User login
COVID-19 infection linked to risk of cutaneous autoimmune and vascular diseases
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
BOSTON – . This predominately favored systemic disease states with cutaneous involvement, rather than skin-limited processes.
The findings come from a large multicenter analysis that Zachary Holcomb, MD, presented during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
“Viral triggers have been implicated in the pathogenesis of rheumatologic disease, but information regarding development of autoimmune disease following SARS-CoV-2 infection is limited,” said Dr. Holcomb, chief resident in the Harvard Combined Internal Medicine–Dermatology Residency, Boston. “Given its proposed thromboinflammatory pathobiology, we hypothesized that SARS-CoV-2 infection increases the risk of development of autoimmune disease with cutaneous manifestations and sought to define incidence rates of newly-diagnosed autoimmune diseases following SARS-CoV-2 infection.”
The researchers drew from the TriNetX Dataworks platform, an online cloud-based system that contains aggregated and deidentified patient information from about 75 million patients across 48 health care organizations. The infected cohort was defined as having a positive lab test for severe SARS-CoV-2 within the study window using Logical Observation Identifiers Names and Codes (LOINCs). Healthy controls consisted of a documented health care contact (inpatient or outpatient visit) during the study window without a positive SARS-CoV-2 lab test. Each cohort included patients aged 18-65 at the time of the study, and patients with previously diagnosed cutaneous autoimmune or vascular diseases were excluded from the analysis.
After propensity matching, the COVID-19 infected cohort and the healthy cohort included 1,904,864 patients each, with no baseline differences in age at index event, ethnicity, race, or sex. The study window was between April 1, 2020, and Oct. 1, 2020. The index event was a COVID-19 infection for the infected group and first documented health care contact in the healthy control group. The researchers looked at a window of 60 days following this index event for new incidence of cutaneous or vascular disease.
In the realm of connective tissue and related diseases, they found the incidence was increased among the COVID-19 infected group compared with controls for dermatomyositis (risk ratio, 2.273; P = .0196), scleroderma (RR, 1.959; P = .0001), and systemic lupus erythematosus (RR, 1.401; P < .0001). They also noted a significant decrease in the new incidence of alopecia areata in the COVID-19 infected group compared with controls (RR, 0.527; P < .0001).
No significant differences in the incidence of bullous and papulosquamous diseases were observed between the two groups. However, sarcoidosis was significantly more common in the COVID-19–infected group compared with controls (RR, 2.086; P < .001). “When taking all of these autoinflammatory diseases as a whole, there was an increased incidence in the COVID-19 infected group overall with a RR of 1.168 (P < .0001),” Dr. Holcomb said.
In the realm of vascular skin diseases, there was an increased incidence in the COVID-19 infected group in acrocyanosis (RR, 2.825; P < .001), Raynaud’s phenomenon (RR, 1.462; P < .0001), cutaneous small vessel vasculitis (RR, 1.714; P < .0001), granulomatosis with polyangiitis (RR, 2.667; P = .0002), and temporal arteritis (RR, 1.900; P = .0038).
“Interestingly, despite the academic and lay press reports of COVID toes, we did not see that in our data related to the COVID-infected group,” he said.
Dr. Holcomb acknowledged certain limitations of the study, including a narrow study window with a relatively short follow-up. “We were able to propensity match based on baseline demographics but not necessarily so based on health status and prior autoimmune disease,” he said. In addition, since the study was limited to those aged 18-65, the results may not be generalizable to pediatric and elderly patients, he said.
He described the study findings as “somewhat hypothesis-generating.” For instance, “why would we have more of a systemic process [at play?]. Our theory is that the severe inflammatory nature of COVID-19 leads to a lot of internal organ damage and exposure of autoantigens in that process, with relative skin sparing.”
One of the session moderators, Robert Paul Dellavalle, MD, PhD, professor of dermatology at the University of Colorado, Aurora, characterized the findings as “intriguing” but preliminary. “It would be interesting to look at more recent cohorts and see how vaccination for COVID-19 would impact the incidence rates of some of these diseases,” he said.
When asked for comment, Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women's Hospital and assistant professor of medicine at Harvard Medical School, both in Boston, said, "This is an interesting study that should be followed up. Viral triggers have been known to precede autoimmune diseases so it will be very important to understand whether COVID-19 also impacts systemic autoimmune rheumatic diseases. I would be interested in differences in surveillance between the infection and control groups early in the pandemic. Many patients were avoiding interaction with the health care system at that point."
Dr. Holcomb reported having no financial disclosures. Dr. Dellavalle disclosed that he is a consultant for Altus Labs and ParaPRO LLC. He has received grants and research funding from Pfizer.
* This story was updated on 3/29/22.
AT AAD 2022
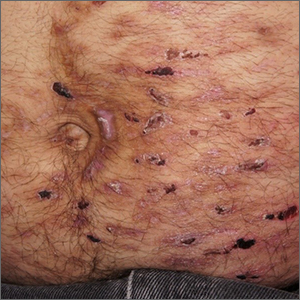
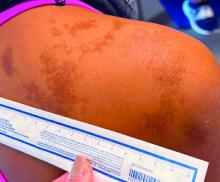
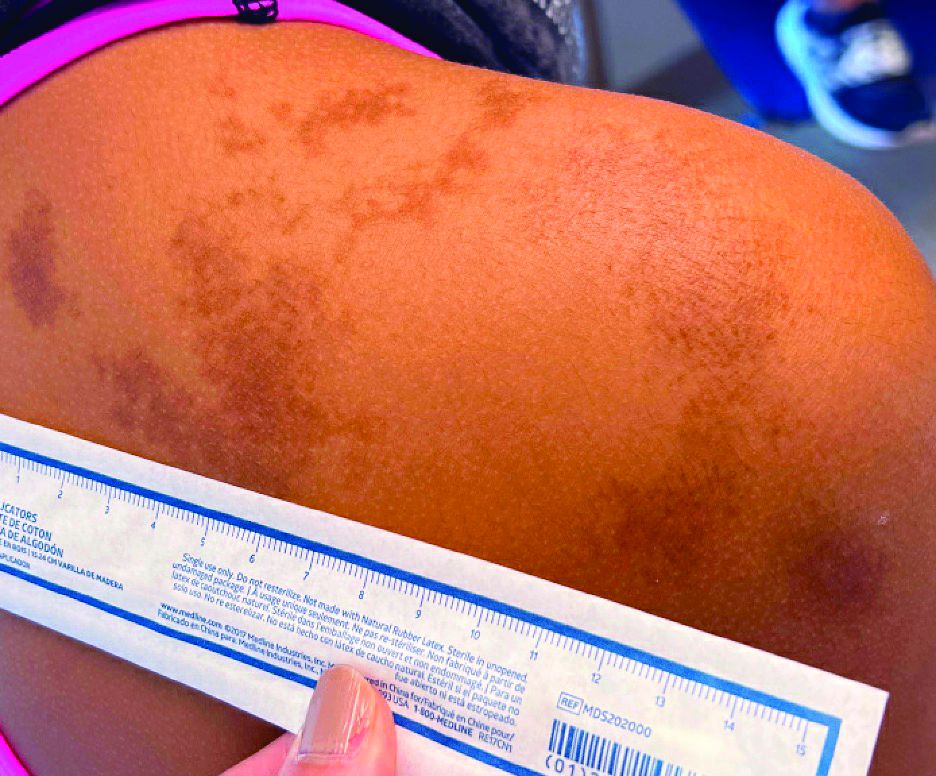
Abdominal rash
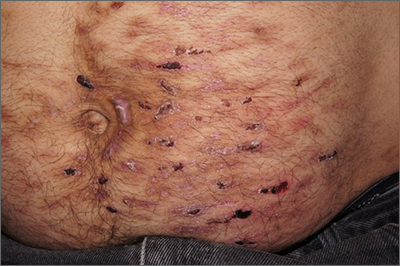
Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006
Trial gives new guidance for choosing initial PsA treatment
For patients with psoriatic arthritis (PsA) whose condition doesn’t respond adequately to methotrexate, addition of the tumor necrosis factor (TNF) inhibitor adalimumab increased the likelihood of achieving minimum disease activity (MDA), compared with escalation of MTX dose, according to results from a phase 4, open-label study.
The new study is one of only a few to compare treatment protocols in a field that has seen new therapeutic options become available in recent years. That lack of evidence can leave patients and physicians uncertain about the next step if the initial results of treatment are disappointing.
“There are some gaps in our database and our understanding of psoriatic arthritis, compared to rheumatoid arthritis, where we have had many more studies over the years,” Arthur Kavanaugh, MD, told this news organization when asked to comment on the study.
The trial provides one answer, at least. “There was a clear-cut signal that it made more sense to add adalimumab at that early juncture where a person is not quite doing well enough on methotrexate to satisfy our goal of getting the patient to low disease activity. It gives us as clinicians some ammunition to speak to our insurance formulary people on this side of the Atlantic, or [for] people in the U.K. to go to their local regulatory board that approves medicines and be able to show them some actual practically derived evidence about this very common question that comes up in practice,” senior and corresponding author Philip Mease, MD, said in an interview. The study was published online in The Lancet Rheumatology.
“When a clinician and patient are making the decision to move on from methotrexate monotherapy, either because of lack of efficacy or safety issues, tolerability issues, it makes most sense to add on a biologic medication such as a TNF inhibitor at that juncture, rather than intensifying methotrexate therapy,” said Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and a clinical professor at the University of Washington, both in Seattle.
Physicians may be tempted to bump up the dose for patients who can tolerate MTX and who may be showing some improvement, but the new study should prompt a different strategy if MDA isn’t achieved, according to Oliver FitzGerald, MD, a professor at the Conway Institute for Biomolecular Research at University College Dublin, who was asked to comment on the study. “This study clearly shows that the early addition of adalimumab is the better choice, and it would change practice. That being said, there are clearly some patients who do respond sufficiently to increasing methotrexate, and it would be useful to be able to predict which patients might do that.” He added that the study focused on adalimumab and that the results might not apply to other biologics.
The study should encourage use of a quantitative treat-to-target measure like MDA, which is a composite measure of patient perspectives, Dr. Mease said. The American College of Rheumatology and National Psoriasis Foundation and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis have recommended the use of MDA as a treat-to-target measure for PsA. The ACR and NPF recommend TNF inhibitors as first-line treatment, and GRAPPA includes it as a first-line option, whereas the European Alliance of Associations for Rheumatology recommends MTX only in the first line.
The study also suggests that there is value to using adalimumab on a weekly basis if an every-other-week schedule doesn’t produce the desired results. This strategy hasn’t been examined in PsA or even RA, according to Dr. Kavanaugh, who is a professor of medicine at the University of California, San Diego. “It did look like raising the dose might be an option for patients who are on every other week and are not doing quite as well as we would have hoped.”
The CONTROL study was a phase 4, two-part, open-label study. It included 245 patients in 14 countries who did not have MDA with MTX. In the first part of the study, patients were randomly assigned to receive weekly 15 mg MTX along with 40 mg adalimumab every other week, or escalation of MTX dose to 20-25 mg/week. MTX could be administered orally or intravenously. After 16 weeks (part 1), for patients who achieved MDA, current therapy was maintained or modified; for patients who did not achieve MDA, therapy was escalated over the following 16 weeks by giving adalimumab every week in the combination group or by adding adalimumab every other week in the MTX escalation arm.
Overall, 95% of the MTX plus adalimumab group completed part 1, as did 90% of the MTX escalation group. A total of 41% of the adalimumab group achieved MDA at 16 weeks versus 13% of the MTX group (P < .0001). The result held after accounting for sex and the interaction between sex and treatment (odds ratio, 4.6; 95% confidence interval, 2.4-8.9).
Among patients who achieved MDA at 16 weeks, 80% in the adalimumab group continued to have MDA at 32 weeks even after MTX had been withdrawn. Of those in the MTX escalation group, 67% continued to have MDA at 32 weeks with continued escalation of MTX.
Of the patients in the MTX escalation group who did not respond, 55% reached MDA following introduction of adalimumab every other week. Of those who did not respond to adalimumab, 30% reached MDA after switching to weekly adalimumab doses.
The study was open label, and patients who received adalimumab may have expected some improvement; that could have skewed the findings, Dr. Kavanaugh said. “I think that’s an important consideration as we interpret the data. The people who got the MTX arm probably had less of an expectation that they were going to do much better than those who switched to the adalimumab, as did the doctors taking care of them.”
The CONTROL study was funded by AbbVie. Dr. Mease has received research grants, consulted for, or received speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Sun Pharma, and UCB. Dr. FitzGerald has received grant support and honoraria from AbbVie. Dr. Kavanaugh has received research support from or consulted for AbbVie, Janssen, Pfizer, Lilly, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
For patients with psoriatic arthritis (PsA) whose condition doesn’t respond adequately to methotrexate, addition of the tumor necrosis factor (TNF) inhibitor adalimumab increased the likelihood of achieving minimum disease activity (MDA), compared with escalation of MTX dose, according to results from a phase 4, open-label study.
The new study is one of only a few to compare treatment protocols in a field that has seen new therapeutic options become available in recent years. That lack of evidence can leave patients and physicians uncertain about the next step if the initial results of treatment are disappointing.
“There are some gaps in our database and our understanding of psoriatic arthritis, compared to rheumatoid arthritis, where we have had many more studies over the years,” Arthur Kavanaugh, MD, told this news organization when asked to comment on the study.
The trial provides one answer, at least. “There was a clear-cut signal that it made more sense to add adalimumab at that early juncture where a person is not quite doing well enough on methotrexate to satisfy our goal of getting the patient to low disease activity. It gives us as clinicians some ammunition to speak to our insurance formulary people on this side of the Atlantic, or [for] people in the U.K. to go to their local regulatory board that approves medicines and be able to show them some actual practically derived evidence about this very common question that comes up in practice,” senior and corresponding author Philip Mease, MD, said in an interview. The study was published online in The Lancet Rheumatology.
“When a clinician and patient are making the decision to move on from methotrexate monotherapy, either because of lack of efficacy or safety issues, tolerability issues, it makes most sense to add on a biologic medication such as a TNF inhibitor at that juncture, rather than intensifying methotrexate therapy,” said Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and a clinical professor at the University of Washington, both in Seattle.
Physicians may be tempted to bump up the dose for patients who can tolerate MTX and who may be showing some improvement, but the new study should prompt a different strategy if MDA isn’t achieved, according to Oliver FitzGerald, MD, a professor at the Conway Institute for Biomolecular Research at University College Dublin, who was asked to comment on the study. “This study clearly shows that the early addition of adalimumab is the better choice, and it would change practice. That being said, there are clearly some patients who do respond sufficiently to increasing methotrexate, and it would be useful to be able to predict which patients might do that.” He added that the study focused on adalimumab and that the results might not apply to other biologics.
The study should encourage use of a quantitative treat-to-target measure like MDA, which is a composite measure of patient perspectives, Dr. Mease said. The American College of Rheumatology and National Psoriasis Foundation and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis have recommended the use of MDA as a treat-to-target measure for PsA. The ACR and NPF recommend TNF inhibitors as first-line treatment, and GRAPPA includes it as a first-line option, whereas the European Alliance of Associations for Rheumatology recommends MTX only in the first line.
The study also suggests that there is value to using adalimumab on a weekly basis if an every-other-week schedule doesn’t produce the desired results. This strategy hasn’t been examined in PsA or even RA, according to Dr. Kavanaugh, who is a professor of medicine at the University of California, San Diego. “It did look like raising the dose might be an option for patients who are on every other week and are not doing quite as well as we would have hoped.”
The CONTROL study was a phase 4, two-part, open-label study. It included 245 patients in 14 countries who did not have MDA with MTX. In the first part of the study, patients were randomly assigned to receive weekly 15 mg MTX along with 40 mg adalimumab every other week, or escalation of MTX dose to 20-25 mg/week. MTX could be administered orally or intravenously. After 16 weeks (part 1), for patients who achieved MDA, current therapy was maintained or modified; for patients who did not achieve MDA, therapy was escalated over the following 16 weeks by giving adalimumab every week in the combination group or by adding adalimumab every other week in the MTX escalation arm.
Overall, 95% of the MTX plus adalimumab group completed part 1, as did 90% of the MTX escalation group. A total of 41% of the adalimumab group achieved MDA at 16 weeks versus 13% of the MTX group (P < .0001). The result held after accounting for sex and the interaction between sex and treatment (odds ratio, 4.6; 95% confidence interval, 2.4-8.9).
Among patients who achieved MDA at 16 weeks, 80% in the adalimumab group continued to have MDA at 32 weeks even after MTX had been withdrawn. Of those in the MTX escalation group, 67% continued to have MDA at 32 weeks with continued escalation of MTX.
Of the patients in the MTX escalation group who did not respond, 55% reached MDA following introduction of adalimumab every other week. Of those who did not respond to adalimumab, 30% reached MDA after switching to weekly adalimumab doses.
The study was open label, and patients who received adalimumab may have expected some improvement; that could have skewed the findings, Dr. Kavanaugh said. “I think that’s an important consideration as we interpret the data. The people who got the MTX arm probably had less of an expectation that they were going to do much better than those who switched to the adalimumab, as did the doctors taking care of them.”
The CONTROL study was funded by AbbVie. Dr. Mease has received research grants, consulted for, or received speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Sun Pharma, and UCB. Dr. FitzGerald has received grant support and honoraria from AbbVie. Dr. Kavanaugh has received research support from or consulted for AbbVie, Janssen, Pfizer, Lilly, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
For patients with psoriatic arthritis (PsA) whose condition doesn’t respond adequately to methotrexate, addition of the tumor necrosis factor (TNF) inhibitor adalimumab increased the likelihood of achieving minimum disease activity (MDA), compared with escalation of MTX dose, according to results from a phase 4, open-label study.
The new study is one of only a few to compare treatment protocols in a field that has seen new therapeutic options become available in recent years. That lack of evidence can leave patients and physicians uncertain about the next step if the initial results of treatment are disappointing.
“There are some gaps in our database and our understanding of psoriatic arthritis, compared to rheumatoid arthritis, where we have had many more studies over the years,” Arthur Kavanaugh, MD, told this news organization when asked to comment on the study.
The trial provides one answer, at least. “There was a clear-cut signal that it made more sense to add adalimumab at that early juncture where a person is not quite doing well enough on methotrexate to satisfy our goal of getting the patient to low disease activity. It gives us as clinicians some ammunition to speak to our insurance formulary people on this side of the Atlantic, or [for] people in the U.K. to go to their local regulatory board that approves medicines and be able to show them some actual practically derived evidence about this very common question that comes up in practice,” senior and corresponding author Philip Mease, MD, said in an interview. The study was published online in The Lancet Rheumatology.
“When a clinician and patient are making the decision to move on from methotrexate monotherapy, either because of lack of efficacy or safety issues, tolerability issues, it makes most sense to add on a biologic medication such as a TNF inhibitor at that juncture, rather than intensifying methotrexate therapy,” said Dr. Mease, who is director of rheumatology research at Swedish Medical Center/Providence St. Joseph Health and a clinical professor at the University of Washington, both in Seattle.
Physicians may be tempted to bump up the dose for patients who can tolerate MTX and who may be showing some improvement, but the new study should prompt a different strategy if MDA isn’t achieved, according to Oliver FitzGerald, MD, a professor at the Conway Institute for Biomolecular Research at University College Dublin, who was asked to comment on the study. “This study clearly shows that the early addition of adalimumab is the better choice, and it would change practice. That being said, there are clearly some patients who do respond sufficiently to increasing methotrexate, and it would be useful to be able to predict which patients might do that.” He added that the study focused on adalimumab and that the results might not apply to other biologics.
The study should encourage use of a quantitative treat-to-target measure like MDA, which is a composite measure of patient perspectives, Dr. Mease said. The American College of Rheumatology and National Psoriasis Foundation and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis have recommended the use of MDA as a treat-to-target measure for PsA. The ACR and NPF recommend TNF inhibitors as first-line treatment, and GRAPPA includes it as a first-line option, whereas the European Alliance of Associations for Rheumatology recommends MTX only in the first line.
The study also suggests that there is value to using adalimumab on a weekly basis if an every-other-week schedule doesn’t produce the desired results. This strategy hasn’t been examined in PsA or even RA, according to Dr. Kavanaugh, who is a professor of medicine at the University of California, San Diego. “It did look like raising the dose might be an option for patients who are on every other week and are not doing quite as well as we would have hoped.”
The CONTROL study was a phase 4, two-part, open-label study. It included 245 patients in 14 countries who did not have MDA with MTX. In the first part of the study, patients were randomly assigned to receive weekly 15 mg MTX along with 40 mg adalimumab every other week, or escalation of MTX dose to 20-25 mg/week. MTX could be administered orally or intravenously. After 16 weeks (part 1), for patients who achieved MDA, current therapy was maintained or modified; for patients who did not achieve MDA, therapy was escalated over the following 16 weeks by giving adalimumab every week in the combination group or by adding adalimumab every other week in the MTX escalation arm.
Overall, 95% of the MTX plus adalimumab group completed part 1, as did 90% of the MTX escalation group. A total of 41% of the adalimumab group achieved MDA at 16 weeks versus 13% of the MTX group (P < .0001). The result held after accounting for sex and the interaction between sex and treatment (odds ratio, 4.6; 95% confidence interval, 2.4-8.9).
Among patients who achieved MDA at 16 weeks, 80% in the adalimumab group continued to have MDA at 32 weeks even after MTX had been withdrawn. Of those in the MTX escalation group, 67% continued to have MDA at 32 weeks with continued escalation of MTX.
Of the patients in the MTX escalation group who did not respond, 55% reached MDA following introduction of adalimumab every other week. Of those who did not respond to adalimumab, 30% reached MDA after switching to weekly adalimumab doses.
The study was open label, and patients who received adalimumab may have expected some improvement; that could have skewed the findings, Dr. Kavanaugh said. “I think that’s an important consideration as we interpret the data. The people who got the MTX arm probably had less of an expectation that they were going to do much better than those who switched to the adalimumab, as did the doctors taking care of them.”
The CONTROL study was funded by AbbVie. Dr. Mease has received research grants, consulted for, or received speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Sun Pharma, and UCB. Dr. FitzGerald has received grant support and honoraria from AbbVie. Dr. Kavanaugh has received research support from or consulted for AbbVie, Janssen, Pfizer, Lilly, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Adverse skin effects of cancer immunotherapy reviewed
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
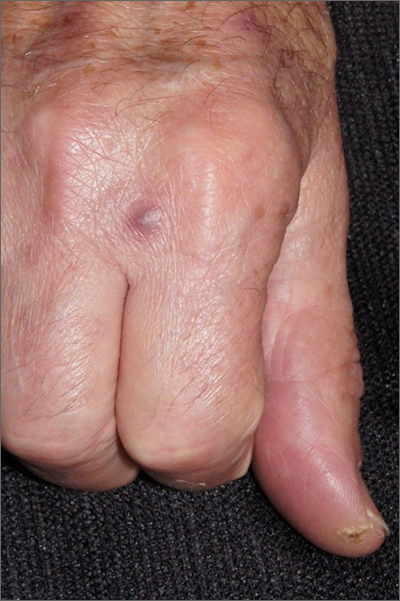
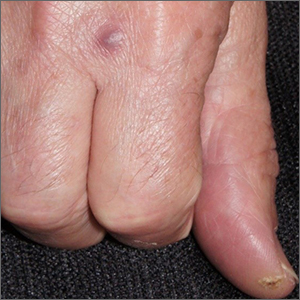
COVID-19–alopecia areata link? Review doesn’t find much evidence
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
FROM JAAD INTERNATIONAL
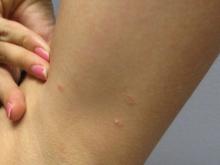
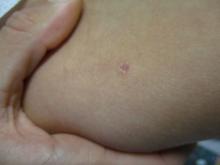
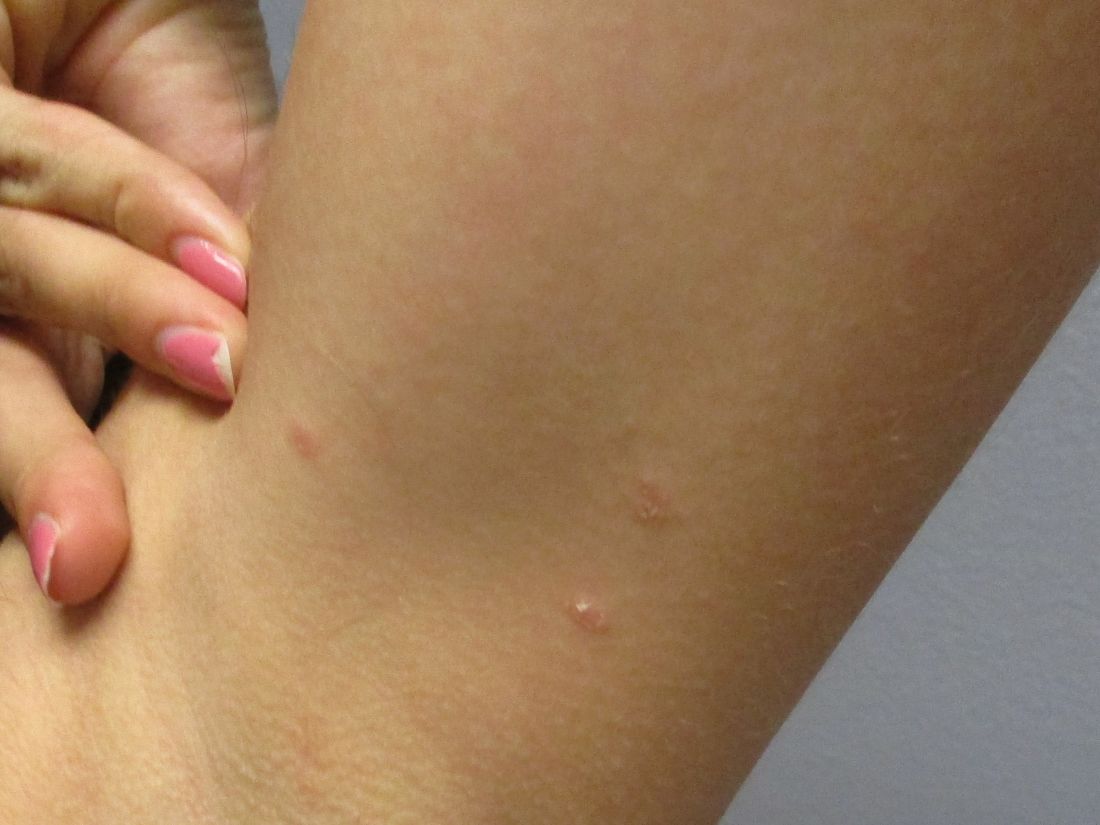
A 31-year-old female presented with a burning rash on upper arms, groin, and axillae
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
Betamethasone cream did not alleviate symptoms.
“Fishy” papule
A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020
A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020
Study links air pollution to psoriasis flares
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
Exposure to air pollution – even short term – may play a role in triggering psoriasis flares, according to new research from Italy, which found a significant association between exposure to higher levels of air pollution prior to patients presenting for psoriasis flares at medical visits, compared with visits unrelated to flares.
“We found that higher concentration of different air pollutants was associated with psoriasis flares in patients living in an industrialized city of the Po Valley” in Verona, Italy, report the authors of the study, published in JAMA Dermatology.
The findings underscore the need for clinicians to “consider environmental/external triggers in patients with chronic inflammatory diseases experiencing flares,” first author Francesco Bellinato, MD, of the Section of Dermatology and Venereology, University of Verona, Italy, told this news organization.
He and his coauthors conducted a case-crossover and cross-sectional longitudinal study that involved a retrospective analysis of data in 957 patients in Verona with chronic plaque psoriasis, who were evaluated every 3-4 months at an outpatient dermatology clinic for a median of 2.7 years.
Over the study period, disease flares, defined as an increase in the Psoriasis Area and Severity Index (PASI) of 5 or more points from the previous visit, occurred in 369 patients (38.6%), consistent with known flare rates in psoriasis. Participants in the study (mean age, 61) had median PASI scores of 12 during visits for psoriatic flares compared with PASI scores of 1 during control (no flare) visits (P < .001).
Evaluations of mean concentrations of several air pollutants within 10 miles of the patients over 4,398 visits showed that concentrations were significantly higher in the 60 days prior to the psoriasis flare, compared with control visits that were not related to flares (P < .05), after adjusting for factors including seasonality (by trimester, to adjust for weather conditions and UV/sunlight exposure) and the type of systemic psoriasis treatments patients were receiving (conventional or biological).
Increases in air pollutant levels prior to flares were observed among the 35.8% of patients who had a flare of at least a 50% increase in the PASI score, as well among the 47.2% of patients who had at least a 100% increase in PASI, compared with control visits not involving flares. In addition, mean and area-under-the-curve concentrations of air pollutants were higher in the 60 days before the visits among those with PASI 5 or greater, compared with those with PASI scores below 5, the authors add.
Dr. Bellinato noted that the associations were not limited to any particular subgroup. “The associations with air pollution and flares were observed in the entire population,” he said in an interview.
Vehicle, industry emissions
The pollutants that were measured were those mainly associated with fossil fuel combustion from vehicle and industry emissions, including carbon monoxide, nitrogen dioxide, other nitrogen oxides, benzene, coarse particulate matter (2.5-10.0 μm in diameter) and fine particulate matter (less than 2.5 μm in diameter).
They note that the risk of having a PASI score of 5 or greater was elevated even at thresholds of exposure that are largely considered safe. “Indeed, the risk for having a PASI score of 5 or greater was 40% to 50% higher at exposures as low as 20 μg/m3” of coarse particulate matter and 15 μg/m3 of fine particulate matter in the 60-day period prior to the visits, they write.
The authors referred to evidence linking air pollution with a worsening of a variety of inflammatory cutaneous diseases, including atopic dermatitis and acne, as well as photoaging. Psoriasis flares are known to be triggered by a variety of environmental factors, including infections or certain drugs; however, evidence of a role of air pollution has been lacking. Potential mechanisms linking the exposures to flares include the possibility that exhaust particles can activate skin resident T-cells, “resulting in abnormal production of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukins (ILs), including IL-1α, IL-1β, IL-6, and IL-8.8,” the authors write.
Their results, though inferring a causal relationship, fall short of showing a clear dose–response relationship between higher pollutant levels and an increased risk of psoriasis flares, possibly the result of a smaller sample size of subjects exposed to higher levels of pollution, they add.
Limitations of the study included the definition of flare, which used a clinical score that could be affected by other measurements, they point out, while strengths of the study included the large cohort of patients followed for over 7 years and the availability of daily measurements of air pollutants.
While the study suggests that environmental air pollutant fluctuations may affect psoriasis course,” the authors concluded, “further study is needed to examine whether these findings generalize to other populations and to better understand the mechanisms by which air pollution may affect psoriasis disease activity.”
Dr. Bellinato and four coauthors had no disclosures; the remaining authors had disclosures that included receiving personal fees from pharmaceutical companies that were outside of the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
An 11-year-old female presented with skin discoloration on her back
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Drug survival study looks at what lasts longest in RA, axSpA, PsA, and psoriasis
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
Survival rates of biologics and other novel immunomodulatory drugs vary substantially across chronic inflammatory diseases, and rates are highest for rituximab in rheumatoid arthritis (RA) and golimumab in axial spondyloarthritis (axSpA), but with similar rates seen for most drugs used in the treatment of psoriasis and psoriatic arthritis (PsA), according to findings from a study of two Danish registries.
Drug survival refers to “the probability that patients will remain on a given drug, and is a proxy for efficacy as well as safety in daily clinical practice,” wrote Alexander Egeberg, MD, PhD, of the department of dermatology at Copenhagen University Hospital–Bispebjerg, and colleagues. Although the use of biologics has expanded for inflammatory diseases, real-world data on drug survival in newer agents such as interleukin (IL)-17, IL-23, and Janus kinase inhibitors are lacking, they said.
In a study published in Seminars in Arthritis and Rheumatism, the researchers reviewed data from the DANBIO and DERMBIO registries of patients in Denmark with inflammatory diseases including rheumatoid arthritis (RA), axial spondyloarthritis (AxSpA), psoriatic arthritis (PsA), and psoriasis.
The study population included 12,089 adults: 5,104 with RA, 2,157 with AxSpA, 2,251 with PsA, and 2,577 with psoriasis. Patients’ mean age at the time of first treatment for these conditions was 57.8 years, 42.3 years, 49 years, and 45 years, respectively. Participants were treated with biologics or novel small molecule therapies for RA, AxSpA, PsA, or psoriasis between January 2015 and May 2021 (from the DANBIO database) and November 2009 to November 2019 (DERMBIO database).
In adjusted models, drug survival in RA was highest for rituximab followed by baricitinib, etanercept, and tocilizumab. Drug survival in AxSpA was highest for golimumab, compared with all other drugs, followed by secukinumab and etanercept. Survival was lowest for infliximab. In PsA, drug survival was roughly equal for most drugs, including golimumab, secukinumab, and ixekizumab, with the lowest survival observed for tofacitinib and infliximab, compared with all other drugs. Drug survival in psoriasis was highest with guselkumab, followed by ustekinumab and IL-17 inhibitors.
However, the number of treatment series “was low for some drugs, and not all differences were statistically significant, which could influence the overall interpretability of these findings,” the researchers noted in their discussion.
Notably, the high treatment persistence for rituximab in RA patients needs further confirmation, the researchers said. “In Denmark, rituximab is often the biologic drug of choice in RA patients with a history of cancer while there is a reluctancy to use TNF [tumor necrosis factor] inhibitors in such patients; this may have prolonged the drug survival for rituximab treated patients due to limited treatment alternatives,” they said.
The findings were limited by several factors, including the observational study design and changes in guidelines over the course of the study, the researchers noted. Other limitations included the inability to adjust for certain variables, such as antibody status, body weight, and smoking, because of missing data, and a lack of data on the underlying reasons for drug discontinuation, they said.
However, the results were strengthened by the large number of patients and completeness of the registries, the researchers emphasized. The range in responses to different drug types across diseases supports the need for individualized treatments with attention to underlying disease, patient profile, and treatment history, they concluded.
The study received no outside funding. Eight coauthors reported financial ties to a number of pharmaceutical companies.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM