User login
A guide to the Tx of cellulitis and other soft-tissue infections
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
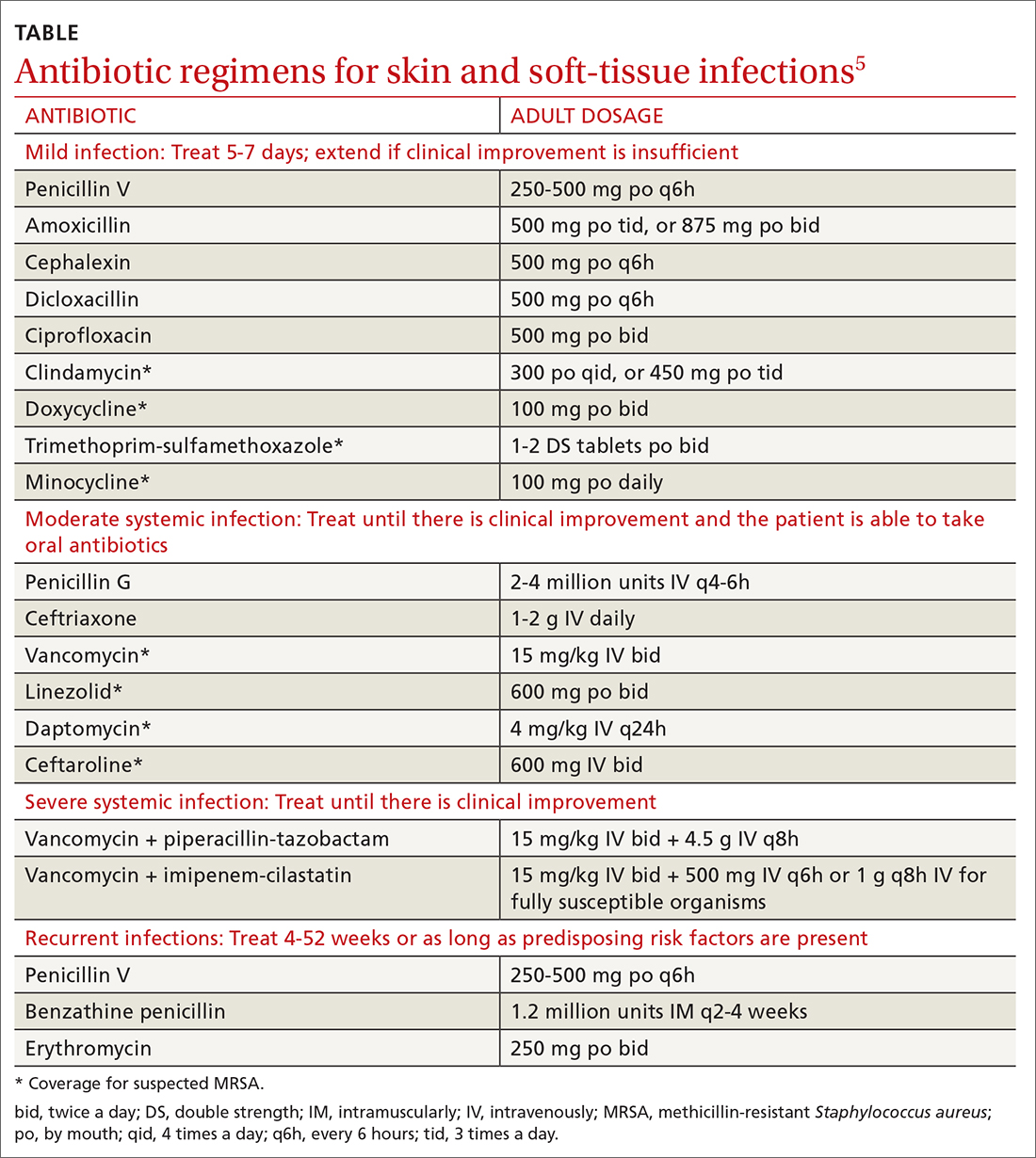
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5
Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
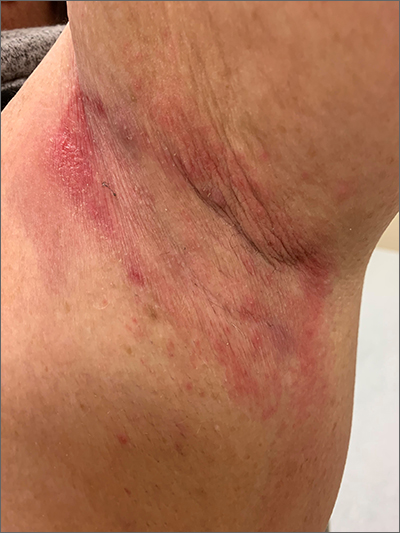
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
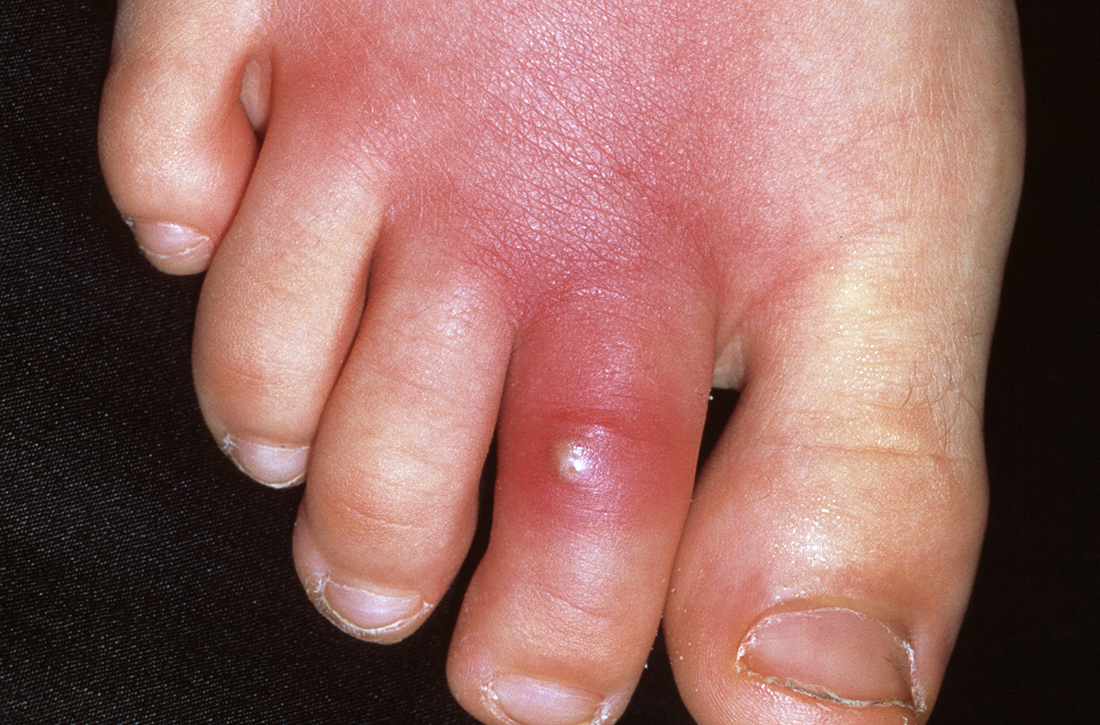
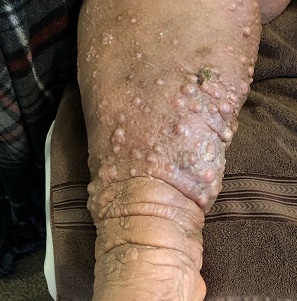
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5
Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5
Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; kclebak@pennstatehealth.psu.edu
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
PRACTICE RECOMMENDATIONS
› Start trimethoprim-sulfamethoxazole, clindamycin, doxycycline, minocycline, or a third- or fourth-generation fluoroquinolone for patients with cellulitis likely caused by community acquired methicillin-resistant Staphylococcus aureus (MRSA). A
› Consider culturing for MRSA and treating with oral doxycycline or trimethoprim-sulfamethoxazole for resistant cases of folliculitis. C
› Perform complete surgical debridement promptly if necrotizing fasciitis is suspected. C
› Prescribe broad-spectrum antibiotics for necrotizing fasciitis, covering both anaerobes and aerobes including MRSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Risankizumab shows efficacy, tolerability in patients with PsA
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
An otherwise healthy 1-month-old female presents with lesions on the face, scalp, and chest
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A 1-month-old, full-term female, born via normal vaginal delivery, presented to the dermatology clinic with a 3-week history of recurrent skin lesions on the scalp, face, and chest. The mother has been treating the lesions with breast milk and most recently with clotrimazole cream without resolution.
The mother of the baby is a healthy 32-year-old female with no past medical history. She had adequate prenatal care, and all the prenatal infectious and genetic tests were normal. The baby has been healthy and growing well. There is no history of associated fevers, chills, or any other symptoms. The family took no recent trips, and the parents are not affected. There are no other children at home and they have a cat and a dog. The family history is noncontributory.
On physical examination the baby was not in acute distress and her vital signs were normal. On skin examination she had several erythematous annular plaques and patches on the face, scalp, and upper chest (Fig. 1). There was no liver or spleen enlargement and no lymphadenopathy was palpated on exam.
All’s Well That Ends Swell(ing)
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
ANSWER
The correct answer is elephantiasis nostras verrucosa (ENV; choice “d”).
DISCUSSION
ENV is a rare condition of advanced cutaneous hypertrophy secondary to a combination of contributing factors including: a sedentary lifestyle, obesity, chronic venous stasis, repeated bouts of lymphangitis, cellulitis, and congestive heart failure (CHF). Most commonly affecting the lower extremities, it is occasionally seen in other dependent areas such as the scrotum and the abdominal pannus. It is, essentially, an exaggerated form of cutaneous lymphedema that causes the skin to become increasingly thick and fibrotic, changes which also reduce blood flow to or from the area.
Despite its name, ENV is not associated with elephantiasis, more commonly known as lymphatic filariasis (choice “b”). Although that condition manifests with similar skin changes, it is typically seen only in those who live in tropical areas where these organisms are endemic—places this patient has never visited.
There was no reason to believe that these skin changes were attributable to warts (choice “a”). Biopsy would have settled that question but also would have run the risk of creating a nonhealing wound, which could easily turn into an ulcer.
Lymphedema (choice “c”) was clearly present, but it was quite advanced—far beyond what is usually seen in venous insufficiency. This diagnosis would not, by itself, explain the nodules or extreme fibrosis.
Other potential causes for these skin changes include postradiation and pretibial myxedema, which had been ruled out prior to the dermatology consult.
TREATMENT
As with simple venous insufficiency, treatment of ENV consists of compression, elevation, and weight loss. For this patient, the diuretics prescribed as part of her CHF treatment might help a bit, but her prognosis is guarded at best.
A 61-year-old black woman presents for worrisome skin changes on her lower extremities. She reports that the condition is generally uncomfortable, but during occasional flares, it causes serious pain. She’s been affected for “many years” without diagnosis or resolution. It was her new primary care provider who, after seeing the lesions, sent her to dermatology.
The patient’s medical history includes diabetes, congestive heart failure, and obesity. All are being managed reasonably well.
Examination, performed while she is in a recumbent position, reveals legs swollen out of proportion to the rest of her body. Little or no erythema is noted. Both legs are affected equally, but only from just below the knees down to and including the feet. These areas, including her feet, are quite swollen, though no pitting edema can be provoked. The skin is quite firm and studded with multiple discrete and confluent 1-2 cm firm nodules. The skin around her ankles feels almost “woody” to the touch. There is no tenderness or increased warmth on palpation, nor is any drainage noted. (She also has a dystrophic great toenail that was partially avulsed by recent trauma.)
Benzene was found in some sunscreens. Now what?
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Erythematous axillary plaques
Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Due to the condition’s persistence and the negative KOH prep, erythrasma was the most likely diagnosis.
Erythrasma is caused by a bacterial infection with Corynebacterium minutissimum. It occurs in intertriginous areas that tend to be moist and irritated by friction. The erythema is usually a dull red, rather than the bright red that one would see with yeast infections. In addition, there is typically central pallor.
Woods lamp examination can confirm the diagnosis by showing coral pink fluorescence. In this patient, however, there was no fluorescence because the patient had recently washed the area and, thus, removed the porphyrins produced by C minutissimum. Biopsy for pathology is not usually necessary.
Erythrasma is treated with topical clindamycin, fusidic acid, or mupirocin. Oral macrolides and tetracyclines are also effective.1 Due to the chronicity of the erythrasma and the discomfort it caused, this patient opted for oral doxycycline 100 mg twice daily for 10 days. At follow-up 2 weeks later, the erythrasma had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Pediatric Dermatology 2021 Supplement
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
- Dupilumab curbed itch intensity, frequency in children with severe eczema
- Vitiligo treatment options abound but consider patient goals
- Beware a pair of dermatologic emergencies in children
- Database offers snapshot of common causes of pediatric allergic contact dermatitis
- Who’s at risk for depression on isotretinoin?
- Expert shares his approach to treating warts in children
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
- Dupilumab curbed itch intensity, frequency in children with severe eczema
- Vitiligo treatment options abound but consider patient goals
- Beware a pair of dermatologic emergencies in children
- Database offers snapshot of common causes of pediatric allergic contact dermatitis
- Who’s at risk for depression on isotretinoin?
- Expert shares his approach to treating warts in children
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
- Dupilumab curbed itch intensity, frequency in children with severe eczema
- Vitiligo treatment options abound but consider patient goals
- Beware a pair of dermatologic emergencies in children
- Database offers snapshot of common causes of pediatric allergic contact dermatitis
- Who’s at risk for depression on isotretinoin?
- Expert shares his approach to treating warts in children
GRAPPA refines recommendations on psoriatic disease treatment
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has included more drugs and data and is moving toward a slightly more stepped approach to treating some forms of psoriatic disease in the latest iteration of their recommendations.
“There’s been an explosion over the last few years in terms of the number of medications,” available to treat psoriasis and psoriatic arthritis, Laura C. Coates, MBChB, PhD, said in an interview ahead of presenting the draft recommendations at the annual European Congress of Rheumatology.
“The good thing about having more drugs is you’ve got more choice, but actually it makes these recommendations even more important because it becomes more complicated to choose optimal treatment for individuals,” added Dr. Coates, a senior clinical research fellow at the University of Oxford (England).
“We’ve been waiting for a while now for the new GRAPPA recommendations,” Laure Gossec, MD, PhD, of Sorbonne University and Pitié-Salpêtrière Hospital in Paris, said in a separate interview.
The last version of the guidelines was developed in 2015 and published in 2016, and since then there have been new data on Janus kinase inhibitors and interleukin-23 inhibitors, for example, which have now been incorporated into the updated recommendations alongside the old stalwarts of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and tumor necrosis factor inhibitors.
“I think that we can see some similarities but also differences compared to the previous version of the recommendations,” Dr. Gossec said.
One similarity is that the recommendations retain their modular or domain-oriented approach, keeping the core way that clinicians can use the recommendations based on their patients’ presentations. So, they still cover the management of peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease individually.
What’s different, however, is that the domain on comorbidities has been split into two to cover general comorbidities and to give more specific guidance on managing inflammatory bowel disease (IBD) and uveitis, “both of which may not ‘strictly speaking’ be treated by rheumatologists or dermatologists, but are manifestations which can appear in psoriatic disease,” Dr. Gossec noted.
IBD and uveitis “are part of the whole spondyloarthritis syndrome and are genetically related,” Dr. Coates said in her interview. “A lot of the drugs have licenses in those particular areas. The evidence is much stronger for which medication you should choose if somebody has psoriatic arthritis and Crohn’s disease or psoriatic arthritis and uveitis,” she noted.
When it comes to the rest of the comorbidities, think “cardiovascular disease, liver disease, infections – all the ‘normal’ comorbidities,” she added, noting “that’s usually where there’s a lot less data” on which drug to use.
New overarching principle and position statements
The goal of the recommendations hasn’t really changed since the first iteration of the guidelines in 2009, Dr. Coates noted in her presentation. They are intended to provide clinicians with recommendations “based on the best available evidence” for the management of patients with psoriatic disease.
To that end, a through process was followed, starting with the setting of PICO (Patient/population/problem; intervention; comparison; outcome) questions followed by systematic literature searches, data extraction, and review that assess the quality of evidence and then grade it accordingly before using it to inform the recommendation statements.
There is a new overarching principle that says: “These recommendations, which include the most current data concerning the optimal assessment of and therapeutic approached to psoriatic arthritis, present contextual considerations to empower shared decision making.”
The other overarching principles remain the same as in the 2015 version, with “minor wording changes particularly around the comorbidities overarching principle,” Dr. Coates said.
Also new are two position statements. “One of them is specifically around biosimilars, because that’s been a big shift since 2015,” Dr. Coates said. “It has basic rules about what evidence there should be, what we should consider when we’re using them, and patient involvement and decision making.”
The second statement covers “similar advice on tapering or discontinuing therapy – what we do when people are doing really well, how we should stop or taper, and which drugs we should choose to stop along with shared decision making with patients.”
GRAPPA intentionally gives clinicians more freedom
While there may be data to show differences in efficacy and side effects between the various drugs cited in the recommendations, “GRAPPA makes the choice to not prioritize one drug over another,” Dr. Gossec said. This decision gives “a lot of freedom then to the physician to make the decision.”
One important change according to Dr. Gossec is that oral “NSAIDs have clearly been put back as first-line treatment, before going on to disease-modifying drugs for most of the musculoskeletal manifestations. She added that for skin manifestations, topical NSAIDs were recommended, but that NSAIDs were more recommended for IBD and uveitis of course.
“I feel that’s a big step towards more of a step-up approach,” Dr. Gossec said. “The old recommendations were not clear that you would precede an NSAID before moving on to a disease-modifying drug. So, I think that makes it a little bit more similar to the 2019 EULAR recommendations.” The use of csDMARDs such as methotrexate has also been “pushed up a notch” in peripheral arthritis, she said.
What’s next?
There are a few fine tunings still to be made before the final recommendations are published. They also have to be discussed at the meeting of the GRAPPA task force, which consists of rheumatologists, dermatologists, and patient representatives.
Besides the recommendations manuscript, there will be individual papers detailing the evidence underpinning the recommendations in each of the eight domains, Dr. Coates noted. Those “will look at relative efficacy in detail,” she said. “There will be a lot more discussion/evidence summary included” to help with drug selection.
“We also plan to have some case studies to illustrate how the recommendations can be used, similar to that included in the 2015 recommendations,” she added.
Paul Studenic, MD, PhD, of the Karolinska Institute in Stockholm and Medical University of Vienna, tweeted that the GRAPPA recommendations showed treatment “needs to be tailored to the patient” taking “comorbidities as well as the heterogeneity of features of the clinical presentation into account.”
He said in an interview: “The third edition of the GRAPPA is a huge collaborative effort.” The new overarching principle put the recommendations in the context of shared decision making and, he added, they emphasize an “integrated management plan taking not only ‘classical’-related manifestations like uveitis into account but [also] a spectrum of comorbidities and reproductive health.”
GRAPPA is a not-for-profit organization and receives funding from multiple pharmaceutical companies. Currently this includes AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, Pfizer, UCB, and Sun Pharma with Galapagos and Nordic Bioscience as Innovation Partners. Dr. Coates acknowledged receiving research funding, honoraria, speaker fees or all of these from most of the aforementioned companies.
Dr. Gossec has received research funding or other support from numerous pharmaceutical companies and is a member of GRAPPA and the task force that developed the EULAR guidelines on the pharmacological management of psoriatic arthritis.
Dr. Studenic had nothing to disclose.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has included more drugs and data and is moving toward a slightly more stepped approach to treating some forms of psoriatic disease in the latest iteration of their recommendations.
“There’s been an explosion over the last few years in terms of the number of medications,” available to treat psoriasis and psoriatic arthritis, Laura C. Coates, MBChB, PhD, said in an interview ahead of presenting the draft recommendations at the annual European Congress of Rheumatology.
“The good thing about having more drugs is you’ve got more choice, but actually it makes these recommendations even more important because it becomes more complicated to choose optimal treatment for individuals,” added Dr. Coates, a senior clinical research fellow at the University of Oxford (England).
“We’ve been waiting for a while now for the new GRAPPA recommendations,” Laure Gossec, MD, PhD, of Sorbonne University and Pitié-Salpêtrière Hospital in Paris, said in a separate interview.
The last version of the guidelines was developed in 2015 and published in 2016, and since then there have been new data on Janus kinase inhibitors and interleukin-23 inhibitors, for example, which have now been incorporated into the updated recommendations alongside the old stalwarts of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and tumor necrosis factor inhibitors.
“I think that we can see some similarities but also differences compared to the previous version of the recommendations,” Dr. Gossec said.
One similarity is that the recommendations retain their modular or domain-oriented approach, keeping the core way that clinicians can use the recommendations based on their patients’ presentations. So, they still cover the management of peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease individually.
What’s different, however, is that the domain on comorbidities has been split into two to cover general comorbidities and to give more specific guidance on managing inflammatory bowel disease (IBD) and uveitis, “both of which may not ‘strictly speaking’ be treated by rheumatologists or dermatologists, but are manifestations which can appear in psoriatic disease,” Dr. Gossec noted.
IBD and uveitis “are part of the whole spondyloarthritis syndrome and are genetically related,” Dr. Coates said in her interview. “A lot of the drugs have licenses in those particular areas. The evidence is much stronger for which medication you should choose if somebody has psoriatic arthritis and Crohn’s disease or psoriatic arthritis and uveitis,” she noted.
When it comes to the rest of the comorbidities, think “cardiovascular disease, liver disease, infections – all the ‘normal’ comorbidities,” she added, noting “that’s usually where there’s a lot less data” on which drug to use.
New overarching principle and position statements
The goal of the recommendations hasn’t really changed since the first iteration of the guidelines in 2009, Dr. Coates noted in her presentation. They are intended to provide clinicians with recommendations “based on the best available evidence” for the management of patients with psoriatic disease.
To that end, a through process was followed, starting with the setting of PICO (Patient/population/problem; intervention; comparison; outcome) questions followed by systematic literature searches, data extraction, and review that assess the quality of evidence and then grade it accordingly before using it to inform the recommendation statements.
There is a new overarching principle that says: “These recommendations, which include the most current data concerning the optimal assessment of and therapeutic approached to psoriatic arthritis, present contextual considerations to empower shared decision making.”
The other overarching principles remain the same as in the 2015 version, with “minor wording changes particularly around the comorbidities overarching principle,” Dr. Coates said.
Also new are two position statements. “One of them is specifically around biosimilars, because that’s been a big shift since 2015,” Dr. Coates said. “It has basic rules about what evidence there should be, what we should consider when we’re using them, and patient involvement and decision making.”
The second statement covers “similar advice on tapering or discontinuing therapy – what we do when people are doing really well, how we should stop or taper, and which drugs we should choose to stop along with shared decision making with patients.”
GRAPPA intentionally gives clinicians more freedom
While there may be data to show differences in efficacy and side effects between the various drugs cited in the recommendations, “GRAPPA makes the choice to not prioritize one drug over another,” Dr. Gossec said. This decision gives “a lot of freedom then to the physician to make the decision.”
One important change according to Dr. Gossec is that oral “NSAIDs have clearly been put back as first-line treatment, before going on to disease-modifying drugs for most of the musculoskeletal manifestations. She added that for skin manifestations, topical NSAIDs were recommended, but that NSAIDs were more recommended for IBD and uveitis of course.
“I feel that’s a big step towards more of a step-up approach,” Dr. Gossec said. “The old recommendations were not clear that you would precede an NSAID before moving on to a disease-modifying drug. So, I think that makes it a little bit more similar to the 2019 EULAR recommendations.” The use of csDMARDs such as methotrexate has also been “pushed up a notch” in peripheral arthritis, she said.
What’s next?
There are a few fine tunings still to be made before the final recommendations are published. They also have to be discussed at the meeting of the GRAPPA task force, which consists of rheumatologists, dermatologists, and patient representatives.
Besides the recommendations manuscript, there will be individual papers detailing the evidence underpinning the recommendations in each of the eight domains, Dr. Coates noted. Those “will look at relative efficacy in detail,” she said. “There will be a lot more discussion/evidence summary included” to help with drug selection.
“We also plan to have some case studies to illustrate how the recommendations can be used, similar to that included in the 2015 recommendations,” she added.
Paul Studenic, MD, PhD, of the Karolinska Institute in Stockholm and Medical University of Vienna, tweeted that the GRAPPA recommendations showed treatment “needs to be tailored to the patient” taking “comorbidities as well as the heterogeneity of features of the clinical presentation into account.”
He said in an interview: “The third edition of the GRAPPA is a huge collaborative effort.” The new overarching principle put the recommendations in the context of shared decision making and, he added, they emphasize an “integrated management plan taking not only ‘classical’-related manifestations like uveitis into account but [also] a spectrum of comorbidities and reproductive health.”
GRAPPA is a not-for-profit organization and receives funding from multiple pharmaceutical companies. Currently this includes AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, Pfizer, UCB, and Sun Pharma with Galapagos and Nordic Bioscience as Innovation Partners. Dr. Coates acknowledged receiving research funding, honoraria, speaker fees or all of these from most of the aforementioned companies.
Dr. Gossec has received research funding or other support from numerous pharmaceutical companies and is a member of GRAPPA and the task force that developed the EULAR guidelines on the pharmacological management of psoriatic arthritis.
Dr. Studenic had nothing to disclose.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has included more drugs and data and is moving toward a slightly more stepped approach to treating some forms of psoriatic disease in the latest iteration of their recommendations.
“There’s been an explosion over the last few years in terms of the number of medications,” available to treat psoriasis and psoriatic arthritis, Laura C. Coates, MBChB, PhD, said in an interview ahead of presenting the draft recommendations at the annual European Congress of Rheumatology.
“The good thing about having more drugs is you’ve got more choice, but actually it makes these recommendations even more important because it becomes more complicated to choose optimal treatment for individuals,” added Dr. Coates, a senior clinical research fellow at the University of Oxford (England).
“We’ve been waiting for a while now for the new GRAPPA recommendations,” Laure Gossec, MD, PhD, of Sorbonne University and Pitié-Salpêtrière Hospital in Paris, said in a separate interview.
The last version of the guidelines was developed in 2015 and published in 2016, and since then there have been new data on Janus kinase inhibitors and interleukin-23 inhibitors, for example, which have now been incorporated into the updated recommendations alongside the old stalwarts of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and tumor necrosis factor inhibitors.
“I think that we can see some similarities but also differences compared to the previous version of the recommendations,” Dr. Gossec said.
One similarity is that the recommendations retain their modular or domain-oriented approach, keeping the core way that clinicians can use the recommendations based on their patients’ presentations. So, they still cover the management of peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease individually.
What’s different, however, is that the domain on comorbidities has been split into two to cover general comorbidities and to give more specific guidance on managing inflammatory bowel disease (IBD) and uveitis, “both of which may not ‘strictly speaking’ be treated by rheumatologists or dermatologists, but are manifestations which can appear in psoriatic disease,” Dr. Gossec noted.
IBD and uveitis “are part of the whole spondyloarthritis syndrome and are genetically related,” Dr. Coates said in her interview. “A lot of the drugs have licenses in those particular areas. The evidence is much stronger for which medication you should choose if somebody has psoriatic arthritis and Crohn’s disease or psoriatic arthritis and uveitis,” she noted.
When it comes to the rest of the comorbidities, think “cardiovascular disease, liver disease, infections – all the ‘normal’ comorbidities,” she added, noting “that’s usually where there’s a lot less data” on which drug to use.
New overarching principle and position statements
The goal of the recommendations hasn’t really changed since the first iteration of the guidelines in 2009, Dr. Coates noted in her presentation. They are intended to provide clinicians with recommendations “based on the best available evidence” for the management of patients with psoriatic disease.
To that end, a through process was followed, starting with the setting of PICO (Patient/population/problem; intervention; comparison; outcome) questions followed by systematic literature searches, data extraction, and review that assess the quality of evidence and then grade it accordingly before using it to inform the recommendation statements.
There is a new overarching principle that says: “These recommendations, which include the most current data concerning the optimal assessment of and therapeutic approached to psoriatic arthritis, present contextual considerations to empower shared decision making.”
The other overarching principles remain the same as in the 2015 version, with “minor wording changes particularly around the comorbidities overarching principle,” Dr. Coates said.
Also new are two position statements. “One of them is specifically around biosimilars, because that’s been a big shift since 2015,” Dr. Coates said. “It has basic rules about what evidence there should be, what we should consider when we’re using them, and patient involvement and decision making.”
The second statement covers “similar advice on tapering or discontinuing therapy – what we do when people are doing really well, how we should stop or taper, and which drugs we should choose to stop along with shared decision making with patients.”
GRAPPA intentionally gives clinicians more freedom
While there may be data to show differences in efficacy and side effects between the various drugs cited in the recommendations, “GRAPPA makes the choice to not prioritize one drug over another,” Dr. Gossec said. This decision gives “a lot of freedom then to the physician to make the decision.”
One important change according to Dr. Gossec is that oral “NSAIDs have clearly been put back as first-line treatment, before going on to disease-modifying drugs for most of the musculoskeletal manifestations. She added that for skin manifestations, topical NSAIDs were recommended, but that NSAIDs were more recommended for IBD and uveitis of course.
“I feel that’s a big step towards more of a step-up approach,” Dr. Gossec said. “The old recommendations were not clear that you would precede an NSAID before moving on to a disease-modifying drug. So, I think that makes it a little bit more similar to the 2019 EULAR recommendations.” The use of csDMARDs such as methotrexate has also been “pushed up a notch” in peripheral arthritis, she said.
What’s next?
There are a few fine tunings still to be made before the final recommendations are published. They also have to be discussed at the meeting of the GRAPPA task force, which consists of rheumatologists, dermatologists, and patient representatives.
Besides the recommendations manuscript, there will be individual papers detailing the evidence underpinning the recommendations in each of the eight domains, Dr. Coates noted. Those “will look at relative efficacy in detail,” she said. “There will be a lot more discussion/evidence summary included” to help with drug selection.
“We also plan to have some case studies to illustrate how the recommendations can be used, similar to that included in the 2015 recommendations,” she added.
Paul Studenic, MD, PhD, of the Karolinska Institute in Stockholm and Medical University of Vienna, tweeted that the GRAPPA recommendations showed treatment “needs to be tailored to the patient” taking “comorbidities as well as the heterogeneity of features of the clinical presentation into account.”
He said in an interview: “The third edition of the GRAPPA is a huge collaborative effort.” The new overarching principle put the recommendations in the context of shared decision making and, he added, they emphasize an “integrated management plan taking not only ‘classical’-related manifestations like uveitis into account but [also] a spectrum of comorbidities and reproductive health.”
GRAPPA is a not-for-profit organization and receives funding from multiple pharmaceutical companies. Currently this includes AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, Pfizer, UCB, and Sun Pharma with Galapagos and Nordic Bioscience as Innovation Partners. Dr. Coates acknowledged receiving research funding, honoraria, speaker fees or all of these from most of the aforementioned companies.
Dr. Gossec has received research funding or other support from numerous pharmaceutical companies and is a member of GRAPPA and the task force that developed the EULAR guidelines on the pharmacological management of psoriatic arthritis.
Dr. Studenic had nothing to disclose.
FROM THE EULAR 2021 CONGRESS
Intravenous immunoglobulin controls dermatomyositis in phase 3 trial
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
Nearly 50% achieve moderate improvement or better
Nearly 50% achieve moderate improvement or better
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
The first multinational, phase 3, placebo-controlled trial conducted with intravenous immunoglobulin therapy (IVIg) for dermatomyositis has confirmed significant efficacy and acceptable safety, according to data presented at the opening plenary abstract session of the annual European Congress of Rheumatology.
At the week 16 evaluation of the trial, called ProDERM, the response rates were 78.7% and 43.8% (P = .0008) for active therapy and placebo, respectively, reported Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh.
ProDERM is a “much-awaited study,” according to session moderator Hendrik Schulze-Koops, MD, PhD, of the division of rheumatology and clinical immunology at Ludwig Maximilian University of Munich (Germany). He was not involved in the study.
“We all have been doing what we have been doing,” Dr. Schulze-Koops said, referring to the use of IVIg for the control of dermatomyositis, “but we had no evidence for support.”
This statement could apply not only to IVIg, which has long been listed among treatment options by the Myositis Association despite the absence of controlled studies, but also to most immunosuppressive therapies and other options used for this challenging disease.
The proprietary IVIg employed in this study, Octagam 10%, has been approved in the United States for the treatment of chronic immune thrombocytopenic purpura. Its manufacturer, Octagam, plans to file a supplemental new drug application with the Food and Drug Administration for the treatment of dermatomyositis. The agent is already approved for dermatomyositis by the European Medicines Agency, according to Dr. Aggarwal.
Multiple response criteria favor IVIg
In the trial, 95 patients with dermatomyositis were randomized to 2 g/kg of IVIg (Octagam 10%) or placebo administered every 4 weeks. In a subsequent open-label extension study in which patients on placebo were switched to active therapy, the same every-4-week treatment schedule was used. The patients’ mean age was 53; 75% were women, and 92% were White.
The primary endpoint was at least minimal improvement on 2016 ACR/EULAR (American College of Rheumatology/European Alliance of Associations for Rheumatology) myositis response criteria, defined as a 20-point or greater gain in the Total Improvement Score (TIS) and no clinical worsening at two consecutive visits. But IVIg also provided a large relative benefit over placebo using more rigorous definitions of improvement. For moderate improvement, defined as at least a 40-point TIS improvement, there was a 45.2% relative advantage for IVIg over placebo (68.1% vs. 22.9%; P < .0001). For major improvement, defined as at least a 60-point TIS improvement, the relative advantage was 23.6% (31.9% vs. 8.3%; P < .0062).
At 16 weeks, the mean TIS score was more than twice as high in those receiving IVIg than in those randomized to placebo (48.4 vs. 21.6). At that point, an open-label extension was initiated. Those in the IVIg group were permitted to remain on therapy for an additional 24 weeks if they had not worsened in the blinded phase.
The mean TIS score in the IVIg group continued to rise during the extension phase. By 12 weeks in this phase, it reached 54.0. Over the same period, mean TIS scores climbed steeply among the placebo-treated patients who had switched to active therapy, reaching 44.4.
At the end of 24 weeks of the extension trial, when patients initiated on IVIg had been on active therapy for 40 weeks, the mean TIS score advantage of starting on IVIg rather than placebo was relatively modest (55.4 vs. 51.1).
Benefit is significant for skin and muscle
Changes in the two major components of dermatomyositis were tracked individually. For skin symptoms, patients were evaluated with the Cutaneous Dermatomyositis Disease Areas and Severity Index (CDASI). For muscle involvement, symptoms were evaluated with the 8-item Manual Muscle Testing (MMT-8) tool.
“The effects of IVIg on the muscle and the skin were both highly statistically significant,” Dr. Aggarwal reported. He said the CDASI score was reduced by almost half at the end of 16 weeks among those treated with IVIg relative to those treated with placebo. Improvement in MMT-8 scores were also clinically as well as statistically significant.
The IVIg therapy was well tolerated. The most common adverse effects in this study, like those reported with IVIg when used to treat other diseases, were headache, pyrexia, and nausea, but Dr. Aggarwal reported that these were generally mild.
Serious adverse events, particularly thromboembolism, did occur over the course of the study, but the rate of events was only slightly higher in the group receiving active therapy (5.8% vs. 4.2%).
Patients who entered the study were permitted to remain on most immunosuppressive therapies, such as methotrexate, mycophenolate, tacrolimus, and glucocorticoids. Dr. Aggarwal said that the majority of patients were taking a glucocorticoid and at least one nonglucocorticoid immunosuppressant.
Effect on associated conditions is planned
The data from this trial have not yet been analyzed for the impact of IVIg on conditions that occur frequently in association with dermatomyositis, such as interstitial lung disease (ILD) and dysphagia, but Dr. Aggarwal reported that there are plans to do so. Although severe ILD was a trial exclusion, the presence of mild to moderate ILD and dysphagia were evaluated at baseline, so the impact of treatment can be assessed.
There are also plans to evaluate how the presence or absence of myositis-specific antibodies, which were also evaluated at baseline, affected response to IVIg.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Dr. Schulze-Koops reported no relevant potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Asymptomatic hyperpigmented skin changes
The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599
The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.
The intertriginous findings, along with results from a punch biopsy showing resolving lichenoid inflammation with post-inflammatory pigmentary alteration, indicated a diagnosis of lichen planus pigmentosus inversus (LPPI).
Insidious onset of usually asymptomatic, sometimes mildly pruritic, well-defined, hyperpigmented macules and patches with occasional Wickham striae of the intertriginous areas is characteristic of LPPI. It is a variant of lichen planus pigmentosus, which conversely occurs on sun-exposed areas. The etiology is unknown and there is no association with medications or sun exposure. Pathophysiology is thought to be chronic inflammation, mediated by T-lymphocyte cytotoxic activity against basal keratinocytes.1
At the time of this patient’s clinical presentation, the differential diagnoses for new onset hyperpigmentation included confluent and reticulated papillomatosis, post-inflammatory hyperpigmentation, and erythema dyschromicum perstans. However, further tests were ordered for diagnostic clarification.
The clinical course of LPPI is variable. In some cases, there is complete resolution of lesions without treatment, while in other cases, lesions may last for years despite treatment and the condition may recur. Current management options include topical calcineurin inhibitors (tacrolimus and pimecrolimus) and topical steroids. Response to these topical medications may be variable. The patient in this case was started on topical tacrolimus 0.1% twice daily, with follow-up in 3 months.
Text courtesy of Rachel Rose, BS, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy of Daniel Stulberg, MD, FAAFP.
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599
Barros HR, de Almeida JRP, Mattos e Dinato SL, et al. Lichen planus pigmentosus inversus. An Bras Dermatol. 2013;88(6 suppl 1):146-149. doi:10.1590/abd1806-4841.20132599