User login
Analysis supports CAC for personalizing statin use
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
FROM JAMA CARDIOLOGY
Prescribe an SGLT2 inhibitor for heart failure in the absence of diabetes?
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
PRACTICE CHANGER
Prescribe dapagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, 10 mg/d in addition to standard therapies for adult patients with heart failure (HF) with a reduced ejection fraction (≤ 40%) and New York Heart Association (NYHA) class II or greater, regardless of type 2 diabetes history, due to improved heart failure and cardiovascular outcomes.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.1
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008.
Transitioning patients with developmental disabilities to adult care
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.
Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
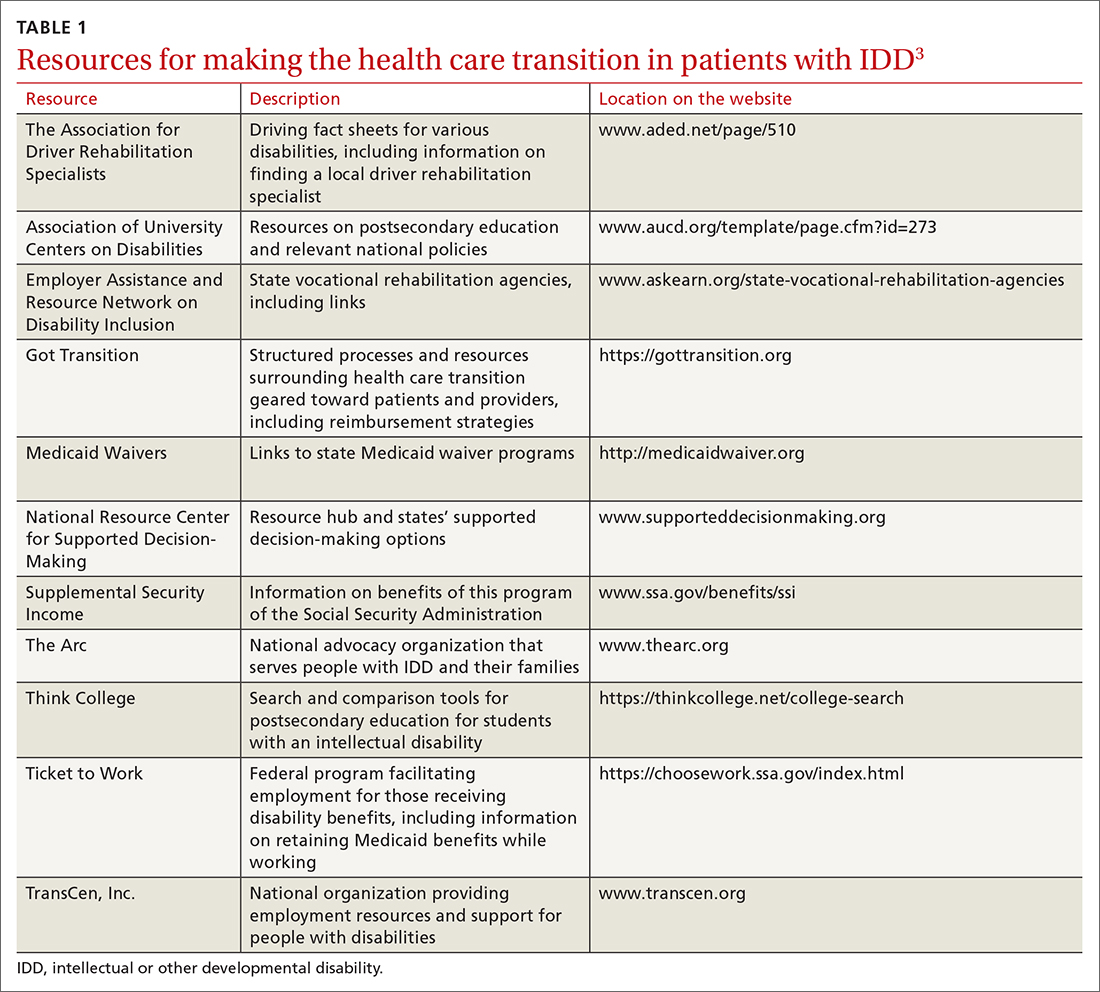
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
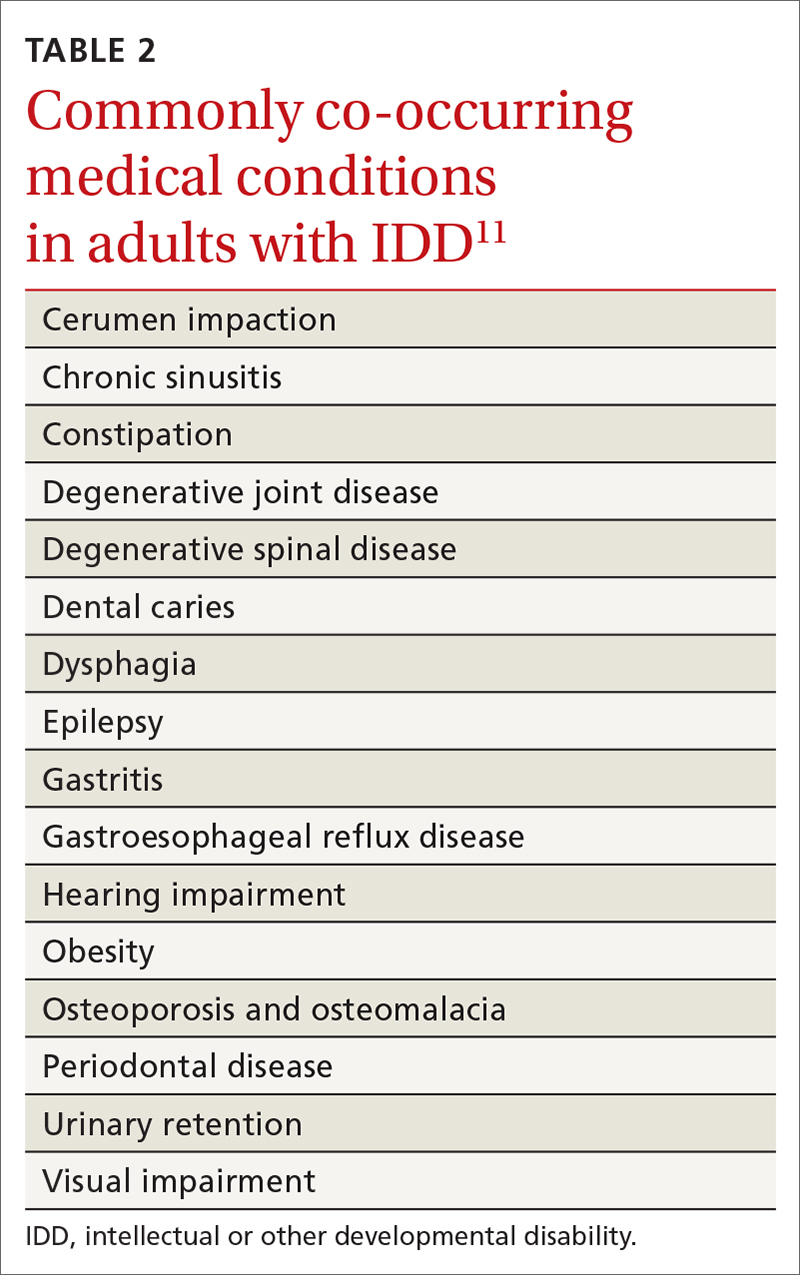
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
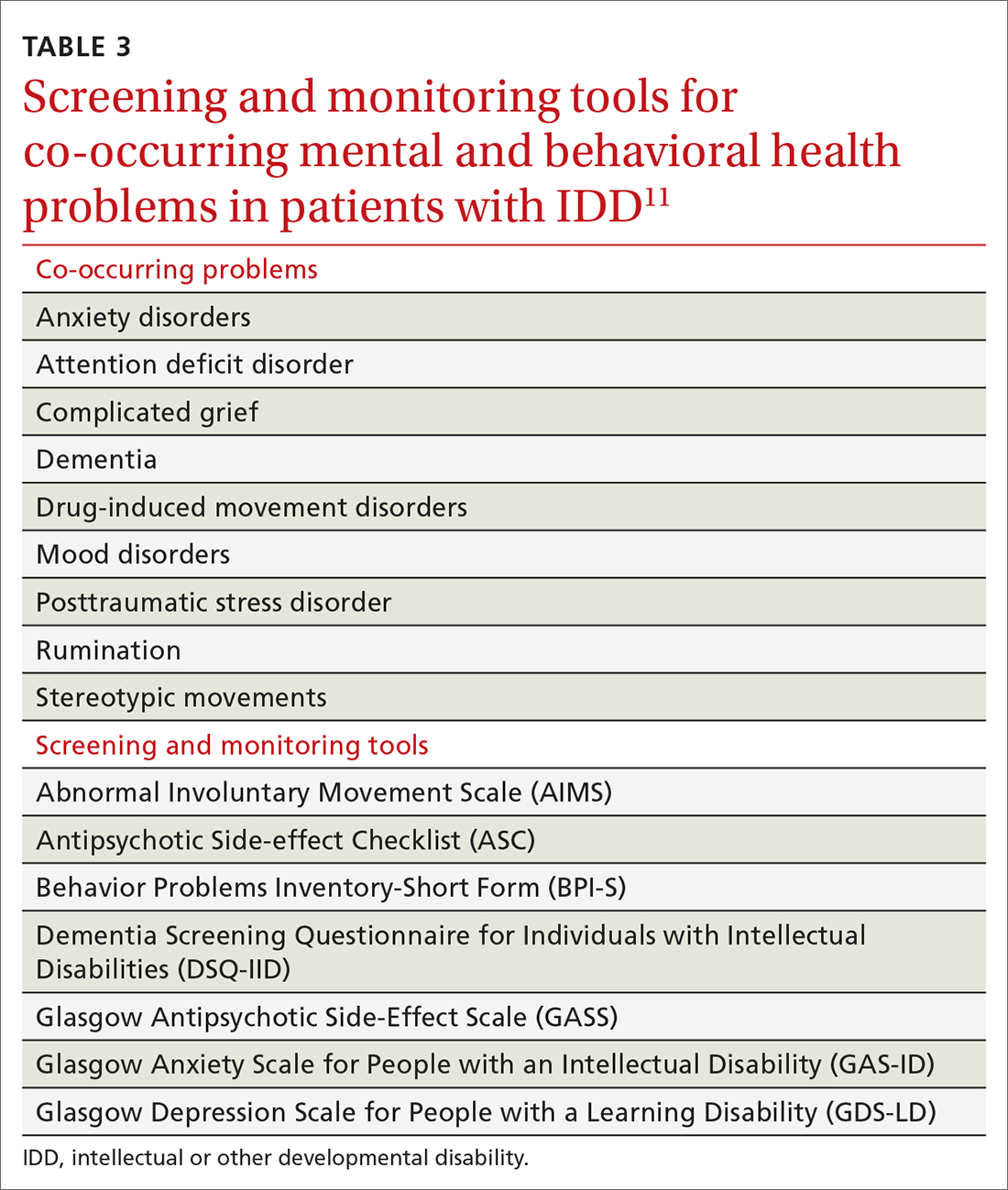
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; catyle@ccf.org
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.
Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; catyle@ccf.org
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.
Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; catyle@ccf.org
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
PRACTICE RECOMMENDATIONS
› Provide young people who have an intellectual or other developmental disability (IDD) with a defined, explicit process for making the transition into the adult health care system. A
› Conduct an annual comprehensive, systematic health assessment for patients who have IDD to improve detection of serious conditions and sensory impairments. A
› Encourage young people and adults with IDD to participate in regular physical activity to reduce psychosocial stressors and counteract metabolic syndromes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cycling linked to longer life in people with type 2 diabetes
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
FROM JAMA INTERNAL MEDICINE
Statin safety, low muscle pain risk upheld in ‘reassuring’ study
Statins are associated with a low risk of adverse events in patients without a history of heart disease, but the potential harms are small and should not deter their use in primary prevention, a new systematic review and meta-analysis concludes.
As reported July 14 in BMJ, the analysis showed a slightly increased risk for self-reported muscle symptoms after treatment with statins but no increased risk for clinically confirmed muscle disorders. Statins were associated with liver dysfunction, renal insufficiency, and eye conditions, but not with diabetes.
“These risks are very, very small and, in fact, the adverse events we’re talking about are potentially quite mild, so if you weigh them against the benefits in terms of reduction in major cardiovascular events, the benefit-to-harm ratio is very much in favor of prescribing treatment for almost all patients,” senior author James P. Sheppard, MD, University of Oxford (England), said in an interview.
Although there’s an abundance of data showing that statins prevent recurrent cardiovascular events, their use is controversial in primary prevention, owing partly to the lower risk for cardiovascular disease (CVD). The absolute benefits of statins are smaller in primary prevention than in those with existing CVD, and the benefit-to-harm balance of treatment might be less favorable, the authors note.
A 2019 review suggested that the use of statins in primary prevention may be an example of “low-value care, having little benefit and potential to cause harm,” and a meta-analysis with more than 94,000 trial participants showed statins significantly increased risks for myopathy, renal dysfunction, and hepatic dysfunction.
Nevertheless, clinical guidelines have recommended wider use of statins for primary prevention, calling on physicians to weigh the benefits and harms.
“This is a reasonable expectation but, at present, the data on the harms of treatment are much less well understood in comparison to the benefits and there’s quite a lot of debate about the extent to which statins are associated with adverse events,” Dr. Sheppard said. “So we wanted to look at this in a bit more detail.”
The investigators analyzed results from 62 randomized controlled trials with 120,456 participants (mean age, 61; 40% women) followed for a mean of 3.9 years. All but two studies enrolled participants with hyperlipidemia or dyslipidemia. Common comorbidities were diabetes (11 studies), asymptomatic atherosclerosis (nine studies), and hypertension (four studies).
Statins increased risks for self-reported muscle symptoms in 21 trials (odds ratio [OR], 1.06), liver dysfunction in 21 trials (OR, 1.33), renal insufficiency in eight trials (OR, 1.14), and cataracts or other eye-related conditions in six trials (OR, 1.23).
At the same time, statins decreased risks for myocardial infarction in 22 trials (OR, 0.72), stroke in 17 trials (OR, 0.80), and CVD death in 22 trials (OR, 0.83).
These risks translated into 15 more events of muscle symptoms, 8 more liver events, 12 more kidney events, and 14 more eye conditions per 10,000 patients treated for a year.
Statins were estimated to prevent 19 myocardial infarctions, 9 strokes, and 8 CVD deaths per 10,000 patients treated for a year.
Dr. Sheppard suggested that the inclusion of previously omitted trials and the decision to classify muscle problems as self-reported symptoms or clinically defined muscle disorders based on changes in creatine kinase might explain why they found the association with statins, whereas most systematic reviews have not.
“Some people would argue that these side effects are so small and so negligible that we shouldn’t talk about them, but the problem with doing that is if you’ve got a patient who has a preconceived idea that statins are harmful,” he added. “So having some empirical data where you can actually say: ‘Look, just 15 people out of 10,000 patients who’ve been treated for a year might experience one of those self-reported muscle symptoms,’ hopefully, will be helpful for physicians having discussions in practice.”
The analysis is “another data point indicating the overall safety and net benefit of statins for patients, even in primary prevention,” Donald M. Lloyd-Jones, MD, ScM, chair of preventive medicine, Northwestern University, Chicago, said in an interview.
He noted that the renal insufficiency findings are difficult to interpret, given that the endpoint was defined as “any decline in renal function,” but that most will have been clinically unimportant. In general, most studies didn’t systematically look to ascertain some of adverse events but relied on participant or physician report. “Nonetheless, there is little reason to suspect bias in the collection of these data among the blinded studies.
“Although not definitive, given the study design and inclusion of very different types of studies and variable ascertainment of adverse events, the findings are reassuring that the risks of adverse events were small, and the potential adverse events identified were not very clinically significant and clearly outweighed by the important beneficial reductions in major cardiovascular events,” said Dr. Lloyd-Jones.
“This study is yet another reminder of the safety of statins,” Ann Marie Navar, MD, PhD, a specialist in preventive cardiology at UT Southwestern Medical School, Dallas, said in an email.
“I’m pleased to have a comprehensive study like this – a well-done, systematic review of randomized trials – to help combat the vast amounts of misinformation about statins circulating on the Internet.”
Dr. Lloyd-Jones also acknowledged the need to address misinformation, pointing out that the loss of contact with physicians and the adverse effects of the pandemic on weight and other health behaviors mean that many patients have had worsening of their cardiovascular risk factors.
“We must continue to help patients and the public understand that statins are beneficial for patients at sufficient risk for cardiovascular disease because of elevated cholesterol or their total burden of risk factors,” Dr. Lloyd-Jones said. “We must also be upfront about the risks of potential side effects, which are uncommon and almost always very easily managed with washout and dose reduction or switching to a different drug in the same class.”
Analyses by type of statin, however, showed few significant differences in adverse events. Rosuvastatin was associated with increased risks for self-reported muscle symptoms, renal insufficiency, diabetes, and eye conditions, whereas atorvastatin and lovastatin increased the risk for liver dysfunction.
In dose-response meta-analyses, a possible modest dose-response relationship was detected only for the effect of atorvastatin on liver dysfunction.
The current data do not support tailoring the type of statin or dosage to reduce adverse events, the authors say, although routine monitoring of liver function during treatment is probably warranted in primary prevention, given the increased risk for liver dysfunction.
To help improve adherence to statins, the investigators said, additional studies are needed to identify patient characteristics crucial to the small risks of adverse events.
Limitations of the research, they said, are that many of the analyses were underpowered to detect between-group differences, many trials had short periods of follow-up, and some trials excluded vulnerable people more likely to have adverse events, such as those with high serum creatinine.
The study was funded by a British Heart Foundation PhD Scholarship held by first author Ting Cai. Dr. Sheppard reports receiving funding from a Wellcome Trust/Royal Society Sir Henry Dale Fellowship. Disclosures for other authors are listed in the paper. Dr. Lloyd-Jones and Dr. Navar report having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Statins are associated with a low risk of adverse events in patients without a history of heart disease, but the potential harms are small and should not deter their use in primary prevention, a new systematic review and meta-analysis concludes.
As reported July 14 in BMJ, the analysis showed a slightly increased risk for self-reported muscle symptoms after treatment with statins but no increased risk for clinically confirmed muscle disorders. Statins were associated with liver dysfunction, renal insufficiency, and eye conditions, but not with diabetes.
“These risks are very, very small and, in fact, the adverse events we’re talking about are potentially quite mild, so if you weigh them against the benefits in terms of reduction in major cardiovascular events, the benefit-to-harm ratio is very much in favor of prescribing treatment for almost all patients,” senior author James P. Sheppard, MD, University of Oxford (England), said in an interview.
Although there’s an abundance of data showing that statins prevent recurrent cardiovascular events, their use is controversial in primary prevention, owing partly to the lower risk for cardiovascular disease (CVD). The absolute benefits of statins are smaller in primary prevention than in those with existing CVD, and the benefit-to-harm balance of treatment might be less favorable, the authors note.
A 2019 review suggested that the use of statins in primary prevention may be an example of “low-value care, having little benefit and potential to cause harm,” and a meta-analysis with more than 94,000 trial participants showed statins significantly increased risks for myopathy, renal dysfunction, and hepatic dysfunction.
Nevertheless, clinical guidelines have recommended wider use of statins for primary prevention, calling on physicians to weigh the benefits and harms.
“This is a reasonable expectation but, at present, the data on the harms of treatment are much less well understood in comparison to the benefits and there’s quite a lot of debate about the extent to which statins are associated with adverse events,” Dr. Sheppard said. “So we wanted to look at this in a bit more detail.”
The investigators analyzed results from 62 randomized controlled trials with 120,456 participants (mean age, 61; 40% women) followed for a mean of 3.9 years. All but two studies enrolled participants with hyperlipidemia or dyslipidemia. Common comorbidities were diabetes (11 studies), asymptomatic atherosclerosis (nine studies), and hypertension (four studies).
Statins increased risks for self-reported muscle symptoms in 21 trials (odds ratio [OR], 1.06), liver dysfunction in 21 trials (OR, 1.33), renal insufficiency in eight trials (OR, 1.14), and cataracts or other eye-related conditions in six trials (OR, 1.23).
At the same time, statins decreased risks for myocardial infarction in 22 trials (OR, 0.72), stroke in 17 trials (OR, 0.80), and CVD death in 22 trials (OR, 0.83).
These risks translated into 15 more events of muscle symptoms, 8 more liver events, 12 more kidney events, and 14 more eye conditions per 10,000 patients treated for a year.
Statins were estimated to prevent 19 myocardial infarctions, 9 strokes, and 8 CVD deaths per 10,000 patients treated for a year.
Dr. Sheppard suggested that the inclusion of previously omitted trials and the decision to classify muscle problems as self-reported symptoms or clinically defined muscle disorders based on changes in creatine kinase might explain why they found the association with statins, whereas most systematic reviews have not.
“Some people would argue that these side effects are so small and so negligible that we shouldn’t talk about them, but the problem with doing that is if you’ve got a patient who has a preconceived idea that statins are harmful,” he added. “So having some empirical data where you can actually say: ‘Look, just 15 people out of 10,000 patients who’ve been treated for a year might experience one of those self-reported muscle symptoms,’ hopefully, will be helpful for physicians having discussions in practice.”
The analysis is “another data point indicating the overall safety and net benefit of statins for patients, even in primary prevention,” Donald M. Lloyd-Jones, MD, ScM, chair of preventive medicine, Northwestern University, Chicago, said in an interview.
He noted that the renal insufficiency findings are difficult to interpret, given that the endpoint was defined as “any decline in renal function,” but that most will have been clinically unimportant. In general, most studies didn’t systematically look to ascertain some of adverse events but relied on participant or physician report. “Nonetheless, there is little reason to suspect bias in the collection of these data among the blinded studies.
“Although not definitive, given the study design and inclusion of very different types of studies and variable ascertainment of adverse events, the findings are reassuring that the risks of adverse events were small, and the potential adverse events identified were not very clinically significant and clearly outweighed by the important beneficial reductions in major cardiovascular events,” said Dr. Lloyd-Jones.
“This study is yet another reminder of the safety of statins,” Ann Marie Navar, MD, PhD, a specialist in preventive cardiology at UT Southwestern Medical School, Dallas, said in an email.
“I’m pleased to have a comprehensive study like this – a well-done, systematic review of randomized trials – to help combat the vast amounts of misinformation about statins circulating on the Internet.”
Dr. Lloyd-Jones also acknowledged the need to address misinformation, pointing out that the loss of contact with physicians and the adverse effects of the pandemic on weight and other health behaviors mean that many patients have had worsening of their cardiovascular risk factors.
“We must continue to help patients and the public understand that statins are beneficial for patients at sufficient risk for cardiovascular disease because of elevated cholesterol or their total burden of risk factors,” Dr. Lloyd-Jones said. “We must also be upfront about the risks of potential side effects, which are uncommon and almost always very easily managed with washout and dose reduction or switching to a different drug in the same class.”
Analyses by type of statin, however, showed few significant differences in adverse events. Rosuvastatin was associated with increased risks for self-reported muscle symptoms, renal insufficiency, diabetes, and eye conditions, whereas atorvastatin and lovastatin increased the risk for liver dysfunction.
In dose-response meta-analyses, a possible modest dose-response relationship was detected only for the effect of atorvastatin on liver dysfunction.
The current data do not support tailoring the type of statin or dosage to reduce adverse events, the authors say, although routine monitoring of liver function during treatment is probably warranted in primary prevention, given the increased risk for liver dysfunction.
To help improve adherence to statins, the investigators said, additional studies are needed to identify patient characteristics crucial to the small risks of adverse events.
Limitations of the research, they said, are that many of the analyses were underpowered to detect between-group differences, many trials had short periods of follow-up, and some trials excluded vulnerable people more likely to have adverse events, such as those with high serum creatinine.
The study was funded by a British Heart Foundation PhD Scholarship held by first author Ting Cai. Dr. Sheppard reports receiving funding from a Wellcome Trust/Royal Society Sir Henry Dale Fellowship. Disclosures for other authors are listed in the paper. Dr. Lloyd-Jones and Dr. Navar report having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Statins are associated with a low risk of adverse events in patients without a history of heart disease, but the potential harms are small and should not deter their use in primary prevention, a new systematic review and meta-analysis concludes.
As reported July 14 in BMJ, the analysis showed a slightly increased risk for self-reported muscle symptoms after treatment with statins but no increased risk for clinically confirmed muscle disorders. Statins were associated with liver dysfunction, renal insufficiency, and eye conditions, but not with diabetes.
“These risks are very, very small and, in fact, the adverse events we’re talking about are potentially quite mild, so if you weigh them against the benefits in terms of reduction in major cardiovascular events, the benefit-to-harm ratio is very much in favor of prescribing treatment for almost all patients,” senior author James P. Sheppard, MD, University of Oxford (England), said in an interview.
Although there’s an abundance of data showing that statins prevent recurrent cardiovascular events, their use is controversial in primary prevention, owing partly to the lower risk for cardiovascular disease (CVD). The absolute benefits of statins are smaller in primary prevention than in those with existing CVD, and the benefit-to-harm balance of treatment might be less favorable, the authors note.
A 2019 review suggested that the use of statins in primary prevention may be an example of “low-value care, having little benefit and potential to cause harm,” and a meta-analysis with more than 94,000 trial participants showed statins significantly increased risks for myopathy, renal dysfunction, and hepatic dysfunction.
Nevertheless, clinical guidelines have recommended wider use of statins for primary prevention, calling on physicians to weigh the benefits and harms.
“This is a reasonable expectation but, at present, the data on the harms of treatment are much less well understood in comparison to the benefits and there’s quite a lot of debate about the extent to which statins are associated with adverse events,” Dr. Sheppard said. “So we wanted to look at this in a bit more detail.”
The investigators analyzed results from 62 randomized controlled trials with 120,456 participants (mean age, 61; 40% women) followed for a mean of 3.9 years. All but two studies enrolled participants with hyperlipidemia or dyslipidemia. Common comorbidities were diabetes (11 studies), asymptomatic atherosclerosis (nine studies), and hypertension (four studies).
Statins increased risks for self-reported muscle symptoms in 21 trials (odds ratio [OR], 1.06), liver dysfunction in 21 trials (OR, 1.33), renal insufficiency in eight trials (OR, 1.14), and cataracts or other eye-related conditions in six trials (OR, 1.23).
At the same time, statins decreased risks for myocardial infarction in 22 trials (OR, 0.72), stroke in 17 trials (OR, 0.80), and CVD death in 22 trials (OR, 0.83).
These risks translated into 15 more events of muscle symptoms, 8 more liver events, 12 more kidney events, and 14 more eye conditions per 10,000 patients treated for a year.
Statins were estimated to prevent 19 myocardial infarctions, 9 strokes, and 8 CVD deaths per 10,000 patients treated for a year.
Dr. Sheppard suggested that the inclusion of previously omitted trials and the decision to classify muscle problems as self-reported symptoms or clinically defined muscle disorders based on changes in creatine kinase might explain why they found the association with statins, whereas most systematic reviews have not.
“Some people would argue that these side effects are so small and so negligible that we shouldn’t talk about them, but the problem with doing that is if you’ve got a patient who has a preconceived idea that statins are harmful,” he added. “So having some empirical data where you can actually say: ‘Look, just 15 people out of 10,000 patients who’ve been treated for a year might experience one of those self-reported muscle symptoms,’ hopefully, will be helpful for physicians having discussions in practice.”
The analysis is “another data point indicating the overall safety and net benefit of statins for patients, even in primary prevention,” Donald M. Lloyd-Jones, MD, ScM, chair of preventive medicine, Northwestern University, Chicago, said in an interview.
He noted that the renal insufficiency findings are difficult to interpret, given that the endpoint was defined as “any decline in renal function,” but that most will have been clinically unimportant. In general, most studies didn’t systematically look to ascertain some of adverse events but relied on participant or physician report. “Nonetheless, there is little reason to suspect bias in the collection of these data among the blinded studies.
“Although not definitive, given the study design and inclusion of very different types of studies and variable ascertainment of adverse events, the findings are reassuring that the risks of adverse events were small, and the potential adverse events identified were not very clinically significant and clearly outweighed by the important beneficial reductions in major cardiovascular events,” said Dr. Lloyd-Jones.
“This study is yet another reminder of the safety of statins,” Ann Marie Navar, MD, PhD, a specialist in preventive cardiology at UT Southwestern Medical School, Dallas, said in an email.
“I’m pleased to have a comprehensive study like this – a well-done, systematic review of randomized trials – to help combat the vast amounts of misinformation about statins circulating on the Internet.”
Dr. Lloyd-Jones also acknowledged the need to address misinformation, pointing out that the loss of contact with physicians and the adverse effects of the pandemic on weight and other health behaviors mean that many patients have had worsening of their cardiovascular risk factors.
“We must continue to help patients and the public understand that statins are beneficial for patients at sufficient risk for cardiovascular disease because of elevated cholesterol or their total burden of risk factors,” Dr. Lloyd-Jones said. “We must also be upfront about the risks of potential side effects, which are uncommon and almost always very easily managed with washout and dose reduction or switching to a different drug in the same class.”
Analyses by type of statin, however, showed few significant differences in adverse events. Rosuvastatin was associated with increased risks for self-reported muscle symptoms, renal insufficiency, diabetes, and eye conditions, whereas atorvastatin and lovastatin increased the risk for liver dysfunction.
In dose-response meta-analyses, a possible modest dose-response relationship was detected only for the effect of atorvastatin on liver dysfunction.
The current data do not support tailoring the type of statin or dosage to reduce adverse events, the authors say, although routine monitoring of liver function during treatment is probably warranted in primary prevention, given the increased risk for liver dysfunction.
To help improve adherence to statins, the investigators said, additional studies are needed to identify patient characteristics crucial to the small risks of adverse events.
Limitations of the research, they said, are that many of the analyses were underpowered to detect between-group differences, many trials had short periods of follow-up, and some trials excluded vulnerable people more likely to have adverse events, such as those with high serum creatinine.
The study was funded by a British Heart Foundation PhD Scholarship held by first author Ting Cai. Dr. Sheppard reports receiving funding from a Wellcome Trust/Royal Society Sir Henry Dale Fellowship. Disclosures for other authors are listed in the paper. Dr. Lloyd-Jones and Dr. Navar report having no conflicts of interest.
A version of this article first appeared on Medscape.com.
ADA/EASD draft guidance aims to bring adults with type 1 diabetes out of shadows
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
Metformin use may curb BCC risk
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
New agents for youth-onset type 2 diabetes ‘finally in sight’
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
Rising rates of T1D in children: Is COVID to blame?
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
Not so crazy: Pancreas transplants in type 2 diabetes rising
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.