User login
Survey spotlights the out-of-pocket burden on Blacks with atopic dermatitis
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
FROM REVOLUTIONIZING AD 2021
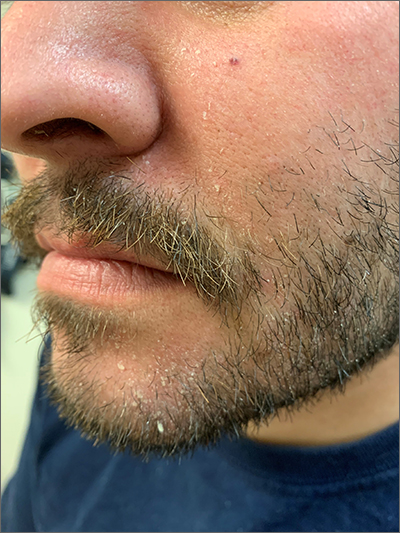
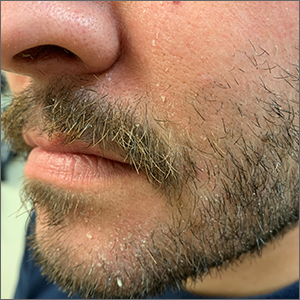
Scaly beard rash
Waxy loose scale with associated erythema on the face and scalp is a classic sign of seborrheic dermatitis (SD).
SD is caused by inflammation related to the presence of Malassezia, which proliferates on sebum-rich areas of skin. Malassezia is normally present on the skin, but some individuals have a heightened sensitivity to it, leading to erythema and scale. It is prudent to examine the scalp, nasolabial folds, and around the ears where it often occurs concomitantly.
There are multiple topical and systemic options which treat the fungal involvement, the subsequent inflammation, and reduce the scale.1 Topical azole antifungals are effective for reducing the amount of Malassezia present. Topical steroids work well to reduce the erythema. Fortunately, low-potency steroids, including hydrocortisone and desonide, are adequate. This is important since SD frequently involves the face and higher-potency steroids can cause skin atrophy or rebound erythema.
Salicylic acid products exfoliate the scale and topical tar products suppress the scale, both leading to clinical improvement. Sunlight and narrow beam UVB light therapy are also effective treatments. As was true with this patient, SD often improves during the summer months (when there is more sunlight) and when patients shave, as this allows for additional sun exposure to the skin.
The patient in this case was told to use ketoconazole shampoo for his scalp, beard, and mustache. He was instructed to use it at least 3 times per week, applying it to the scalp as the first part of his bathing routine and then waiting until the end to rinse it off. This technique maximizes the antifungal shampoo’s contact time on the skin. He was also given a prescription for ketoconazole cream to apply twice daily to the areas of facial erythema and scale. He was counseled that shaving his beard and mustache might help reduce the SD in those areas.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169. doi: 10.1080/09546634.2018.1473554
Waxy loose scale with associated erythema on the face and scalp is a classic sign of seborrheic dermatitis (SD).
SD is caused by inflammation related to the presence of Malassezia, which proliferates on sebum-rich areas of skin. Malassezia is normally present on the skin, but some individuals have a heightened sensitivity to it, leading to erythema and scale. It is prudent to examine the scalp, nasolabial folds, and around the ears where it often occurs concomitantly.
There are multiple topical and systemic options which treat the fungal involvement, the subsequent inflammation, and reduce the scale.1 Topical azole antifungals are effective for reducing the amount of Malassezia present. Topical steroids work well to reduce the erythema. Fortunately, low-potency steroids, including hydrocortisone and desonide, are adequate. This is important since SD frequently involves the face and higher-potency steroids can cause skin atrophy or rebound erythema.
Salicylic acid products exfoliate the scale and topical tar products suppress the scale, both leading to clinical improvement. Sunlight and narrow beam UVB light therapy are also effective treatments. As was true with this patient, SD often improves during the summer months (when there is more sunlight) and when patients shave, as this allows for additional sun exposure to the skin.
The patient in this case was told to use ketoconazole shampoo for his scalp, beard, and mustache. He was instructed to use it at least 3 times per week, applying it to the scalp as the first part of his bathing routine and then waiting until the end to rinse it off. This technique maximizes the antifungal shampoo’s contact time on the skin. He was also given a prescription for ketoconazole cream to apply twice daily to the areas of facial erythema and scale. He was counseled that shaving his beard and mustache might help reduce the SD in those areas.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Waxy loose scale with associated erythema on the face and scalp is a classic sign of seborrheic dermatitis (SD).
SD is caused by inflammation related to the presence of Malassezia, which proliferates on sebum-rich areas of skin. Malassezia is normally present on the skin, but some individuals have a heightened sensitivity to it, leading to erythema and scale. It is prudent to examine the scalp, nasolabial folds, and around the ears where it often occurs concomitantly.
There are multiple topical and systemic options which treat the fungal involvement, the subsequent inflammation, and reduce the scale.1 Topical azole antifungals are effective for reducing the amount of Malassezia present. Topical steroids work well to reduce the erythema. Fortunately, low-potency steroids, including hydrocortisone and desonide, are adequate. This is important since SD frequently involves the face and higher-potency steroids can cause skin atrophy or rebound erythema.
Salicylic acid products exfoliate the scale and topical tar products suppress the scale, both leading to clinical improvement. Sunlight and narrow beam UVB light therapy are also effective treatments. As was true with this patient, SD often improves during the summer months (when there is more sunlight) and when patients shave, as this allows for additional sun exposure to the skin.
The patient in this case was told to use ketoconazole shampoo for his scalp, beard, and mustache. He was instructed to use it at least 3 times per week, applying it to the scalp as the first part of his bathing routine and then waiting until the end to rinse it off. This technique maximizes the antifungal shampoo’s contact time on the skin. He was also given a prescription for ketoconazole cream to apply twice daily to the areas of facial erythema and scale. He was counseled that shaving his beard and mustache might help reduce the SD in those areas.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169. doi: 10.1080/09546634.2018.1473554
Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169. doi: 10.1080/09546634.2018.1473554
Allergic conjunctivitis severely affects children’s quality of life
Allergic conjunctivitis harms quality of life for children and their parents, apparently causing greater day-to-day worries than potentially blinding diseases, researchers report.
Parents worry especially that treatments might not be effective, according to Shi-yao Zhang, MD, and colleagues from Sun Yat-sen University, Guangzhou, China. “This finding suggests that more communication with parents regarding treatment and prognosis is needed,” they write in an article published online June 10 in JAMA Ophthalmology.
One of the most prevalent eye disorders in children, allergic conjunctivitis is often chronic, leading patients to ask repeatedly for help from physicians. It can take an emotional toll and can cause children to miss school.
“With any sign of a slightly pink eye [or a] runny nose, which are very common with allergies, children are being sent home, because everyone’s concerned about COVID,” said Yi Ning J. Strube, MD, an associate professor of ophthalmology and pediatrics at Queen’s University, Kingston, Canada, whose commentary appears in the same issue of JAMA Ophthalmology.
Adolescents are also sometimes accused of smoking cannabis because of their red eyes, she said.
However, little research has examined the effects of allergic conjunctivitis on the quality of life of children and their guardians, Dr. Zhang and colleagues write. To fill that gap, the researchers administered the Pediatric Quality of Life Inventory (PedsQL) to 92 children with allergic conjunctivitis and their parents. The children were aged 5 to 18 years.
The researchers administered the same questionnaire to 96 healthy children of the same ages, along with their parents. These participants served as a control group.
On a scale of 0 to 100, in which a higher score signifies a better quality of life, the median total PedsQL score was 69.6 for children with allergic conjunctivitis versus 96.7 for the control group.
Subscores of physical, emotional, social, and especially school functioning were all significantly lower for the children with allergic conjunctivitis than for the control persons. “Because children generally spend most of their time in the school environment, this outcome raises an issue regarding whether children have a poorer performance in their education,” Dr. Zhang and colleagues write.
Dr. Strube recommends that physicians educate their patients about allergic conjunctivitis using handouts or high-quality websites. She often refers patients and their families to the allergic conjunctivitis webpage of the American Academy of Pediatric Ophthalmology and Strabismus.
She tells parents to have their child “take a shower and wash their hair when they get home before they rub their pollen-filled hair on their pillowcase and make their allergy symptoms worse.”
Parents and schools should try to filter pollen and other allergens from indoor air, she added.
Parents of the children with allergic conjunctivitis in the study also reported lower quality of life; they scored 68.8, versus 96.5 for parents of children in the control group. The differences for both parents and children were statistically significant (P < .001). Overall, the parents’ quality-of-life scores correlated with their children’s (correlation coefficient, r = 0.59; P < .001).
Children with vernal or atopic keratoconjunctivitis scored 3.3 points lower on health-related quality of life than those with seasonal allergic conjunctivitis.
Children with higher corneal fluorescein staining scores also had lower quality-of-life scores. Parents whose children had higher corneal fluorescein staining scores and also those who had multiple consultations with health care practitioners also reported lower quality of life.
The quality-of-life scores of the children with allergic conjunctivitis were lower than scores in previous studies for children with vision-threatening diseases, such as glaucoma and congenital cataract. This may be because glaucoma and cataracts do not typically cause discomfort even if they impair the patient’s vision, said Dr. Strube.
She pointed out one potential flaw in the study: In the cohort with allergic conjunctivitis, 83.7% were boys, compared to 42.7% of the control group. Vernal keratoconjunctivitis affects more boys than girls, and not controlling for this factor could have confounded the data, Dr. Strube said.
It could also be useful to replicate the study in other countries to see whether geographic or cultural factors affected the results, she said. “A lot of these big centers around the world, including in China, have poor air quality, so that may be contributing to patients’ symptoms,” she said. “With regards to reported health quality of life and impact on education, results from different parts of the world may be different, due to parenting styles and education styles,” she said.
The study was supported by the National Natural Science Foundation of China and the Science Foundation of Guangdong Province. Dr. Zhang and colleagues reported no relevant financial relationships. Dr. Strube reported receiving personal fees from Santen Canada Advisory Board Consultant outside the submitted work.
A version of this article first appeared on Medscape.com.
Allergic conjunctivitis harms quality of life for children and their parents, apparently causing greater day-to-day worries than potentially blinding diseases, researchers report.
Parents worry especially that treatments might not be effective, according to Shi-yao Zhang, MD, and colleagues from Sun Yat-sen University, Guangzhou, China. “This finding suggests that more communication with parents regarding treatment and prognosis is needed,” they write in an article published online June 10 in JAMA Ophthalmology.
One of the most prevalent eye disorders in children, allergic conjunctivitis is often chronic, leading patients to ask repeatedly for help from physicians. It can take an emotional toll and can cause children to miss school.
“With any sign of a slightly pink eye [or a] runny nose, which are very common with allergies, children are being sent home, because everyone’s concerned about COVID,” said Yi Ning J. Strube, MD, an associate professor of ophthalmology and pediatrics at Queen’s University, Kingston, Canada, whose commentary appears in the same issue of JAMA Ophthalmology.
Adolescents are also sometimes accused of smoking cannabis because of their red eyes, she said.
However, little research has examined the effects of allergic conjunctivitis on the quality of life of children and their guardians, Dr. Zhang and colleagues write. To fill that gap, the researchers administered the Pediatric Quality of Life Inventory (PedsQL) to 92 children with allergic conjunctivitis and their parents. The children were aged 5 to 18 years.
The researchers administered the same questionnaire to 96 healthy children of the same ages, along with their parents. These participants served as a control group.
On a scale of 0 to 100, in which a higher score signifies a better quality of life, the median total PedsQL score was 69.6 for children with allergic conjunctivitis versus 96.7 for the control group.
Subscores of physical, emotional, social, and especially school functioning were all significantly lower for the children with allergic conjunctivitis than for the control persons. “Because children generally spend most of their time in the school environment, this outcome raises an issue regarding whether children have a poorer performance in their education,” Dr. Zhang and colleagues write.
Dr. Strube recommends that physicians educate their patients about allergic conjunctivitis using handouts or high-quality websites. She often refers patients and their families to the allergic conjunctivitis webpage of the American Academy of Pediatric Ophthalmology and Strabismus.
She tells parents to have their child “take a shower and wash their hair when they get home before they rub their pollen-filled hair on their pillowcase and make their allergy symptoms worse.”
Parents and schools should try to filter pollen and other allergens from indoor air, she added.
Parents of the children with allergic conjunctivitis in the study also reported lower quality of life; they scored 68.8, versus 96.5 for parents of children in the control group. The differences for both parents and children were statistically significant (P < .001). Overall, the parents’ quality-of-life scores correlated with their children’s (correlation coefficient, r = 0.59; P < .001).
Children with vernal or atopic keratoconjunctivitis scored 3.3 points lower on health-related quality of life than those with seasonal allergic conjunctivitis.
Children with higher corneal fluorescein staining scores also had lower quality-of-life scores. Parents whose children had higher corneal fluorescein staining scores and also those who had multiple consultations with health care practitioners also reported lower quality of life.
The quality-of-life scores of the children with allergic conjunctivitis were lower than scores in previous studies for children with vision-threatening diseases, such as glaucoma and congenital cataract. This may be because glaucoma and cataracts do not typically cause discomfort even if they impair the patient’s vision, said Dr. Strube.
She pointed out one potential flaw in the study: In the cohort with allergic conjunctivitis, 83.7% were boys, compared to 42.7% of the control group. Vernal keratoconjunctivitis affects more boys than girls, and not controlling for this factor could have confounded the data, Dr. Strube said.
It could also be useful to replicate the study in other countries to see whether geographic or cultural factors affected the results, she said. “A lot of these big centers around the world, including in China, have poor air quality, so that may be contributing to patients’ symptoms,” she said. “With regards to reported health quality of life and impact on education, results from different parts of the world may be different, due to parenting styles and education styles,” she said.
The study was supported by the National Natural Science Foundation of China and the Science Foundation of Guangdong Province. Dr. Zhang and colleagues reported no relevant financial relationships. Dr. Strube reported receiving personal fees from Santen Canada Advisory Board Consultant outside the submitted work.
A version of this article first appeared on Medscape.com.
Allergic conjunctivitis harms quality of life for children and their parents, apparently causing greater day-to-day worries than potentially blinding diseases, researchers report.
Parents worry especially that treatments might not be effective, according to Shi-yao Zhang, MD, and colleagues from Sun Yat-sen University, Guangzhou, China. “This finding suggests that more communication with parents regarding treatment and prognosis is needed,” they write in an article published online June 10 in JAMA Ophthalmology.
One of the most prevalent eye disorders in children, allergic conjunctivitis is often chronic, leading patients to ask repeatedly for help from physicians. It can take an emotional toll and can cause children to miss school.
“With any sign of a slightly pink eye [or a] runny nose, which are very common with allergies, children are being sent home, because everyone’s concerned about COVID,” said Yi Ning J. Strube, MD, an associate professor of ophthalmology and pediatrics at Queen’s University, Kingston, Canada, whose commentary appears in the same issue of JAMA Ophthalmology.
Adolescents are also sometimes accused of smoking cannabis because of their red eyes, she said.
However, little research has examined the effects of allergic conjunctivitis on the quality of life of children and their guardians, Dr. Zhang and colleagues write. To fill that gap, the researchers administered the Pediatric Quality of Life Inventory (PedsQL) to 92 children with allergic conjunctivitis and their parents. The children were aged 5 to 18 years.
The researchers administered the same questionnaire to 96 healthy children of the same ages, along with their parents. These participants served as a control group.
On a scale of 0 to 100, in which a higher score signifies a better quality of life, the median total PedsQL score was 69.6 for children with allergic conjunctivitis versus 96.7 for the control group.
Subscores of physical, emotional, social, and especially school functioning were all significantly lower for the children with allergic conjunctivitis than for the control persons. “Because children generally spend most of their time in the school environment, this outcome raises an issue regarding whether children have a poorer performance in their education,” Dr. Zhang and colleagues write.
Dr. Strube recommends that physicians educate their patients about allergic conjunctivitis using handouts or high-quality websites. She often refers patients and their families to the allergic conjunctivitis webpage of the American Academy of Pediatric Ophthalmology and Strabismus.
She tells parents to have their child “take a shower and wash their hair when they get home before they rub their pollen-filled hair on their pillowcase and make their allergy symptoms worse.”
Parents and schools should try to filter pollen and other allergens from indoor air, she added.
Parents of the children with allergic conjunctivitis in the study also reported lower quality of life; they scored 68.8, versus 96.5 for parents of children in the control group. The differences for both parents and children were statistically significant (P < .001). Overall, the parents’ quality-of-life scores correlated with their children’s (correlation coefficient, r = 0.59; P < .001).
Children with vernal or atopic keratoconjunctivitis scored 3.3 points lower on health-related quality of life than those with seasonal allergic conjunctivitis.
Children with higher corneal fluorescein staining scores also had lower quality-of-life scores. Parents whose children had higher corneal fluorescein staining scores and also those who had multiple consultations with health care practitioners also reported lower quality of life.
The quality-of-life scores of the children with allergic conjunctivitis were lower than scores in previous studies for children with vision-threatening diseases, such as glaucoma and congenital cataract. This may be because glaucoma and cataracts do not typically cause discomfort even if they impair the patient’s vision, said Dr. Strube.
She pointed out one potential flaw in the study: In the cohort with allergic conjunctivitis, 83.7% were boys, compared to 42.7% of the control group. Vernal keratoconjunctivitis affects more boys than girls, and not controlling for this factor could have confounded the data, Dr. Strube said.
It could also be useful to replicate the study in other countries to see whether geographic or cultural factors affected the results, she said. “A lot of these big centers around the world, including in China, have poor air quality, so that may be contributing to patients’ symptoms,” she said. “With regards to reported health quality of life and impact on education, results from different parts of the world may be different, due to parenting styles and education styles,” she said.
The study was supported by the National Natural Science Foundation of China and the Science Foundation of Guangdong Province. Dr. Zhang and colleagues reported no relevant financial relationships. Dr. Strube reported receiving personal fees from Santen Canada Advisory Board Consultant outside the submitted work.
A version of this article first appeared on Medscape.com.
Americans’ sun protection practices fall short of intentions
commissioned by the American Academy of Dermatology.
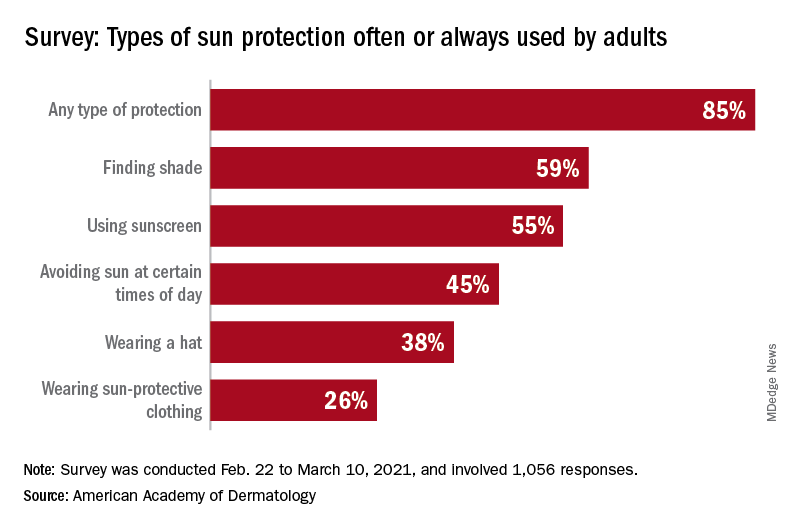
With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
12-month follow-up shows monthly maintenance dose of tralokinumab maintains response in some AD patients
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
FROM REVOLUTIONIZING AD 2021
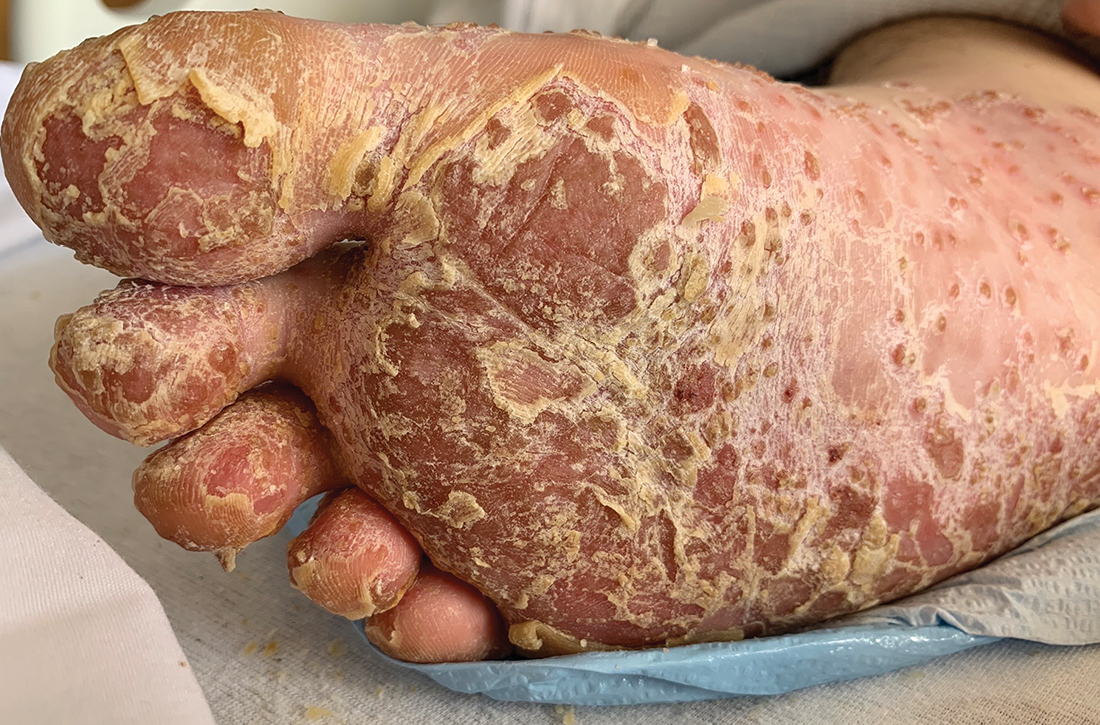
Foot rash and joint pain
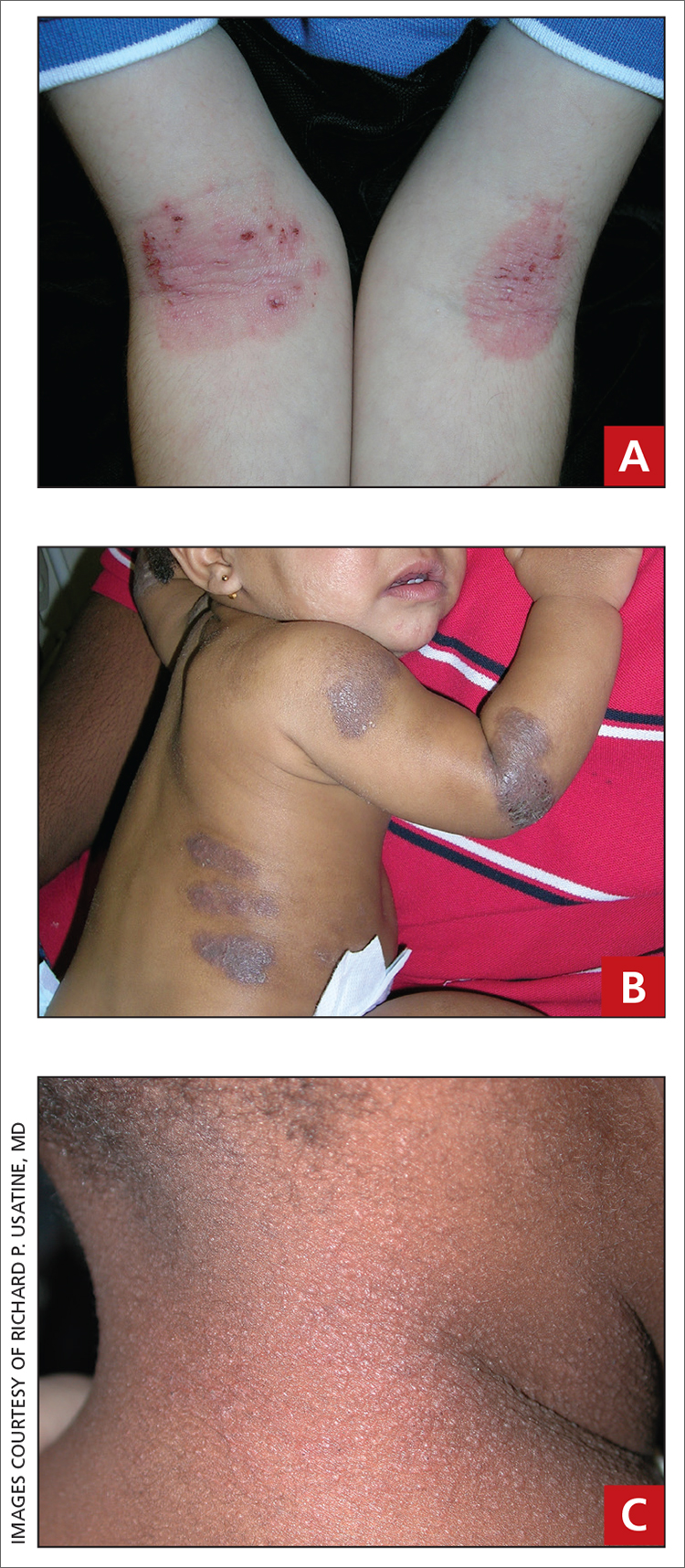
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.
An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.
An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.
An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
Trial offers first look at how tralokinumab-treated patients weather COVID-19
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
FROM REVOLUTIONIZING AD 2021
Lupus images fall short on diverse examples
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
Rapidly growing hand nodule
Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18
Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18
Atopic dermatitis
THE COMPARISON
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
THE COMPARISON
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
THE COMPARISON
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432