User login
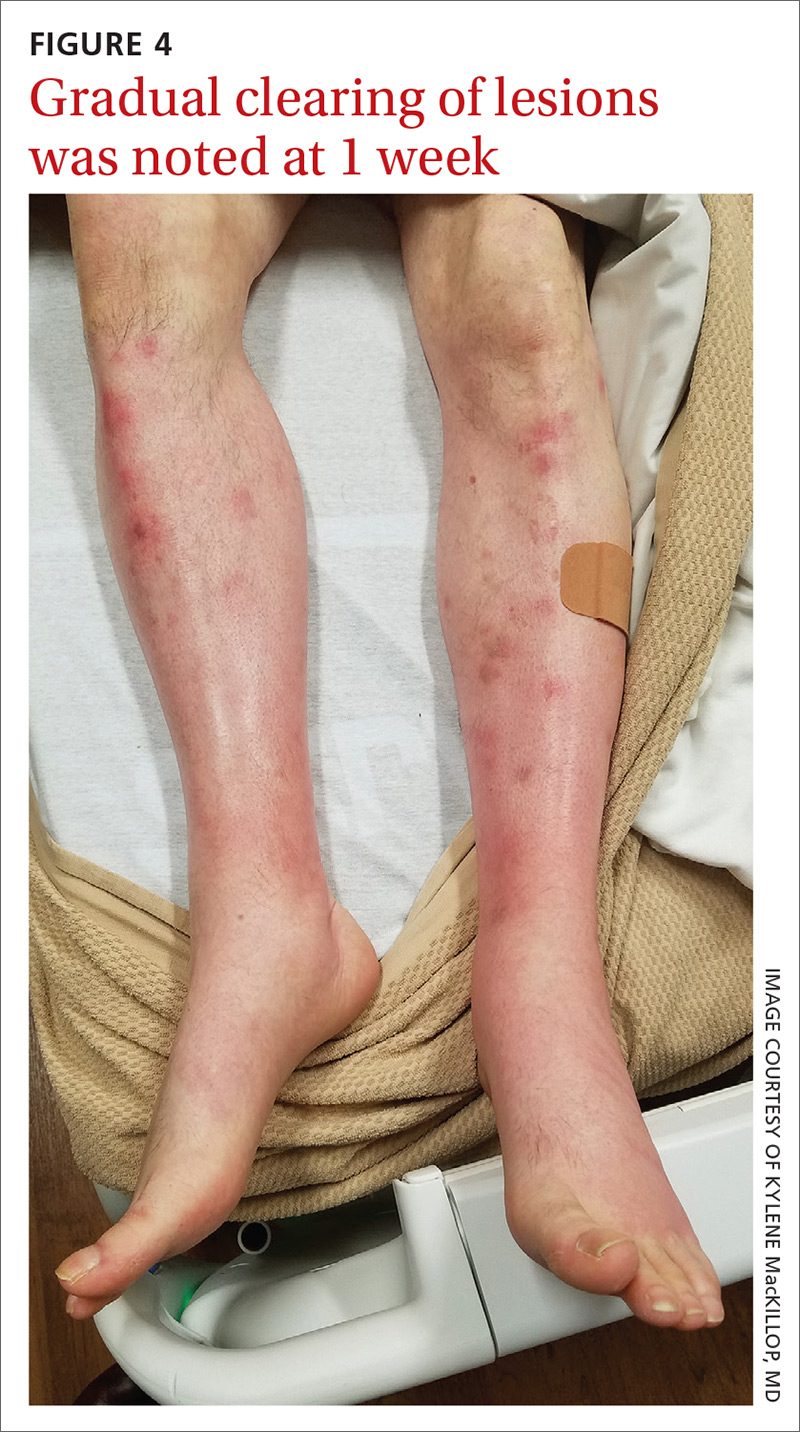
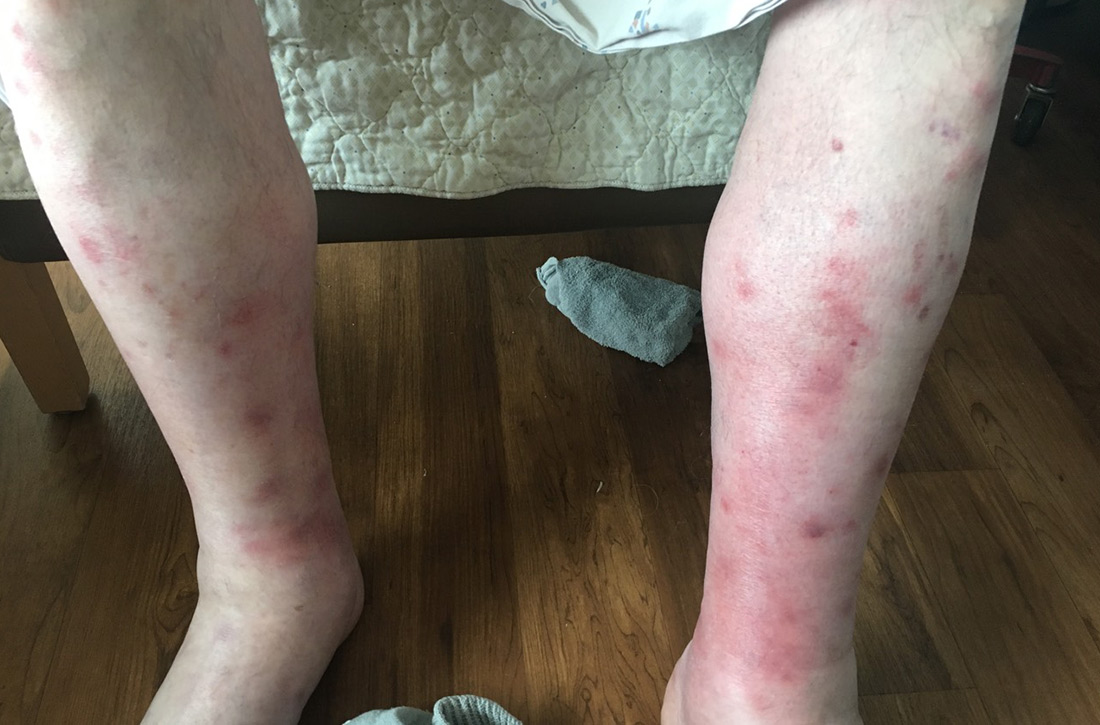
A case of neutrophilic eccrine hidradenitis attributed to HIV treatment
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
Expert discusses her approach to using systemic agents in children and adolescents with severe skin disease
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
When to suspect calciphylaxis and what to do about it
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
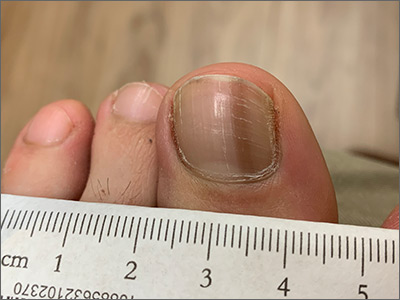
Dark toenail line
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Dupilumab hits the mark for severe AD in younger children
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
FROM REVOLUTIONIZING AD 2020
D.C.-area blacks face increased risk of mortality from SJS/TEN
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
Is There an Association Between Hidradenitis Suppurativa and Fibromyalgia?
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
Practice Point
- Although fibromyalgia does not occur more frequently in hidradenitis suppurativa (HS) patients, it is important to recognize that HS patients can have comorbidities that should be addressed when possible to improve overall quality of life.
Belimumab may improve skin in scleroderma
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
Red painful nodules in a hospitalized patient
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A decade of telemedicine policy has advanced in just 2 weeks
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.