User login
Novel cardiac troponin protocol rapidly rules out MI
PARIS – An accelerated rule-out pathway, reliant upon a single high-sensitivity cardiac troponin test upon presentation to the ED with suspected acute coronary syndrome, reduced length of stay and hospital admission rates without increasing cardiac events at 30 days or 1 year in a major Scottish study.
“We conclude that implementation of this early rule-out pathway is both effective and safe, and adoption of this pathway will have major benefits for patients and health care systems,” Nicholas L. Mills, MBChB, PhD, said in presenting the results of the HiSTORIC (High-Sensitivity Cardiac Troponin at Presentation to Rule Out Myocardial Infarction) trial at the annual congress of the European Society of Cardiology.
Indeed, in the Unites States, where more than 20 million people per year present to EDs with suspected ACS, the 3.3-hour reduction in length of stay achieved in the HiSTORIC trial by implementing the accelerated rule-out pathway would add up to a $3.6 billion annual savings in bed occupancy alone, according to Dr. Mills, who is chair of cardiology at the University of Edinburgh.
The HiSTORIC pathway incorporates separate thresholds for risk stratification and diagnosis. This strategy is based on an accumulation of persuasive evidence that the major advantage of high-sensitivity cardiac troponin testing is to rule out MI, rather than to rule it in, Dr. Mills explained.
HiSTORIC was a 2-year, prospective, stepped-wedge, cluster-randomized, controlled trial including 31,492 consecutive patients with suspected ACS who presented to seven participating hospitals in Scotland. Patients were randomized, at the hospital level, to one of two management pathways. The control group got a standard guideline-recommended strategy involving high-sensitivity cardiac troponin I testing upon presentation and again 6-12 hours later, with MI being ruled out if the troponin levels were not above the 99th percentile.
In contrast, the novel early rule-out strategy worked as follows: If the patient presented with at least 2 hours of symptoms and the initial troponin I level was below 5 ng/L, then MI was ruled out and the patient was triaged straightaway for outpatient management. If the level was above the 99th percentile, the patient was admitted for serial testing to be done 6-12 hours after symptom onset. And for an intermediate test result – that is, a troponin level between 5 ng/L and the 99th percentile – patients remained in the ED for retesting 3 hours from the time of presentation, and were subsequently admitted only if their troponin level was rising.
Using the accelerated rule-out strategy, two-thirds of patients were quickly discharged from the ED on the basis of a troponin level below 5 ng/mL, and another 7% were ruled out for MI and discharged from the ED after a 3-hour stay on the basis of their second test.
The primary efficacy outcome was length of stay from initial presentation to the ED to discharge. The duration was 10.1 hours with the guideline-recommended pathway and 6.8 hours with the accelerated rule-out pathway, for a statistically significant and clinically meaningful 3.3-hour difference. Moreover, the proportion of patients discharged directly from the ED without hospital admission increased from 53% to 74%, a 57% jump.
The primary safety outcome was the rate of MI or cardiac death post discharge. The rates at 30 days and 1 year were 0.4% and 2.6%, respectively, in the standard-pathway group, compared with 0.3% and 1.8% with the early rule-out pathway. Those between-group differences favoring the accelerated rule-out pathway weren’t statistically significant, but they provided reassurance that the novel pathway was safe.
Of note, this was the first-ever randomized trial to evaluate the safety and efficacy of an early rule-out pathway. Other rapid diagnostic pathways are largely based on observational experience and expert opinion, Dr. Mills said.
The assay utilized in the HiSTORIC trial was the Abbott Diagnostics Architect high sensitivity assay. The 5-ng/L threshold for early rule-out was chosen for the trial because an earlier study by Dr. Mills and coinvestigators showed that a level below that cutoff had a 99.6% negative predictive value for MI (Lancet. 2015 Dec 19;386[10012]:2481-8)
The early rule-out pathway was deliberately designed to be simple and pragmatic, according to the cardiologist. “One of the most remarkable observations in this trial was the adherence to the pathway. We prespecified three criteria to evaluate this and demonstrated adherence rates of 86%-92% for each of these criteria. This was despite the pathway being implemented in all consecutive patients at seven different hospitals and used by many hundreds of different clinicians.”
Discussant Hugo A. Katus, MD, called the HiSTORIC study “a really urgently needed and very well-conducted trial.”
“There were very consistently low MI and cardiac death rates at 30 days and 1 year. So this really works,” commented Dr. Katus, who is chief of internal medicine and director of the department of cardiovascular medicine at Heidelberg (Germany) University.
“Accelerated rule-out high-sensitivity cardiac troponin protocols are here to stay,” he declared.
However, Dr. Katus voiced a concern: “By early discharge as rule out, are other life-threatening conditions ignored?”
He raised this issue because of what he views as the substantial 1-year all-cause mortality and return-to-hospital rates of 5.8% and 39.2% in the standard-pathway group and 5.2% and 38.9% in the accelerated rule-out patients in HiSTORIC. An accelerated rule-out strategy should not prohibit a careful clinical work-up, he emphasized.
Dr. Mills discussed the results in a video interview.
The HiSTORIC trial was funded by the British Heart Foundation. Dr. Mills reported receiving research grants from Abbott Diagnostics and Siemens.
Simultaneous with Dr. Mills’ presentation of the HiSTORIC trial results at the ESC congress, an earlier study that formed the scientific basis for the investigators’ decision to employ distinct risk stratification and diagnostic thresholds for cardiac troponin testing was published online (Circulation. 2019 Sep 1. doi: 10.1161/CIRCULATIONAHA.119.042866). The actual HiSTORIC trial results will be published later.
Dr. Katus reported holding a patent for a cardiac troponin T test and serving as a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk.
PARIS – An accelerated rule-out pathway, reliant upon a single high-sensitivity cardiac troponin test upon presentation to the ED with suspected acute coronary syndrome, reduced length of stay and hospital admission rates without increasing cardiac events at 30 days or 1 year in a major Scottish study.
“We conclude that implementation of this early rule-out pathway is both effective and safe, and adoption of this pathway will have major benefits for patients and health care systems,” Nicholas L. Mills, MBChB, PhD, said in presenting the results of the HiSTORIC (High-Sensitivity Cardiac Troponin at Presentation to Rule Out Myocardial Infarction) trial at the annual congress of the European Society of Cardiology.
Indeed, in the Unites States, where more than 20 million people per year present to EDs with suspected ACS, the 3.3-hour reduction in length of stay achieved in the HiSTORIC trial by implementing the accelerated rule-out pathway would add up to a $3.6 billion annual savings in bed occupancy alone, according to Dr. Mills, who is chair of cardiology at the University of Edinburgh.
The HiSTORIC pathway incorporates separate thresholds for risk stratification and diagnosis. This strategy is based on an accumulation of persuasive evidence that the major advantage of high-sensitivity cardiac troponin testing is to rule out MI, rather than to rule it in, Dr. Mills explained.
HiSTORIC was a 2-year, prospective, stepped-wedge, cluster-randomized, controlled trial including 31,492 consecutive patients with suspected ACS who presented to seven participating hospitals in Scotland. Patients were randomized, at the hospital level, to one of two management pathways. The control group got a standard guideline-recommended strategy involving high-sensitivity cardiac troponin I testing upon presentation and again 6-12 hours later, with MI being ruled out if the troponin levels were not above the 99th percentile.
In contrast, the novel early rule-out strategy worked as follows: If the patient presented with at least 2 hours of symptoms and the initial troponin I level was below 5 ng/L, then MI was ruled out and the patient was triaged straightaway for outpatient management. If the level was above the 99th percentile, the patient was admitted for serial testing to be done 6-12 hours after symptom onset. And for an intermediate test result – that is, a troponin level between 5 ng/L and the 99th percentile – patients remained in the ED for retesting 3 hours from the time of presentation, and were subsequently admitted only if their troponin level was rising.
Using the accelerated rule-out strategy, two-thirds of patients were quickly discharged from the ED on the basis of a troponin level below 5 ng/mL, and another 7% were ruled out for MI and discharged from the ED after a 3-hour stay on the basis of their second test.
The primary efficacy outcome was length of stay from initial presentation to the ED to discharge. The duration was 10.1 hours with the guideline-recommended pathway and 6.8 hours with the accelerated rule-out pathway, for a statistically significant and clinically meaningful 3.3-hour difference. Moreover, the proportion of patients discharged directly from the ED without hospital admission increased from 53% to 74%, a 57% jump.
The primary safety outcome was the rate of MI or cardiac death post discharge. The rates at 30 days and 1 year were 0.4% and 2.6%, respectively, in the standard-pathway group, compared with 0.3% and 1.8% with the early rule-out pathway. Those between-group differences favoring the accelerated rule-out pathway weren’t statistically significant, but they provided reassurance that the novel pathway was safe.
Of note, this was the first-ever randomized trial to evaluate the safety and efficacy of an early rule-out pathway. Other rapid diagnostic pathways are largely based on observational experience and expert opinion, Dr. Mills said.
The assay utilized in the HiSTORIC trial was the Abbott Diagnostics Architect high sensitivity assay. The 5-ng/L threshold for early rule-out was chosen for the trial because an earlier study by Dr. Mills and coinvestigators showed that a level below that cutoff had a 99.6% negative predictive value for MI (Lancet. 2015 Dec 19;386[10012]:2481-8)
The early rule-out pathway was deliberately designed to be simple and pragmatic, according to the cardiologist. “One of the most remarkable observations in this trial was the adherence to the pathway. We prespecified three criteria to evaluate this and demonstrated adherence rates of 86%-92% for each of these criteria. This was despite the pathway being implemented in all consecutive patients at seven different hospitals and used by many hundreds of different clinicians.”
Discussant Hugo A. Katus, MD, called the HiSTORIC study “a really urgently needed and very well-conducted trial.”
“There were very consistently low MI and cardiac death rates at 30 days and 1 year. So this really works,” commented Dr. Katus, who is chief of internal medicine and director of the department of cardiovascular medicine at Heidelberg (Germany) University.
“Accelerated rule-out high-sensitivity cardiac troponin protocols are here to stay,” he declared.
However, Dr. Katus voiced a concern: “By early discharge as rule out, are other life-threatening conditions ignored?”
He raised this issue because of what he views as the substantial 1-year all-cause mortality and return-to-hospital rates of 5.8% and 39.2% in the standard-pathway group and 5.2% and 38.9% in the accelerated rule-out patients in HiSTORIC. An accelerated rule-out strategy should not prohibit a careful clinical work-up, he emphasized.
Dr. Mills discussed the results in a video interview.
The HiSTORIC trial was funded by the British Heart Foundation. Dr. Mills reported receiving research grants from Abbott Diagnostics and Siemens.
Simultaneous with Dr. Mills’ presentation of the HiSTORIC trial results at the ESC congress, an earlier study that formed the scientific basis for the investigators’ decision to employ distinct risk stratification and diagnostic thresholds for cardiac troponin testing was published online (Circulation. 2019 Sep 1. doi: 10.1161/CIRCULATIONAHA.119.042866). The actual HiSTORIC trial results will be published later.
Dr. Katus reported holding a patent for a cardiac troponin T test and serving as a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk.
PARIS – An accelerated rule-out pathway, reliant upon a single high-sensitivity cardiac troponin test upon presentation to the ED with suspected acute coronary syndrome, reduced length of stay and hospital admission rates without increasing cardiac events at 30 days or 1 year in a major Scottish study.
“We conclude that implementation of this early rule-out pathway is both effective and safe, and adoption of this pathway will have major benefits for patients and health care systems,” Nicholas L. Mills, MBChB, PhD, said in presenting the results of the HiSTORIC (High-Sensitivity Cardiac Troponin at Presentation to Rule Out Myocardial Infarction) trial at the annual congress of the European Society of Cardiology.
Indeed, in the Unites States, where more than 20 million people per year present to EDs with suspected ACS, the 3.3-hour reduction in length of stay achieved in the HiSTORIC trial by implementing the accelerated rule-out pathway would add up to a $3.6 billion annual savings in bed occupancy alone, according to Dr. Mills, who is chair of cardiology at the University of Edinburgh.
The HiSTORIC pathway incorporates separate thresholds for risk stratification and diagnosis. This strategy is based on an accumulation of persuasive evidence that the major advantage of high-sensitivity cardiac troponin testing is to rule out MI, rather than to rule it in, Dr. Mills explained.
HiSTORIC was a 2-year, prospective, stepped-wedge, cluster-randomized, controlled trial including 31,492 consecutive patients with suspected ACS who presented to seven participating hospitals in Scotland. Patients were randomized, at the hospital level, to one of two management pathways. The control group got a standard guideline-recommended strategy involving high-sensitivity cardiac troponin I testing upon presentation and again 6-12 hours later, with MI being ruled out if the troponin levels were not above the 99th percentile.
In contrast, the novel early rule-out strategy worked as follows: If the patient presented with at least 2 hours of symptoms and the initial troponin I level was below 5 ng/L, then MI was ruled out and the patient was triaged straightaway for outpatient management. If the level was above the 99th percentile, the patient was admitted for serial testing to be done 6-12 hours after symptom onset. And for an intermediate test result – that is, a troponin level between 5 ng/L and the 99th percentile – patients remained in the ED for retesting 3 hours from the time of presentation, and were subsequently admitted only if their troponin level was rising.
Using the accelerated rule-out strategy, two-thirds of patients were quickly discharged from the ED on the basis of a troponin level below 5 ng/mL, and another 7% were ruled out for MI and discharged from the ED after a 3-hour stay on the basis of their second test.
The primary efficacy outcome was length of stay from initial presentation to the ED to discharge. The duration was 10.1 hours with the guideline-recommended pathway and 6.8 hours with the accelerated rule-out pathway, for a statistically significant and clinically meaningful 3.3-hour difference. Moreover, the proportion of patients discharged directly from the ED without hospital admission increased from 53% to 74%, a 57% jump.
The primary safety outcome was the rate of MI or cardiac death post discharge. The rates at 30 days and 1 year were 0.4% and 2.6%, respectively, in the standard-pathway group, compared with 0.3% and 1.8% with the early rule-out pathway. Those between-group differences favoring the accelerated rule-out pathway weren’t statistically significant, but they provided reassurance that the novel pathway was safe.
Of note, this was the first-ever randomized trial to evaluate the safety and efficacy of an early rule-out pathway. Other rapid diagnostic pathways are largely based on observational experience and expert opinion, Dr. Mills said.
The assay utilized in the HiSTORIC trial was the Abbott Diagnostics Architect high sensitivity assay. The 5-ng/L threshold for early rule-out was chosen for the trial because an earlier study by Dr. Mills and coinvestigators showed that a level below that cutoff had a 99.6% negative predictive value for MI (Lancet. 2015 Dec 19;386[10012]:2481-8)
The early rule-out pathway was deliberately designed to be simple and pragmatic, according to the cardiologist. “One of the most remarkable observations in this trial was the adherence to the pathway. We prespecified three criteria to evaluate this and demonstrated adherence rates of 86%-92% for each of these criteria. This was despite the pathway being implemented in all consecutive patients at seven different hospitals and used by many hundreds of different clinicians.”
Discussant Hugo A. Katus, MD, called the HiSTORIC study “a really urgently needed and very well-conducted trial.”
“There were very consistently low MI and cardiac death rates at 30 days and 1 year. So this really works,” commented Dr. Katus, who is chief of internal medicine and director of the department of cardiovascular medicine at Heidelberg (Germany) University.
“Accelerated rule-out high-sensitivity cardiac troponin protocols are here to stay,” he declared.
However, Dr. Katus voiced a concern: “By early discharge as rule out, are other life-threatening conditions ignored?”
He raised this issue because of what he views as the substantial 1-year all-cause mortality and return-to-hospital rates of 5.8% and 39.2% in the standard-pathway group and 5.2% and 38.9% in the accelerated rule-out patients in HiSTORIC. An accelerated rule-out strategy should not prohibit a careful clinical work-up, he emphasized.
Dr. Mills discussed the results in a video interview.
The HiSTORIC trial was funded by the British Heart Foundation. Dr. Mills reported receiving research grants from Abbott Diagnostics and Siemens.
Simultaneous with Dr. Mills’ presentation of the HiSTORIC trial results at the ESC congress, an earlier study that formed the scientific basis for the investigators’ decision to employ distinct risk stratification and diagnostic thresholds for cardiac troponin testing was published online (Circulation. 2019 Sep 1. doi: 10.1161/CIRCULATIONAHA.119.042866). The actual HiSTORIC trial results will be published later.
Dr. Katus reported holding a patent for a cardiac troponin T test and serving as a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk.
REPORTING FROM THE ESC CONGRESS 2019
Clinical Pharmacists Improve Patient Outcomes and Expand Access to Care
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
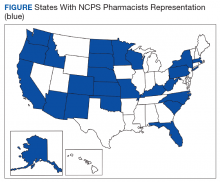
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
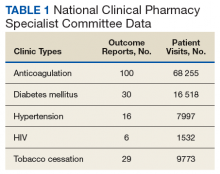
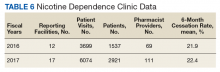
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
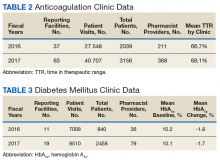
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
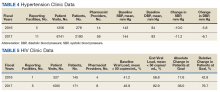
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
Smoking, inactivity most powerful post-MI lifestyle risk factors
PARIS – All lifestyle-related cardiovascular risk factors aren’t equal in power when it comes to secondary prevention after a first acute MI, according to a massive Swedish registry study.
Insufficient physical activity and current smoking were consistently the strongest risk factors for all-cause mortality, major adverse cardiovascular events, and other key adverse outcomes in an analysis from the SWEDEHEART registry. The study included 65,002 patients discharged after a first MI and 325,010 age- and sex-matched controls with no prior MI followed for a median of 5.5 years and maximum of 12, Emil Hagstrom, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Strongest lifestyle risk factors
The study examined the long-term relative importance of control of six major lifestyle risk factors for secondary cardiovascular prevention: current smoking, insufficient physical activity, blood pressure of 140/90 mm Hg or more, obesity, a fasting blood glucose of at least 126 mg/dL, and an LDL cholesterol of 70 mg/dL or more. Notably, two risk factors that physicians often emphasize in working with their patients with known coronary heart disease – an elevated LDL cholesterol and obesity – barely moved the needle. Out of the six risk factors scrutinized, those two consistently showed the weakest association with long-term risk of adverse outcomes. Occupying the middle ground in terms of predictive strength were hypertension and elevated blood glucose, according to Dr. Hagstrom, a cardiologist at Uppsala (Sweden) University.
Risk factor status was assessed 6-10 weeks post MI. Insufficient physical activity was defined as not engaging in at least 30 minutes of moderate-intensity exercise on at least 5 days per week. And when Dr. Hagstrom recalculated the risk of adverse outcomes using an LDL cholesterol threshold of 55 mg/dL rather than using 70 mg/dL, as recommended in new ESC secondary prevention guidelines released during the congress, the study results remained unchanged.
Cumulative effects
A key SWEDEHEART finding underscoring the importance of lifestyle in secondary prevention was that a linear stepwise relationship existed between the number of risk factors at target levels and the risk of all of the various adverse outcomes assessed, including stroke and heart failure hospitalization as well as all-cause mortality, cardiovascular mortality, and major bleeding.
Moreover, patients with none of the six risk factors outside of target when assessed after their MI had the same risks of all-cause mortality, cardiovascular mortality, and stroke as the matched controls.
For example, in an analysis adjusted for comorbid cancer, chronic obstructive pulmonary disease, and dementia, post-MI patients with zero risk factors had the same long-term risk of cardiovascular mortality as controls without a history of MI at baseline. With one risk factor not at target, a patient had a 41% increased risk compared with controls, a statistically significant difference. With two out-of-whack risk factors, the risk climbed to 102%. With three, 185%. With four risk factors not at target, the all-cause mortality risk jumped to 291%. And patients with more than four of the six risk factors not at target had a 409% greater risk of all-cause mortality than controls who had never had a heart attack.
When Dr. Hagstrom stratified subjects by age at baseline – up to 55, 56-64, 65-70, and 70-75 years – he discovered that, regardless of age, patients with zero risk factors had the same risk of all-cause mortality and other adverse outcomes as controls. However, when risk factors were present, younger patients consistently had a higher risk of all adverse outcomes than older patients with the same number of risk factors. When asked for an explanation of this phenomenon, Dr. Hagstrom noted that younger patients with multiple risk factors have a longer time to be exposed to and accumulate risk.
Follow-up of the study cohort will continue for years to come, the cardiologist promised.
At an ESC congress highlights session that closed out the meeting, Eva Prescott, MD, put the SWEDEHEART study at the top of her list of important developments in preventive cardiology arising from the congress.
“This is an excellent national registry I think we’re all envious of,” commented Dr. Prescott, a cardiologist at Copenhagen University. “The conclusion of this registry-based data, I think, is that lifestyle really remains at the core of prevention of cardiovascular events still today.”
The SWEDEHEART study analysis was funded free of commercial support. Dr. Hagstrom reported serving as a consultant to or receiving speakers’ fees from Amgen, AstraZeneca, Bayer, Novo Nordisk, and Sanofi.
PARIS – All lifestyle-related cardiovascular risk factors aren’t equal in power when it comes to secondary prevention after a first acute MI, according to a massive Swedish registry study.
Insufficient physical activity and current smoking were consistently the strongest risk factors for all-cause mortality, major adverse cardiovascular events, and other key adverse outcomes in an analysis from the SWEDEHEART registry. The study included 65,002 patients discharged after a first MI and 325,010 age- and sex-matched controls with no prior MI followed for a median of 5.5 years and maximum of 12, Emil Hagstrom, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Strongest lifestyle risk factors
The study examined the long-term relative importance of control of six major lifestyle risk factors for secondary cardiovascular prevention: current smoking, insufficient physical activity, blood pressure of 140/90 mm Hg or more, obesity, a fasting blood glucose of at least 126 mg/dL, and an LDL cholesterol of 70 mg/dL or more. Notably, two risk factors that physicians often emphasize in working with their patients with known coronary heart disease – an elevated LDL cholesterol and obesity – barely moved the needle. Out of the six risk factors scrutinized, those two consistently showed the weakest association with long-term risk of adverse outcomes. Occupying the middle ground in terms of predictive strength were hypertension and elevated blood glucose, according to Dr. Hagstrom, a cardiologist at Uppsala (Sweden) University.
Risk factor status was assessed 6-10 weeks post MI. Insufficient physical activity was defined as not engaging in at least 30 minutes of moderate-intensity exercise on at least 5 days per week. And when Dr. Hagstrom recalculated the risk of adverse outcomes using an LDL cholesterol threshold of 55 mg/dL rather than using 70 mg/dL, as recommended in new ESC secondary prevention guidelines released during the congress, the study results remained unchanged.
Cumulative effects
A key SWEDEHEART finding underscoring the importance of lifestyle in secondary prevention was that a linear stepwise relationship existed between the number of risk factors at target levels and the risk of all of the various adverse outcomes assessed, including stroke and heart failure hospitalization as well as all-cause mortality, cardiovascular mortality, and major bleeding.
Moreover, patients with none of the six risk factors outside of target when assessed after their MI had the same risks of all-cause mortality, cardiovascular mortality, and stroke as the matched controls.
For example, in an analysis adjusted for comorbid cancer, chronic obstructive pulmonary disease, and dementia, post-MI patients with zero risk factors had the same long-term risk of cardiovascular mortality as controls without a history of MI at baseline. With one risk factor not at target, a patient had a 41% increased risk compared with controls, a statistically significant difference. With two out-of-whack risk factors, the risk climbed to 102%. With three, 185%. With four risk factors not at target, the all-cause mortality risk jumped to 291%. And patients with more than four of the six risk factors not at target had a 409% greater risk of all-cause mortality than controls who had never had a heart attack.
When Dr. Hagstrom stratified subjects by age at baseline – up to 55, 56-64, 65-70, and 70-75 years – he discovered that, regardless of age, patients with zero risk factors had the same risk of all-cause mortality and other adverse outcomes as controls. However, when risk factors were present, younger patients consistently had a higher risk of all adverse outcomes than older patients with the same number of risk factors. When asked for an explanation of this phenomenon, Dr. Hagstrom noted that younger patients with multiple risk factors have a longer time to be exposed to and accumulate risk.
Follow-up of the study cohort will continue for years to come, the cardiologist promised.
At an ESC congress highlights session that closed out the meeting, Eva Prescott, MD, put the SWEDEHEART study at the top of her list of important developments in preventive cardiology arising from the congress.
“This is an excellent national registry I think we’re all envious of,” commented Dr. Prescott, a cardiologist at Copenhagen University. “The conclusion of this registry-based data, I think, is that lifestyle really remains at the core of prevention of cardiovascular events still today.”
The SWEDEHEART study analysis was funded free of commercial support. Dr. Hagstrom reported serving as a consultant to or receiving speakers’ fees from Amgen, AstraZeneca, Bayer, Novo Nordisk, and Sanofi.
PARIS – All lifestyle-related cardiovascular risk factors aren’t equal in power when it comes to secondary prevention after a first acute MI, according to a massive Swedish registry study.
Insufficient physical activity and current smoking were consistently the strongest risk factors for all-cause mortality, major adverse cardiovascular events, and other key adverse outcomes in an analysis from the SWEDEHEART registry. The study included 65,002 patients discharged after a first MI and 325,010 age- and sex-matched controls with no prior MI followed for a median of 5.5 years and maximum of 12, Emil Hagstrom, MD, PhD, reported at the annual congress of the European Society of Cardiology.
Strongest lifestyle risk factors
The study examined the long-term relative importance of control of six major lifestyle risk factors for secondary cardiovascular prevention: current smoking, insufficient physical activity, blood pressure of 140/90 mm Hg or more, obesity, a fasting blood glucose of at least 126 mg/dL, and an LDL cholesterol of 70 mg/dL or more. Notably, two risk factors that physicians often emphasize in working with their patients with known coronary heart disease – an elevated LDL cholesterol and obesity – barely moved the needle. Out of the six risk factors scrutinized, those two consistently showed the weakest association with long-term risk of adverse outcomes. Occupying the middle ground in terms of predictive strength were hypertension and elevated blood glucose, according to Dr. Hagstrom, a cardiologist at Uppsala (Sweden) University.
Risk factor status was assessed 6-10 weeks post MI. Insufficient physical activity was defined as not engaging in at least 30 minutes of moderate-intensity exercise on at least 5 days per week. And when Dr. Hagstrom recalculated the risk of adverse outcomes using an LDL cholesterol threshold of 55 mg/dL rather than using 70 mg/dL, as recommended in new ESC secondary prevention guidelines released during the congress, the study results remained unchanged.
Cumulative effects
A key SWEDEHEART finding underscoring the importance of lifestyle in secondary prevention was that a linear stepwise relationship existed between the number of risk factors at target levels and the risk of all of the various adverse outcomes assessed, including stroke and heart failure hospitalization as well as all-cause mortality, cardiovascular mortality, and major bleeding.
Moreover, patients with none of the six risk factors outside of target when assessed after their MI had the same risks of all-cause mortality, cardiovascular mortality, and stroke as the matched controls.
For example, in an analysis adjusted for comorbid cancer, chronic obstructive pulmonary disease, and dementia, post-MI patients with zero risk factors had the same long-term risk of cardiovascular mortality as controls without a history of MI at baseline. With one risk factor not at target, a patient had a 41% increased risk compared with controls, a statistically significant difference. With two out-of-whack risk factors, the risk climbed to 102%. With three, 185%. With four risk factors not at target, the all-cause mortality risk jumped to 291%. And patients with more than four of the six risk factors not at target had a 409% greater risk of all-cause mortality than controls who had never had a heart attack.
When Dr. Hagstrom stratified subjects by age at baseline – up to 55, 56-64, 65-70, and 70-75 years – he discovered that, regardless of age, patients with zero risk factors had the same risk of all-cause mortality and other adverse outcomes as controls. However, when risk factors were present, younger patients consistently had a higher risk of all adverse outcomes than older patients with the same number of risk factors. When asked for an explanation of this phenomenon, Dr. Hagstrom noted that younger patients with multiple risk factors have a longer time to be exposed to and accumulate risk.
Follow-up of the study cohort will continue for years to come, the cardiologist promised.
At an ESC congress highlights session that closed out the meeting, Eva Prescott, MD, put the SWEDEHEART study at the top of her list of important developments in preventive cardiology arising from the congress.
“This is an excellent national registry I think we’re all envious of,” commented Dr. Prescott, a cardiologist at Copenhagen University. “The conclusion of this registry-based data, I think, is that lifestyle really remains at the core of prevention of cardiovascular events still today.”
The SWEDEHEART study analysis was funded free of commercial support. Dr. Hagstrom reported serving as a consultant to or receiving speakers’ fees from Amgen, AstraZeneca, Bayer, Novo Nordisk, and Sanofi.
REPORTING FROM THE ESC CONGRESS 2019
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
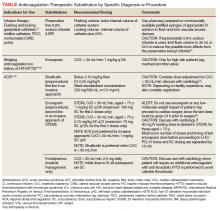
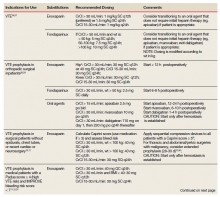
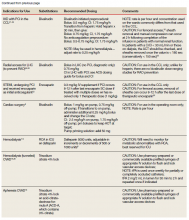
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Moderate aortic stenosis just as deadly as severe AS
PARIS – The 5-year mortality rate associated with untreated moderate aortic stenosis is just as grim as it is for severe aortic stenosis, according to new findings from the largest-ever study of the natural history of aortic stenosis.
“These data provide a clear signal of the expected adverse outcomes for individuals presenting across the globe with a mean aortic valve gradient greater than 20.0 mm Hg or a peak aortic valve velocity above 3.0 m/sec,” Geoff Strange, PhD, said in presenting an analysis from NEDA, the National Echocardiography Database of Australia, at the annual congress of the European Society of Cardiology.
These results, if confirmed in other large datasets, could potentially have enormous implications for the use of transcatheter and surgical aortic valve replacement, interventions which until now have been restricted to patients with severe aortic stenosis (AS) as defined by an aortic valve (AV) mean gradient in excess of 40 mm Hg or a peak AV velocity greater than 4.0 m/sec. This restriction was based on what Dr. Strange considers rather limited and flimsy evidence suggesting that the mortality associated with AS was negligible except in severe AS.
“Cut points used to stratify for interventional strategies are based on very small numbers,” observed Dr. Strange, professor of medicine at the University of Notre Dame in Fremantle, Australia.
The NEDA findings, he added, constitute a call to action: “These data provide the impetus for a contemporary evaluation of the risk-to-benefit ratio of intervention in the moderate AS population,” Dr. Strange declared.
He and his NEDA coinvestigators analyzed echocardiographic data on 241,303 individuals in the Australian database, zeroing in on the 25,827 with untreated mild, moderate, or severe native valve AS. To place the size and scope of this project into perspective, the next-largest study of the natural history of untreated AS included 1,375 individuals – and that study was in turn roughly 10-fold bigger than the handful of other published studies addressing this issue.
A key finding in the NEDA study was that the 5-year all-cause mortality rate of 61.4% in the group with moderate AS wasn’t significantly different from the 64.6% rate in those with severe AS (see graphic).
The investigators performed additional analyses, analyzing peak velocity and mean gradient as continuous variables and stratifying patients into quintiles on that basis. They found that the top quintile for AV velocity started very low, at 1.73 m/sec, while the top quintile for mean AV gradient also started at a surprisingly low level: greater than 9.6 mm Hg. They noted that both all-cause and cardiovascular-specific mortality rates were basically flat until taking what Dr. Strange described as “a sharp pivot point upward” right around 20 mm Hg or 3 m/sec.
“No matter how we looked at these data – whether we looked at patients with or without left heart disease, whether we used the dimensionless index, whether we adjusted for stroke volume index, whether we stratified between age above or below 65, whether we used the gradient, the velocity, or the AV area – this threshold of increasing mortality at around 20 mm Hg or 3 m/sec continued to emerge,” according to Dr. Strange.
He noted that this study used real-world data with hard endpoints – actuarial patient mortality outcomes obtained through linkage to the national database – rather than hypothetical projections based upon Kaplan-Meier curves. A study limitation was that comorbidity data couldn’t be obtained for the AS patients.
Session cochair Patrizio Lancellotti, MD, PhD, commented, “I think this study will change a bit our consideration about patients with moderate AS.”
However, Dr. Lancellotti, who was lead author of the second-largest study of the natural history of aortic stenosis (JAMA Cardiol. 2018 Nov 1;3[11]:1060-8), expressed misgivings about the NEDA system’s lack of a core echocardiographic laboratory for imaging adjudication. That’s a study weakness given that image quality and the accuracy of echocardiographic interpretation are so highly dependent upon an individual cardiologist’s skill, observed Dr. Lancellotti, who is head of cardiology at the University of Liege (Belgium).
Dr. Strange replied that he and his coinvestigators analyzed a random subset of the NEDA data and found very little interlaboratory variability in results.
“All I can say is that the labs that contributed to this study are the most eminent labs across Australia,” he added.
Simultaneously with Dr. Strange’s presentation at the congress, the NEDA study results were published online (J Am Coll Cardiol. Sep 2019. doi: 10.1016/j.jacc.2019.08.004).
Dr. Strange reported having no financial conflicts of interest regarding the NEDA project, which is funded by GlaxoSmithKline, Bayer, and Actelion.
PARIS – The 5-year mortality rate associated with untreated moderate aortic stenosis is just as grim as it is for severe aortic stenosis, according to new findings from the largest-ever study of the natural history of aortic stenosis.
“These data provide a clear signal of the expected adverse outcomes for individuals presenting across the globe with a mean aortic valve gradient greater than 20.0 mm Hg or a peak aortic valve velocity above 3.0 m/sec,” Geoff Strange, PhD, said in presenting an analysis from NEDA, the National Echocardiography Database of Australia, at the annual congress of the European Society of Cardiology.
These results, if confirmed in other large datasets, could potentially have enormous implications for the use of transcatheter and surgical aortic valve replacement, interventions which until now have been restricted to patients with severe aortic stenosis (AS) as defined by an aortic valve (AV) mean gradient in excess of 40 mm Hg or a peak AV velocity greater than 4.0 m/sec. This restriction was based on what Dr. Strange considers rather limited and flimsy evidence suggesting that the mortality associated with AS was negligible except in severe AS.
“Cut points used to stratify for interventional strategies are based on very small numbers,” observed Dr. Strange, professor of medicine at the University of Notre Dame in Fremantle, Australia.
The NEDA findings, he added, constitute a call to action: “These data provide the impetus for a contemporary evaluation of the risk-to-benefit ratio of intervention in the moderate AS population,” Dr. Strange declared.
He and his NEDA coinvestigators analyzed echocardiographic data on 241,303 individuals in the Australian database, zeroing in on the 25,827 with untreated mild, moderate, or severe native valve AS. To place the size and scope of this project into perspective, the next-largest study of the natural history of untreated AS included 1,375 individuals – and that study was in turn roughly 10-fold bigger than the handful of other published studies addressing this issue.
A key finding in the NEDA study was that the 5-year all-cause mortality rate of 61.4% in the group with moderate AS wasn’t significantly different from the 64.6% rate in those with severe AS (see graphic).
The investigators performed additional analyses, analyzing peak velocity and mean gradient as continuous variables and stratifying patients into quintiles on that basis. They found that the top quintile for AV velocity started very low, at 1.73 m/sec, while the top quintile for mean AV gradient also started at a surprisingly low level: greater than 9.6 mm Hg. They noted that both all-cause and cardiovascular-specific mortality rates were basically flat until taking what Dr. Strange described as “a sharp pivot point upward” right around 20 mm Hg or 3 m/sec.
“No matter how we looked at these data – whether we looked at patients with or without left heart disease, whether we used the dimensionless index, whether we adjusted for stroke volume index, whether we stratified between age above or below 65, whether we used the gradient, the velocity, or the AV area – this threshold of increasing mortality at around 20 mm Hg or 3 m/sec continued to emerge,” according to Dr. Strange.
He noted that this study used real-world data with hard endpoints – actuarial patient mortality outcomes obtained through linkage to the national database – rather than hypothetical projections based upon Kaplan-Meier curves. A study limitation was that comorbidity data couldn’t be obtained for the AS patients.
Session cochair Patrizio Lancellotti, MD, PhD, commented, “I think this study will change a bit our consideration about patients with moderate AS.”
However, Dr. Lancellotti, who was lead author of the second-largest study of the natural history of aortic stenosis (JAMA Cardiol. 2018 Nov 1;3[11]:1060-8), expressed misgivings about the NEDA system’s lack of a core echocardiographic laboratory for imaging adjudication. That’s a study weakness given that image quality and the accuracy of echocardiographic interpretation are so highly dependent upon an individual cardiologist’s skill, observed Dr. Lancellotti, who is head of cardiology at the University of Liege (Belgium).
Dr. Strange replied that he and his coinvestigators analyzed a random subset of the NEDA data and found very little interlaboratory variability in results.
“All I can say is that the labs that contributed to this study are the most eminent labs across Australia,” he added.
Simultaneously with Dr. Strange’s presentation at the congress, the NEDA study results were published online (J Am Coll Cardiol. Sep 2019. doi: 10.1016/j.jacc.2019.08.004).
Dr. Strange reported having no financial conflicts of interest regarding the NEDA project, which is funded by GlaxoSmithKline, Bayer, and Actelion.
PARIS – The 5-year mortality rate associated with untreated moderate aortic stenosis is just as grim as it is for severe aortic stenosis, according to new findings from the largest-ever study of the natural history of aortic stenosis.
“These data provide a clear signal of the expected adverse outcomes for individuals presenting across the globe with a mean aortic valve gradient greater than 20.0 mm Hg or a peak aortic valve velocity above 3.0 m/sec,” Geoff Strange, PhD, said in presenting an analysis from NEDA, the National Echocardiography Database of Australia, at the annual congress of the European Society of Cardiology.
These results, if confirmed in other large datasets, could potentially have enormous implications for the use of transcatheter and surgical aortic valve replacement, interventions which until now have been restricted to patients with severe aortic stenosis (AS) as defined by an aortic valve (AV) mean gradient in excess of 40 mm Hg or a peak AV velocity greater than 4.0 m/sec. This restriction was based on what Dr. Strange considers rather limited and flimsy evidence suggesting that the mortality associated with AS was negligible except in severe AS.
“Cut points used to stratify for interventional strategies are based on very small numbers,” observed Dr. Strange, professor of medicine at the University of Notre Dame in Fremantle, Australia.
The NEDA findings, he added, constitute a call to action: “These data provide the impetus for a contemporary evaluation of the risk-to-benefit ratio of intervention in the moderate AS population,” Dr. Strange declared.
He and his NEDA coinvestigators analyzed echocardiographic data on 241,303 individuals in the Australian database, zeroing in on the 25,827 with untreated mild, moderate, or severe native valve AS. To place the size and scope of this project into perspective, the next-largest study of the natural history of untreated AS included 1,375 individuals – and that study was in turn roughly 10-fold bigger than the handful of other published studies addressing this issue.
A key finding in the NEDA study was that the 5-year all-cause mortality rate of 61.4% in the group with moderate AS wasn’t significantly different from the 64.6% rate in those with severe AS (see graphic).
The investigators performed additional analyses, analyzing peak velocity and mean gradient as continuous variables and stratifying patients into quintiles on that basis. They found that the top quintile for AV velocity started very low, at 1.73 m/sec, while the top quintile for mean AV gradient also started at a surprisingly low level: greater than 9.6 mm Hg. They noted that both all-cause and cardiovascular-specific mortality rates were basically flat until taking what Dr. Strange described as “a sharp pivot point upward” right around 20 mm Hg or 3 m/sec.
“No matter how we looked at these data – whether we looked at patients with or without left heart disease, whether we used the dimensionless index, whether we adjusted for stroke volume index, whether we stratified between age above or below 65, whether we used the gradient, the velocity, or the AV area – this threshold of increasing mortality at around 20 mm Hg or 3 m/sec continued to emerge,” according to Dr. Strange.
He noted that this study used real-world data with hard endpoints – actuarial patient mortality outcomes obtained through linkage to the national database – rather than hypothetical projections based upon Kaplan-Meier curves. A study limitation was that comorbidity data couldn’t be obtained for the AS patients.
Session cochair Patrizio Lancellotti, MD, PhD, commented, “I think this study will change a bit our consideration about patients with moderate AS.”
However, Dr. Lancellotti, who was lead author of the second-largest study of the natural history of aortic stenosis (JAMA Cardiol. 2018 Nov 1;3[11]:1060-8), expressed misgivings about the NEDA system’s lack of a core echocardiographic laboratory for imaging adjudication. That’s a study weakness given that image quality and the accuracy of echocardiographic interpretation are so highly dependent upon an individual cardiologist’s skill, observed Dr. Lancellotti, who is head of cardiology at the University of Liege (Belgium).
Dr. Strange replied that he and his coinvestigators analyzed a random subset of the NEDA data and found very little interlaboratory variability in results.
“All I can say is that the labs that contributed to this study are the most eminent labs across Australia,” he added.
Simultaneously with Dr. Strange’s presentation at the congress, the NEDA study results were published online (J Am Coll Cardiol. Sep 2019. doi: 10.1016/j.jacc.2019.08.004).
Dr. Strange reported having no financial conflicts of interest regarding the NEDA project, which is funded by GlaxoSmithKline, Bayer, and Actelion.
REPORTING FROM THE ESC CONGRESS 2019
Treatment guided by remote readings works when used as intended
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
REPORTING FROM HFSA 2019
Cardiovascular complications of systemic sclerosis: What to look for
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
KEY POINTS
- Pulmonary hypertension is common in systemic sclerosis and carries a poor prognosis. Patients with systemic sclerosis should be screened regularly with echocardiography, followed, when necessary, by right heart catheterization to detect it early.
- Myocardial infarction and stroke are more common in patients with systemic sclerosis, and preventive measures are the same as for the general population.
- Right ventricular dysfunction secondary to pulmonary hypertension is common in systemic sclerosis; left ventricular dysfunction is less so. Routine echocardiography should include assessment of right and left ventricular function.
- Electrocardiography should be performed periodically, and urgently when indicated, to look for potentially dangerous arrhythmias.
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
Panel releases guidelines for red meat, processed meat consumption
according to recent guidelines from an international panel that were recently published in the Annals of Internal Medicine.
This recommendation was based on the panel having found “low- to very-low-certainty evidence that diets lower in unprocessed red meat may have little or no effect on the risk for major cardiometabolic outcomes and cancer mortality and incidence.” Additionally, meta-analysis results from 23 cohort studies provided low- to very-low-certainty evidence that decreasing unprocessed red meat intake may result in a very small reduction in the risk for major cardiovascular outcomes and type 2 diabetes, with no statistically differences in all-cause mortality and cardiovascular mortality, the guidelines say.
“Our weak recommendation that people continue their current meat consumption highlights both the uncertainty associated with possible harmful effects and the very small magnitude of effect, even if the best estimates represent true causation, which we believe to be implausible,” Bradley C. Johnston, PhD, of the department of community health and epidemiology at Dalhousie University, Halifax, N.S., and colleagues wrote in their paper summarizing the panel’s guidelines.
The evidence Dr. Johnston and colleagues examined were from four systematic reviews analyzing the health effects of red meat and processed meat consumption in randomized trials and meta-analyses of cohort studies as well as one systematic review that identified how people viewed their consumption of meat and values surrounding meat consumption.
In one review of 12 randomized trials examining diets of high and low red meat consumption, a diet consisting of low red meat had little effect on cardiovascular mortality (hazard ratio, 0.98; 95% confidence interval, 0.91-1.06), cardiovascular disease (HR, 0.99; 95% CI, 0.94-1.05), all-cause mortality (0.99; 95% CI, 0.95-1.03) and total cancer mortality (HR, 0.95; 95% CI, 0.89-1.01), including on colorectal cancer or breast cancer (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0622). A different review of observational cohort studies with more than 1,000 participants found “very-small or possibly small decreases” in all-cause mortality, incidence, and all-cause mortality of cancer, cardiovascular mortality, nonfatal coronary heart disease and MI, and type 2 diabetes for patients who had a diet low in red meat or processed meat (Vernooij R et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1583); a second review by Zeraatkar and colleagues of 55 observational cohort studies with more than 4 million participants found three servings of unprocessed red meat and processed meat per week was associated with a “very small reduction” in risk for MI, stroke, type 2 diabetes, cardiovascular mortality, and all-cause mortality (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1326). Another systematic review of 56 observational cohort studies found three servings of unprocessed red meat per week was associated with a slight reduction in overall cancer mortality (Han MA et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0699).
The authors also performed a systematic review of participant preferences and values regarding meat consumption. The evidence from 54 qualitative studies showed omnivores preferred eating meat, considered it part of a healthy diet, “lack[ed] the skills needed” to prepare meals without meat, and were mostly unwilling to change their meat consumption (Valli C et al. Ann Intern Med. 2019. doi: 10.7326/M19-1326).
“There was a very small and often trivial absolute risk reduction based on a realistic decrease of three servings of red or processed meat per week,” Dr. Johnston and colleagues wrote in their guidelines. If the very-small exposure effect is true, given peoples’ attachment to their meat-based diet, the associated risk reduction is not likely to provide sufficient motivation to reduce consumption of red meat or processed meat in fully informed individuals, and the weak, rather than strong, recommendation is based on the large variability in peoples’ values and preferences related to meat.”
The authors noted they did not examine factors such as cost, acceptability, feasibility, equity, environmental impact, and views on animal welfare when creating the guidelines. In addition, the low level of evidence from the randomized trials and observational studies means that the potential benefits of reducing red meat or processed meat intake may not outweigh the cultural and personal preferences or quality of life issues that could arise from changing one’s diet.
“This assessment may be excessively pessimistic; indeed, we hope that is the case,” they said. “What is certain is that generating higher-quality evidence regarding the magnitude of any causal effect of meat consumption on health outcomes will test the ingenuity and imagination of health science investigators.”
Dr. El Dib reported receiving funding from the São Paulo Research Foundation, the National Council for Scientific and Technological Development, and the faculty of medicine at Dalhousie University. Dr. de Souza reports relationships with the Canadian Institutes of Health Research/Health Canada, the Canadian Foundation for Dietetic Research and the World Health Organization in the forms of personal fees, grants, and speakers bureau and board of directorship appointments. Dr. Patel reports receiving grants and person fees from the National Institutes of Health, Sanofi, the National Science Foundation, XY.health, doc.ai, Janssen, and the CDC.
SOURCE: Johnston B et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
The new guidelines for red meat and processed meat consumption will be controversial. Since it is based on a review of all available data on red meat and processed meat consumption; however, it will be difficult to find evidence to argue against it, wrote Aaron E. Carroll, MD, MS; and Tiffany S. Doherty, PhD, in a related editorial.
Further, many participants in a systematic review by Valli and colleagues expressed beliefs that they had already reduced their meat consumption. Additionally, some cited mistrust of the information presented by studies as their explanation for not reducing meat consumption, according to Dr. Carroll and Dr. Doherty (Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620). “It’s not even clear that those who disbelieve what they hear about meat are wrong,” they added. “We have saturated the market with warnings about the dangers of red meat. It would be hard to find someone who doesn’t ‘know’ that experts think we should all eat less. Continuing to broadcast that fact, with more and more shaky studies touting potential small relative risks, is not changing anyone’s mind.”
Dr. Carroll and Dr. Doherty proposed that more study in this area with smaller cohorts may be of limited value, and randomized trials should be conducted in areas where we “don’t already know” the information.
The authors also called for efforts to be made to discuss reasons to reduce meat consumption unrelated to health.
“Ethical concerns about animal welfare can be important, as can concerns about the effects of meat consumption on the environment,” they concluded. “Both of these issues might be more likely to sway people, and they have the added benefit of empirical evidence behind them. And if they result in reducing meat consumption, and some receive a small health benefit as a side effect, everyone wins.”
Dr. Carroll and Dr. Doherty are from the Center for Pediatric and Adolescent Comparative Effectiveness Research, Indiana University, Indianapolis. These comments reflect their editorial in response to Johnston et al. Dr. Carroll reports receiving royalties for a book he wrote on nutrition; Dr. Doherty reports no relevant conflicts of interest.
The new guidelines for red meat and processed meat consumption will be controversial. Since it is based on a review of all available data on red meat and processed meat consumption; however, it will be difficult to find evidence to argue against it, wrote Aaron E. Carroll, MD, MS; and Tiffany S. Doherty, PhD, in a related editorial.
Further, many participants in a systematic review by Valli and colleagues expressed beliefs that they had already reduced their meat consumption. Additionally, some cited mistrust of the information presented by studies as their explanation for not reducing meat consumption, according to Dr. Carroll and Dr. Doherty (Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620). “It’s not even clear that those who disbelieve what they hear about meat are wrong,” they added. “We have saturated the market with warnings about the dangers of red meat. It would be hard to find someone who doesn’t ‘know’ that experts think we should all eat less. Continuing to broadcast that fact, with more and more shaky studies touting potential small relative risks, is not changing anyone’s mind.”
Dr. Carroll and Dr. Doherty proposed that more study in this area with smaller cohorts may be of limited value, and randomized trials should be conducted in areas where we “don’t already know” the information.
The authors also called for efforts to be made to discuss reasons to reduce meat consumption unrelated to health.
“Ethical concerns about animal welfare can be important, as can concerns about the effects of meat consumption on the environment,” they concluded. “Both of these issues might be more likely to sway people, and they have the added benefit of empirical evidence behind them. And if they result in reducing meat consumption, and some receive a small health benefit as a side effect, everyone wins.”
Dr. Carroll and Dr. Doherty are from the Center for Pediatric and Adolescent Comparative Effectiveness Research, Indiana University, Indianapolis. These comments reflect their editorial in response to Johnston et al. Dr. Carroll reports receiving royalties for a book he wrote on nutrition; Dr. Doherty reports no relevant conflicts of interest.
The new guidelines for red meat and processed meat consumption will be controversial. Since it is based on a review of all available data on red meat and processed meat consumption; however, it will be difficult to find evidence to argue against it, wrote Aaron E. Carroll, MD, MS; and Tiffany S. Doherty, PhD, in a related editorial.
Further, many participants in a systematic review by Valli and colleagues expressed beliefs that they had already reduced their meat consumption. Additionally, some cited mistrust of the information presented by studies as their explanation for not reducing meat consumption, according to Dr. Carroll and Dr. Doherty (Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620). “It’s not even clear that those who disbelieve what they hear about meat are wrong,” they added. “We have saturated the market with warnings about the dangers of red meat. It would be hard to find someone who doesn’t ‘know’ that experts think we should all eat less. Continuing to broadcast that fact, with more and more shaky studies touting potential small relative risks, is not changing anyone’s mind.”
Dr. Carroll and Dr. Doherty proposed that more study in this area with smaller cohorts may be of limited value, and randomized trials should be conducted in areas where we “don’t already know” the information.
The authors also called for efforts to be made to discuss reasons to reduce meat consumption unrelated to health.
“Ethical concerns about animal welfare can be important, as can concerns about the effects of meat consumption on the environment,” they concluded. “Both of these issues might be more likely to sway people, and they have the added benefit of empirical evidence behind them. And if they result in reducing meat consumption, and some receive a small health benefit as a side effect, everyone wins.”
Dr. Carroll and Dr. Doherty are from the Center for Pediatric and Adolescent Comparative Effectiveness Research, Indiana University, Indianapolis. These comments reflect their editorial in response to Johnston et al. Dr. Carroll reports receiving royalties for a book he wrote on nutrition; Dr. Doherty reports no relevant conflicts of interest.
according to recent guidelines from an international panel that were recently published in the Annals of Internal Medicine.
This recommendation was based on the panel having found “low- to very-low-certainty evidence that diets lower in unprocessed red meat may have little or no effect on the risk for major cardiometabolic outcomes and cancer mortality and incidence.” Additionally, meta-analysis results from 23 cohort studies provided low- to very-low-certainty evidence that decreasing unprocessed red meat intake may result in a very small reduction in the risk for major cardiovascular outcomes and type 2 diabetes, with no statistically differences in all-cause mortality and cardiovascular mortality, the guidelines say.
“Our weak recommendation that people continue their current meat consumption highlights both the uncertainty associated with possible harmful effects and the very small magnitude of effect, even if the best estimates represent true causation, which we believe to be implausible,” Bradley C. Johnston, PhD, of the department of community health and epidemiology at Dalhousie University, Halifax, N.S., and colleagues wrote in their paper summarizing the panel’s guidelines.
The evidence Dr. Johnston and colleagues examined were from four systematic reviews analyzing the health effects of red meat and processed meat consumption in randomized trials and meta-analyses of cohort studies as well as one systematic review that identified how people viewed their consumption of meat and values surrounding meat consumption.
In one review of 12 randomized trials examining diets of high and low red meat consumption, a diet consisting of low red meat had little effect on cardiovascular mortality (hazard ratio, 0.98; 95% confidence interval, 0.91-1.06), cardiovascular disease (HR, 0.99; 95% CI, 0.94-1.05), all-cause mortality (0.99; 95% CI, 0.95-1.03) and total cancer mortality (HR, 0.95; 95% CI, 0.89-1.01), including on colorectal cancer or breast cancer (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0622). A different review of observational cohort studies with more than 1,000 participants found “very-small or possibly small decreases” in all-cause mortality, incidence, and all-cause mortality of cancer, cardiovascular mortality, nonfatal coronary heart disease and MI, and type 2 diabetes for patients who had a diet low in red meat or processed meat (Vernooij R et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1583); a second review by Zeraatkar and colleagues of 55 observational cohort studies with more than 4 million participants found three servings of unprocessed red meat and processed meat per week was associated with a “very small reduction” in risk for MI, stroke, type 2 diabetes, cardiovascular mortality, and all-cause mortality (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1326). Another systematic review of 56 observational cohort studies found three servings of unprocessed red meat per week was associated with a slight reduction in overall cancer mortality (Han MA et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0699).
The authors also performed a systematic review of participant preferences and values regarding meat consumption. The evidence from 54 qualitative studies showed omnivores preferred eating meat, considered it part of a healthy diet, “lack[ed] the skills needed” to prepare meals without meat, and were mostly unwilling to change their meat consumption (Valli C et al. Ann Intern Med. 2019. doi: 10.7326/M19-1326).
“There was a very small and often trivial absolute risk reduction based on a realistic decrease of three servings of red or processed meat per week,” Dr. Johnston and colleagues wrote in their guidelines. If the very-small exposure effect is true, given peoples’ attachment to their meat-based diet, the associated risk reduction is not likely to provide sufficient motivation to reduce consumption of red meat or processed meat in fully informed individuals, and the weak, rather than strong, recommendation is based on the large variability in peoples’ values and preferences related to meat.”
The authors noted they did not examine factors such as cost, acceptability, feasibility, equity, environmental impact, and views on animal welfare when creating the guidelines. In addition, the low level of evidence from the randomized trials and observational studies means that the potential benefits of reducing red meat or processed meat intake may not outweigh the cultural and personal preferences or quality of life issues that could arise from changing one’s diet.
“This assessment may be excessively pessimistic; indeed, we hope that is the case,” they said. “What is certain is that generating higher-quality evidence regarding the magnitude of any causal effect of meat consumption on health outcomes will test the ingenuity and imagination of health science investigators.”
Dr. El Dib reported receiving funding from the São Paulo Research Foundation, the National Council for Scientific and Technological Development, and the faculty of medicine at Dalhousie University. Dr. de Souza reports relationships with the Canadian Institutes of Health Research/Health Canada, the Canadian Foundation for Dietetic Research and the World Health Organization in the forms of personal fees, grants, and speakers bureau and board of directorship appointments. Dr. Patel reports receiving grants and person fees from the National Institutes of Health, Sanofi, the National Science Foundation, XY.health, doc.ai, Janssen, and the CDC.
SOURCE: Johnston B et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
according to recent guidelines from an international panel that were recently published in the Annals of Internal Medicine.
This recommendation was based on the panel having found “low- to very-low-certainty evidence that diets lower in unprocessed red meat may have little or no effect on the risk for major cardiometabolic outcomes and cancer mortality and incidence.” Additionally, meta-analysis results from 23 cohort studies provided low- to very-low-certainty evidence that decreasing unprocessed red meat intake may result in a very small reduction in the risk for major cardiovascular outcomes and type 2 diabetes, with no statistically differences in all-cause mortality and cardiovascular mortality, the guidelines say.
“Our weak recommendation that people continue their current meat consumption highlights both the uncertainty associated with possible harmful effects and the very small magnitude of effect, even if the best estimates represent true causation, which we believe to be implausible,” Bradley C. Johnston, PhD, of the department of community health and epidemiology at Dalhousie University, Halifax, N.S., and colleagues wrote in their paper summarizing the panel’s guidelines.
The evidence Dr. Johnston and colleagues examined were from four systematic reviews analyzing the health effects of red meat and processed meat consumption in randomized trials and meta-analyses of cohort studies as well as one systematic review that identified how people viewed their consumption of meat and values surrounding meat consumption.
In one review of 12 randomized trials examining diets of high and low red meat consumption, a diet consisting of low red meat had little effect on cardiovascular mortality (hazard ratio, 0.98; 95% confidence interval, 0.91-1.06), cardiovascular disease (HR, 0.99; 95% CI, 0.94-1.05), all-cause mortality (0.99; 95% CI, 0.95-1.03) and total cancer mortality (HR, 0.95; 95% CI, 0.89-1.01), including on colorectal cancer or breast cancer (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0622). A different review of observational cohort studies with more than 1,000 participants found “very-small or possibly small decreases” in all-cause mortality, incidence, and all-cause mortality of cancer, cardiovascular mortality, nonfatal coronary heart disease and MI, and type 2 diabetes for patients who had a diet low in red meat or processed meat (Vernooij R et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1583); a second review by Zeraatkar and colleagues of 55 observational cohort studies with more than 4 million participants found three servings of unprocessed red meat and processed meat per week was associated with a “very small reduction” in risk for MI, stroke, type 2 diabetes, cardiovascular mortality, and all-cause mortality (Zeraatkar D et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1326). Another systematic review of 56 observational cohort studies found three servings of unprocessed red meat per week was associated with a slight reduction in overall cancer mortality (Han MA et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-0699).
The authors also performed a systematic review of participant preferences and values regarding meat consumption. The evidence from 54 qualitative studies showed omnivores preferred eating meat, considered it part of a healthy diet, “lack[ed] the skills needed” to prepare meals without meat, and were mostly unwilling to change their meat consumption (Valli C et al. Ann Intern Med. 2019. doi: 10.7326/M19-1326).
“There was a very small and often trivial absolute risk reduction based on a realistic decrease of three servings of red or processed meat per week,” Dr. Johnston and colleagues wrote in their guidelines. If the very-small exposure effect is true, given peoples’ attachment to their meat-based diet, the associated risk reduction is not likely to provide sufficient motivation to reduce consumption of red meat or processed meat in fully informed individuals, and the weak, rather than strong, recommendation is based on the large variability in peoples’ values and preferences related to meat.”
The authors noted they did not examine factors such as cost, acceptability, feasibility, equity, environmental impact, and views on animal welfare when creating the guidelines. In addition, the low level of evidence from the randomized trials and observational studies means that the potential benefits of reducing red meat or processed meat intake may not outweigh the cultural and personal preferences or quality of life issues that could arise from changing one’s diet.
“This assessment may be excessively pessimistic; indeed, we hope that is the case,” they said. “What is certain is that generating higher-quality evidence regarding the magnitude of any causal effect of meat consumption on health outcomes will test the ingenuity and imagination of health science investigators.”
Dr. El Dib reported receiving funding from the São Paulo Research Foundation, the National Council for Scientific and Technological Development, and the faculty of medicine at Dalhousie University. Dr. de Souza reports relationships with the Canadian Institutes of Health Research/Health Canada, the Canadian Foundation for Dietetic Research and the World Health Organization in the forms of personal fees, grants, and speakers bureau and board of directorship appointments. Dr. Patel reports receiving grants and person fees from the National Institutes of Health, Sanofi, the National Science Foundation, XY.health, doc.ai, Janssen, and the CDC.
SOURCE: Johnston B et al. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
FROM ANNALS OF INTERNAL MEDICINE