User login
Team-Based Hypertension Management in Outpatient Settings
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
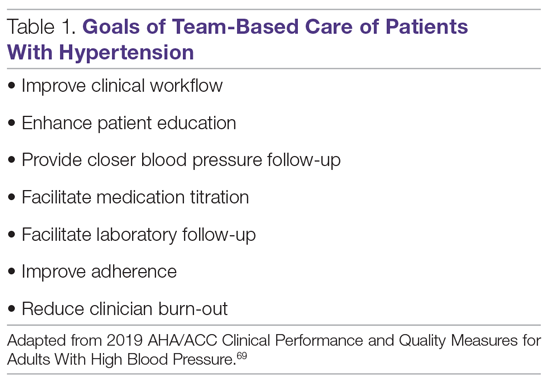
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
Timing of Surgery in Patients With Asymptomatic Severe Aortic Stenosis
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
Today’s top news highlights: ACE inhibitors in COVID patients, fewer AMI admissions, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Is anemia due to folate deficiency a myth?
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
ACE inhibitors and severe COVID-19: Protective in older patients?
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
As visits for AMI drop during pandemic, deaths rise
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
FROM THE EUROPEAN HEART JOURNAL
To fast or not to fast before elective cardiac catheterization
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
REPORTING FROM SCAI 2020
New diagnostic CT scan model predicts pulmonary hypertension
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
A new CT scan pulmonary angiography model may help optimize the diagnostic work-up process for patients with suspected pulmonary hypertension (PH), according to a recent study.
The diagnostic and prognostic utility of the model was validated in a tertiary referral population of treatment-naive patients who had a high pretest probability of PH.
“The aim of this study was to (a) build a diagnostic CT model in patients with suspected PH using the current guideline definition of PH (mPAP [mean pulmonary arterial pressure] ≥25 mm Hg) and the recent proposed definition of >20 mm Hg and (b) test its prognostic significance,” wrote Andrew J. Swift, MBChB, PhD, of the University of Sheffield (England) and colleagues in European Radiology.
The study cohort included 491 patients with suspected PH who underwent routine CT pulmonary angiography and right-heart catheterization between April 2012 and March 2016. CT metrics for patients with PH were developed using axial and reconstructed images.
The researchers identified the derivation (n = 247) and validation (n = 244) cohorts using random patient selection. In the derivation cohort, multivariate regression analysis was conducted to develop a model with the ability to predict mPAP ≥25 mm Hg and >20 mm Hg.
In the validation cohort, receiver operating characteristic analysis was performed to establish compromise CT thresholds, as well as sensitivity and specificity. The prognostic utility of the model was evaluated using Kaplan-Meier analysis.
Derivation cohort
Among the 247 patients in the derivation cohort, a CT regression model was identified, which included right-ventricle outflow tract thickness, main pulmonary artery diameter, and left ventricular area and interventricular septal angle; the area under the curve (AUC) in this cohort was 0.92.
Validation cohort
Among the 244 patients in the validation cohort, the model demonstrated strong diagnostic utility for the detection of PH, with an AUC of 0.91 and 0.94 for mPAP >20 mm Hg and ≥25 mm Hg, respectively.
With respect to the prognostic utility of the model, the researchers found that the diagnostic thresholds were prognostic in the CT model (all P < .01).
“The diagnostic CT thresholds are also of prognostic value; patients found not to have PH on CT have an excellent outcome,” they explained.
Dr. Swift and colleagues acknowledged that positive and negative predictive values will change based on the diagnostic setting. As a result, the findings from the current study may only be applicable to tertiary referral patient populations.
“This data may be particularly helpful when triaging patients with suspected severe PH for consideration of targeted pulmonary vascular therapies,” they concluded.
The study was supported by Wellcome Trust, the National Institute for Health Research, MRC POLARIS, and Bayer. The authors reported having no conflicts of interest with any companies related to the publication.
SOURCE: Swift AJ et al. Eur Radiol. 2020 Apr 27. doi: 10.1007/s00330-020-06846-1.
FROM EUROPEAN RADIOLOGY
DOACs linked to lower fracture risk versus warfarin in AFib patients
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
results of a recent population-based cohort study show.
The choice of direct oral anticoagulant (DOAC) didn’t appear to have an impact, as each individual agent yielded a substantially lower risk of fracture versus the vitamin K antagonist, with risk reductions ranging from 38% to 48%, according to the study authors.
This is one of the latest reports to suggest DOACs could have an edge over warfarin for preventing fractures, providing new evidence that “may help inform the benefit risk assessment” when it comes to choosing an anticoagulant for a patient with atrial fibrillation (AFib) in the clinic, wrote the authors, led by Wallis C.Y. Lau, PhD, with the University College London.
“There exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” Dr. Lau and coauthors wrote in a report on the study that appears in Annals of Internal Medicine.
The case is especially compelling since fracture risk is “often neglected” when choosing an anticoagulant, the authors wrote. Surgeries to treat fracture are difficult because of the need for perioperative management of anticoagulation as “a balance between the risk for stroke and excessive bleeding must be achieved,” they added.
Based on these data, physicians should strongly consider DOACs as an alternative to vitamin K antagonists to reduce the risk of osteoporosis over the long term in patients with AFib, according to Victor Lawrence Roberts, MD, a Florida endocrinologist.
“Osteoporosis takes years, sometimes decades to develop, and if you then overlay warfarin on top of a readily evolving metabolic bone disease, you probably accelerate that process, said Dr. Roberts, professor of internal medicine at the University of Central Florida, Orlando, and editorial advisory board member of Internal Medicine News.
There’s a considerable amount of concerning preclinical data that warfarin could increase osteoporotic fracture risk. Of note, vitamin K antagonists modulate osteocalcin, a calcium-binding bone matrix protein, Dr. Roberts said.
“Osteocalcin is important for bone metabolism and health, and inhibiting osteocalcin will inhibit the ability to have a healthy bone matrix,” he explained.
The impact of anticoagulants on fracture risk is particularly relevant to patients with AFib, according to Dr. Lau and colleagues, who referenced one 2017 report showing a higher incidence of hip fracture among AFib patients versus those without AFib.
In their more recent study, Dr. Lau and colleagues reviewed electronic health records in a Hong Kong database for 23,515 older adults with a new diagnosis of AFib who received a new prescription of warfarin or DOACs including apixaban, dabigatran, or rivaroxaban.
DOAC use was consistently associated with a lower risk of osteoporotic fractures versus warfarin, regardless of the DOAC considered. The hazard ratios were 0.62 (95% confidence interval, 0.41-0.94) for apixaban, 0.65 (95% CI, 0.49-0.86) for dabigatran, and 0.52 (95% CI, 0.37-0.73) for rivaroxaban versus warfarin, the report showed.
Head-to-head comparisons between DOACS didn’t yield any statistically significant differences, though the analyses were underpowered in this respect, according to the investigators.
“This study can only rule out more than a twofold higher or a 50% lower relative risk for osteoporotic fractures between individual DOACs,” they wrote. “However, any absolute risk differences were small and would likely be of minor clinical significance.”
The reduced risk of fracture for DOACs versus warfarin was consistent in men and women with AFib, suggesting that women may particularly benefit from DOACs, given that they have a higher risk of fracture than men, the investigators added.
The results of this study suggest yet another benefit of DOACs over warfarin in patients with AFib, according to internist Noel Deep, MD, who is the chief medical officer of Aspirus Langlade Hospital in Antigo, Wisconsin.
“The lower risk of osteoporotic fractures with DOACS, in addition to other advantages such as lower risk of intracranial bleeding, once- or twice-daily consistent dosing, no dietary restrictions, and no blood tests to regulate the dose might be another reason that physicians may favor them over warfarin in older individuals requiring anticoagulation,” Dr. Deep said in an interview.
Results of this and several other recent studies may help in recommending DOACs to internal medicine patients who have a diagnosis of AFib requiring anticoagulation, according to Dr. Deep, who is also a physician at Aspirus Antigo Clinic and a member of Internal Medicine News’ editorial advisory board. These include a 2019 U.S.-based study of more than 167,000 patients with AFib (JAMA Intern Med. 2019;180[2]:245‐253) showing that use of DOACs, particularly apixaban, were linked to lower fracture risk versus warfarin use. Similarly, a Danish national registry study also published in 2019 showed that the absolute risk of osteoporotic fractures was low overall and significantly lower in patients who received DOACs (J Am Coll Cardiol. 2019;74[17]:2150-2158).
Funding for the study came from the University of Hong Kong and University College London Strategic Planning Fund. The study authors reported disclosures related to Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, IQVIA, and others.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 18. doi: 10.7326/M19-3671.
FROM ANNALS OF INTERNAL MEDICINE
Blood pressure lowering lessens risk of dementia, cognitive decline
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
FROM JAMA