User login
For pemphigus, rituximab is first line, expert says
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Dupilumab treats itch and clears lesions in prurigo nodularis patients
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Skin reactions to first COVID-19 vaccine don’t justify forgoing second dose
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Melanoma increasing, but is this overdiagnosis?
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
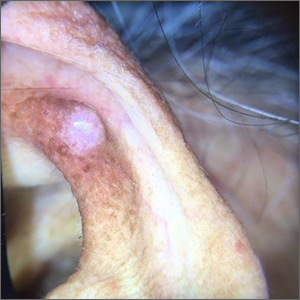
Ear growth
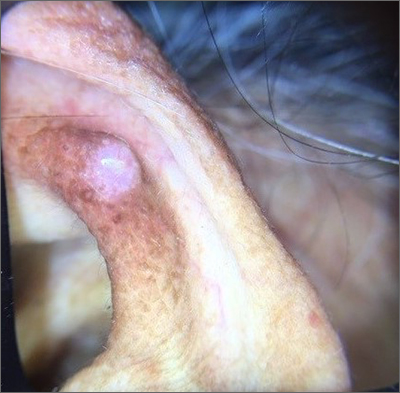
A shave biopsy of the lesion was performed and it confirmed the diagnosis of chondrodermatitis nodularis helicis (CNH).
CNH is an inflammatory process that most commonly occurs on the helix of the ear but can also occur on the antihelix and, rarely, on other areas of the ear. It generally manifests as a firm nodule with surrounding erythema that may be painful only when pressure is applied. Patients may describe bleeding, ulceration, and exudate. They will usually report discomfort from sleeping on the affected side.
The pathogenesis of CNH is poorly understood but is thought to be related to vasculitis and inflammation from prolonged pressure to the affected ear during sleep or from devices that are worn in or around the ear (eg, hearing aids, headphones). Other factors such as actinic damage or ear trauma have also been described. Histopathologic studies have identified arteriolar narrowing with ischemic changes and necrosis of cartilage causing localized inflammation.1
The differential diagnosis for this lesion includes nonmelanoma skin cancer, as well as tophaceous gout and seborrheic keratosis.
There are multiple conservative treatment options. One option is to relieve pressure by sleeping on the unaffected side or using commercially available pillows with a cutout or window where the affected ear can rest. Pharmacologic treatments include topical nitroglycerin1 and intralesional collagen or corticosteroid injections. If previous treatments are unsuccessful, consider surgical excision of the affected tissue and curettage of the underlying abnormal cartilage. Recurrence is possible with both conservative and surgical treatment.
This patient was counseled on the benign nature of her biopsy findings and treatment options were discussed. She elected to proceed with pressure-relieving measures when sleeping and planned to follow up if there was no improvement.
Image courtesy of Marion Cook, MD, First Choice Community Healthcare, Albuquerque, New Mexico. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Nielsen LJ, Olsen CH, Lock-Anderson J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi: 10.1155/2016/4340168

A shave biopsy of the lesion was performed and it confirmed the diagnosis of chondrodermatitis nodularis helicis (CNH).
CNH is an inflammatory process that most commonly occurs on the helix of the ear but can also occur on the antihelix and, rarely, on other areas of the ear. It generally manifests as a firm nodule with surrounding erythema that may be painful only when pressure is applied. Patients may describe bleeding, ulceration, and exudate. They will usually report discomfort from sleeping on the affected side.
The pathogenesis of CNH is poorly understood but is thought to be related to vasculitis and inflammation from prolonged pressure to the affected ear during sleep or from devices that are worn in or around the ear (eg, hearing aids, headphones). Other factors such as actinic damage or ear trauma have also been described. Histopathologic studies have identified arteriolar narrowing with ischemic changes and necrosis of cartilage causing localized inflammation.1
The differential diagnosis for this lesion includes nonmelanoma skin cancer, as well as tophaceous gout and seborrheic keratosis.
There are multiple conservative treatment options. One option is to relieve pressure by sleeping on the unaffected side or using commercially available pillows with a cutout or window where the affected ear can rest. Pharmacologic treatments include topical nitroglycerin1 and intralesional collagen or corticosteroid injections. If previous treatments are unsuccessful, consider surgical excision of the affected tissue and curettage of the underlying abnormal cartilage. Recurrence is possible with both conservative and surgical treatment.
This patient was counseled on the benign nature of her biopsy findings and treatment options were discussed. She elected to proceed with pressure-relieving measures when sleeping and planned to follow up if there was no improvement.
Image courtesy of Marion Cook, MD, First Choice Community Healthcare, Albuquerque, New Mexico. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A shave biopsy of the lesion was performed and it confirmed the diagnosis of chondrodermatitis nodularis helicis (CNH).
CNH is an inflammatory process that most commonly occurs on the helix of the ear but can also occur on the antihelix and, rarely, on other areas of the ear. It generally manifests as a firm nodule with surrounding erythema that may be painful only when pressure is applied. Patients may describe bleeding, ulceration, and exudate. They will usually report discomfort from sleeping on the affected side.
The pathogenesis of CNH is poorly understood but is thought to be related to vasculitis and inflammation from prolonged pressure to the affected ear during sleep or from devices that are worn in or around the ear (eg, hearing aids, headphones). Other factors such as actinic damage or ear trauma have also been described. Histopathologic studies have identified arteriolar narrowing with ischemic changes and necrosis of cartilage causing localized inflammation.1
The differential diagnosis for this lesion includes nonmelanoma skin cancer, as well as tophaceous gout and seborrheic keratosis.
There are multiple conservative treatment options. One option is to relieve pressure by sleeping on the unaffected side or using commercially available pillows with a cutout or window where the affected ear can rest. Pharmacologic treatments include topical nitroglycerin1 and intralesional collagen or corticosteroid injections. If previous treatments are unsuccessful, consider surgical excision of the affected tissue and curettage of the underlying abnormal cartilage. Recurrence is possible with both conservative and surgical treatment.
This patient was counseled on the benign nature of her biopsy findings and treatment options were discussed. She elected to proceed with pressure-relieving measures when sleeping and planned to follow up if there was no improvement.
Image courtesy of Marion Cook, MD, First Choice Community Healthcare, Albuquerque, New Mexico. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Nielsen LJ, Olsen CH, Lock-Anderson J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi: 10.1155/2016/4340168
1. Nielsen LJ, Olsen CH, Lock-Anderson J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi: 10.1155/2016/4340168
Cellulitis care costly from misdiagnosis, needless hospitalizations
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Global registry tracks COVID-19 outcomes in atopic dermatitis patients
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
AT AAD 22
Clinical clarity grows about toenail disorder, experts report
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
AT AAD 2022
New trial data show hair growth in more alopecia areata patients
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
BOSTON – according to updated results from two phase 3 trials presented at the annual meeting of the American Academy of Dermatology.
The results indicate improved response rates and hair growth among trial participants, said Brett King, MD, PhD, an associate professor of dermatology at Yale University, New Haven, Conn. He is the lead author of the analyses and presented the research.
Dr. King presented 36-week results from the clinical trials at the 2021 annual meeting of the European Academy of Dermatology and Venereology. The same results were also published March 26, 2022, in the New England Journal of Medicine.
“Every bit of data we’ve had is hugely important,” Dr. King said in an interview. “Every time we add 16 weeks of data across hundreds of patients, we are making a huge step forward toward the goal of [Food and Drug Administration approval for a medication for alopecia areata.”
All patients enrolled in the two trials, called BRAVE-AA1 and BRAVE-AA2, had severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) score of at least 50, meaning 50% or less scalp coverage. The score ranges from 0 (no hair loss) to 100 (complete hair loss). The primary endpoint was a SALT score of 20 or less (80% scalp hair coverage).
The researchers pooled data from both clinical trials, with a combined enrollment of 1,200, for the 52-week results presented at the meeting. The placebo group stopped at 36 weeks, and these patients were randomly reassigned to either the 4-mg or 2-mg once-daily baricitinib treatment groups.
At baseline, patients enrolled in the trial had a mean SALT score of 85.5. After 52 weeks, 39.0% of patients who received 4 mg of baricitinib had at least 80% scalp coverage. Of this group, nearly three out of four (74.1%) had at least 90% scalp coverage, or a SALT score of 10 or less.
In patients who received 2 mg of baricitinib, 22.6% had a SALT score of 20 or less 20 (at least 80% scalp hair coverage) at 52 weeks, and two-thirds of that group (67.5%) had at least 90% scalp hair coverage at 52 weeks.
Comparatively, at 36 weeks, 35.2% of participants in BRAVE-AA1 and 32.5% of participants in BRAVE-AA2 receiving 4 mg of baricitinib had at least 80% scalp coverage. In the group taking the lower dose, 21.7% and 17.3% of patients in the BRAVE-AA1 and BRAVE-AA2 trials, respectively, had achieved at least 80% scalp coverage at 36 weeks. (These percentages differ slightly from the NEJM article because of a different analysis of missing data, Dr. King said. For comparison of both 36- and 52-week results, the percentages from the EADV are used above.)
The results indicate that 5% more patients reached the primary endpoint in the additional 16 weeks of the trial, Dr. King said.
Alopecia areata is an autoimmune condition where immune cells attack hair follicles, causing the hair to fall out, and is associated with emotional and psychological distress. Any hair follicle can be attacked, but they are rarely destroyed, so hair can regrow.
"Many underestimate the impact of this autoimmune hair loss condition," Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, told this news organization. He was not involved with the trial. "The burden of the disease, which certainly is an emotional but also a physical one, definitely needs to be addressed with indicated FDA-approved drugs," he noted, which is the goal of these trials.
The BRAVE-AA1 and BRAVE-AA2 trials focused on scalp hair regrowth.
Eyebrow and eyelash growth, secondary outcomes, also improved between 36 and 52 weeks in both groups, calculated using the proportion of participants who had achieved full regrowth or regrowth with minimal gaps. At 36 weeks, about 31%-35% of patients who received 4 mg of baricitinib regrew eyebrow and eyelash hair. By 52 weeks, more than two out of five patients regrew eyebrow (44.1%) and eyelash (45.3%) hair.
“It’s a fantastic achievement and a major step forward in alopecia areata, especially for patients with the most severe and refractory cases,” said Arash Mostaghimi, MD, MPH, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. Dr. Mostaghimi is on the advisory board for Eli Lilly, which manufactures baricitinib, and Brigham and Women’s was one of the clinical sites of the trial.
While dermatologists have been aware of how JAK inhibitors can affect hair regrowth in alopecia patients, they have been using these drugs off label, Dr. Friedman said. Therefore, these drugs are expensive and more difficult to access. These trials provide "data that proves the efficacy and safety of [baricitinib] under the umbrella of the FDA portal," he added, which will hopefully lead to an approved indication for alopecia areata, so it can be more accessible to patients.
Adverse events at 52 weeks were consistent with data from 36 weeks, which found that none of these adverse events occurred in more than 10% of participants. The most common adverse events were headache, acne, and increases in muscle-related blood markers. The most common infections reported were pneumonia, herpes zoster, and urinary tract infection.
In February 2022, the FDA granted priority review for baricitinib for the treatment of severe alopecia areata. Lilly expects a regulatory decision by the end of 2022, they said in a press release.
Lilly provided funding for the BRAVE-AA1 and BRAVE-AA2 trials. Dr. King reported financial relationships with Aclaris, Arena Pharmaceuticals, Bristol-Myers Squibb, Concert Pharmaceutics, Dermavant, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Viela Bio. Dr. Mostaghimi has reported serving on an advisory board for Lilly. Dr. Friedman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This article was updated on 3/28/2022 to include Dr. Friedman's comments, and on 3/31/2022 to correct the statement regarding adverse events reported in the study
AT AAD 2022
Aluminum named allergen of the year
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The . Aluminum salts, which are the major cause of allergic reactions, are “ubiquitous,” Donald Belsito, MD, professor of dermatology at Columbia University, New York, said at the annual meeting of the American Contact Dermatitis Society.
These salts can be found in sunscreen, cosmetics, dental restorations, and food, to name a few, though the most commonly identified reactions are from aluminum hydroxide, which can be found in some vaccines or preparations for allergen-specific immunotherapy. “It’s the aluminum hydroxide that seems to be more allergenic than other aluminum salts,” Dr. Belsito said in an interview.
“It’s not a dangerous allergy; It’s not a threat,” he said, “but it’s something that dermatologists need to be aware of.”
These reactions normally present as itchy nodules that can last for months and even years, like some reactions from patch testing. “We’re not talking about a vaccine allergy in such a way where people are getting anaphylaxis,” JiaDe Yu, MD, a pediatric dermatologist specializing in allergic contact dermatitis at Massachusetts General Hospital, Boston, said in an interview. “An itchy rash is what we tend to see.”
There have also been occasional reports of atopic dermatitis from aluminum in antiperspirants, astringents, as well as from the metallic aluminum.
Dr. Yu noted that aluminum allergies are not thought to be very common, but the overall prevalence is not known. Studies do suggest, however, that the allergy may be more prevalent in children. In one recent study in Sweden, 5% of children and 0.9% of adults who underwent patch testing had an aluminum contact allergy.
Recommendations for testing
Aluminum is not included in baseline patch testing in the United States, though a recent report about the allergen in the journal Dermatitis argued for its inclusion for pediatric patch testing. Both Dr. Belsito and Dr. Yu agreed that the best approach is to do targeted testing. “If there is a suspicion for it, absolutely test for it,” Dr. Yu said, but if a patient comes in with something like eyelid dermatitis or a rash after a hair care appointment, an aluminum allergy is not very likely.
Because aluminum is also present in Finn Chambers for patch testing, Dr. Belsito advised using plastic chambers in people suspected of having an aluminum allergy. He now uses only plastic chambers in children, he said, as some patients have had reactions to the Finn Chambers even if they have no history of reactions to vaccines or other aluminum-containing products.
While aluminum chloride hexahydrate (ACH) 2% in petrolatum is the commercially available preparation in patch testing, a preparation with ACH 10% is more sensitive, Dr. Belsito said. If a physician strongly suspects an aluminum allergy in a patient but the test with the ACH 2% is negative, he or she should then try a 10% solution, he noted, adding that 7-day readings are also necessary to maximize accuracy.
Vaccine safety
One of the concerns about naming aluminum as the allergen of the year is the potential to cause anxiety around vaccines. “We want to make sure that we’re not giving more fuel to people who have an excuse not to get a vaccine,” Dr. Yu said. “We certainly want to reinforce that fact that it is safe.” Dr. Belsito noted that COVID-19 vaccines do not contain aluminum.
Even on the rare chance that a patient does have a reaction to an aluminum-containing vaccine, these subcutaneous nodules resolve over time, Dr. Belsito said. In his own clinical experience, “99.99% of the time they resolve and there is no residual.” He did add that overreacting to the rash by prescribing injectable steroids can lead to steroid atrophy. In these cases, a topical steroid may be more appropriate.
All unexpected or clinically significant vaccine reactions should be reported to the Vaccine Adverse Event Reporting System, cosponsored by the Centers for Disease Control and Prevention and the Food and Drug Administration. The Clinical Immunization Project Safety Assessment Project, from the CDC, also can provide expertise and advice on aluminum-free alternatives for some vaccines.
Dr. Belsito and Dr. Yu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACDS 2022