User login
Hypertension—or not? Looking beyond office BP readings
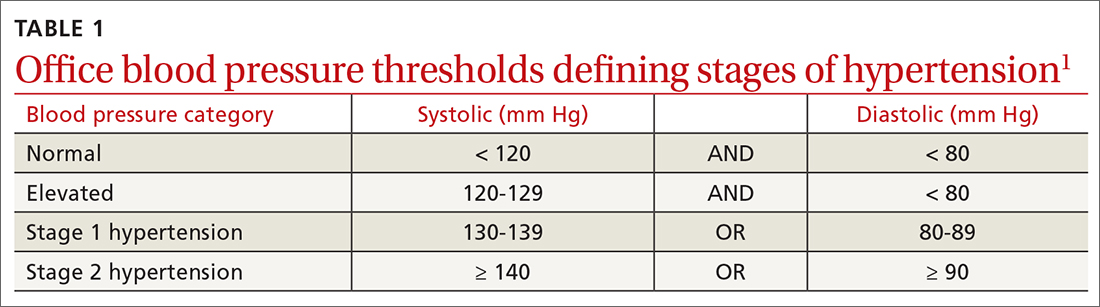
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
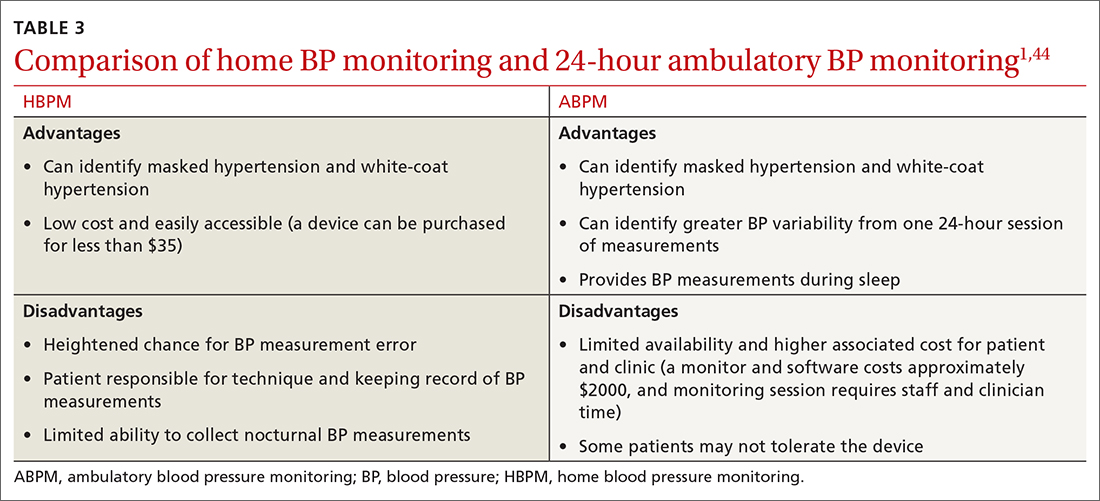
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
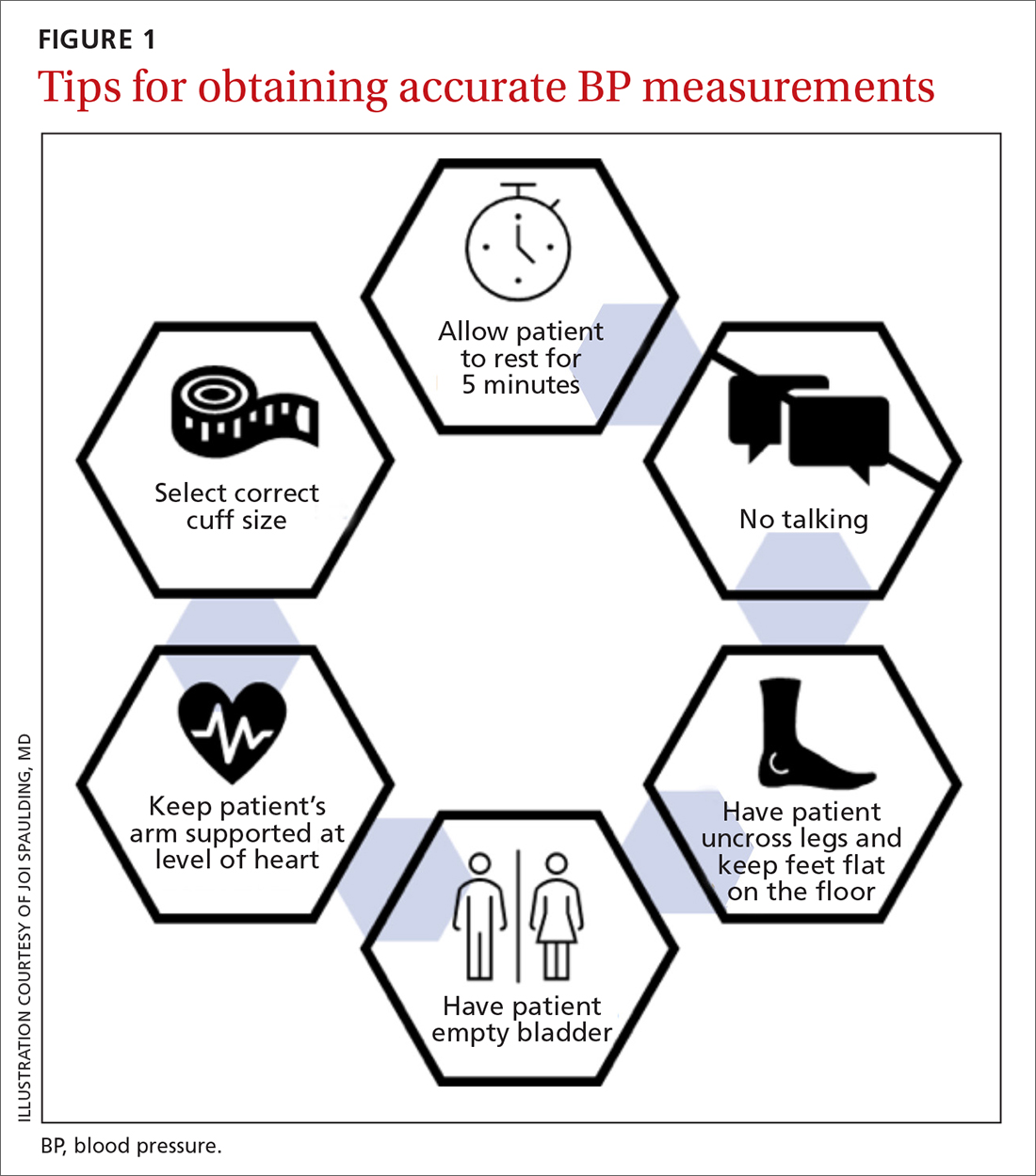
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
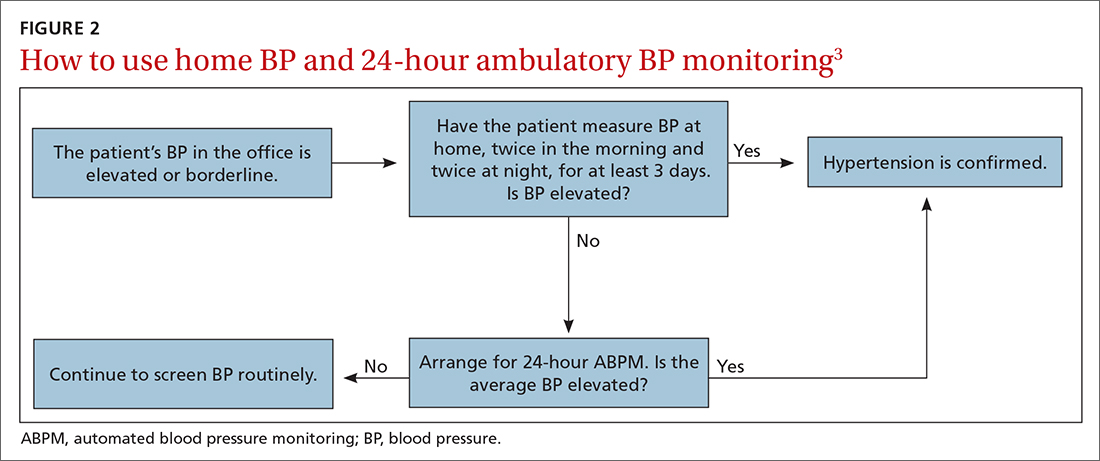
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
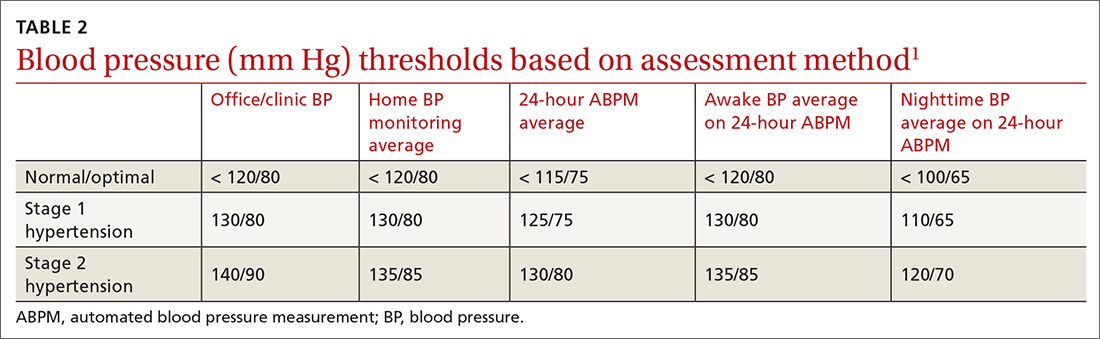
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
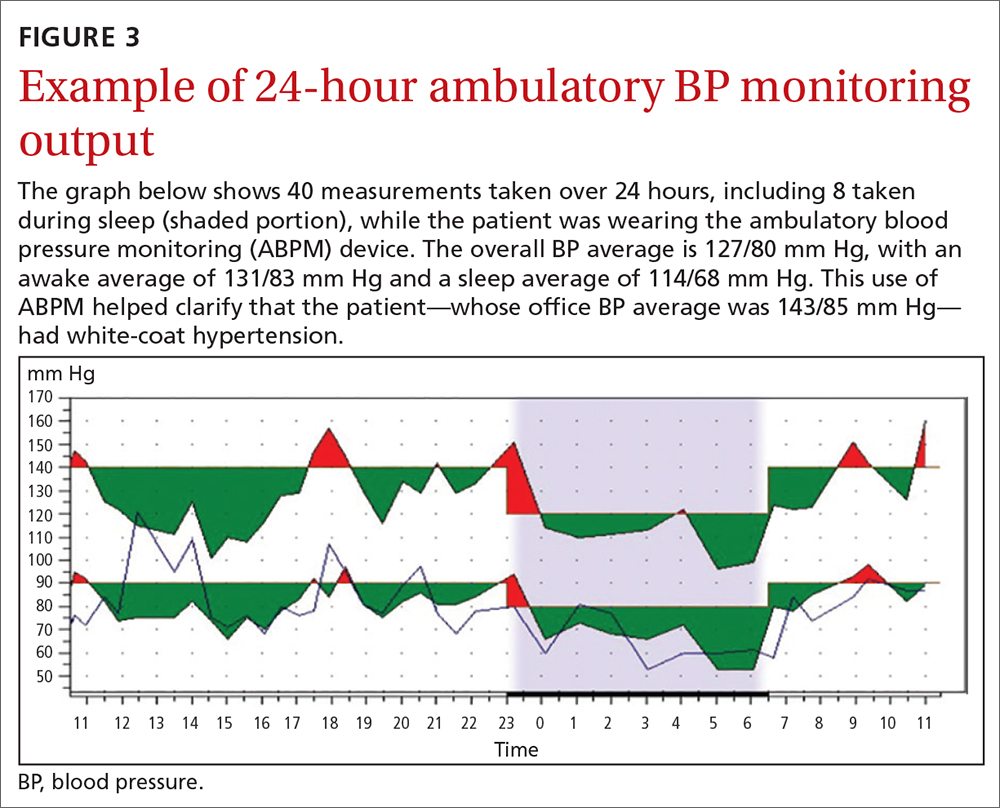
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; ajv18@duke.edu
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; ajv18@duke.edu
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; ajv18@duke.edu
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
PRACTICE RECOMMENDATIONS
› Use home blood pressure measurement (HBPM) for initial out-of-office evaluation to confirm hypertension. A
› Use 24-hour ambulatory measurement only when the results between office and HBPM are discordant. A
› Instruct patients to record their home BP measurements twice in the morning and twice at night for a minimum of 3 days. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Endovascular benefit finally confirmed for basilar artery stroke
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
Antithrombotic therapies shifting for Watchman LAA occlusion
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
Screening for hypertensive disorders of pregnancy is often incomplete
Nearly three-quarters of clinicians reported screening patients for hypertensive disorders of pregnancy, but only one-quarter comprehensively identified cardiovascular risk, based on survey data from approximately 1,500 clinicians in the United States.
Rates of hypertensive disorders of pregnancy have been on the rise in the United States for the past decade, and women with a history of these disorders require cardiovascular risk monitoring during the postpartum period and beyond, wrote Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Specifically, the American College of Obstetricians and Gynecologists recommends cardiovascular risk evaluation and lifestyle modification for these individuals, the researchers said.
The most effective management of women with a history of hypertensive disorders of pregnancy will likely involve a team effort by primary care, ob.gyns., and cardiologists, but data on clinician screening and referrals are limited, they added.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from a cross-sectional, web-based survey of clinicians practicing in the United States (Fall DocStyles 2020). The study population of 1,502 respondents with complete surveys included 1,000 primary care physicians, 251 ob.gyns., and 251 nurse practitioners or physician assistants. Approximately 60% of the respondents were male, and approximately 65% had been in practice for at least 10 years.
Overall, 73.6% of clinicians reported screening patients for a history of hypertensive disorders of pregnancy. The screening rates were highest among ob.gyns. (94.8%).
However, although 93.9% of clinicians overall correctly identified at least one potential risk associated with hypertensive disorders of pregnancy, only 24.8% correctly identified all cardiovascular risks associated with hypertensive disorders of pregnancy listed in the survey, the researchers noted.
Screening rates ranged from 49% to 91% for pregnant women, 34%-75% for postpartum women, 26%-61% for nonpregnant reproductive-age women, 20%-45% for perimenopausal or menopausal women, and 1%-4% for others outside of these categories.
The most often–cited barriers to referral were lack of patient follow-through (51.5%) and patient refusal (33.6%). To improve and facilitate referrals, respondents’ most frequent resource request was for more referral options (42.9%), followed by patient education materials (36.2%), and professional guidelines (34.1%).
In a multivariate analysis, primary care physicians were more than five times as likely to report not screening patients for hypertensive disorders of pregnancy (adjusted prevalence ratio, 5.54); nurse practitioners and physician assistants were more than seven times as likely (adjusted prevalence ratio, 7.42).
The researchers also found that clinicians who saw fewer than 80 patients per week were almost twice as likely not to screen for hypertensive disorders of pregnancy than those who saw 110 or more patients per week (adjusted prevalence ratio, 1.81).
“Beyond the immediate postpartum period, there is a lack of clear guidance on CVD [cardiovascular disease] evaluation and ongoing monitoring in women with history of hypertensive disorders of pregnancy,” the researchers wrote in their discussion. “Recognizing hypertensive disorders of pregnancy as a risk factor for CVD may allow clinicians to identify women requiring early evaluation and intervention,” they said.
The study findings were limited by several factors including potentially biased estimates of screening practices, and the potential for selection bias because of the convenience sample used to recruit survey participants, the researchers noted.
However, the results were strengthened by the inclusion of data from several clinician types and the relatively large sample size, and are consistent with those of previous studies, they said. Based on the findings, addressing barriers at both the patient and clinician level and increasing both patient and clinician education about the long-term risks of hypertensive disorders of pregnancy might increase cardiovascular screening and subsequent referrals, they concluded.
More education, improved screening tools needed
“Unfortunately, most CVD risk stratification scores such as the Framingham score do not include pregnancy complications, despite excellent evidence that pregnancy complications increase risk of CVD,” said Catherine M. Albright, MD, MS, of the University of Washington, Seattle, in an interview. “This is likely because these scores were developed primarily to screen for CVD risk in men. Given the rising incidence of hypertensive disorders of pregnancy and the clear evidence that this is a risk factor for future CVD, more studies like this one are needed in order to help guide patient and provider education,” said Dr. Albright, who was not involved in the study.
“It is generally well reported within the ob.gyn. literature about the increased lifetime CVD risk related to hypertensive disorders of pregnancy and we, as ob.gyns., always ask about pregnancy history because of our specialty, which gives us the opportunity to counsel about future risks,” she said.
“Women’s health [including during pregnancy] has been undervalued and underresearched for a long time,” with limited focus on pregnancy-related issues until recently, Dr. Albright noted. “This is clear in the attitudes and education of the primary care providers in this study,” she said.
A major barrier to screening in clinical practice has been that the standard screening guidelines for CVD (for example, those published by the United States Preventive Services Taskforce) have not included pregnancy history, said Dr. Albright. “Subsequently, these questions are not asked during routine annual visits,” she said. Ideally, “we should be able to leverage the electronic medical record to prompt providers to view a previously recorded pregnancy history or to ask about pregnancy history as a routine part of CVD risk assessment, and, of course, additional education outside of ob.gyn. and cardiology is needed,” she said.
The clinical takeaway from the current study is that “every annual visit with a person who has been pregnant is an opportunity to ask about and document pregnancy history,” Dr. Albright said. “After the completion of childbearing, many patients no longer see an ob.gyn., so other providers need to feel comfortable asking about and counseling about risks related to pregnancy complications,” she added.
“It is clear that adverse pregnancy outcomes pose lifetime health risks,” said Dr. Albright. “We will continue to look into the mechanisms of this through research. However, right now the additional research that is needed is to determine the optimal screening and follow-up for patients with a history of hypertensive disorders of pregnancy, as well as to examine how existing CVD-screening algorithms can be modified to include adverse pregnancy outcomes,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
Nearly three-quarters of clinicians reported screening patients for hypertensive disorders of pregnancy, but only one-quarter comprehensively identified cardiovascular risk, based on survey data from approximately 1,500 clinicians in the United States.
Rates of hypertensive disorders of pregnancy have been on the rise in the United States for the past decade, and women with a history of these disorders require cardiovascular risk monitoring during the postpartum period and beyond, wrote Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Specifically, the American College of Obstetricians and Gynecologists recommends cardiovascular risk evaluation and lifestyle modification for these individuals, the researchers said.
The most effective management of women with a history of hypertensive disorders of pregnancy will likely involve a team effort by primary care, ob.gyns., and cardiologists, but data on clinician screening and referrals are limited, they added.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from a cross-sectional, web-based survey of clinicians practicing in the United States (Fall DocStyles 2020). The study population of 1,502 respondents with complete surveys included 1,000 primary care physicians, 251 ob.gyns., and 251 nurse practitioners or physician assistants. Approximately 60% of the respondents were male, and approximately 65% had been in practice for at least 10 years.
Overall, 73.6% of clinicians reported screening patients for a history of hypertensive disorders of pregnancy. The screening rates were highest among ob.gyns. (94.8%).
However, although 93.9% of clinicians overall correctly identified at least one potential risk associated with hypertensive disorders of pregnancy, only 24.8% correctly identified all cardiovascular risks associated with hypertensive disorders of pregnancy listed in the survey, the researchers noted.
Screening rates ranged from 49% to 91% for pregnant women, 34%-75% for postpartum women, 26%-61% for nonpregnant reproductive-age women, 20%-45% for perimenopausal or menopausal women, and 1%-4% for others outside of these categories.
The most often–cited barriers to referral were lack of patient follow-through (51.5%) and patient refusal (33.6%). To improve and facilitate referrals, respondents’ most frequent resource request was for more referral options (42.9%), followed by patient education materials (36.2%), and professional guidelines (34.1%).
In a multivariate analysis, primary care physicians were more than five times as likely to report not screening patients for hypertensive disorders of pregnancy (adjusted prevalence ratio, 5.54); nurse practitioners and physician assistants were more than seven times as likely (adjusted prevalence ratio, 7.42).
The researchers also found that clinicians who saw fewer than 80 patients per week were almost twice as likely not to screen for hypertensive disorders of pregnancy than those who saw 110 or more patients per week (adjusted prevalence ratio, 1.81).
“Beyond the immediate postpartum period, there is a lack of clear guidance on CVD [cardiovascular disease] evaluation and ongoing monitoring in women with history of hypertensive disorders of pregnancy,” the researchers wrote in their discussion. “Recognizing hypertensive disorders of pregnancy as a risk factor for CVD may allow clinicians to identify women requiring early evaluation and intervention,” they said.
The study findings were limited by several factors including potentially biased estimates of screening practices, and the potential for selection bias because of the convenience sample used to recruit survey participants, the researchers noted.
However, the results were strengthened by the inclusion of data from several clinician types and the relatively large sample size, and are consistent with those of previous studies, they said. Based on the findings, addressing barriers at both the patient and clinician level and increasing both patient and clinician education about the long-term risks of hypertensive disorders of pregnancy might increase cardiovascular screening and subsequent referrals, they concluded.
More education, improved screening tools needed
“Unfortunately, most CVD risk stratification scores such as the Framingham score do not include pregnancy complications, despite excellent evidence that pregnancy complications increase risk of CVD,” said Catherine M. Albright, MD, MS, of the University of Washington, Seattle, in an interview. “This is likely because these scores were developed primarily to screen for CVD risk in men. Given the rising incidence of hypertensive disorders of pregnancy and the clear evidence that this is a risk factor for future CVD, more studies like this one are needed in order to help guide patient and provider education,” said Dr. Albright, who was not involved in the study.
“It is generally well reported within the ob.gyn. literature about the increased lifetime CVD risk related to hypertensive disorders of pregnancy and we, as ob.gyns., always ask about pregnancy history because of our specialty, which gives us the opportunity to counsel about future risks,” she said.
“Women’s health [including during pregnancy] has been undervalued and underresearched for a long time,” with limited focus on pregnancy-related issues until recently, Dr. Albright noted. “This is clear in the attitudes and education of the primary care providers in this study,” she said.
A major barrier to screening in clinical practice has been that the standard screening guidelines for CVD (for example, those published by the United States Preventive Services Taskforce) have not included pregnancy history, said Dr. Albright. “Subsequently, these questions are not asked during routine annual visits,” she said. Ideally, “we should be able to leverage the electronic medical record to prompt providers to view a previously recorded pregnancy history or to ask about pregnancy history as a routine part of CVD risk assessment, and, of course, additional education outside of ob.gyn. and cardiology is needed,” she said.
The clinical takeaway from the current study is that “every annual visit with a person who has been pregnant is an opportunity to ask about and document pregnancy history,” Dr. Albright said. “After the completion of childbearing, many patients no longer see an ob.gyn., so other providers need to feel comfortable asking about and counseling about risks related to pregnancy complications,” she added.
“It is clear that adverse pregnancy outcomes pose lifetime health risks,” said Dr. Albright. “We will continue to look into the mechanisms of this through research. However, right now the additional research that is needed is to determine the optimal screening and follow-up for patients with a history of hypertensive disorders of pregnancy, as well as to examine how existing CVD-screening algorithms can be modified to include adverse pregnancy outcomes,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
Nearly three-quarters of clinicians reported screening patients for hypertensive disorders of pregnancy, but only one-quarter comprehensively identified cardiovascular risk, based on survey data from approximately 1,500 clinicians in the United States.
Rates of hypertensive disorders of pregnancy have been on the rise in the United States for the past decade, and women with a history of these disorders require cardiovascular risk monitoring during the postpartum period and beyond, wrote Nicole D. Ford, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues. Specifically, the American College of Obstetricians and Gynecologists recommends cardiovascular risk evaluation and lifestyle modification for these individuals, the researchers said.
The most effective management of women with a history of hypertensive disorders of pregnancy will likely involve a team effort by primary care, ob.gyns., and cardiologists, but data on clinician screening and referrals are limited, they added.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from a cross-sectional, web-based survey of clinicians practicing in the United States (Fall DocStyles 2020). The study population of 1,502 respondents with complete surveys included 1,000 primary care physicians, 251 ob.gyns., and 251 nurse practitioners or physician assistants. Approximately 60% of the respondents were male, and approximately 65% had been in practice for at least 10 years.
Overall, 73.6% of clinicians reported screening patients for a history of hypertensive disorders of pregnancy. The screening rates were highest among ob.gyns. (94.8%).
However, although 93.9% of clinicians overall correctly identified at least one potential risk associated with hypertensive disorders of pregnancy, only 24.8% correctly identified all cardiovascular risks associated with hypertensive disorders of pregnancy listed in the survey, the researchers noted.
Screening rates ranged from 49% to 91% for pregnant women, 34%-75% for postpartum women, 26%-61% for nonpregnant reproductive-age women, 20%-45% for perimenopausal or menopausal women, and 1%-4% for others outside of these categories.
The most often–cited barriers to referral were lack of patient follow-through (51.5%) and patient refusal (33.6%). To improve and facilitate referrals, respondents’ most frequent resource request was for more referral options (42.9%), followed by patient education materials (36.2%), and professional guidelines (34.1%).
In a multivariate analysis, primary care physicians were more than five times as likely to report not screening patients for hypertensive disorders of pregnancy (adjusted prevalence ratio, 5.54); nurse practitioners and physician assistants were more than seven times as likely (adjusted prevalence ratio, 7.42).
The researchers also found that clinicians who saw fewer than 80 patients per week were almost twice as likely not to screen for hypertensive disorders of pregnancy than those who saw 110 or more patients per week (adjusted prevalence ratio, 1.81).
“Beyond the immediate postpartum period, there is a lack of clear guidance on CVD [cardiovascular disease] evaluation and ongoing monitoring in women with history of hypertensive disorders of pregnancy,” the researchers wrote in their discussion. “Recognizing hypertensive disorders of pregnancy as a risk factor for CVD may allow clinicians to identify women requiring early evaluation and intervention,” they said.
The study findings were limited by several factors including potentially biased estimates of screening practices, and the potential for selection bias because of the convenience sample used to recruit survey participants, the researchers noted.
However, the results were strengthened by the inclusion of data from several clinician types and the relatively large sample size, and are consistent with those of previous studies, they said. Based on the findings, addressing barriers at both the patient and clinician level and increasing both patient and clinician education about the long-term risks of hypertensive disorders of pregnancy might increase cardiovascular screening and subsequent referrals, they concluded.
More education, improved screening tools needed
“Unfortunately, most CVD risk stratification scores such as the Framingham score do not include pregnancy complications, despite excellent evidence that pregnancy complications increase risk of CVD,” said Catherine M. Albright, MD, MS, of the University of Washington, Seattle, in an interview. “This is likely because these scores were developed primarily to screen for CVD risk in men. Given the rising incidence of hypertensive disorders of pregnancy and the clear evidence that this is a risk factor for future CVD, more studies like this one are needed in order to help guide patient and provider education,” said Dr. Albright, who was not involved in the study.
“It is generally well reported within the ob.gyn. literature about the increased lifetime CVD risk related to hypertensive disorders of pregnancy and we, as ob.gyns., always ask about pregnancy history because of our specialty, which gives us the opportunity to counsel about future risks,” she said.
“Women’s health [including during pregnancy] has been undervalued and underresearched for a long time,” with limited focus on pregnancy-related issues until recently, Dr. Albright noted. “This is clear in the attitudes and education of the primary care providers in this study,” she said.
A major barrier to screening in clinical practice has been that the standard screening guidelines for CVD (for example, those published by the United States Preventive Services Taskforce) have not included pregnancy history, said Dr. Albright. “Subsequently, these questions are not asked during routine annual visits,” she said. Ideally, “we should be able to leverage the electronic medical record to prompt providers to view a previously recorded pregnancy history or to ask about pregnancy history as a routine part of CVD risk assessment, and, of course, additional education outside of ob.gyn. and cardiology is needed,” she said.
The clinical takeaway from the current study is that “every annual visit with a person who has been pregnant is an opportunity to ask about and document pregnancy history,” Dr. Albright said. “After the completion of childbearing, many patients no longer see an ob.gyn., so other providers need to feel comfortable asking about and counseling about risks related to pregnancy complications,” she added.
“It is clear that adverse pregnancy outcomes pose lifetime health risks,” said Dr. Albright. “We will continue to look into the mechanisms of this through research. However, right now the additional research that is needed is to determine the optimal screening and follow-up for patients with a history of hypertensive disorders of pregnancy, as well as to examine how existing CVD-screening algorithms can be modified to include adverse pregnancy outcomes,” she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
‘Critical window’ to intervene for weight issues in early childhood
Signs of cardiometabolic damage in children who are overweight appear as early as 6-8 years of age, but were not evident in preschoolers, providing a window of opportunity for intervention, show the latest results from a long-running Danish study of childhood weight.
The proportion of children who were overweight (nearly 14% in 2015) was similar between the two groups – those of preschool age (2-5 years) and school age (6-8 years) – but only the latter showed significant signs of cardiometabolic abnormalities.
The results, published in Obesity Research & Clinical Practice, are the latest in a series of many findings from the HOLBAEK study (formerly known as The Danish Childhood Obesity Biobank) that have emerged since it began in 2007. They were presented, along with a meta-analysis of much of their work, at the European Congress on Obesity (ECO) 2022.
“When comparing children with and without overweight, there were only barely significant differences among the preschool children,” said investigator Christine Frithioff-Bøjsøe, MD, but in contrast, “the school children with overweight exhibited significantly higher systolic blood pressure, glucose, insulin, and higher HDL cholesterol,” among other markers, she noted.
“Detection needs to start as early as age 2-5 years because if you wait just a few years longer these children will show early signs of disease starting to take hold. This could provide a critical window to detect and manage overweight,” said Frithioff-Bøjsøe, PhD, of the Children’s Obesity Clinic, Copenhagen University, Hospital Holbaek, Denmark.
Asked to comment, Aaron S. Kelly, PhD, professor of pediatrics, codirector, University of Minnesota Center for Pediatric Obesity Medicine in Minneapolis, said: “Recent results from HOLBAEK highlight the critical importance of identifying obesity early in life, before its complications spring up.
“Ideally, we should be in the business of managing and reducing excess adiposity as soon as it surfaces with the goal of preventing the onset of cardiometabolic risk factors, not watchful waiting and hoping for the best.”
Routine dental visits checked overweight
In the newest study, the researchers trained dental assistants to measure weight and height and carried out body mass index assessments during routine appointments.
A total of 335 preschool and 657 school-age children were recruited for the study. Of these, 40% attended additional hospital-based examinations including blood pressure measurement and a blood sample. Children were reexamined approximately 1 year later.
Systolic blood pressure, for example, was significantly higher in 6- to 8-year-olds with overweight compared to those of normal weight (P = .001). There was no significant difference between systolic blood pressure of 2.5- to 5-year-olds without and with overweight.
Likewise, with insulin resistance, there was no significant difference between preschoolers with and without overweight. However, in schoolchildren, homoeostasis model of assessment–insulin resistance (HOMA-IR) was significantly higher in those with overweight, at 2.2, compared to those without, at 0.9 (P < .001).
Also, during follow-up (around a year later), the prevalence of overweight did not change in preschool children but increased from 13.7% to 17.0% in schoolchildren.
The researchers noted that, in Europe, it is the primary health care sector that has continuous contact with the pediatric population, with the potential for early evaluation of children at risk. Their decision to use dental health care assistants to assess weight in this particular study is novel, but feasible, they observed.
Danish model for treating overweight and obesity is ‘game-changing’
As part of the HOLBAEK initiative, clinical data and biological samples have been collected from children and adolescents receiving treatment at The Children’s Obesity Clinic, Holbaek Hospital, using a population-based cohort as a reference group. Data have been collected on about 8,000 children and adolescents so far.
Jens-Christian Holm, PhD, along with colleague and research assistant Maria Frauland, both from Copenhagen University, Hospital Holbaek, presented a review of the HOLBAEK studies (2007-2021) at ECO 2022. They said the results highlight the importance of taking an integrated approach to managing children and adolescents with obesity.
The review, which included 82 papers, found a wide variety of obesity-related complications already present at a young age in some of the cross-sectional studies, including dyslipidemia in 28% of children with obesity, hepatic steatosis in 31%, obstructive sleep apnea in 45%, and prehypertension or hypertension in 52%.
The family-based interventional weight management programs adopted by HOLBAEK showed a 75% reduction in the “degree of obesity,” which comprised a measure of dyslipidemia, hypertension, hepatic steatosis, sleep apnea, and parental obesity.
“The HOLBAEK method is a holistic approach where we integrate everything,” Dr. Holm told this news organization.
Ms. Frauland said: “The HOLBAEK study has provided important insights into childhood overweight. It has highlighted that obesity is a serious multisystem disease that can be managed and treated effectively, reducing the degree of overweight and improving overweight-related complications.”
Dr. Kelly, the U.S. pediatrician, applauded the HOLBAEK philosophy, which emphasizes that obesity is not the fault of the child or parent, but rather the manifestation of dysregulated energy metabolism. “The recognition that obesity is a biologically driven, chronic, refractory, and relapsing disease is interwoven into the approach, which shifts the responsibility to the care provider for ensuring positive outcomes of treatment.
“Highlighting this fact to the parents and child can be game-changing since it removes the blame and shame associated with obesity and unburdens the family by framing the problem in a different light,” Dr. Kelly stressed.
Dr. Frithioff-Bøjsøe has reported no relevant financial relationships. Dr. Holm has an obesity management company called Holm. Dr. Kelly serves as an unpaid consultant for Novo Nordisk, Vivus, Eli Lilly, and Boehringer Ingelheim and receives donated drug/placebo from Vivus for a clinical trial funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Signs of cardiometabolic damage in children who are overweight appear as early as 6-8 years of age, but were not evident in preschoolers, providing a window of opportunity for intervention, show the latest results from a long-running Danish study of childhood weight.
The proportion of children who were overweight (nearly 14% in 2015) was similar between the two groups – those of preschool age (2-5 years) and school age (6-8 years) – but only the latter showed significant signs of cardiometabolic abnormalities.
The results, published in Obesity Research & Clinical Practice, are the latest in a series of many findings from the HOLBAEK study (formerly known as The Danish Childhood Obesity Biobank) that have emerged since it began in 2007. They were presented, along with a meta-analysis of much of their work, at the European Congress on Obesity (ECO) 2022.
“When comparing children with and without overweight, there were only barely significant differences among the preschool children,” said investigator Christine Frithioff-Bøjsøe, MD, but in contrast, “the school children with overweight exhibited significantly higher systolic blood pressure, glucose, insulin, and higher HDL cholesterol,” among other markers, she noted.
“Detection needs to start as early as age 2-5 years because if you wait just a few years longer these children will show early signs of disease starting to take hold. This could provide a critical window to detect and manage overweight,” said Frithioff-Bøjsøe, PhD, of the Children’s Obesity Clinic, Copenhagen University, Hospital Holbaek, Denmark.
Asked to comment, Aaron S. Kelly, PhD, professor of pediatrics, codirector, University of Minnesota Center for Pediatric Obesity Medicine in Minneapolis, said: “Recent results from HOLBAEK highlight the critical importance of identifying obesity early in life, before its complications spring up.
“Ideally, we should be in the business of managing and reducing excess adiposity as soon as it surfaces with the goal of preventing the onset of cardiometabolic risk factors, not watchful waiting and hoping for the best.”
Routine dental visits checked overweight
In the newest study, the researchers trained dental assistants to measure weight and height and carried out body mass index assessments during routine appointments.
A total of 335 preschool and 657 school-age children were recruited for the study. Of these, 40% attended additional hospital-based examinations including blood pressure measurement and a blood sample. Children were reexamined approximately 1 year later.
Systolic blood pressure, for example, was significantly higher in 6- to 8-year-olds with overweight compared to those of normal weight (P = .001). There was no significant difference between systolic blood pressure of 2.5- to 5-year-olds without and with overweight.
Likewise, with insulin resistance, there was no significant difference between preschoolers with and without overweight. However, in schoolchildren, homoeostasis model of assessment–insulin resistance (HOMA-IR) was significantly higher in those with overweight, at 2.2, compared to those without, at 0.9 (P < .001).
Also, during follow-up (around a year later), the prevalence of overweight did not change in preschool children but increased from 13.7% to 17.0% in schoolchildren.
The researchers noted that, in Europe, it is the primary health care sector that has continuous contact with the pediatric population, with the potential for early evaluation of children at risk. Their decision to use dental health care assistants to assess weight in this particular study is novel, but feasible, they observed.
Danish model for treating overweight and obesity is ‘game-changing’
As part of the HOLBAEK initiative, clinical data and biological samples have been collected from children and adolescents receiving treatment at The Children’s Obesity Clinic, Holbaek Hospital, using a population-based cohort as a reference group. Data have been collected on about 8,000 children and adolescents so far.
Jens-Christian Holm, PhD, along with colleague and research assistant Maria Frauland, both from Copenhagen University, Hospital Holbaek, presented a review of the HOLBAEK studies (2007-2021) at ECO 2022. They said the results highlight the importance of taking an integrated approach to managing children and adolescents with obesity.
The review, which included 82 papers, found a wide variety of obesity-related complications already present at a young age in some of the cross-sectional studies, including dyslipidemia in 28% of children with obesity, hepatic steatosis in 31%, obstructive sleep apnea in 45%, and prehypertension or hypertension in 52%.
The family-based interventional weight management programs adopted by HOLBAEK showed a 75% reduction in the “degree of obesity,” which comprised a measure of dyslipidemia, hypertension, hepatic steatosis, sleep apnea, and parental obesity.
“The HOLBAEK method is a holistic approach where we integrate everything,” Dr. Holm told this news organization.
Ms. Frauland said: “The HOLBAEK study has provided important insights into childhood overweight. It has highlighted that obesity is a serious multisystem disease that can be managed and treated effectively, reducing the degree of overweight and improving overweight-related complications.”
Dr. Kelly, the U.S. pediatrician, applauded the HOLBAEK philosophy, which emphasizes that obesity is not the fault of the child or parent, but rather the manifestation of dysregulated energy metabolism. “The recognition that obesity is a biologically driven, chronic, refractory, and relapsing disease is interwoven into the approach, which shifts the responsibility to the care provider for ensuring positive outcomes of treatment.
“Highlighting this fact to the parents and child can be game-changing since it removes the blame and shame associated with obesity and unburdens the family by framing the problem in a different light,” Dr. Kelly stressed.
Dr. Frithioff-Bøjsøe has reported no relevant financial relationships. Dr. Holm has an obesity management company called Holm. Dr. Kelly serves as an unpaid consultant for Novo Nordisk, Vivus, Eli Lilly, and Boehringer Ingelheim and receives donated drug/placebo from Vivus for a clinical trial funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Signs of cardiometabolic damage in children who are overweight appear as early as 6-8 years of age, but were not evident in preschoolers, providing a window of opportunity for intervention, show the latest results from a long-running Danish study of childhood weight.
The proportion of children who were overweight (nearly 14% in 2015) was similar between the two groups – those of preschool age (2-5 years) and school age (6-8 years) – but only the latter showed significant signs of cardiometabolic abnormalities.
The results, published in Obesity Research & Clinical Practice, are the latest in a series of many findings from the HOLBAEK study (formerly known as The Danish Childhood Obesity Biobank) that have emerged since it began in 2007. They were presented, along with a meta-analysis of much of their work, at the European Congress on Obesity (ECO) 2022.
“When comparing children with and without overweight, there were only barely significant differences among the preschool children,” said investigator Christine Frithioff-Bøjsøe, MD, but in contrast, “the school children with overweight exhibited significantly higher systolic blood pressure, glucose, insulin, and higher HDL cholesterol,” among other markers, she noted.
“Detection needs to start as early as age 2-5 years because if you wait just a few years longer these children will show early signs of disease starting to take hold. This could provide a critical window to detect and manage overweight,” said Frithioff-Bøjsøe, PhD, of the Children’s Obesity Clinic, Copenhagen University, Hospital Holbaek, Denmark.
Asked to comment, Aaron S. Kelly, PhD, professor of pediatrics, codirector, University of Minnesota Center for Pediatric Obesity Medicine in Minneapolis, said: “Recent results from HOLBAEK highlight the critical importance of identifying obesity early in life, before its complications spring up.
“Ideally, we should be in the business of managing and reducing excess adiposity as soon as it surfaces with the goal of preventing the onset of cardiometabolic risk factors, not watchful waiting and hoping for the best.”
Routine dental visits checked overweight
In the newest study, the researchers trained dental assistants to measure weight and height and carried out body mass index assessments during routine appointments.
A total of 335 preschool and 657 school-age children were recruited for the study. Of these, 40% attended additional hospital-based examinations including blood pressure measurement and a blood sample. Children were reexamined approximately 1 year later.
Systolic blood pressure, for example, was significantly higher in 6- to 8-year-olds with overweight compared to those of normal weight (P = .001). There was no significant difference between systolic blood pressure of 2.5- to 5-year-olds without and with overweight.
Likewise, with insulin resistance, there was no significant difference between preschoolers with and without overweight. However, in schoolchildren, homoeostasis model of assessment–insulin resistance (HOMA-IR) was significantly higher in those with overweight, at 2.2, compared to those without, at 0.9 (P < .001).
Also, during follow-up (around a year later), the prevalence of overweight did not change in preschool children but increased from 13.7% to 17.0% in schoolchildren.
The researchers noted that, in Europe, it is the primary health care sector that has continuous contact with the pediatric population, with the potential for early evaluation of children at risk. Their decision to use dental health care assistants to assess weight in this particular study is novel, but feasible, they observed.
Danish model for treating overweight and obesity is ‘game-changing’
As part of the HOLBAEK initiative, clinical data and biological samples have been collected from children and adolescents receiving treatment at The Children’s Obesity Clinic, Holbaek Hospital, using a population-based cohort as a reference group. Data have been collected on about 8,000 children and adolescents so far.
Jens-Christian Holm, PhD, along with colleague and research assistant Maria Frauland, both from Copenhagen University, Hospital Holbaek, presented a review of the HOLBAEK studies (2007-2021) at ECO 2022. They said the results highlight the importance of taking an integrated approach to managing children and adolescents with obesity.
The review, which included 82 papers, found a wide variety of obesity-related complications already present at a young age in some of the cross-sectional studies, including dyslipidemia in 28% of children with obesity, hepatic steatosis in 31%, obstructive sleep apnea in 45%, and prehypertension or hypertension in 52%.
The family-based interventional weight management programs adopted by HOLBAEK showed a 75% reduction in the “degree of obesity,” which comprised a measure of dyslipidemia, hypertension, hepatic steatosis, sleep apnea, and parental obesity.
“The HOLBAEK method is a holistic approach where we integrate everything,” Dr. Holm told this news organization.
Ms. Frauland said: “The HOLBAEK study has provided important insights into childhood overweight. It has highlighted that obesity is a serious multisystem disease that can be managed and treated effectively, reducing the degree of overweight and improving overweight-related complications.”
Dr. Kelly, the U.S. pediatrician, applauded the HOLBAEK philosophy, which emphasizes that obesity is not the fault of the child or parent, but rather the manifestation of dysregulated energy metabolism. “The recognition that obesity is a biologically driven, chronic, refractory, and relapsing disease is interwoven into the approach, which shifts the responsibility to the care provider for ensuring positive outcomes of treatment.
“Highlighting this fact to the parents and child can be game-changing since it removes the blame and shame associated with obesity and unburdens the family by framing the problem in a different light,” Dr. Kelly stressed.
Dr. Frithioff-Bøjsøe has reported no relevant financial relationships. Dr. Holm has an obesity management company called Holm. Dr. Kelly serves as an unpaid consultant for Novo Nordisk, Vivus, Eli Lilly, and Boehringer Ingelheim and receives donated drug/placebo from Vivus for a clinical trial funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM OBESITY RESEARCH & CLINICAL PRACTICE
Natriuretic Peptide Screening for Primary Prevention or Early Detection of Heart Failure: A Pharmacist-Driven Team-Based Approach
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Topline results for dapagliflozin in HFpEF: DELIVER
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Bone, breath, heart, guts: Eight essential papers in primary care
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Porcine virus a suspect in man’s death after pig heart transplant
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
FROM MIT TECHNOLOGY REVIEW
Takotsubo syndrome also linked to happy life events
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The findings show that although Takotsubo syndrome, a type of acute heart failure related to atypical patterns of transient left ventricular contraction abnormalities, is often triggered by negative emotional stressors, it can also stem from positive life events, something the researchers are calling “happy heart syndrome.”
In this registry study, males were more likely to experience Takotsubo syndrome from a positive life event, as were those with atypical, nonapical ballooning, reported Thomas Stiermaier, MD, of University Hospital Schleswig-Holstein in Lübeck, Germany, and colleagues.
Patients with negative and positive emotional triggers experienced similar short- and long-term outcomes, they found.
The results were published online in JACC: Heart Failure.
Previous studies have shown that Takotsubo syndrome can be related to negative emotional triggers, physical triggers such as heavy physical activity, or medical procedures (or, in some cases, neither of these), or even a combination of emotional and physical triggers, the authors said. Research shows that physical triggers are most often linked to poor outcomes.
A vast number of clinical scenarios may lead up to Takotsubo syndrome, noted Jason H. Rogers, MD, professor of cardiovascular medicine at the University of California, Davis, who commented on these findings.
“Examples would include other medical illness, such as infection or recent surgery, having a heated argument with someone, running to catch a flight at the airport, and even being awakened suddenly by a sick pet,” Dr. Rogers told this news organization.
But not all patients experience unhappy life stressors before these events occur, he added. “It is possible for patients to have happy life stressors that can lead to Takotsubo syndrome also.”
For this analysis, the research team evaluated 2,482 patients using data from the multicenter German-Italian-Spanish Takotsubo (GEIST) Registry, one of the largest of its kind. Of these patients, 910 experienced an emotional trigger; of these, 873 had negative preceding events, and 37 had pleasant preceding events. The mean age was 70 years in both groups.
The study team then compared patients with negative emotional triggers to those with positive emotional triggers, which included weddings, the birth of grandchildren, birthday parties, or anticipation of a trip or Christmas.
There was a 1.5% incidence of pleasant emotional triggers among all Takotsubo syndrome patients.
Among patients with positive prior triggers, there was a higher incidence of atypical ballooning (27.0% vs. 12.5%; P = .01), and a higher percentage of these patients were male (18.9% vs. 5.0%; P < .01) in comparison with those with negative events prior to Takotsubo syndrome.
Long-term death rates (8.8% vs. 2.7%; P = .20) and rates of in-hospital complication outcomes, including cardiogenic shock, stroke, death, or pulmonary edema (12.3% vs. 8.1%; P = .45), were similar for patients with negative preceding events and for those with positive preceding events.
Study limitations included that it cannot provide insight into the specific mechanisms of Takotsubo syndrome, it was observational, the sample size of patients in the positive events group was small, and the contributing research facilities assessed cardiac biomarker levels differently.
“Additional research efforts are needed to explore whether numerically lower cardiac-related event rates in patients with happy heart syndrome would be statistically significant in a larger sample size,” the researchers concluded.
Dr. Stiermaier reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
JACC: HEART FAILURE