User login
Independent vascular surgery board called key to self-determination
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
NEW YORK – Numerous experts, including a past president of the Society for Clinical Vascular Surgery, lined up at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation to explain why there is a critical need for a separate vascular surgery board with membership on the American Board of Medical Specialties.
Not least of the reasons is, “We need to control our destiny by training more residents and fellows in vascular surgery,” said Alan M. Dietzek, MD, who served as SCVS president in 2018 and is an active vascular surgeon in Danbury, Conn.
According to Dr. Dietzek, the American Board of Surgeons recently rejected an application for a vascular resident review committee (RRC), which develops and controls training within a specialty.
“A vascular surgery RRC would facilitate more applications [for training] as well as innovations in specialized programs, which we could certainly use in aortic and venous fellowships,” Dr. Dietzek said. “All ABMS-recognized boards have their own RRC.”
Other speakers, including Timothy M. Sullivan, MD, a professor of surgery at the University of Minnesota, Minneapolis, made the same point. He also believes that a vascular surgery RRC is pivotal in establishing recognition for the specialty and what it offers.
From his perspective, O. William Brown, MD, a vascular surgeon from Bingham Falls, Minn., believes that creating a vascular surgery board will increase recognition in general. Like the others, he maintained that an independent board could draw attention to the specific skills of vascular surgeons, creating a basis for attracting patients, advocating for their needs, and lobbying for resources.
Many experts, including Dr. Brown, believe that the specialty of vascular surgery already meets the qualifications for creating an independent board. However, Dr. Dietzek said that membership in ABMS is dependent on support from the Society of Vascular Surgeons, “and we don’t have that yet.”
In the title of his talk on creating a vascular surgery board, Dr. Brown called for the SVS Executive Committee to “recognize this need and go after it with full force.”
Dr. Dietzek believes the SVS should survey the membership. If there is support for an independent board, it should move ahead with the appropriate support.
“Can we afford it? Other small boards have done just fine,” said Dr. Dietzek, citing the American Board of Colorectal Surgery and the American Board of Thoracic Surgery. He said both are doing well financially, and he provided estimates suggesting that a vascular surgery board would also achieve firm financial footing.
The value of an independent board in exercising control over training programs is part of a larger issue of self-determination, according to Dr. Dietzek. For example, vascular surgeons have “little or no control over the priorities or the budget” at most institutions where they work. An established and recognized vascular surgery board could help these specialists define their identity and separate from other surgical specialties to create their own divisions or departments.
Others who spoke on this topic agreed. Many expressed concern about marginalization by hospital administrators who are often unclear on what vascular surgeons do. A vascular surgery board has the potential to provide a degree of stature that is now lacking.
“We need to build relationships with hospital administrators, politicians, and the insurance industry. This is critical,” Dr. Dietzek said. He believes a vascular surgery board offers an opportunity to achieve these goals and “help us control our own destiny.”
Dr. Dietzek and the other participants report no financial conflicts of interest relevant to this topic.
REPORTING FROM VEITHsymposium
Open enrollment 2020: Activity down on Healthcare.gov
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.
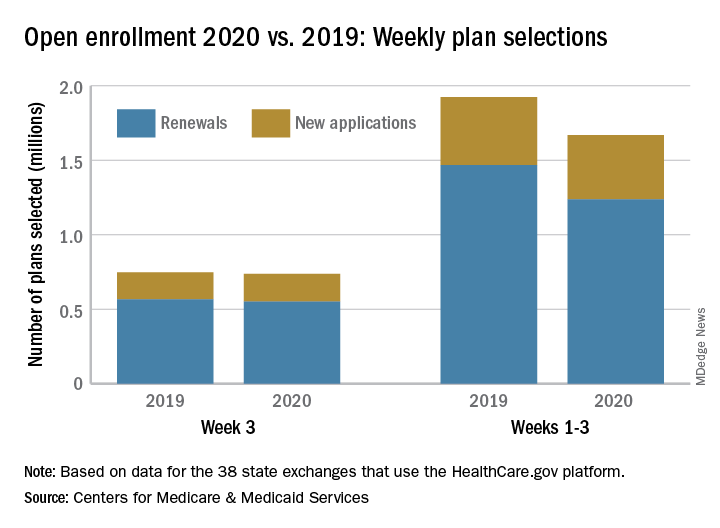
CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.

CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.

CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
Vaping front and center at Hahn’s first FDA confirmation hearing
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
REPORTING FROM A SENATE SUBCOMMITTEE HEARING
Telehealth consults for vascular surgery reimbursed at par with office visits
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
NEW YORK – Telehealth should be embraced by vascular surgeons for their own self-interest independent of the evidence that it is well accepted and more convenient for patients, according to an update on an evolution that is already underway.
“One of the great advantages of telehealth is the efficacy of time for the clinician,” John W. Hallett, MD, professor of vascular surgery at the Medical University of South Carolina, Charleston, said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
This efficiency is purchased with no loss of revenue, he added. He said that many clinicians are unaware of the opportunity this affords.
“Almost every payer reimburses telehealth visit at the same rate as that of an office visit,” Dr. Hallett explained. The only additional step is adding a “GT” modifier when billing Medicare or a “95” modifier when billing private payers.
Telemedicine is not a new concept. Published studies date back decades, but this interaction is increasingly understood to be the future. Along with an increasing array of sensors employing smartphone technology to allow physicians remote access to vital signs and other clinical data, patient attitudes have changed.
“Patients like telemedicine. It is convenient for them,” said Dr. Hallett, who noted that many providers are recognizing telemedicine as a potential marketing tool.
“On my way in from the airport yesterday, there was an advertisement for telemedicine from NYU on the television in the cab,” said Dr. Hallett, referring to the New York University health system.
The data supporting the benefits of telemedicine even include studies undertaken in vascular surgery patients. In one recent retrospective study cited by Dr. Hallett, substantial time and travel costs were saved for every vascular surgery consult conducted by telemedicine rather than in an office visit (Paquette S et al. Ann Vasc Surg. 2019;59:167-172).
“There was no difference in the rate of complications, and 94% of the patients considered the telehealth consultation adequate,” Dr. Hallett said.
He said there is urgency for vascular surgeons to pursue telemedicine. With the number of individuals over the age of 65 growing by thousands in the United States every day, there will be increasing pressure on the relatively fixed pool of vascular surgeons to improve their efficiency.
In addition, telemedicine is coming whether vascular surgeons like it or not.
“Patients are becoming more interested in looking at an app on their smartphone than coming to the office,” said Tony S. Das, MD, an interventional cardiologist who practices in Dallas. Dr. Das also spoke about the value of telemedicine for the vascular and cardiovascular surgeon at the VIETHsymposium.
In his overview, Dr. Das spoke about telehealth in the context of the estimated $12 billion dollars that will be spent on digital health in vascular medicine by 2021. The growth in digital health in vascular medicine is a reflection of a global change in clinical care. According to Dr. Das, there were more than 600 vendors of wearable sensors to monitor disease and health at a recent consumer electronics convention.
“This technology is here to stay,” said Dr. Das, who, appropriately, was not present at the symposium but delivered his presentation remotely.
Both the Centers for Medicare and Medicaid Services and the Food and Drug Administration have digital health action plans, according to Dr. Das. The CMS has already developed reimbursement codes to pay for remote monitoring services and more are expected.
Calling this type of telehealth “untethered vascular care,” Dr. Das agreed with Dr. Hallett that an evolution is coming whether vascular surgeons choose to get on board now or are forced to take action later.
SOURCE: VIETHsymposium
REPORTING FROM VIETH SYMPOSIUM
Frontline ibrutinib saves money over chemoimmunotherapy
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Health policy Q&A: Oncology Care Model
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
Inpatient care declining among family physicians
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.
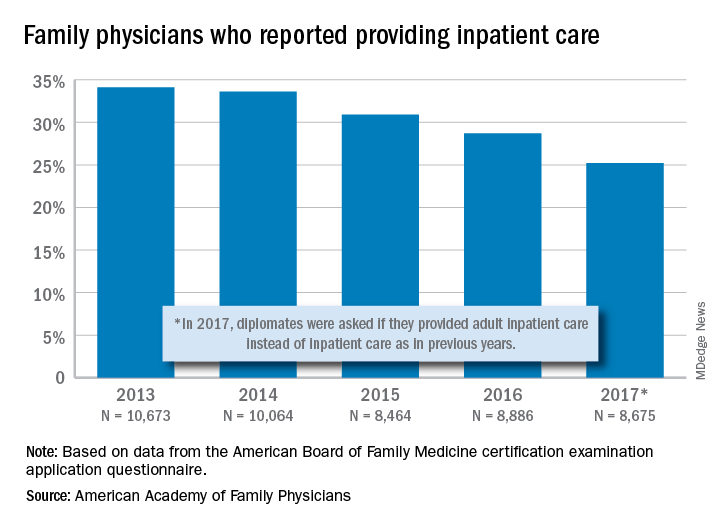
The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.

The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.

The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
Rand analysis of proposed Medicare buy-in uncovers surprising findings
Early buy-in by people aged 50-64 might raise insurance premiums
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
Early buy-in by people aged 50-64 might raise insurance premiums
Early buy-in by people aged 50-64 might raise insurance premiums
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
Cleveland Clinic grants award, welcomes new talent
Brian Bolwell, MD, chairman of Cleveland Clinic Taussig Cancer Institute in Ohio, has received the 2019 Alfred and Norma Lerner Humanitarian Award.
Dr. Bolwell received the award “in honor of his 32 years of compassionate service as a physician leader and educator, along with his contributions to clinical and translational cancer research throughout the world,” according to a statement from Cleveland Clinic. The award recognizes “Cleveland Clinic physicians whose selfless dedication, boundless compassion, and tireless work have made the most profound and singular contribution to the good of humankind.”
In addition to serving as chairman of Taussig Cancer Institute, Dr. Bolwell is a professor of medicine for Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and deputy director of the Case Comprehensive Cancer Center. He was director of the Cleveland Clinic bone marrow transplant program for 24 years and chairman of the department of hematologic oncology & blood disorders from 2006 to 2011.
In other Cleveland Clinic news, Halle Moore, MD, has been named director of breast medical oncology and codirector for the comprehensive breast cancer program at the clinic. She will assume this role in January 2020.
Dr. Moore is currently a staff physician at Taussig Cancer Institute and an associate professor of medicine at Lerner College of Medicine. Her clinical expertise is the management of breast cancer, and her research is focused on breast cancer treatment and cancer survivorship.
Cleveland Clinic has also added four new staff physicians to the department of hematology and medical oncology.
Shilpa Gupta, MD, has joined the clinic’s genitourinary cancer program. Dr. Gupta previously worked in the division of hematology, oncology, and transplantation at the University of Minnesota in Minneapolis, where she led the interdisciplinary solid tumor phase 1 program .
Khaled Hassan, MD, has joined the thoracic cancer program at Cleveland Clinic. Dr. Hassan was previously an assistant professor in the department of internal medicine, division of hematology/oncology at the University of Michigan in Ann Arbor. Suneel Kamath, MD, has joined the gastrointestinal cancer program at the clinic. Dr. Kamath was previously the hematology and medical oncology chief fellow at Northwestern University in Evanston, Ill.
Brian Bolwell, MD, chairman of Cleveland Clinic Taussig Cancer Institute in Ohio, has received the 2019 Alfred and Norma Lerner Humanitarian Award.
Dr. Bolwell received the award “in honor of his 32 years of compassionate service as a physician leader and educator, along with his contributions to clinical and translational cancer research throughout the world,” according to a statement from Cleveland Clinic. The award recognizes “Cleveland Clinic physicians whose selfless dedication, boundless compassion, and tireless work have made the most profound and singular contribution to the good of humankind.”
In addition to serving as chairman of Taussig Cancer Institute, Dr. Bolwell is a professor of medicine for Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and deputy director of the Case Comprehensive Cancer Center. He was director of the Cleveland Clinic bone marrow transplant program for 24 years and chairman of the department of hematologic oncology & blood disorders from 2006 to 2011.
In other Cleveland Clinic news, Halle Moore, MD, has been named director of breast medical oncology and codirector for the comprehensive breast cancer program at the clinic. She will assume this role in January 2020.
Dr. Moore is currently a staff physician at Taussig Cancer Institute and an associate professor of medicine at Lerner College of Medicine. Her clinical expertise is the management of breast cancer, and her research is focused on breast cancer treatment and cancer survivorship.
Cleveland Clinic has also added four new staff physicians to the department of hematology and medical oncology.
Shilpa Gupta, MD, has joined the clinic’s genitourinary cancer program. Dr. Gupta previously worked in the division of hematology, oncology, and transplantation at the University of Minnesota in Minneapolis, where she led the interdisciplinary solid tumor phase 1 program .
Khaled Hassan, MD, has joined the thoracic cancer program at Cleveland Clinic. Dr. Hassan was previously an assistant professor in the department of internal medicine, division of hematology/oncology at the University of Michigan in Ann Arbor. Suneel Kamath, MD, has joined the gastrointestinal cancer program at the clinic. Dr. Kamath was previously the hematology and medical oncology chief fellow at Northwestern University in Evanston, Ill.
Brian Bolwell, MD, chairman of Cleveland Clinic Taussig Cancer Institute in Ohio, has received the 2019 Alfred and Norma Lerner Humanitarian Award.
Dr. Bolwell received the award “in honor of his 32 years of compassionate service as a physician leader and educator, along with his contributions to clinical and translational cancer research throughout the world,” according to a statement from Cleveland Clinic. The award recognizes “Cleveland Clinic physicians whose selfless dedication, boundless compassion, and tireless work have made the most profound and singular contribution to the good of humankind.”
In addition to serving as chairman of Taussig Cancer Institute, Dr. Bolwell is a professor of medicine for Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and deputy director of the Case Comprehensive Cancer Center. He was director of the Cleveland Clinic bone marrow transplant program for 24 years and chairman of the department of hematologic oncology & blood disorders from 2006 to 2011.
In other Cleveland Clinic news, Halle Moore, MD, has been named director of breast medical oncology and codirector for the comprehensive breast cancer program at the clinic. She will assume this role in January 2020.
Dr. Moore is currently a staff physician at Taussig Cancer Institute and an associate professor of medicine at Lerner College of Medicine. Her clinical expertise is the management of breast cancer, and her research is focused on breast cancer treatment and cancer survivorship.
Cleveland Clinic has also added four new staff physicians to the department of hematology and medical oncology.
Shilpa Gupta, MD, has joined the clinic’s genitourinary cancer program. Dr. Gupta previously worked in the division of hematology, oncology, and transplantation at the University of Minnesota in Minneapolis, where she led the interdisciplinary solid tumor phase 1 program .
Khaled Hassan, MD, has joined the thoracic cancer program at Cleveland Clinic. Dr. Hassan was previously an assistant professor in the department of internal medicine, division of hematology/oncology at the University of Michigan in Ann Arbor. Suneel Kamath, MD, has joined the gastrointestinal cancer program at the clinic. Dr. Kamath was previously the hematology and medical oncology chief fellow at Northwestern University in Evanston, Ill.
Are you operating in the black when it comes to vaccine administration?
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19



















