User login
FDA approves secukinumab in psoriasis patients age six and older
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
Benzene found in some sunscreen products, online pharmacy says
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
Valisure, an online pharmacy known for testing every batch of medication it sells, announced that it has
The company tested 294 batches from 69 companies and found benzene in 27% – many in major national brands like Neutrogena and Banana Boat. Some batches contained as much as three times the emergency FDA limit of 2 parts per million.
Long-term exposure to benzene is known to cause cancer in humans.
“This is especially concerning with sunscreen because multiple FDA studies have shown that sunscreen ingredients absorb through the skin and end up in the blood at high levels,” said David Light, CEO of Valisure.
The FDA is seeking more information about the potential risks from common sunscreen ingredients.
“There is not a safe level of benzene that can exist in sunscreen products,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in Valisure’s FDA petition. “The total mass of sunscreen required to cover and protect the human body, in single daily application or repeated applications daily, means that even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Valisure’s testing previously led to FDA recalls of heartburn medications and hand sanitizers.
Examining sunscreen’s environmental impact
Chemicals in sunscreen may be harmful to other forms of life, too. For years, scientists have been examining whether certain chemicals in sunscreen could be causing damage to marine life, in particular the world’s coral reefs. Specific ingredients, including oxybenzone, benzophenone-1, benzophenone-8, OD-PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene, have been identified as potential risks.
Earlier this year, the National Academies of Sciences, Engineering, and Medicine created a committee to review the existing science about the potential environmental hazards. Over the next 2 years, they’ll also consider the public health implications if people stopped using sunscreen.
Valisure’s announcement included this message: “It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.”
Using sunscreen with SPF 15 every day can lower risk of squamous cell carcinoma by around 40% and melanoma by 50%. The American Academy of Dermatology recommends a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher.
A version of this article first appeared on WebMD.com.
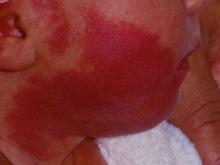
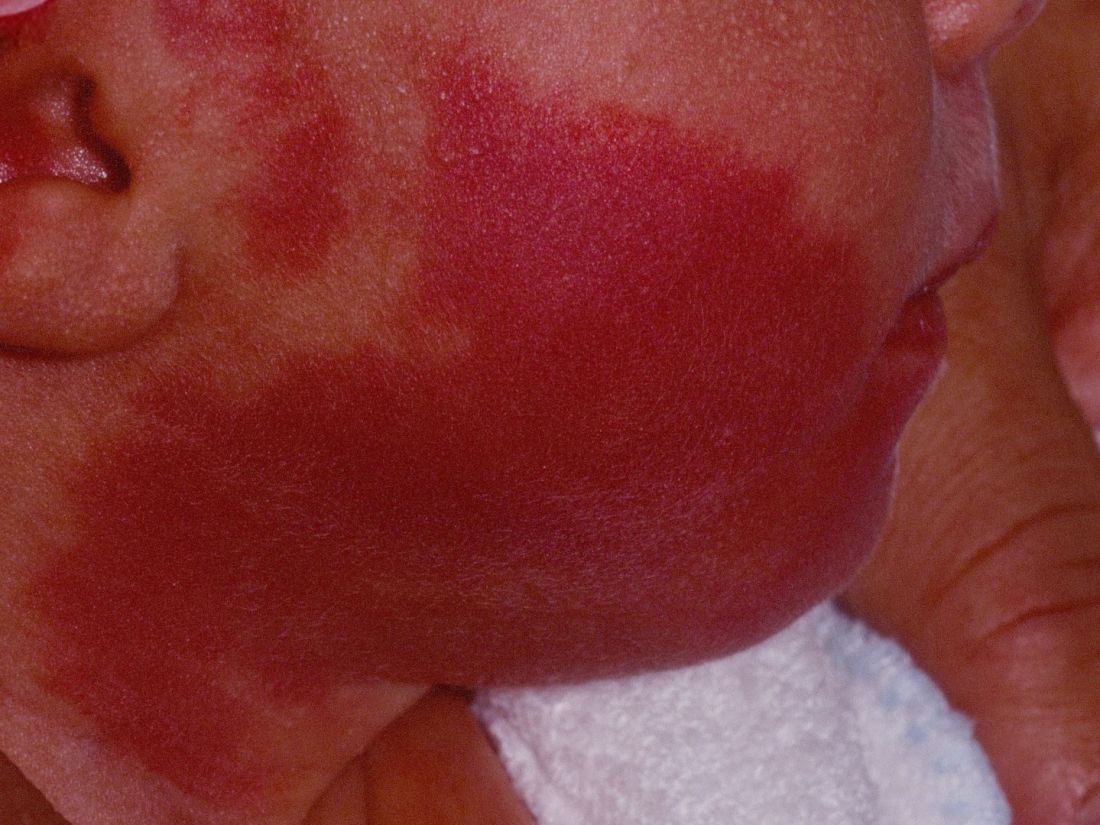
How early can laser treatment for port wine stains in infants be initiated?
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
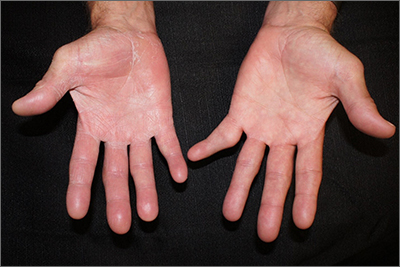
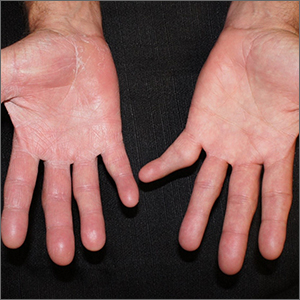
Scaly patches on hand and feet
A potassium hydroxide (KOH) mount of skin scrapings from the patient’s feet and hand confirmed a diagnosis of unilateral tinea manuum and bilateral tinea pedis—the so-called 2-foot, 1-hand syndrome. Additionally, nail clippings from the patient’s right hand confirmed onychomycosis.
Tinea is common and caused by various dermatophytes that are ubiquitous in soil. Often there is a history of atopic dermatitis or xerosis leading to skin barrier dysfunction. Immunosuppression and diabetes mellitus are also predisposing factors. Trichophyton rubrum is a commonly isolated cause.
On the hands, tinea may be challenging to distinguish from irritant dermatitis, atopic dermatitis, and contact dermatitis. A KOH prep test should be considered for any red, scaly rash, especially on the hands and feet. Curiously, the incidence of unilateral tinea manuum and bilateral tinea pedis occurs relatively frequently and can affect either the dominant or nondominant hand. The cause of this asymmetry is speculative.
Topical therapy with various antifungals—terbinafine, clotrimazole, ketoconazole, ciclopirox—can be effective, but challenging to apply to affected areas. For the treatment of nail disease, oral therapy with terbinafine or itraconazole is usually indicated (6 weeks for fingernails and 12 weeks for toenails). Terbinafine is generally tolerated very well for both 6- and 12-week courses. Some clinicians consider lab monitoring unnecessary because the risk of hepatic injury from terbinafine is uncertain; others consider it worthwhile to check liver function test results prior to initiation of terbinafine and after 6 weeks of therapy, with either a 6- or 12-week course.1
Since the patient in this case had skin and nail disease, oral therapy with terbinafine 250 mg/d for 6 weeks was prescribed. His skin cleared within 3 weeks and his nails cleared after 6 months. It is important to counsel patients with nail disease that treatment will end before they see a clinical improvement. Fingernails typically require 6 months to see clearance and toenails require 18 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
1. Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections. JAMA Dermatol. 2018;154:1409-1416. doi:10.1001/jamadermatol.2018.3578
A potassium hydroxide (KOH) mount of skin scrapings from the patient’s feet and hand confirmed a diagnosis of unilateral tinea manuum and bilateral tinea pedis—the so-called 2-foot, 1-hand syndrome. Additionally, nail clippings from the patient’s right hand confirmed onychomycosis.
Tinea is common and caused by various dermatophytes that are ubiquitous in soil. Often there is a history of atopic dermatitis or xerosis leading to skin barrier dysfunction. Immunosuppression and diabetes mellitus are also predisposing factors. Trichophyton rubrum is a commonly isolated cause.
On the hands, tinea may be challenging to distinguish from irritant dermatitis, atopic dermatitis, and contact dermatitis. A KOH prep test should be considered for any red, scaly rash, especially on the hands and feet. Curiously, the incidence of unilateral tinea manuum and bilateral tinea pedis occurs relatively frequently and can affect either the dominant or nondominant hand. The cause of this asymmetry is speculative.
Topical therapy with various antifungals—terbinafine, clotrimazole, ketoconazole, ciclopirox—can be effective, but challenging to apply to affected areas. For the treatment of nail disease, oral therapy with terbinafine or itraconazole is usually indicated (6 weeks for fingernails and 12 weeks for toenails). Terbinafine is generally tolerated very well for both 6- and 12-week courses. Some clinicians consider lab monitoring unnecessary because the risk of hepatic injury from terbinafine is uncertain; others consider it worthwhile to check liver function test results prior to initiation of terbinafine and after 6 weeks of therapy, with either a 6- or 12-week course.1
Since the patient in this case had skin and nail disease, oral therapy with terbinafine 250 mg/d for 6 weeks was prescribed. His skin cleared within 3 weeks and his nails cleared after 6 months. It is important to counsel patients with nail disease that treatment will end before they see a clinical improvement. Fingernails typically require 6 months to see clearance and toenails require 18 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
A potassium hydroxide (KOH) mount of skin scrapings from the patient’s feet and hand confirmed a diagnosis of unilateral tinea manuum and bilateral tinea pedis—the so-called 2-foot, 1-hand syndrome. Additionally, nail clippings from the patient’s right hand confirmed onychomycosis.
Tinea is common and caused by various dermatophytes that are ubiquitous in soil. Often there is a history of atopic dermatitis or xerosis leading to skin barrier dysfunction. Immunosuppression and diabetes mellitus are also predisposing factors. Trichophyton rubrum is a commonly isolated cause.
On the hands, tinea may be challenging to distinguish from irritant dermatitis, atopic dermatitis, and contact dermatitis. A KOH prep test should be considered for any red, scaly rash, especially on the hands and feet. Curiously, the incidence of unilateral tinea manuum and bilateral tinea pedis occurs relatively frequently and can affect either the dominant or nondominant hand. The cause of this asymmetry is speculative.
Topical therapy with various antifungals—terbinafine, clotrimazole, ketoconazole, ciclopirox—can be effective, but challenging to apply to affected areas. For the treatment of nail disease, oral therapy with terbinafine or itraconazole is usually indicated (6 weeks for fingernails and 12 weeks for toenails). Terbinafine is generally tolerated very well for both 6- and 12-week courses. Some clinicians consider lab monitoring unnecessary because the risk of hepatic injury from terbinafine is uncertain; others consider it worthwhile to check liver function test results prior to initiation of terbinafine and after 6 weeks of therapy, with either a 6- or 12-week course.1
Since the patient in this case had skin and nail disease, oral therapy with terbinafine 250 mg/d for 6 weeks was prescribed. His skin cleared within 3 weeks and his nails cleared after 6 months. It is important to counsel patients with nail disease that treatment will end before they see a clinical improvement. Fingernails typically require 6 months to see clearance and toenails require 18 months.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
1. Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections. JAMA Dermatol. 2018;154:1409-1416. doi:10.1001/jamadermatol.2018.3578
1. Stolmeier DA, Stratman HB, McIntee TJ, et al. Utility of laboratory test result monitoring in patients taking oral terbinafine or griseofulvin for dermatophyte infections. JAMA Dermatol. 2018;154:1409-1416. doi:10.1001/jamadermatol.2018.3578
Lower SARS-CoV-2 vaccine responses seen in patients with immune-mediated inflammatory diseases
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
FROM ANNALS OF THE RHEUMATIC DISEASES
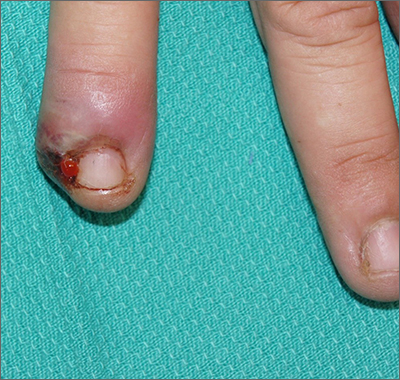
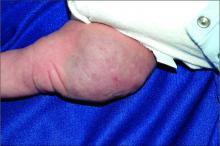
Painful ingrown nail
Paronychia, or an ingrown nail, is caused by the introduction of bacteria into the nail fold, which can cause cellulitis and/or abscess formation. Typically, a single nail is involved, although multiple nails may be affected in rare, chemotherapy-induced cases. Generally, nail grooming behaviors, abnormal or scarred nail matrix architecture, nail biting, and wet work are the causes of acute or chronic disease.1
Conservative options are a reasonable first choice when there is no abscess. These include soaks 2 to 3 times daily for 15 to 20 minutes in hot water or water treated with an antiseptic, such as chlorhexidine. Topical or oral antibiotics aimed at suspected organisms may be helpful. Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, is a common isolate. Psuedomonas is characteristic in cases involving trauma to the hand and frequent workplace water exposure, as can happen in foodservice, farming, and marine industries.
When an abscess has formed (as in this case), or when conservative treatments fail, incision and drainage or partial nail avulsion can be curative and successful with, or without, antibiotics. Patients with recurrent paronychia in the same location may benefit from partial nail avulsion with partial matrix removal. This can be done surgically or with phenol.
In this case, the patient underwent lateral partial nail avulsion, which also served as an incision and drainage. She was not given antibiotics and her nail regrew within 6 months; there was no recurrence. She was counseled not to cut the nail plate too aggressively.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Rigopoulos D, Larios G, Gregoriou S, et al. Acute and chronic paronychia. Am Fam Physician. 2008;77:339-346.
Paronychia, or an ingrown nail, is caused by the introduction of bacteria into the nail fold, which can cause cellulitis and/or abscess formation. Typically, a single nail is involved, although multiple nails may be affected in rare, chemotherapy-induced cases. Generally, nail grooming behaviors, abnormal or scarred nail matrix architecture, nail biting, and wet work are the causes of acute or chronic disease.1
Conservative options are a reasonable first choice when there is no abscess. These include soaks 2 to 3 times daily for 15 to 20 minutes in hot water or water treated with an antiseptic, such as chlorhexidine. Topical or oral antibiotics aimed at suspected organisms may be helpful. Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, is a common isolate. Psuedomonas is characteristic in cases involving trauma to the hand and frequent workplace water exposure, as can happen in foodservice, farming, and marine industries.
When an abscess has formed (as in this case), or when conservative treatments fail, incision and drainage or partial nail avulsion can be curative and successful with, or without, antibiotics. Patients with recurrent paronychia in the same location may benefit from partial nail avulsion with partial matrix removal. This can be done surgically or with phenol.
In this case, the patient underwent lateral partial nail avulsion, which also served as an incision and drainage. She was not given antibiotics and her nail regrew within 6 months; there was no recurrence. She was counseled not to cut the nail plate too aggressively.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Paronychia, or an ingrown nail, is caused by the introduction of bacteria into the nail fold, which can cause cellulitis and/or abscess formation. Typically, a single nail is involved, although multiple nails may be affected in rare, chemotherapy-induced cases. Generally, nail grooming behaviors, abnormal or scarred nail matrix architecture, nail biting, and wet work are the causes of acute or chronic disease.1
Conservative options are a reasonable first choice when there is no abscess. These include soaks 2 to 3 times daily for 15 to 20 minutes in hot water or water treated with an antiseptic, such as chlorhexidine. Topical or oral antibiotics aimed at suspected organisms may be helpful. Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, is a common isolate. Psuedomonas is characteristic in cases involving trauma to the hand and frequent workplace water exposure, as can happen in foodservice, farming, and marine industries.
When an abscess has formed (as in this case), or when conservative treatments fail, incision and drainage or partial nail avulsion can be curative and successful with, or without, antibiotics. Patients with recurrent paronychia in the same location may benefit from partial nail avulsion with partial matrix removal. This can be done surgically or with phenol.
In this case, the patient underwent lateral partial nail avulsion, which also served as an incision and drainage. She was not given antibiotics and her nail regrew within 6 months; there was no recurrence. She was counseled not to cut the nail plate too aggressively.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Rigopoulos D, Larios G, Gregoriou S, et al. Acute and chronic paronychia. Am Fam Physician. 2008;77:339-346.
Rigopoulos D, Larios G, Gregoriou S, et al. Acute and chronic paronychia. Am Fam Physician. 2008;77:339-346.
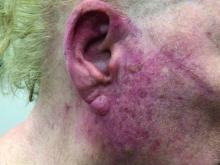
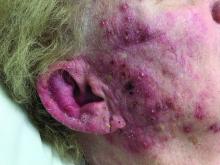
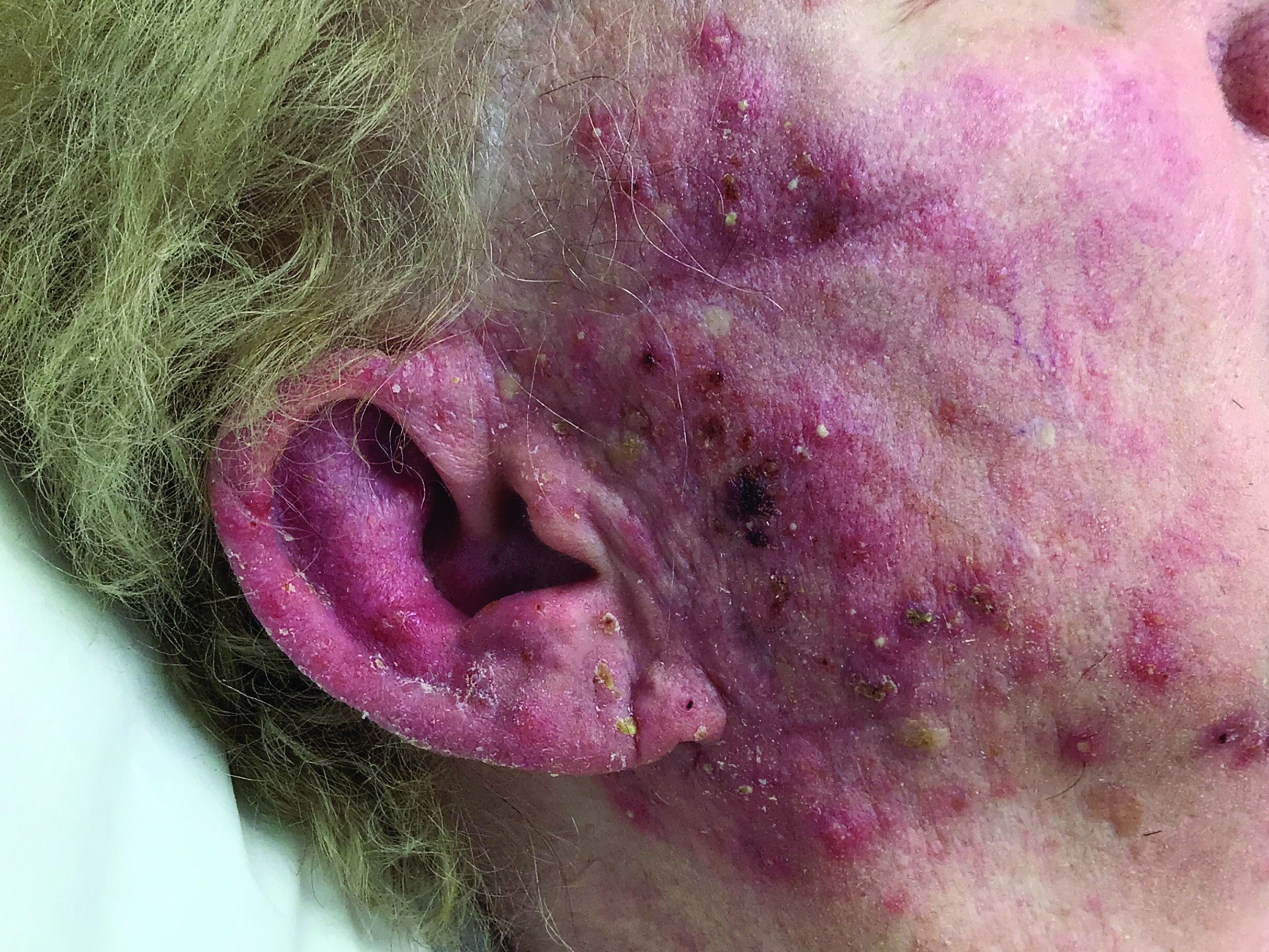
A woman with scaling, and painful, crusted, erythematous papules and pustules on her face
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
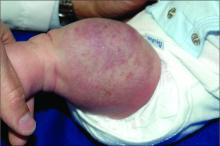
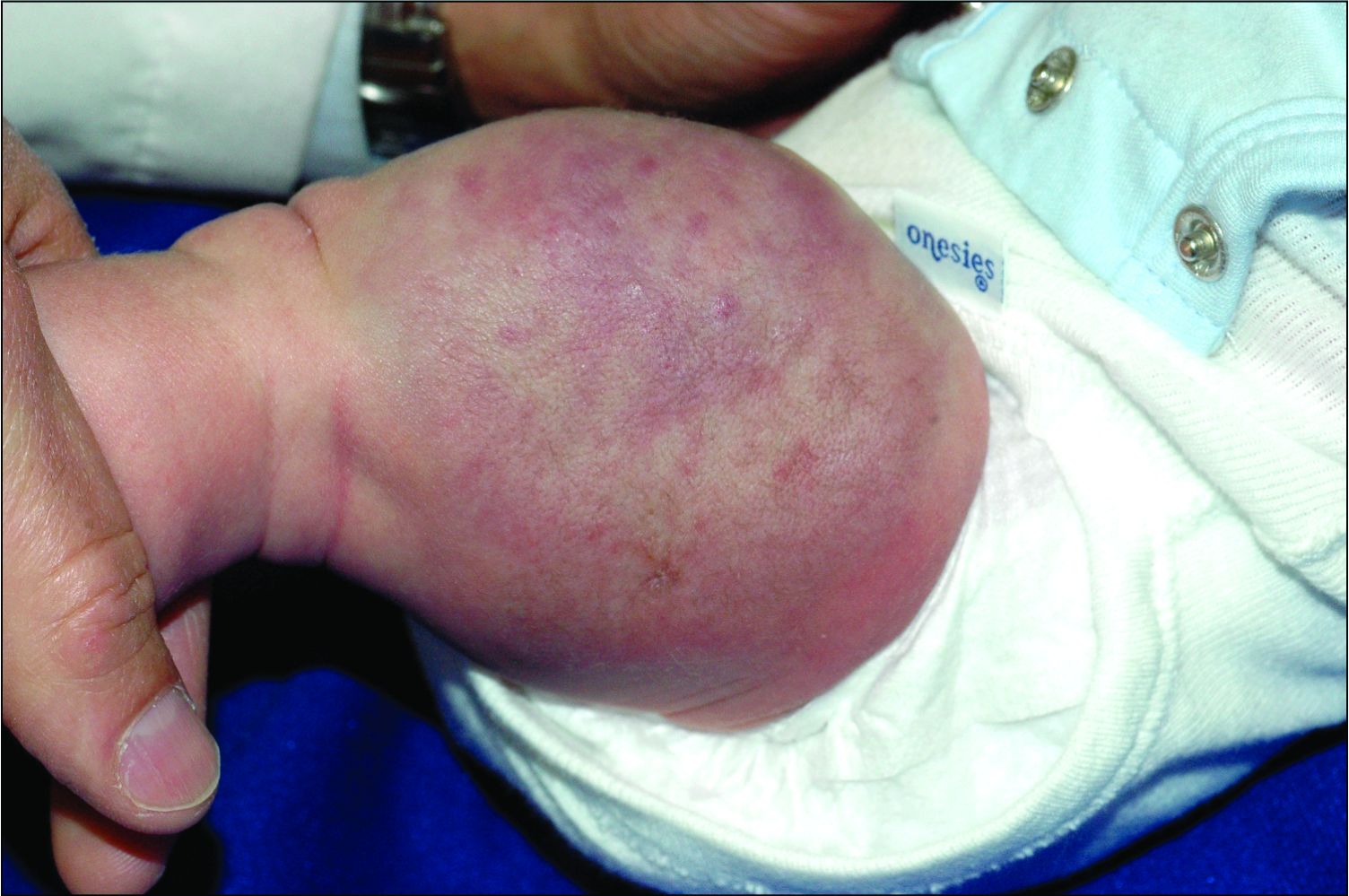
An infant girl presents with a growing pink-red leg nodule
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
On physical exam, you see an infant with a mass of the left lower extremity. Close examination reveals an approximately 7 cm x 8 cm poorly defined mass with overlying central erythematous to violaceous color of the left anterior upper leg with a lumpy texture. The lesion is moderately firm and mildly tender on palpation.
The case for molecular classification of vascular anomalies presented
according to Beth Drolet, MD.
“We now know that 75%-80% of vascular malformations have gene mutations that make the cells either live longer, grow faster, or make them bigger in size,” Dr. Drolet, professor and chair of dermatology at the University of Wisconsin–Madison, said during the Society for Pediatric Dermatology pre-AAD meeting. “The basic binary premise of the current ISSVA [International Society for the Study of Vascular Anomalies] classification dividing vascular anomalies into tumors and malformations is wrong; the biology is not that straightforward. It may be helpful to differentiate between an infantile hemangioma and a capillary malformation during infancy as the hemangioma will grow in the next month, but we now know that patients with capillary malformations also have significant overgrowth of their tissue. We’ve all seen that; it just takes years, not months for us to notice it.”
The change in thinking about the root causes of vascular anomalies, she noted, stems from scientific advances in the understanding of embryonic mosaicism, DNA variation that happens after the zygote is formed, but before birth. “We know that each cell in a zygote will undergo 40 cell divisions before a baby is born,” she said. “Those cell divisions are not as neat as we thought they were. That cell and DNA duplication is actually quite messy, so there are mutations that happen purely because of embryonic cell division.”
Everyone is born with 120 somatic mutations per cell, she continued, “so we have multiple genomes in one human. Not all of those mutations are going to cause disease. Not all of those are going to be functional. About 10% of those mutations will actually be in a coding region of the gene and have the potential to change the function of the protein. If it changes the function of the protein so that the cell can’t survive, that cell dies off, but it gives the cell an advantage. It grows a little bit faster, let’s say. That cell survives, divides, producing a line of cells that can cause disease.”
In 2011, Dr. Drolet and colleagues from the Hemangioma Investigator Group and the Pediatric Dermatology Research Alliance (PeDRA) launched a multisite collaborative group to investigate the role of mosaic genetics in patients with vascular anomalies and discrepancy of growth. To date, 365 patients are enrolled, and the researchers have sequenced 97 of 165 affected tissue samples collected. “What’s nice about the registry is that we enrolled a wide spectrum of diseases: very mild diseases that might be treated by dermatologists to complex, syndromic diseases that might end up in an interdisciplinary vascular anomalies clinic,” she said.
For gene sequencing, the researchers drew from solid tumor biology and used next-generation sequencing with semi-target hybrid capture, “so we’re only looking at a subset of genes,” she said. “Right now, the chip we’re using has 180 cancer-related genes. It sequences the entire exome of the gene with a high depth of coverage, usually over 1,000 X. We use a specific pipeline that can detect very low allele frequency mutation: down to 1%, and robust criteria to determine variant pathogenicity.”
In 75% of tissue samples so far, the researchers have found a gene mutation in one of 13 genes: AKT1, AKT3, BRAF, GNA11, GNAQ, KRAS, MAP2K1, NRAS, PIK3CA, PIK3R1, PTPN11, RASA1, and TEK. According to Dr. Drolet, the common thread in these 13 genes is that they are implicated in cancer and have direct control over the cell cycle. “They’re intracellular proteins that control the cell cycle,” she explained. “These are proteins that are in the cell but interact with transmembrane proteins that receive extracellular messengers of cell growth”.
Understanding and recognizing genetic conditions is complicated, she said, because it involves determining which gene is altered, where in the DNA the gene is altered, how the gene variation will influence the function of the protein, and what tissue expresses that gene. “Then you get your phenotype,” Dr. Drolet said. “If you add mosaicism onto that, you have several additional variables. You need to know: When in embryogenesis did the mutation occur? What region of the body is affected? What cell lineage is affected? That predicts what phenotype you’re going to have.”
While molecular classification efforts continue to be refined, Dr. Drolet incorporates genotyping at every opportunity, like when she counsels parents of a baby born with a vascular stain on its face. “What can we tell them about what else might be wrong? What can we tell them about how this will change over time? What can we tell them about how we can treat it? I think genotyping absolutely helps to clarify that for me,” she said. “I can’t use that alone, but it gives me another piece of evidence to help do a better job in predicting when I need to screen, what I need to screen for, and what might happen in the future. If you combine your genotype with your clinical exam, I really do believe we can start to offer some prognostication for our families, to say, ‘this is the degree of overgrowth we may see over time; these are the complications I predict that you might have.’ ”
Even the vascular stain can give you a clue. “If it’s light and lacey, you probably don’t have a lot of cell cycle activation,” Dr. Drolet said. “If it’s dark and there’s blebs and you’ve got some bleeding at a young age, you’ve got a highly activated mutation, and there’s everything in between.”
Dr. Drolet disclosed that she is a consultant for Venthera and Novartis and is a board member for the Isthmus Project. She also holds intellectual property rights in and is a patent holder for Peds Derm Development Group. Dr. Drolet has also received funding from the Spirit Foundation, Kayleigh’s Crew Endowment, the SPD, PeDRA, and the National Institutes of Health.
according to Beth Drolet, MD.
“We now know that 75%-80% of vascular malformations have gene mutations that make the cells either live longer, grow faster, or make them bigger in size,” Dr. Drolet, professor and chair of dermatology at the University of Wisconsin–Madison, said during the Society for Pediatric Dermatology pre-AAD meeting. “The basic binary premise of the current ISSVA [International Society for the Study of Vascular Anomalies] classification dividing vascular anomalies into tumors and malformations is wrong; the biology is not that straightforward. It may be helpful to differentiate between an infantile hemangioma and a capillary malformation during infancy as the hemangioma will grow in the next month, but we now know that patients with capillary malformations also have significant overgrowth of their tissue. We’ve all seen that; it just takes years, not months for us to notice it.”
The change in thinking about the root causes of vascular anomalies, she noted, stems from scientific advances in the understanding of embryonic mosaicism, DNA variation that happens after the zygote is formed, but before birth. “We know that each cell in a zygote will undergo 40 cell divisions before a baby is born,” she said. “Those cell divisions are not as neat as we thought they were. That cell and DNA duplication is actually quite messy, so there are mutations that happen purely because of embryonic cell division.”
Everyone is born with 120 somatic mutations per cell, she continued, “so we have multiple genomes in one human. Not all of those mutations are going to cause disease. Not all of those are going to be functional. About 10% of those mutations will actually be in a coding region of the gene and have the potential to change the function of the protein. If it changes the function of the protein so that the cell can’t survive, that cell dies off, but it gives the cell an advantage. It grows a little bit faster, let’s say. That cell survives, divides, producing a line of cells that can cause disease.”
In 2011, Dr. Drolet and colleagues from the Hemangioma Investigator Group and the Pediatric Dermatology Research Alliance (PeDRA) launched a multisite collaborative group to investigate the role of mosaic genetics in patients with vascular anomalies and discrepancy of growth. To date, 365 patients are enrolled, and the researchers have sequenced 97 of 165 affected tissue samples collected. “What’s nice about the registry is that we enrolled a wide spectrum of diseases: very mild diseases that might be treated by dermatologists to complex, syndromic diseases that might end up in an interdisciplinary vascular anomalies clinic,” she said.
For gene sequencing, the researchers drew from solid tumor biology and used next-generation sequencing with semi-target hybrid capture, “so we’re only looking at a subset of genes,” she said. “Right now, the chip we’re using has 180 cancer-related genes. It sequences the entire exome of the gene with a high depth of coverage, usually over 1,000 X. We use a specific pipeline that can detect very low allele frequency mutation: down to 1%, and robust criteria to determine variant pathogenicity.”
In 75% of tissue samples so far, the researchers have found a gene mutation in one of 13 genes: AKT1, AKT3, BRAF, GNA11, GNAQ, KRAS, MAP2K1, NRAS, PIK3CA, PIK3R1, PTPN11, RASA1, and TEK. According to Dr. Drolet, the common thread in these 13 genes is that they are implicated in cancer and have direct control over the cell cycle. “They’re intracellular proteins that control the cell cycle,” she explained. “These are proteins that are in the cell but interact with transmembrane proteins that receive extracellular messengers of cell growth”.
Understanding and recognizing genetic conditions is complicated, she said, because it involves determining which gene is altered, where in the DNA the gene is altered, how the gene variation will influence the function of the protein, and what tissue expresses that gene. “Then you get your phenotype,” Dr. Drolet said. “If you add mosaicism onto that, you have several additional variables. You need to know: When in embryogenesis did the mutation occur? What region of the body is affected? What cell lineage is affected? That predicts what phenotype you’re going to have.”
While molecular classification efforts continue to be refined, Dr. Drolet incorporates genotyping at every opportunity, like when she counsels parents of a baby born with a vascular stain on its face. “What can we tell them about what else might be wrong? What can we tell them about how this will change over time? What can we tell them about how we can treat it? I think genotyping absolutely helps to clarify that for me,” she said. “I can’t use that alone, but it gives me another piece of evidence to help do a better job in predicting when I need to screen, what I need to screen for, and what might happen in the future. If you combine your genotype with your clinical exam, I really do believe we can start to offer some prognostication for our families, to say, ‘this is the degree of overgrowth we may see over time; these are the complications I predict that you might have.’ ”
Even the vascular stain can give you a clue. “If it’s light and lacey, you probably don’t have a lot of cell cycle activation,” Dr. Drolet said. “If it’s dark and there’s blebs and you’ve got some bleeding at a young age, you’ve got a highly activated mutation, and there’s everything in between.”
Dr. Drolet disclosed that she is a consultant for Venthera and Novartis and is a board member for the Isthmus Project. She also holds intellectual property rights in and is a patent holder for Peds Derm Development Group. Dr. Drolet has also received funding from the Spirit Foundation, Kayleigh’s Crew Endowment, the SPD, PeDRA, and the National Institutes of Health.
according to Beth Drolet, MD.
“We now know that 75%-80% of vascular malformations have gene mutations that make the cells either live longer, grow faster, or make them bigger in size,” Dr. Drolet, professor and chair of dermatology at the University of Wisconsin–Madison, said during the Society for Pediatric Dermatology pre-AAD meeting. “The basic binary premise of the current ISSVA [International Society for the Study of Vascular Anomalies] classification dividing vascular anomalies into tumors and malformations is wrong; the biology is not that straightforward. It may be helpful to differentiate between an infantile hemangioma and a capillary malformation during infancy as the hemangioma will grow in the next month, but we now know that patients with capillary malformations also have significant overgrowth of their tissue. We’ve all seen that; it just takes years, not months for us to notice it.”
The change in thinking about the root causes of vascular anomalies, she noted, stems from scientific advances in the understanding of embryonic mosaicism, DNA variation that happens after the zygote is formed, but before birth. “We know that each cell in a zygote will undergo 40 cell divisions before a baby is born,” she said. “Those cell divisions are not as neat as we thought they were. That cell and DNA duplication is actually quite messy, so there are mutations that happen purely because of embryonic cell division.”
Everyone is born with 120 somatic mutations per cell, she continued, “so we have multiple genomes in one human. Not all of those mutations are going to cause disease. Not all of those are going to be functional. About 10% of those mutations will actually be in a coding region of the gene and have the potential to change the function of the protein. If it changes the function of the protein so that the cell can’t survive, that cell dies off, but it gives the cell an advantage. It grows a little bit faster, let’s say. That cell survives, divides, producing a line of cells that can cause disease.”
In 2011, Dr. Drolet and colleagues from the Hemangioma Investigator Group and the Pediatric Dermatology Research Alliance (PeDRA) launched a multisite collaborative group to investigate the role of mosaic genetics in patients with vascular anomalies and discrepancy of growth. To date, 365 patients are enrolled, and the researchers have sequenced 97 of 165 affected tissue samples collected. “What’s nice about the registry is that we enrolled a wide spectrum of diseases: very mild diseases that might be treated by dermatologists to complex, syndromic diseases that might end up in an interdisciplinary vascular anomalies clinic,” she said.
For gene sequencing, the researchers drew from solid tumor biology and used next-generation sequencing with semi-target hybrid capture, “so we’re only looking at a subset of genes,” she said. “Right now, the chip we’re using has 180 cancer-related genes. It sequences the entire exome of the gene with a high depth of coverage, usually over 1,000 X. We use a specific pipeline that can detect very low allele frequency mutation: down to 1%, and robust criteria to determine variant pathogenicity.”
In 75% of tissue samples so far, the researchers have found a gene mutation in one of 13 genes: AKT1, AKT3, BRAF, GNA11, GNAQ, KRAS, MAP2K1, NRAS, PIK3CA, PIK3R1, PTPN11, RASA1, and TEK. According to Dr. Drolet, the common thread in these 13 genes is that they are implicated in cancer and have direct control over the cell cycle. “They’re intracellular proteins that control the cell cycle,” she explained. “These are proteins that are in the cell but interact with transmembrane proteins that receive extracellular messengers of cell growth”.
Understanding and recognizing genetic conditions is complicated, she said, because it involves determining which gene is altered, where in the DNA the gene is altered, how the gene variation will influence the function of the protein, and what tissue expresses that gene. “Then you get your phenotype,” Dr. Drolet said. “If you add mosaicism onto that, you have several additional variables. You need to know: When in embryogenesis did the mutation occur? What region of the body is affected? What cell lineage is affected? That predicts what phenotype you’re going to have.”
While molecular classification efforts continue to be refined, Dr. Drolet incorporates genotyping at every opportunity, like when she counsels parents of a baby born with a vascular stain on its face. “What can we tell them about what else might be wrong? What can we tell them about how this will change over time? What can we tell them about how we can treat it? I think genotyping absolutely helps to clarify that for me,” she said. “I can’t use that alone, but it gives me another piece of evidence to help do a better job in predicting when I need to screen, what I need to screen for, and what might happen in the future. If you combine your genotype with your clinical exam, I really do believe we can start to offer some prognostication for our families, to say, ‘this is the degree of overgrowth we may see over time; these are the complications I predict that you might have.’ ”
Even the vascular stain can give you a clue. “If it’s light and lacey, you probably don’t have a lot of cell cycle activation,” Dr. Drolet said. “If it’s dark and there’s blebs and you’ve got some bleeding at a young age, you’ve got a highly activated mutation, and there’s everything in between.”
Dr. Drolet disclosed that she is a consultant for Venthera and Novartis and is a board member for the Isthmus Project. She also holds intellectual property rights in and is a patent holder for Peds Derm Development Group. Dr. Drolet has also received funding from the Spirit Foundation, Kayleigh’s Crew Endowment, the SPD, PeDRA, and the National Institutes of Health.
FROM THE SPD PRE-AAD MEETING
Numerous large nodules on scalp
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
A 31-year-old Hispanic man presented for evaluation of numerous disfiguring growths on his scalp. They first appeared when he was 19 years old. A review of his family history revealed that his father had 2 “cysts” on his body.
The patient had 10 nodules on his scalp and upper back (Figures 1A and 1B). The ones on his scalp lacked puncta and appeared in a “turban tumor” configuration. The lesions were pink, smooth, and semisoft, and ranged in size from 1 to 6 cm.
Six years earlier, the patient had been seen for evaluation of 20 protuberant nodules. At the time, he had been referred to plastic surgery, where 15 lesions were excised. No other treatment was reported by the patient during the 6-year gap between exams.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pilar cysts
Pilar cysts (PC), also known as trichilemma cysts, wen, or isthmus-catagen cysts, are benign cysts that manifest as smooth, firm, well-circumscribed, pink nodules. PCs originate from the follicular isthmus of the hair’s external root sheath1 and are found in 5% to 10% of the US population.2 Possible sites of appearance include the face, neck, trunk, and extremities, although 90% of PCs develop on the scalp.1 They tend to have an autosomal dominant pattern of inheritance with linkages to the short arm of chromosome 3.3 PCs can occasionally become inflamed following infection or trauma.
Characteristic histology of PCs demonstrates semisolid, keratin-filled, subepidermal cysts lined by stratified epithelium without a granular layer (trichilemmal keratinization). Lesions excised from this patient’s scalp showed 2 subtypes of PCs: nonproliferating (FIGURE 2A) and proliferating (FIGURE 2B). Subtypes appear similar on exam but can be differentiated on histology.
With gradual growth, proliferating PCs can reach up to 25 cm in diameter.1 Rapid growth, size > 5 cm, infiltration, or a non-scalp location may indicate malignancy.4
Differential diagnosis includes lipomas
The differential diagnosis for a lesion such as this includes epidermal inclusion cysts, dermoid cysts, and lipomas. Epidermal inclusion cysts have a punctum, whereas PCs do not. Dermoid cysts are single congenital lesions that manifest much earlier than PCs. Lipomas are easily movable rubbery bulges that appear more frequently in lipid-dense areas of the body.
For this patient, the striking turban tumor–like presentation, with numerous large cysts on the scalp, initially inspired a differential diagnosis including several genetic tumor syndromes. However, unlike the association between Gardner syndrome and numerous epidermoid cysts or Brooke-Spiegler syndrome and spiradenomas, no syndromes have been linked to numerous trichilemmal cysts.
Continue to: Excision is effective
Excision is effective
Excision is the treatment of choice for both proliferating and nonproliferating PCs.5 The local recurrence rate of proliferating PCs is 3.7% with a rare likelihood of transformation to trichilemmal carcinoma.6
Our patient continues to be followed in clinic for monitoring and periodic excision of bothersome cysts.
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50
1. Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. http://doi.org/10.4103/1947-2714.107532
2. Ibrahim AE, Barikian A, Janom H, et al. Numerous recurrent trichilemmal cysts of the scalp: differential diagnosis and surgical management. J Craniofac Surg. 2012;23:e164-168. http://doi.org/10.1097/SCS.0b013e31824cdbd2
3. Adya KA, Inamadar AC, Palit A. Multiple firm mobile swellings over the scalp. Int J Trichology. 2012;4:98-99. http://doi.org/10.4103/0974-7753.96906
4. Folpe AL, Reisenauer AK, Mentzel T, et al. Proliferating trichilemmal tumors: clinicopathologic evaluation is a guide to biologic behavior. J Cutan Pathol. 2003;30:492-498. http://doi.org/10.1034/j.1600-0560.2003.00041.x
5. Leppard BJ, Sanderson KV. The natural history of trichilemmal cysts. Br J Dermatol. 1976;94:379-390. http://doi.org/10.1111/j.1365-2133.1976.tb06115.x
6. Kim UG, Kook DB, Kim TH, et al. Trichilemmal carcinoma from proliferating trichilemmal cyst on the posterior neck. Arch Craniofac Surg. 2017;18:50-53. http://doi.org/10.7181/acfs.2017.18.1.50