User login
Study highlights impact of acne in adult women on quality of life, mental health
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
FROM JAMA DERMATOLOGY
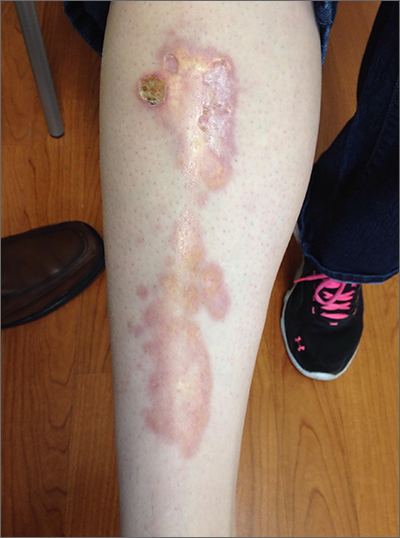
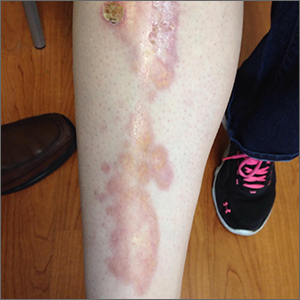
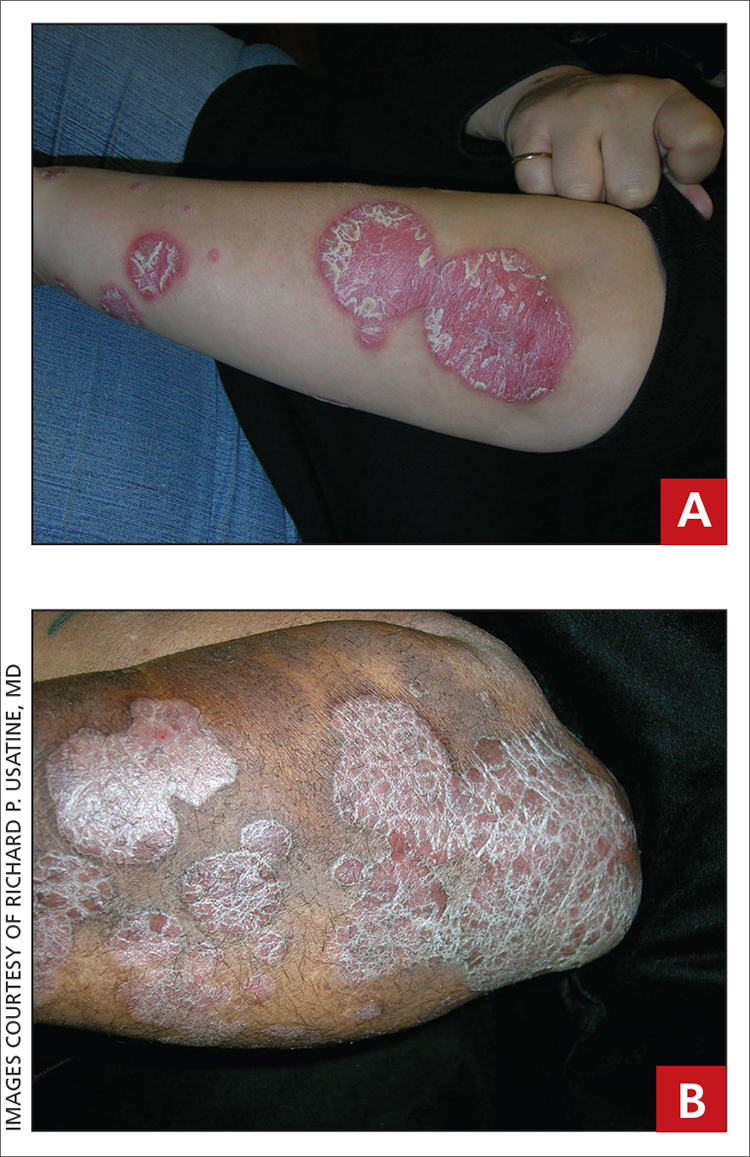
Leg rash
The appearance and location of this rash are classic signs for necrobiosis lipoidica, a chronic granulomatous skin disease commonly associated with diabetes. The patient’s initial hemoglobin A1c was 12.4%, confirming a diagnosis of type 2 diabetes, and a punch biopsy of the lesion demonstrated a broad zone of necrobiosis in the mid to lower dermis and a chronic inflammatory infiltrate, including plasma cells.
Necrobiosis lipoidica is rare, typically affects middle-aged adults, and is more common in women than in men.1 Although commonly associated with diabetes (hence the historical name necrobiosis lipoidica diabeticorum), a significant number of cases occur in patients without diabetes.2 The pathogenesis is not fully understood. The condition first appears as asymptomatic yellow to red-brown papules and plaques, most commonly on the anterior legs. The lesions then flatten over time, forming broad, yellow-pink patches and plaques.1,2
Generally, the diagnosis can be made clinically but, if uncertain, a punch biopsy is the preferred technique for confirmation. The differential diagnosis includes chronic cutaneous lupus erythematosus (LE), sarcoidosis, and dermatophytosis. Although the appearance of lesions associated with LE or sarcoidosis can vary, neither one manifests with the yellow coloring seen here. Dermatophytosis typically demonstrates scale and pruritus with an active border; viewing a potassium hydroxide preparation of a skin scraping is usually sufficient to make a diagnosis.
Treatment for necrobiosis lipoidica includes counseling patients to avoid trauma to the affected areas, high-potency topical corticosteroids, and photodynamic therapy.3 Often, lesions are permanent.
For this patient’s diabetes treatment, she was prescribed metformin and insulin glargine and counseled extensively on weight loss, regular exercise, and appropriate diet adjustments. The rash was treated topically with triamcinolone 0.1% cream bid. At her 4-month follow-up, the patient’s hemoglobin A1c value had dropped to 5.4%, and the rash had become less prominent and widespread. The patient was pleased with the cosmetic outcome and declined referral to a dermatologist for further treatment.
Photo and text courtesy of Samuel Dickmann, MD, and James Medley, MD, University of Florida College of Medicine, Gainesville.
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. O’Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286. doi: 10.1046/j.1365-2133.1999.02663.x
3. Heidenheim M, Jemec GBE. Successful treatment of necrobiosis lipoidica diabeticorum with photodynamic therapy. Arch Dermatol. 2006;142:1548-1550. doi: 10.1001/archderm.142.12.1548
The appearance and location of this rash are classic signs for necrobiosis lipoidica, a chronic granulomatous skin disease commonly associated with diabetes. The patient’s initial hemoglobin A1c was 12.4%, confirming a diagnosis of type 2 diabetes, and a punch biopsy of the lesion demonstrated a broad zone of necrobiosis in the mid to lower dermis and a chronic inflammatory infiltrate, including plasma cells.
Necrobiosis lipoidica is rare, typically affects middle-aged adults, and is more common in women than in men.1 Although commonly associated with diabetes (hence the historical name necrobiosis lipoidica diabeticorum), a significant number of cases occur in patients without diabetes.2 The pathogenesis is not fully understood. The condition first appears as asymptomatic yellow to red-brown papules and plaques, most commonly on the anterior legs. The lesions then flatten over time, forming broad, yellow-pink patches and plaques.1,2
Generally, the diagnosis can be made clinically but, if uncertain, a punch biopsy is the preferred technique for confirmation. The differential diagnosis includes chronic cutaneous lupus erythematosus (LE), sarcoidosis, and dermatophytosis. Although the appearance of lesions associated with LE or sarcoidosis can vary, neither one manifests with the yellow coloring seen here. Dermatophytosis typically demonstrates scale and pruritus with an active border; viewing a potassium hydroxide preparation of a skin scraping is usually sufficient to make a diagnosis.
Treatment for necrobiosis lipoidica includes counseling patients to avoid trauma to the affected areas, high-potency topical corticosteroids, and photodynamic therapy.3 Often, lesions are permanent.
For this patient’s diabetes treatment, she was prescribed metformin and insulin glargine and counseled extensively on weight loss, regular exercise, and appropriate diet adjustments. The rash was treated topically with triamcinolone 0.1% cream bid. At her 4-month follow-up, the patient’s hemoglobin A1c value had dropped to 5.4%, and the rash had become less prominent and widespread. The patient was pleased with the cosmetic outcome and declined referral to a dermatologist for further treatment.
Photo and text courtesy of Samuel Dickmann, MD, and James Medley, MD, University of Florida College of Medicine, Gainesville.
The appearance and location of this rash are classic signs for necrobiosis lipoidica, a chronic granulomatous skin disease commonly associated with diabetes. The patient’s initial hemoglobin A1c was 12.4%, confirming a diagnosis of type 2 diabetes, and a punch biopsy of the lesion demonstrated a broad zone of necrobiosis in the mid to lower dermis and a chronic inflammatory infiltrate, including plasma cells.
Necrobiosis lipoidica is rare, typically affects middle-aged adults, and is more common in women than in men.1 Although commonly associated with diabetes (hence the historical name necrobiosis lipoidica diabeticorum), a significant number of cases occur in patients without diabetes.2 The pathogenesis is not fully understood. The condition first appears as asymptomatic yellow to red-brown papules and plaques, most commonly on the anterior legs. The lesions then flatten over time, forming broad, yellow-pink patches and plaques.1,2
Generally, the diagnosis can be made clinically but, if uncertain, a punch biopsy is the preferred technique for confirmation. The differential diagnosis includes chronic cutaneous lupus erythematosus (LE), sarcoidosis, and dermatophytosis. Although the appearance of lesions associated with LE or sarcoidosis can vary, neither one manifests with the yellow coloring seen here. Dermatophytosis typically demonstrates scale and pruritus with an active border; viewing a potassium hydroxide preparation of a skin scraping is usually sufficient to make a diagnosis.
Treatment for necrobiosis lipoidica includes counseling patients to avoid trauma to the affected areas, high-potency topical corticosteroids, and photodynamic therapy.3 Often, lesions are permanent.
For this patient’s diabetes treatment, she was prescribed metformin and insulin glargine and counseled extensively on weight loss, regular exercise, and appropriate diet adjustments. The rash was treated topically with triamcinolone 0.1% cream bid. At her 4-month follow-up, the patient’s hemoglobin A1c value had dropped to 5.4%, and the rash had become less prominent and widespread. The patient was pleased with the cosmetic outcome and declined referral to a dermatologist for further treatment.
Photo and text courtesy of Samuel Dickmann, MD, and James Medley, MD, University of Florida College of Medicine, Gainesville.
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. O’Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286. doi: 10.1046/j.1365-2133.1999.02663.x
3. Heidenheim M, Jemec GBE. Successful treatment of necrobiosis lipoidica diabeticorum with photodynamic therapy. Arch Dermatol. 2006;142:1548-1550. doi: 10.1001/archderm.142.12.1548
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. O’Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286. doi: 10.1046/j.1365-2133.1999.02663.x
3. Heidenheim M, Jemec GBE. Successful treatment of necrobiosis lipoidica diabeticorum with photodynamic therapy. Arch Dermatol. 2006;142:1548-1550. doi: 10.1001/archderm.142.12.1548
When is MRI useful in the management of congenital melanocytic nevi?
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
FROM SPD 2021
Study estimates carbon footprint reduction of virtual isotretinoin visits
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
FROM PEDIATRIC DERMATOLOGY
Common outcome measures for AD lack adequate reporting of race, skin tone
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2021
Genetic testing for neurofibromatosis 1: An imperfect science
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
FROM SPD 2021
Is your patient a candidate for Mohs micrographic surgery?
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.
Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.
Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; agarcia326@gmail.com
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.
Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.
Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; agarcia326@gmail.com
Mohs micrographic surgery (MMS) is a unique dermatologic surgery technique that allows the dermatologist to fill the concomitant roles of surgeon and pathologist. It is utilized for the extirpation of skin malignancy, with an emphasis on tissue preservation and immediate surgical margin evaluation. In MMS, the Mohs surgeon acts as the surgeon for physical removal of the lesion and the pathologist during evaluation of frozen section margins.1
Primary care providers (PCPs) are on the frontlines of management of cutaneous malignancy. Whether referring to Dermatology for biopsy or performing a biopsy themselves, PCPs can assure optimal treatment outcomes by guiding patients to evidence-based treatments, while still respecting the patient’s wishes. In this evidence-based review of the advantages, improved outcomes, and safety of Mohs surgery for the treatment of common and rare skin neoplasms, we provide our primary care colleagues with information on the indications, process (the order in which steps of the procedure are performed), and techniques used for treating cutaneous malignancies with Mohs surgery.
When is Mohs surgery appropriate?
MMS has typically been reserved for treatment of cutaneous malignancy in cosmetically sensitive areas where tissue preservation is key. In 2012, Connolly et al released appropriate use criteria (AUC) for MMS.2 (See “An app that helps clinicians apply the criteria for Mohs surgery.”) Within the AUC, there are 4 major qualitative and quantitative categories when considering referral for MMS:
- area of the body in which the lesion manifests
- the patient’s medical characteristics
- tumor characteristics
- the size of the lesion to be treated.2
Areas of the body are divided into 3 categories by the AUC according to how challenging tumor extirpation is expected to be and how critical tissue preservation is. Areas termed “H” receive the highest score for appropriate Mohs usage, followed by areas “M” and “L.”
SIDEBAR
An app that helps clinicians apply the criteria for Mohs surgery
“Mohs Surgery Appropriate Use Criteria” is a free and easy-to-use smartphone application to help determine whether Mohs micrographic surgery (MMS) is appropriate for a particular patient. Clinicians can enter the details of a recent skin cancer biopsy along with patient information into the app and it will calculate a score automatically categorized into 1 of 3 categories: “appropriate,” “uncertain,” and “not appropriate” for MMS. The clinician can then talk to the patient about a possible referral to a Mohs surgeon, depending on the appropriateness of the procedure for the patient and their tumor.
Patient medical characteristics that should be taken into account when referring for Mohs surgery are the patient’s immune status, genetic syndromes that may predispose the patient to cutaneous malignancies (eg, xeroderma pigmentosa), history of radiation to the area of involvement, and the patient’s history of aggressive cutaneous malignancies.
Tumor characteristics. The most common malignancies treated with MMS include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These malignancies are further delineated through histologic evaluation by a pathologist or dermatopathologist. Aggressive features of a BCC on any area of the body that warrant referral to a Mohs surgeon include morpheaform/fibrosing/sclerosing histologic findings, as well as micronodular architecture and perineural invasion. Concerning histologic SCC findings that warrant Mohs surgery through the AUC include sclerosing, basosquamous, and small cell histology, as well as poorly differentiated and/or undifferentiated SCC.
Melanoma in situ and lentigo maligna, which are variants of melanoma limited to the epidermis without invasion into the underlying dermis, are included within the AUC for MMS. For invasive melanoma (melanoma that has invaded into the dermis or subcutaneous tissue), MMS has been shown to have marginal benefit but currently is not included within the AUC.3
Continue to: Due to excellent margin control...
Due to excellent margin control via immediate microscopic evaluation of surgical margins, MMS is an appropriate treatment choice and indicated for many more uncommon cutaneous malignancies, including sebaceous and mucinous carcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, leiomyosarcoma, dermatofibrosarcoma protuberans, atypical fibroxanthoma, angiosarcoma, and other more rarely encountered clinical malignancies.2
Tumor size. When considering a referral to MMS for cancer extirpation, the size of the tumor does play a role; however, size depends on the type of tumor as well as the location on the body. In general, most skin cancers of any size on the face, perianal area, genitalia, nipples, hands, feet and ankles, or pretibial surface are appropriate for Mohs surgery. Skin cancers on the trunk and extremities are also appropriate if they are above a certain size specified by the AUC. Tumor type and whether they are recurrences also factor into the equation.
Who will do the procedure?
A recent review showed that PCPs were more likely to refer patients to plastic surgery rather than Mohs surgery for skin cancer removal, especially among younger female patients.4 This is likely because of the perception that plastic surgeons do more complex closures and have more experience removing difficult cancers. Interestingly, this same study showed that Mohs surgeons may actually be doing several-fold more complex closures (flaps and grafts) on the nose and ears than plastic surgeons at similar practice settings.4
Aside from Mohs surgeons doing more closures, perhaps the biggest difference between Mohs surgeons and plastic surgeons is the pathology training of the Mohs surgeon. Mohs surgeons evaluate 100% of the tissue margins at the time of the procedure to both ensure complete tumor removal and to preserve as much tumor-free skin as possible, ultimately resulting in decreased recurrences and smaller scars. In contrast, the plastic surgeon’s rigorous training typically does not include extensive dermatopathology training, particularly the pathology of cutaneous neoplasms. Plastic surgeons will often send pathologic specimens for evaluation, meaning patients have to wait for outside histologic confirmation before their wounds can be closed. Additionally, the histologic evaluation is often not a full-margin assessment, as not all labs are equipped for this technique.
Consider early consultation with a Mohs surgeon for tumor extirpation to keep the defect size as small as possible, as MMS does not require taking margins of healthy surrounding tissue, in contrast to wide local excisions (WLEs; FIGURE 1). A smaller initial incision will result in a smaller scar, which is likely to have better cosmetic outcomes and decreased risk for wound infection.
Continue to: Before consultation...
Before consultation, include a picture of the surgical site with the patient’s referral documentation or have the patient present a photo from his or her phone to the Mohs surgeon. (If a camera or cell phone is not available, triangulation of the site’s location using cosmetic landmarks can be documented in the patient’s chart.)
What the patient can expect during preop visits
During an initial consultation, patients can expect an evaluation by the surgeon that will include more photo taking, a discussion of the surgery, and possibly, performance of an in-clinic biopsy of suspicious lesions. Many practices, including the authors’, use a photo capturing add-on for the EMR in the office.5-7
During the consent process, MMS is described to the patient using lay language and, often, pictorial depictions of the procedure. While explaining that the procedure helps preserve healthy tissue and limit the size of the resulting scar, the surgeon will typically manage the expectations of the patient prior to the first incision. Many clinically small lesions can have significant subclinical extension adjacent to, or on top of, cosmetic landmarks, requiring a flap or graft to close the surgical defect with acceptable cosmetic outcomes.8
One more time. Immediately before surgery, the surgeon will again review the procedure with the patient, using photos of the biopsy site taken during the initial consult, in conjunction with patient verification of the biopsy site, to verify the surgical site and confirm that the patient understands and agrees to the surgery.
A look at how Mohs surgery is performed
MMS typically is performed in the outpatient setting but can also be performed in an operating room or outpatient surgical center. MMS can be performed in a nonsterile procedure room with surgeons and assistants typically utilizing clean, nonsterile gloves, although many Mohs surgeons prefer to perform part, or all, of the technique using sterile gloves.9 A recent systematic review and large meta-analysis showed no significant difference in postsurgical site infections when comparing the use of sterile vs nonsterile gloves.10
Continue to: Prior to initial incision...
Prior to initial incision, the site is marked with a surgical pen and given 1-mm margins around the clinically visualized lesion. The site is then cleansed with an antiseptic, typically a chlorhexidine solution. Local anesthesia is employed, most commonly with a 1:100,000 lidocaine and epinephrine injection. Marking of the tumor prior to numbing is imperative, as the boundaries of the tumor are typically obscured when the local cutaneous vasculature constricts and causes visualized blanching of adjacent skin. Many Mohs surgeons perform a brief curettage of the lesion with a nondisposable, dull curette to better define the tumor edges and to debulk any obvious exophytic tumor noted by the naked eye.
Prior to the first incision, the surgical site is scored in a variety of ways in order to properly orient the tissue after it has been removed from the patient. Mohs surgeons have differing opinions on how to score and/or mark the tissue, but a common practice is to make a nick at the 12 o’clock position. Following removal of the first stage, the nick will be visible on both the extirpated tissue and the tissue just above the surgical defect. This prevents potential confusion regarding orientation during tissue processing.
The majority of all WLEs are performed utilizing the scalpel blade at an angle 90° perpendicular to the plane of the skin. In MMS, a signature 45° angle with the tip of the scalpel pointing toward, and the handle pointing away from, the lesion is commonly used in order to bevel the tissue being excised (FIGURE 2). Once the tissue is excised, hemostasis is obtained using electrodessication/electrofulguration or electrocoagulation.
Tissue processing and microscopic evaluation
The technique of beveling allows the epidermis, dermis, and subcutaneous tissue to lie flat on the tissue block, so the Mohs surgeon can evaluate 100% of the excised tissue’s margins. The tissue is transported to a nearby lab for staining and processing. Even if near-perfect beveling is achieved, many stages will require bisecting, quadrissecting, or relaxing cuts in order to allow the margins to lie flat on the tissue block.
Using the scoring system made prior to incision, the tissue is oriented and stained with colored ink. Subsequently, a map is made with sections highlighting the colors used to stain designated areas of the tissue. This step is imperative for orientation during microscopic evaluation. Additionally, the map serves as a guide and log, should a section of the specimen have an involved margin and require another stage.
Continue to: Once fixed to the block...
Once fixed to the block, the tissue is engulfed in appropriate embedding medium and placed within the cryostat. The block is slowly cut to produce several micron-thin wafers of tissue that are then mounted on glass slides and processed with hematoxylin and eosin (H&E) or various stains. The first wafers of tissue that come from the tissue block are those that are closest to the margin that was excised. Thus, 100% of the epidermis and deep margin can be visualized. “Deeper sections” are those that come from deeper cuts within the tissue and are more likely to show the malignant neoplasm.
The evaluation of immediate margins at the very edge of the tissue is in contrast to the technique of “bread-loafing,” which is the standard of evaluating margins after a WLE.11 With this process, the pathologist examines sections that are cut 2- to 4-mm apart. This process only allows the pathologist to examine roughly 1% of the total tissue that was excised, and large variability in cutaneous representation can occur depending on the individual who cuts and processes the tissue.11
Closing the defect
Once the site is deemed clear of residual tumor, the Mohs surgeon approaches the defect and determines the most appropriate way to close the surgical wound. Mohs surgeons are trained to close wounds using a variety of methods, including complex linear closures, flaps, and full-thickness skin grafts. Thoughtful consideration of local anatomy, cosmetic landmarks that may be affected by the closure method, and local tissue laxity are evaluated.
Depending on the location, a secondary intention closure may prove to be just as effective and cosmetically satisfying as a primary intention closure. In light of the many methods of closure, a complex or large surface area defect may better be suited for evaluation and closure by another specialist such as an ENT physician, ophthalmologist, or plastic surgeon.12
Lower recurrence rates for patients who undergo Mohs surgery
As noted earlier, the cutaneous malignancies most commonly treated with MMS are BCCs, followed by SCCs.13 Comparison studies between WLE and MMS show clinically significant differences in terms of recurrence rates between the 2 procedures.
Continue to: For BCCs
For BCCs, recurrence rates for excisions vs MMS are 10% and 1%, respectively.14-16 A randomized trial reviewing 10-year recurrence of primary BCCs on the face showed recurrence rates for MMS of 4.4% compared to 12.2% for WLE.17 This study also showed recurrence rates for recurrent facial BCCs treated with MMS to be 3.9% vs 13.5% for standard WLE.17
SCC. The evidence similarly supports the efficacy of MMS for SCCs. A recent study showed primary T2a tumors had a 1.2% local recurrence rate with Mohs vs a 4% recurrence rate with WLE at an average follow-up of 2.8 years.18 Another study showed that primary tumors that were < 2 cm in diameter had a 5-year cure rate of 99% with Mohs surgery.11
Melanoma in situ. A few studies have shown no clinically significant benefit of MMS compared to WLE when it comes to melanoma in situ.19,20 However, a more recent article by Etzkom et al noted the ability to potentially upstage melanoma in situ and invasive melanoma after reviewing peripheral and deep margins during MMS.21 In this study, the authors uniquely delayed wound closure if upstaging was established and the need for a sentinel lymph node biopsy was warranted. This approach to MMS with delayed closure ultimately paved the way for very low recurrence rates.
CORRESPONDENCE
Andres Garcia, MD, 2612 112th Street, Lubbock, TX 79423; agarcia326@gmail.com
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
1. Dim-Jamora KC, Perone JB. Management of cutaneous tumors with Mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
2. Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. Published correction appears in J Am Acad Dermatol. 2015;72:748.
3. Cheraghlou S, Christensen S, Agogo G, et al. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol. 2019;155:1252-1259.
4. Hill D, Kim K, Mansouri B, et al. Quantity and characteristics of flap or graft repairs for skin cancer on the nose or ears: a comparison between Mohs micrographic surgery and plastic surgery. Cutis. 2019;103:284-287.
5. McGinness JL, Goldstein G. The value of preoperative biopsy-site photography for identifying cutaneous lesions. Dermatol Surg. 2010;36:194-197.
6. Ke M, Moul D, Camouse M, et al. Where is it? The utility of biopsy-site photography. Dermatol Surg. 2010;36:198-202.
7. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies—a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41:499-504
8. Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
9. Rhinehart BM, Murphy Me, Farley MF, et al. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg. 2006;32:170-176.
10. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
11. Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
12. Gladstone HB, Stewart D. An algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6-9.
13. Robinson JK. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149-156.
14. Swanson NA. Mohs surgery. Technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
15. Robins P. Chemosurgery: my 15 years of experience. J Dermatol Surg Oncol. 1981;7:779-789.
16. Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
17. van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
18. Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82: 1195-1204.
19. Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44:481-492.
20. Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153:436-441.
21. Etzkom JR, Sobanko JF, Elenitsas R, et al. Low recurrences for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
PRACTICE RECOMMENDATIONS
› Consider Mohs surgery for patients who have lesions located mainly in regions of the face that make excision difficult without significant scarring. A
› Consider Mohs surgery for basal cell carcinoma and squamous cell carcinoma that typically involve (but are not necessarily limited to) the face, as the procedure significantly reduces recurrence rates and leads to cure rates of up to 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hand ulceration
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
Psoriasis
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.
Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.
Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
THE COMPARISON
A Elbow and forearm with erythematous, well-demarcated, pink plaques with mild micaceous scale in a 42-year-old White woman.
B Elbow and forearm with violaceous, well-demarcated plaques with micaceous scale and hyperpigmented patches around the active plaques in a 58-year-old Black man.
Epidemiology
Psoriasis prevalence in the United States has been estimated at 3.7%.1-3 If broken down by race or ethnicity, the prevalence of psoriasis varies: 2.5% to 3.7% in White adults1-4; 1.3% to 2% in Black adults1-4; 1.6% in Hispanics/other adults1-3; 1% in children overall; 0.29% in White children1,5; and 0.06% in Black children.1,5
Key clinical features in people with darker skin tones include:
- plaques that may appear more violaceous in color instead of pink or erythematous
- higher body surface area of involvement4 and thicker, more scaly plaques6
- increased likelihood of postinflammatory hyperpigmentation (PIH).
Worth noting
Although individuals of all skin tones may experience the psychosocial impact of psoriasis, quality-of-life measures have been found to be worse in those with skin of color (SOC) compared to White patients. 1,4 This may be due to the lingering PIH and hypopigmentation that occurs even after inflammatory plaques are treated. Of course, lack of access to care contributes to greater disease burden and more devastating psychological impact.
Health disparity highlight
Psoriasis may be underreported and underdiagnosed in individuals with SOC, as factors contributing to health care disparities may play a role, such as access to health care in general,1,7 and access to clinicians proficient in diagnosing cutaneous diseases in SOC may be delayed.8
Biologic medications are used less often in Black patients than in White patients, despite biologic medications being very efficacious for treatment of psoriasis.1,9,10
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
1. Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
2. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
5. Wu JJ, Black MH, Smith N, et al. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65:957-964.
6. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
7. Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
8. Mundluru SN, Ramalingam ND, Tran HN. Addressing internal medicine residents’ discomfort with basic dermatology in persons of color in the primary care clinic. Am J Med Qual. 2019;34:513-513.
9. Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
The first signs of elusive dysautonomia may appear on the skin
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
FROM SPD 2021