technology can extend the care and support of our patients beyond the four walls of the hospital.”
Some companies, such as HealthTap, have been working on artificial intelligence to build a platform to allow physicians and patients to interact online. HealthTap is leveraging the wisdom of those interactions to power a deep learning system called Dr. A.I. Available for Alexa and mobile devices, it attempts to assess patients’ symptoms and provide personalized medical explanations and health recommendations. In the developer’s own words: “Dr. A.I. engages with you in an empathetic conversation about your symptoms and overall health ... then gives you appropriate doctor-recommended insights as well as the best possible courses of action you can take on the road to feeling good.”
Some physicians may find this movement troubling, but we believe it represents an early glimpse of what is to come. with no shortage of companies stepping up to meet the demand. While it’s doubtful the robots they create can be easily reprogrammed to steal jewelry, it won’t stop them from trying to steal our jobs. We as physicians will need to continue to hone our skills in compassion and empathy to provide something a computer never can: true care for our patients.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
1. Robot-enabled support of daily activities in smart home environments. Cogn Syst Res. 2019 May. doi: 10.1016/j.cogsys.2018.10.032.
In the 2012 movie “Robot and Frank,” an aging ex-jewel thief named Frank receives a robotic home assistant from his well-meaning son. Frank lives alone and suffers from dementia, and his son hopes that the friendly electronic companion will help keep his father safe, assisting him with housework and improving his cognitive health. Frank initially rejects the idea but changes his mind when he realizes the robot’s talents aren’t limited to domestic chores. He begins teaching the robot new skills, and an unlikely partnership develops. With Frank’s penchant for pilfering and the robot’s digital dexterity, the two of them pull off a multimillion-dollar jewelry heist – and Frank’s outlook improves in ways his son never dreamed possible!
“Robot and Frank” takes place “in the near future,” and while we don’t yet have robotic home companions as capable as the one in the movie, we need not look very far to realize that robotics and artificial intelligence may revolutionize the delivery of health care.
With an aging population and an industry shift toward value-based care, new research has focused on novel ways of avoiding hospitalization and reducing hospital readmission. We have seen a resurgence of home visits and the development of telemedicine and remote monitoring.
To stay healthy, patients need to be safe in their home environment and at a minimum need to be able to navigate their activities of daily living. Research published last year by Washington University’s Center for Advanced Studies in Adaptive Systems (CASAS) describes a technology that aims to help patients in their own homes.
The Robot Activity Support system, or RAS, interacts with intelligent sensors in a home environment “to detect and assist with activity errors that may occur in everyday settings.”1 If sensors in the home indicate that a person is experiencing difficulty completing a certain task such as taking a medication or finding a bathroom, a robot can navigate to the person in need and show an instructional video, or lead the patient to the next step in the process.
Another manufacturer is taking a ‘softer’ approach to activity support in the elderly. Toymaker Hasbro has developed a line of robotic cats that provide companionship and comfort. While currently limited to tactile stimulation and simple responses, the manufacturer is working in collaboration with researchers at Brown University to add artificial intelligence capabilities. The goal of the program – Project ARIES (Affordable Robotic Intelligence for Elderly Support) – is to give the cats useful skills such as being able to provide medication and safety reminders while keeping their price point accessible to all.
Other organizations are attempting to take the robotic home health aide idea to the next level. “RUDY,” a robotic companion developed by INF Robotics, is capable of much more than just educating patients and leading them around the house. About the size of small child and wearing a huge smile, Rudy can detect falls, ensure medication adherence, provide social interaction, and even offer remote patient monitoring. According to the manufacturer, it uses natural language processing, machine learning, and a smart social interface to “facilitate trusting relationships between RUDY and older adults.” The idea is to promote acceptance from patients and offer peace of mind to their loved ones. This can be particularly valuable in assisting those with dementia, as a heavy emphasis is placed on socialization and maintaining cognitive stimulation.
Instead of developing novel artificial intelligence platforms, many groups are attempting to leverage existing technologies to assist patients in their homes. One such technology is Amazon’s Echo smart speakers, which became more attractive to health care providers on April 4 of this year with the launch of the Alexa Healthcare Skills Kit. This is Amazon’s HIPAA-compliant application programming interface (API) that allows developers to create ‘skills’ (apps for Echo devices) that can securely handle protected health information.
At launch, Amazon announced six partner organizations who have already written skills for patients. One organization, Boston Children’s Hospital, developed a skill called My Children’s Enhanced Recovery After Surgery (ERAS). According to John Brownstein, the hospital’s Chief Innovation Officer, it “allows patients and caregivers to easily share recovery progress with their care team post surgery ... it is just one example of how voice technology can extend the care and support of our patients beyond the four walls of the hospital.”
Some companies, such as HealthTap, have been working on artificial intelligence to build a platform to allow physicians and patients to interact online. HealthTap is leveraging the wisdom of those interactions to power a deep learning system called Dr. A.I. Available for Alexa and mobile devices, it attempts to assess patients’ symptoms and provide personalized medical explanations and health recommendations. In the developer’s own words: “Dr. A.I. engages with you in an empathetic conversation about your symptoms and overall health ... then gives you appropriate doctor-recommended insights as well as the best possible courses of action you can take on the road to feeling good.”
Some physicians may find this movement troubling, but we believe it represents an early glimpse of what is to come. with no shortage of companies stepping up to meet the demand. While it’s doubtful the robots they create can be easily reprogrammed to steal jewelry, it won’t stop them from trying to steal our jobs. We as physicians will need to continue to hone our skills in compassion and empathy to provide something a computer never can: true care for our patients.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
1. Robot-enabled support of daily activities in smart home environments. Cogn Syst Res. 2019 May. doi: 10.1016/j.cogsys.2018.10.032.
ASCO releases revised version of its Patient-Centered Oncology Payment model
The American Society of Clinical Oncology has made some adjustments to its Patient-Centered Oncology Payment (PCOP) advanced alternative payment model and will now be looking to gain approval from the Physician-Focused Payment Model Technical Advisory Committee (PTAC).
PTAC reviews physician-developed advanced alternative payment models and sends those APMs that are approved to the Centers for Medicare & Medicaid Services’ Center for Medicare & Medicaid Innovation (CMMI) to determine if there will be further testing and possible implementation for use by physicians as part of the APM track of the Quality Payment Program.
Even if approved by PTAC, the ASCO model faces an uphill battle. CMS already has its own Oncology Care Model (OCM), and given the agency’s track record of not testing APMs that have been approved by PTAC, the deck may be stacked against ASCO in terms of getting its model into Medicare.
But officials at ASCO are hoping a PTAC vetting and approval will open the door to its implementation by commercial payers.
“We are now ready to take this to PTAC,” Jeffery Ward, MD, past chair of ASCO’s government relations committee, said in an interview. “I don’t expect any trouble getting through PTAC. The other question is whether CMMI would decide to actually test a model like this, and so far, they have seemed pretty much a one-trick pony, as in they’ve got their Oncology Care Model and they are going to run with it.”
However, Dr. Ward, a medical oncologist at Swedish Cancer Institute of Edmonds, Wash., said that PCOP has been designed with the commercial market in mind and the PTAC recommendation will help validate the model and make it more appealing to commercial payers.
A key feature of the adjusted payment model is that it has taken a lesson learned from CMS’s Oncology Care Model in terms of how to account for the price of drugs. Including the price of drugs in a value-based payment model – the approach taken by the OCM – creates too much variability, Dr. Ward noted. He used lung cancer as an example, noting that the type of lung cancer could have more of an impact on physician spending than any specific treatment decisions a physician can make because the treatment choices may not be there.
“When that happens, whether you are successful or not depends on the luck of the draw instead of the choices you make,” he said.
In an effort not to penalize practices because of their patient mix, PCOP takes the cost of drugs out of the value mix.
“What this model does is it doesn’t hold practices responsible for the cost of the drugs. It holds them responsible for how they utilize the drugs,” he said. “It really begins to make me responsible for making value-based decisions as opposed to just getting lucky and having a cheaper panel of patients.”
Overall, Dr. Ward described the PCOP model as accomplishing the goals of CMS’s APMs by encouraging physicians to treat patients through high-value treatments.
“This model does that by including quality measures that play a role in how much you get paid,” he said. “There are pathways that are value-based pathways and your ability to follow those pathways plays a role in how much you get paid. The amount of care that you send to the ER or the hospital plays a role in how much you get paid. Those things are then used in adjusting what is our fee-for-service model of medicine. So you are still getting paid a base rate based on the care that you give on a fee-for-service model, but that gets adjusted up or down depending on those other characteristics.”
But for the model to have the biggest impact, it needs to be adopted by all payers in a given state or region and used by all physicians, who by the design of the payment model, will have a role in shaping the implementation details.
Savings could come from lower administrative costs at the physician office because there would be no need for separate contracts between payers, something that could generate savings of up to 8%, compared with the current system, Dr. Ward noted.
“This is designed to be a multipayer and multipractice initiative,” he said. “In its grandest form, a state or states would implement this and it would be participated in by all the oncology practices and all of the payers.”
The American Society of Clinical Oncology has made some adjustments to its Patient-Centered Oncology Payment (PCOP) advanced alternative payment model and will now be looking to gain approval from the Physician-Focused Payment Model Technical Advisory Committee (PTAC).
PTAC reviews physician-developed advanced alternative payment models and sends those APMs that are approved to the Centers for Medicare & Medicaid Services’ Center for Medicare & Medicaid Innovation (CMMI) to determine if there will be further testing and possible implementation for use by physicians as part of the APM track of the Quality Payment Program.
Even if approved by PTAC, the ASCO model faces an uphill battle. CMS already has its own Oncology Care Model (OCM), and given the agency’s track record of not testing APMs that have been approved by PTAC, the deck may be stacked against ASCO in terms of getting its model into Medicare.
But officials at ASCO are hoping a PTAC vetting and approval will open the door to its implementation by commercial payers.
“We are now ready to take this to PTAC,” Jeffery Ward, MD, past chair of ASCO’s government relations committee, said in an interview. “I don’t expect any trouble getting through PTAC. The other question is whether CMMI would decide to actually test a model like this, and so far, they have seemed pretty much a one-trick pony, as in they’ve got their Oncology Care Model and they are going to run with it.”
However, Dr. Ward, a medical oncologist at Swedish Cancer Institute of Edmonds, Wash., said that PCOP has been designed with the commercial market in mind and the PTAC recommendation will help validate the model and make it more appealing to commercial payers.
A key feature of the adjusted payment model is that it has taken a lesson learned from CMS’s Oncology Care Model in terms of how to account for the price of drugs. Including the price of drugs in a value-based payment model – the approach taken by the OCM – creates too much variability, Dr. Ward noted. He used lung cancer as an example, noting that the type of lung cancer could have more of an impact on physician spending than any specific treatment decisions a physician can make because the treatment choices may not be there.
“When that happens, whether you are successful or not depends on the luck of the draw instead of the choices you make,” he said.
In an effort not to penalize practices because of their patient mix, PCOP takes the cost of drugs out of the value mix.
“What this model does is it doesn’t hold practices responsible for the cost of the drugs. It holds them responsible for how they utilize the drugs,” he said. “It really begins to make me responsible for making value-based decisions as opposed to just getting lucky and having a cheaper panel of patients.”
Overall, Dr. Ward described the PCOP model as accomplishing the goals of CMS’s APMs by encouraging physicians to treat patients through high-value treatments.
“This model does that by including quality measures that play a role in how much you get paid,” he said. “There are pathways that are value-based pathways and your ability to follow those pathways plays a role in how much you get paid. The amount of care that you send to the ER or the hospital plays a role in how much you get paid. Those things are then used in adjusting what is our fee-for-service model of medicine. So you are still getting paid a base rate based on the care that you give on a fee-for-service model, but that gets adjusted up or down depending on those other characteristics.”
But for the model to have the biggest impact, it needs to be adopted by all payers in a given state or region and used by all physicians, who by the design of the payment model, will have a role in shaping the implementation details.
Savings could come from lower administrative costs at the physician office because there would be no need for separate contracts between payers, something that could generate savings of up to 8%, compared with the current system, Dr. Ward noted.
“This is designed to be a multipayer and multipractice initiative,” he said. “In its grandest form, a state or states would implement this and it would be participated in by all the oncology practices and all of the payers.”
The American Society of Clinical Oncology has made some adjustments to its Patient-Centered Oncology Payment (PCOP) advanced alternative payment model and will now be looking to gain approval from the Physician-Focused Payment Model Technical Advisory Committee (PTAC).
PTAC reviews physician-developed advanced alternative payment models and sends those APMs that are approved to the Centers for Medicare & Medicaid Services’ Center for Medicare & Medicaid Innovation (CMMI) to determine if there will be further testing and possible implementation for use by physicians as part of the APM track of the Quality Payment Program.
Even if approved by PTAC, the ASCO model faces an uphill battle. CMS already has its own Oncology Care Model (OCM), and given the agency’s track record of not testing APMs that have been approved by PTAC, the deck may be stacked against ASCO in terms of getting its model into Medicare.
But officials at ASCO are hoping a PTAC vetting and approval will open the door to its implementation by commercial payers.
“We are now ready to take this to PTAC,” Jeffery Ward, MD, past chair of ASCO’s government relations committee, said in an interview. “I don’t expect any trouble getting through PTAC. The other question is whether CMMI would decide to actually test a model like this, and so far, they have seemed pretty much a one-trick pony, as in they’ve got their Oncology Care Model and they are going to run with it.”
However, Dr. Ward, a medical oncologist at Swedish Cancer Institute of Edmonds, Wash., said that PCOP has been designed with the commercial market in mind and the PTAC recommendation will help validate the model and make it more appealing to commercial payers.
A key feature of the adjusted payment model is that it has taken a lesson learned from CMS’s Oncology Care Model in terms of how to account for the price of drugs. Including the price of drugs in a value-based payment model – the approach taken by the OCM – creates too much variability, Dr. Ward noted. He used lung cancer as an example, noting that the type of lung cancer could have more of an impact on physician spending than any specific treatment decisions a physician can make because the treatment choices may not be there.
“When that happens, whether you are successful or not depends on the luck of the draw instead of the choices you make,” he said.
In an effort not to penalize practices because of their patient mix, PCOP takes the cost of drugs out of the value mix.
“What this model does is it doesn’t hold practices responsible for the cost of the drugs. It holds them responsible for how they utilize the drugs,” he said. “It really begins to make me responsible for making value-based decisions as opposed to just getting lucky and having a cheaper panel of patients.”
Overall, Dr. Ward described the PCOP model as accomplishing the goals of CMS’s APMs by encouraging physicians to treat patients through high-value treatments.
“This model does that by including quality measures that play a role in how much you get paid,” he said. “There are pathways that are value-based pathways and your ability to follow those pathways plays a role in how much you get paid. The amount of care that you send to the ER or the hospital plays a role in how much you get paid. Those things are then used in adjusting what is our fee-for-service model of medicine. So you are still getting paid a base rate based on the care that you give on a fee-for-service model, but that gets adjusted up or down depending on those other characteristics.”
But for the model to have the biggest impact, it needs to be adopted by all payers in a given state or region and used by all physicians, who by the design of the payment model, will have a role in shaping the implementation details.
Savings could come from lower administrative costs at the physician office because there would be no need for separate contracts between payers, something that could generate savings of up to 8%, compared with the current system, Dr. Ward noted.
“This is designed to be a multipayer and multipractice initiative,” he said. “In its grandest form, a state or states would implement this and it would be participated in by all the oncology practices and all of the payers.”