User login
Rivaroxaban trends toward higher thrombotic risk than vitamin K antagonists in APS
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
FROM ANNALS OF INTERNAL MEDICINE
Automated ventilation outperformed nurses in post-op cardiac care
MADRID – In patients managed on mechanical ventilation in an intensive care unit following cardiac surgery, a fully automated system provides more reliable ventilatory support than highly experienced ICU nurses, suggest results of a randomized trial.
The study’s control group received usual care, which means that nurses adjusted mechanical ventilation manually in response to respiratory rate, tidal volume, positive end-respiratory pressure (PEEP), and other factors to maintain ventilation within parameters associated with safe respiration. The experimental group was managed with a fully automated closed-loop system to make these adjustments without any nurse intervention.
For those in the experimental group “the proportion of time in the optimal zone was increased and the proportion of time in the unsafe zone was decreased” relative to those randomized to conventional nursing care, Marcus J. Schultz, MD, reported at the annual congress of the European Respiratory Society.
Conducted at a hospital with an experienced ICU staff, the study had a control arm that was managed by “dedicated nurses who, I can tell you, are very eager to provide the best level of care possible,” said Dr. Schultz, professor of experimental intensive care, University of Amsterdam, the Netherlands..
The investigator-initiated POSITiVE trial randomized 220 cardiac surgery patients scheduled to receive postoperative mechanical ventilation in the ICU. Exclusions included those with class III or higher chronic obstructive pulmonary disease (COPD), a requirement for extracorporeal membrane oxygenation (ECMO), or a history of lung surgery.
The primary endpoint was the proportion of time spent in an optimal zone, an acceptable zone, or a dangerous zone of ventilation based on predefined values for tidal volume, maximum airway pressure, end-tidal CO2, and oxygen saturation (SpO2).
The greatest between-group difference was seen in the proportion of time spent in the optimal zone. This climbed from approximately 35% in the control arm to slightly more than 70% in the experimental arm, a significant difference. The proportion of time in the dangerous zone was reduced from approximately 6% in the control arm to 3% in the automated arm. On average nurse-managed patients spent nearly 60% of the time in the acceptable zone versus less than 30% of those in the automated experimental arm.
A heat map using green, yellow, and red to represent optimal, acceptable, and dangerous zones, respectively, for individual participants in the trial provided a more stark global impression. For the control group, the heat map was primarily yellow with scattered dashes of green and red. For the experimental group, the map was primarily green with dashes of yellow and a much smaller number of red dashes relative to the control group.
In addition, the time to spontaneous breathing was 38% shorter for those randomized to automated ventilation than to conventional care, a significant difference.
There are now many devices marketed for automated ventilation, according to Dr. Schultz. The device used in this study was the proprietary INTELLiVENT-ASV system, marketed by Hamilton Medical, which was selected based on prior satisfactory experience. Although not unique, this system has sophisticated software to adjust ventilation to reach targets set by the clinician on the basis of information it is receiving from physiologic sensors for such variables as respiratory rate, tidal volume, and inspiratory pressure.
“It is frequently adjusting the PEEP levels to reach the lowest driving pressure,” said Dr. Schultz. Among its many other features, it also “gives spontaneous breathing trials automatically.”
Uncomplicated patients were selected purposefully to test this system, but Dr. Schultz said that a second trial, called POSITiVE 2, is now being planned that will enroll more complex patients. Keeping complex patients within the optimal zone as defined by tidal volume and other critical variables has the potential to reduce the lung damage that is known to occur when these are not optimized.
“Applying safe ventilatory support in clinical practice remains a serious challenge and is extremely time consuming,” Dr. Schultz said. He reported that fully automated ventilation appears to be reliable, and “it takes out the human factor” in regard to diligence in monitoring and potential for error.
Overall, these results support the potential for a fully automated system to improve optimal ventilatory support, reduce risk of lung injury, and reduce staffing required for monitoring of mechanical ventilation, according to Dr. Schultz.
Relative costs were not evaluated in this analysis, but might be another factor relevant to the value of fully automated ventilation in ICU patients.
MADRID – In patients managed on mechanical ventilation in an intensive care unit following cardiac surgery, a fully automated system provides more reliable ventilatory support than highly experienced ICU nurses, suggest results of a randomized trial.
The study’s control group received usual care, which means that nurses adjusted mechanical ventilation manually in response to respiratory rate, tidal volume, positive end-respiratory pressure (PEEP), and other factors to maintain ventilation within parameters associated with safe respiration. The experimental group was managed with a fully automated closed-loop system to make these adjustments without any nurse intervention.
For those in the experimental group “the proportion of time in the optimal zone was increased and the proportion of time in the unsafe zone was decreased” relative to those randomized to conventional nursing care, Marcus J. Schultz, MD, reported at the annual congress of the European Respiratory Society.
Conducted at a hospital with an experienced ICU staff, the study had a control arm that was managed by “dedicated nurses who, I can tell you, are very eager to provide the best level of care possible,” said Dr. Schultz, professor of experimental intensive care, University of Amsterdam, the Netherlands..
The investigator-initiated POSITiVE trial randomized 220 cardiac surgery patients scheduled to receive postoperative mechanical ventilation in the ICU. Exclusions included those with class III or higher chronic obstructive pulmonary disease (COPD), a requirement for extracorporeal membrane oxygenation (ECMO), or a history of lung surgery.
The primary endpoint was the proportion of time spent in an optimal zone, an acceptable zone, or a dangerous zone of ventilation based on predefined values for tidal volume, maximum airway pressure, end-tidal CO2, and oxygen saturation (SpO2).
The greatest between-group difference was seen in the proportion of time spent in the optimal zone. This climbed from approximately 35% in the control arm to slightly more than 70% in the experimental arm, a significant difference. The proportion of time in the dangerous zone was reduced from approximately 6% in the control arm to 3% in the automated arm. On average nurse-managed patients spent nearly 60% of the time in the acceptable zone versus less than 30% of those in the automated experimental arm.
A heat map using green, yellow, and red to represent optimal, acceptable, and dangerous zones, respectively, for individual participants in the trial provided a more stark global impression. For the control group, the heat map was primarily yellow with scattered dashes of green and red. For the experimental group, the map was primarily green with dashes of yellow and a much smaller number of red dashes relative to the control group.
In addition, the time to spontaneous breathing was 38% shorter for those randomized to automated ventilation than to conventional care, a significant difference.
There are now many devices marketed for automated ventilation, according to Dr. Schultz. The device used in this study was the proprietary INTELLiVENT-ASV system, marketed by Hamilton Medical, which was selected based on prior satisfactory experience. Although not unique, this system has sophisticated software to adjust ventilation to reach targets set by the clinician on the basis of information it is receiving from physiologic sensors for such variables as respiratory rate, tidal volume, and inspiratory pressure.
“It is frequently adjusting the PEEP levels to reach the lowest driving pressure,” said Dr. Schultz. Among its many other features, it also “gives spontaneous breathing trials automatically.”
Uncomplicated patients were selected purposefully to test this system, but Dr. Schultz said that a second trial, called POSITiVE 2, is now being planned that will enroll more complex patients. Keeping complex patients within the optimal zone as defined by tidal volume and other critical variables has the potential to reduce the lung damage that is known to occur when these are not optimized.
“Applying safe ventilatory support in clinical practice remains a serious challenge and is extremely time consuming,” Dr. Schultz said. He reported that fully automated ventilation appears to be reliable, and “it takes out the human factor” in regard to diligence in monitoring and potential for error.
Overall, these results support the potential for a fully automated system to improve optimal ventilatory support, reduce risk of lung injury, and reduce staffing required for monitoring of mechanical ventilation, according to Dr. Schultz.
Relative costs were not evaluated in this analysis, but might be another factor relevant to the value of fully automated ventilation in ICU patients.
MADRID – In patients managed on mechanical ventilation in an intensive care unit following cardiac surgery, a fully automated system provides more reliable ventilatory support than highly experienced ICU nurses, suggest results of a randomized trial.
The study’s control group received usual care, which means that nurses adjusted mechanical ventilation manually in response to respiratory rate, tidal volume, positive end-respiratory pressure (PEEP), and other factors to maintain ventilation within parameters associated with safe respiration. The experimental group was managed with a fully automated closed-loop system to make these adjustments without any nurse intervention.
For those in the experimental group “the proportion of time in the optimal zone was increased and the proportion of time in the unsafe zone was decreased” relative to those randomized to conventional nursing care, Marcus J. Schultz, MD, reported at the annual congress of the European Respiratory Society.
Conducted at a hospital with an experienced ICU staff, the study had a control arm that was managed by “dedicated nurses who, I can tell you, are very eager to provide the best level of care possible,” said Dr. Schultz, professor of experimental intensive care, University of Amsterdam, the Netherlands..
The investigator-initiated POSITiVE trial randomized 220 cardiac surgery patients scheduled to receive postoperative mechanical ventilation in the ICU. Exclusions included those with class III or higher chronic obstructive pulmonary disease (COPD), a requirement for extracorporeal membrane oxygenation (ECMO), or a history of lung surgery.
The primary endpoint was the proportion of time spent in an optimal zone, an acceptable zone, or a dangerous zone of ventilation based on predefined values for tidal volume, maximum airway pressure, end-tidal CO2, and oxygen saturation (SpO2).
The greatest between-group difference was seen in the proportion of time spent in the optimal zone. This climbed from approximately 35% in the control arm to slightly more than 70% in the experimental arm, a significant difference. The proportion of time in the dangerous zone was reduced from approximately 6% in the control arm to 3% in the automated arm. On average nurse-managed patients spent nearly 60% of the time in the acceptable zone versus less than 30% of those in the automated experimental arm.
A heat map using green, yellow, and red to represent optimal, acceptable, and dangerous zones, respectively, for individual participants in the trial provided a more stark global impression. For the control group, the heat map was primarily yellow with scattered dashes of green and red. For the experimental group, the map was primarily green with dashes of yellow and a much smaller number of red dashes relative to the control group.
In addition, the time to spontaneous breathing was 38% shorter for those randomized to automated ventilation than to conventional care, a significant difference.
There are now many devices marketed for automated ventilation, according to Dr. Schultz. The device used in this study was the proprietary INTELLiVENT-ASV system, marketed by Hamilton Medical, which was selected based on prior satisfactory experience. Although not unique, this system has sophisticated software to adjust ventilation to reach targets set by the clinician on the basis of information it is receiving from physiologic sensors for such variables as respiratory rate, tidal volume, and inspiratory pressure.
“It is frequently adjusting the PEEP levels to reach the lowest driving pressure,” said Dr. Schultz. Among its many other features, it also “gives spontaneous breathing trials automatically.”
Uncomplicated patients were selected purposefully to test this system, but Dr. Schultz said that a second trial, called POSITiVE 2, is now being planned that will enroll more complex patients. Keeping complex patients within the optimal zone as defined by tidal volume and other critical variables has the potential to reduce the lung damage that is known to occur when these are not optimized.
“Applying safe ventilatory support in clinical practice remains a serious challenge and is extremely time consuming,” Dr. Schultz said. He reported that fully automated ventilation appears to be reliable, and “it takes out the human factor” in regard to diligence in monitoring and potential for error.
Overall, these results support the potential for a fully automated system to improve optimal ventilatory support, reduce risk of lung injury, and reduce staffing required for monitoring of mechanical ventilation, according to Dr. Schultz.
Relative costs were not evaluated in this analysis, but might be another factor relevant to the value of fully automated ventilation in ICU patients.
REPORTING FROM ERS 2019
Short-term statin use linked to risk of skin and soft tissue infections
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
BP screening nearly universal among Medicare enrollees
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
Congenital heart disease in children linked to increased autism risk
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
FROM PEDIATRICS
Key clinical point: Children born with congenital heart disease have higher odds of developing autism, especially with certain forms of CHD, such as atrial and ventricular septal defects.
Major finding: After sensitivity analysis, children with congenital heart disease had increased odds of autism, compared with controls (odds ratio, 1.32; 95% confidence interval, 1.10-1.59).
Study details: A case-control study of children enrolled in the U.S. Military Health System from 2001 to 2013.
Disclosures: The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
Source: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
Poll: New Algorithm for PE
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: A Better Approach to the Diagnosis of PE.
[polldaddy:10428150]
Click on page 2 below to find out what the correct answer is...
The correct answer is b.) 14%
To learn more, see this month's PURLs: A Better Approach to the Diagnosis of PE.
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: A Better Approach to the Diagnosis of PE.
[polldaddy:10428150]
Click on page 2 below to find out what the correct answer is...
The correct answer is b.) 14%
To learn more, see this month's PURLs: A Better Approach to the Diagnosis of PE.
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: A Better Approach to the Diagnosis of PE.
[polldaddy:10428150]
Click on page 2 below to find out what the correct answer is...
The correct answer is b.) 14%
To learn more, see this month's PURLs: A Better Approach to the Diagnosis of PE.
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
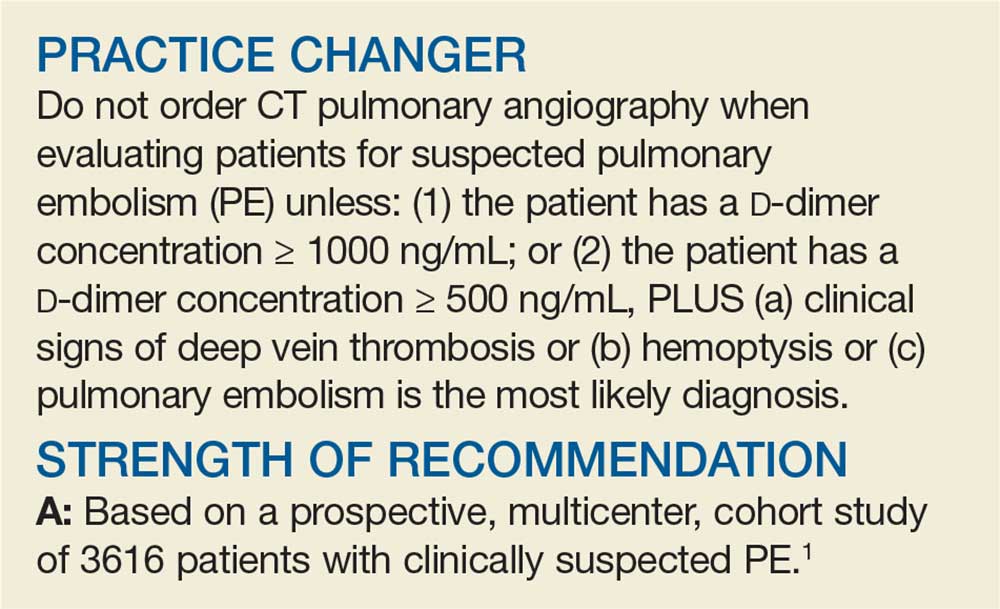
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Study questions preemptive TEVAR for extended type A dissections
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Enough Fuss; She Wants Lunch!
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
During morning rounds at a skilled nursing facility (SNF), a 74-year-old woman is found to have a rapid heart rate. She is placed on telemetry, which reveals a wide complex tachycardia. Concerned about possible ventricular tachycardia, the charge nurse contacts the on-call physician, who recommends calling 911. The patient is transferred via ACLS ambulance to your facility.
When you see her, she seems embarrassed by all the attention she’s receiving and expresses her desire to return to the SNF before she misses lunch. She is in no pain or discomfort, is not particularly short of breath, and does not feel dizzy or lightheaded. According to reports, she was friendly and conversive with both the nursing staff at the SNF and the paramedics during transport.
History is remarkable for several transient ischemic attacks with no residual sequelae, hypertension (under good control), and hypothyroidism (treatedwith medication). Surgical history includes a hyster-ectomy, a cholecystectomy, and an open reduction and metal plate fixation of a high (right) ankle break—all of which were performed more than 10 years ago.
Her medications include warfarin, hydrochlorothiazide, atorvastatin, and levothyroxine. She has no known drug allergies.
The patient is a retired junior high school principal. Her husband died of lung cancer 4 years ago. She has 3 adult children who are all in good health. She has never smoked but does enjoy a daily nightcap. She denies alcohol abuse or illicit drug use.
Family history reveals her parents died in a train accident and her paternal grandparents died of tuberculosis. She does not know her maternal grandparents’ medical history.
Review of systems is positive for chronic constipation and chronic hip and knee discomfort. Vital signs include a blood pressure of 124/88 mm Hg; pulse, 140 beats/min; respiratory rate, 14 breaths/min; and temperature, 97.6°F. Her weight is 158 lb, and her height is not measured.
Physical exam reveals a pleasant elderly woman in no distress. She is dressed appropriately, her hair is styled, and she is wearing makeup as she usually does. The HEENT exam reveals hearing aids and corrective lenses. Her neck has no jugular venous distention, carotid bruits, or thyromegaly.
Her lungs are clear in all fields. Her heart has a rapid and questionably irregular rhythm. There are no appreciable murmurs or rubs. Her abdominal exam is normal, with the exception of well-healed surgical scars. There is no peripheral edema, and all pulses are equal bilaterally in both upper and lower extremities. The neurologic exam is grossly normal with normal affect and mood.
An ECG reveals a ventricular rate of 152 beats/min; PR interval, 128 ms; QRS duration, 88 ms; QT/QTc interval, 280/445 ms; P axis, 27°; R axis, 23°; and T axis, 232°. What is your interpretation?
TAVR, SAVR share same infective endocarditis risk
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
REPORTING FROM THE ESC CONGRESS 2019